Abstract
Background
Elevated blood pressure (BP) is closely related to stroke and its subtypes. However, different time periods changes in BP may result in differential risk of stroke.
Hypothesis
Short‐term blood pressure changes have a more strong impact on stroke and its subtypes than long‐term blood pressure changes.
Methods
We designed the study on the effects of short‐ (2008‐2010) and long‐term (2004‐2010) BP changes on stroke events (2011‐2017), including 22 842 and 28 456 subjects, respectively. The difference in β coefficients between short‐ and long‐term BP changes on the effects of stroke were examined using the Fisher Z test.
Results
During a median 12.5‐year follow‐up period, 1014 and 1505 strokes occurred in short‐ and long‐term groups. In short‐term group, going from prehypertension to hypertension, the risk of stroke events increased (stroke: hazard ratio [HR] = 1.537 [1.248‐1.894], ischemic stroke: 1.456 [1.134‐1.870] and hemorrhagic stroke: 1.630 [1.099‐2.415]); going from hypertension to prehypertension, the risk of stroke events decreased (stroke:0.757 [0.619‐0.927] and hemorrhagic stroke:0.569 [0.388‐0.835]). Similarly, in long‐term group, going from prehypertension to hypertension, individuals had an increased risk of stroke (1.291, 1.062‐1.569) and hemorrhagic stroke (1.818, 1.261‐2.623); going from hypertension to prehypertension, participants had a decreased risk of stroke (0.825, 0.707‐0.963) and hemorrhagic stroke (0.777, 0.575‐0.949). Furthermore, the effects of BP changes during short‐term period on stroke events were greater than that in long‐term period.
Conclusions
Short‐ and long‐terms BP changes were both associated with the risk of stroke events. Furthermore, short‐term BP changes had a stronger impact than did long‐term changes on risk of stroke events.
Keywords: blood pressure, blood pressure changes, cohort study, stroke
1. INTRODUCTION
Stroke is the leading cause of death in China,1, 2 and high blood pressure (BP) is a major risk factor for stroke.3 Worldwide, approximately 54% of strokes are attributable to hypertension.4 Hence, lowering BP can effectively reduce the incidence of stroke. In the past decade or so, the prevalence of hypertension in rural areas of northeast China has changed considerably,5, 6 and the impact of this rising trend on the incidence, mortality of stroke is worrisome.
In addition, previous traditional studies have been linked to the association of BP values at one point in time with cardiovascular disease (CVD) risk. However, intraindividual BP fluctuates dynamically over time, and measurements at a single point in time may not effectively predict disease risk, to achieve early intervention.7 Gosmanova et al determined that traditional analysis would underestimate the true relationship between elevated BP levels and disease, as a result of a “regression dilution” effect.7 Vascular damage leading to stroke is a complex dynamic process. Dynamic BP changes include more critical information for prediction of stroke risk, having higher accuracy and sensitivity.8 Several lines of evidence that visit‐to‐visit variability (VVV) of BP or trajectory changes in BP are associated with a higher incidence of CVD9, 10, 11, 12 and a higher risk of mortality.13, 14, 15 However, few studies have concentrated on the effects of BP changes on stroke and its subtypes, and most of those studies were conducted either in Western countries7, 10 or focused on the adverse outcomes affected by BP changes during the same time period16, 17 or same multiple time periods.9 There is rare evidence of the effects of short‐ and long‐term BP changes on stroke and its subtypes in the general population, while the effects of long‐ and short‐term BP changes may be different.
We hypothesized that BP changes will affect to stroke and its subtypes according to three aspects: first, baseline BP levels are positively correlated with the risk of stroke for the same degree of BP changes; second, the direction of BP change (increase or decrease) is an important aspect of responding to BP changes; third, the rate of BP changes (high‐increasing or low‐increasing, high‐decreasing or low‐decreasing) have different effects on stroke in the same BP level changes. Therefore, we aimed to analyze and compare the effects of same level BP changes over a short‐ and long‐term period on the risk of stroke and its subtypes.
2. METHODS
2.1. Study population
We carried out a large‐scale epidemiological follow‐up study.18, 19 Briefly, from 2004 to 2006, a multistage, random cluster sampling process was used to select a representative sample aged ≥35 years in rural areas of Fuxin County in Liaoning Province, China. All study participants were invited to return for follow‐up: from January to July 2008 (follow‐up 1); from July to December 2010 (follow‐up 2); and from March to December 2017 (follow‐up 3). The study population inclusion and exclusion process is illustrated in Figure S1. Of the 45 925 participants at baseline, 3883 subjects had missing contact information or refused to attend the follow‐up, and 42 042 (91.5%) participants were eligible to attend the follow‐up at least one time.
The study protocol was approved by the China Medical University Research Ethics Committee, and written informed consent was obtained from all subjects or their caregivers.
2.2. Study design
We have completed a baseline survey and three follow‐up surveys (Figure 1A). To investigate the effects of BP changes over short‐ and long‐term time intervals on stroke events, we collected new cases of stroke during the same time period between the short‐ and long‐term groups. Therefore, the study designs used for analysis of the associations between long‐ (from January 2004 to December 2010) and short‐term (from January 2008 to December 2010) BP changes and stroke events is shown in Figure 1B,C.
Figure 1.
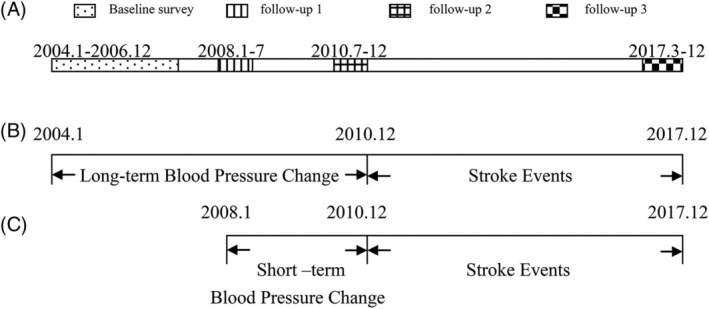
Study designs used for analysis of the associations between short‐ and long‐term blood pressure changes and stroke events. Description of baseline and three follow‐up surveys A. Effects of short‐term B, and long‐term C, blood pressure changes on stroke events
2.3. Data collection and measurements
Using a standardized questionnaire to collect demographic variables (gender, age, and ethnicity), lifestyle factors (current smoking, current drinking status, and physical activity), history of disease (stroke, coronary heart disease [CHD], family history of hypertension, diabetes, or hyperlipidemia), and information on antihypertensive medications.18, 19
When measuring weight and height, subjects did not wear coats and shoes. Body mass index (BMI) was calculated as the weight divided by the height squared (kg/m2). BP measurements were performed using a standard electronic automated sphygmomanometer (HEM‐741C; Omron, Tokyo, Japan). A trained and certified observer used an American Heart Association protocol to perform BP measurements.20 Participants were advised to avoid alcohol consumption, cigarette smoking, drinking coffee/tea, and exercise for at least 30 minutes, and to rest at least 5 minutes before BP measurement. The individual's BP value used for analyses was the average of three BP measurements. According to the JNC7 criteria,21 subjects were categorized as having normotension (SBP < 120 and DBP < 80 mm Hg), prehypertension (120 ≤SBP < 140 mm Hg or 80 ≤ DBP < 90 mm Hg) or hypertension (SBP/DBP ≥ 140/90 mm Hg or receiving antihypertensive treatment). The subject's BP classifications may change from one classification to another or maintain stabilization. All subjects were divided into nine categories according to the BP classifications changes before and after.
2.4. Study outcomes
The present study outcome was stroke events, including ischemic stroke, hemorrhage stroke and uncategorized stroke. Stroke events were confirmed according to the WHO Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) criteria: a sudden onset of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death) with no apparent nonvascular cause.22 The classification of ischemic or hemorrhagic was done on the basis of clinical presentation, and confirmation by CT or MRI was required, with reference to the MONICA criteria. The information about the main outcome was collected by qualified investigators every regular period of time (regular every 3 months). The relevant information about new stroke cases was obtained by direct reference to medical records. All materials obtained by investigators were independently reviewed by the Endpoint Evaluation Committee.
2.5. Statistical methods
Data were presented as the means ± SD, and number (percentages). The rates of events were presented as the number of events per 1000 person‐years. The proportional hazards assumption of the Cox proportional hazards models was confirmed using Schoenfeld residuals. We used multivariable Cox proportional hazards models to calculate the hazard ratio (HR) with 95% confidence intervals (CIs) for the associations between BP categories and stroke events, adjusted for age, gender, ethnicity, SBP, DBP, BMI, current smoking, current drinking, physical activity, education level, antihypertensive treatment, family history of hypertension, history of diabetes and hyperlipidemia, and the duration of hypertension. All analyses were performed using SPSS 22.0 (IBM Inc., Chicago, Illinois). The difference in β coefficients between short‐ and long‐term groups derived from the Z value was examined using the Fisher Z test.23 P < .05 was considered statistically significant.
3. RESULTS
Our results included 22 842 short‐term group and 28 456 long‐term group study population, of which 49.2% and 50.8% were women. The mean age were 51.54 (SD: 10.65) and 49.73 (SD: 10.91) years. Table 1 shows the mean values and percentages of study variables before BP changes for the two groups. In short‐ and long‐term groups, the mean values of SBP were 130.62 (SD: 14.34) mm Hg and 132.20 (SD: 21.07) mm Hg, the mean values of DBP were 81.12 (SD: 9.76) mm Hg and 81.71 (SD: 12.06) mm Hg, respectively. In addition, in the short‐ and long‐term groups, there were statistical significant differences in age, BMI, SBP, and DBP, gender, education level, physical activity, current smoking, current drinking, family history of hypertension, history of hyperlipidemia, antihypertensive treatment, and BP classification (all P < .05), except for ethnicity and history of diabetes.
Table 1.
Baseline characteristics of short‐ and long‐term blood pressure changes groups
| >Characteristics | >Short‐term BP changes group (n = 22 842) | >Long‐term BP changes group (n = 28 456) | >t or χ 2 | >P‐value |
|---|---|---|---|---|
| Women, n (%) | 11 246 (49.2) | 14 450 (50.8) | 12.12 | <.001 |
| Age, mean (SD), year | 51.54 (10.65) | 49.73 (10.91) | 18.84 | <.001 |
| Ethnicity, n (%) | 0.38 | .827 | ||
| Han populations | 17 677 (77.4) | 22 086 (77.6) | ||
| Mongolian | 4863 (21.3) | 6000 (21.1) | ||
| Others | 302 (1.3) | 370 (1.3) | ||
| BMI, mean (SD), kg/m2 | 23.63 (2.48) | 23.19 (2.72) | 18.71 | <.001 |
| SBP, mean (SD), mmHg | 130.62 (14.34) | 132.20 (21.07) | −9.67 | <.001 |
| DBP, mean( SD), mmHg | 81.12 (9.76) | 81.71 (12.06) | −5.95 | <.001 |
| Education level, n (%) | 25.20 | <.001 | ||
| Never or <5 years | 9086 (39.8) | 11 922 (41.9) | ||
| Primary school | 12 423 (54.4) | 14 858 (52.2) | ||
| Tertiary high school or higher education | 1333 (5.8) | 1676 (5.9) | ||
| Physical activity, n (%) | 13.08 | .001 | ||
| Low | 5640 (24.7) | 7397 (26.0) | ||
| Moderate | 10 606(46.4) | 13 122 (46.1) | ||
| High | 6596 (28.9) | 7937 (27.9) | ||
| Current drinking, n (%) | 8413 (36.8) | 9091 (31.9) | 27 391.25 | <.001 |
| Current smoking, n (%) | 9151 (40.1) | 11 808 (41.5) | 27 553.65 | <.001 |
| Family history of hypertension, n (%) | 2611 (11.4) | 3542 (12.4) | 12.41 | <.001 |
| History of diabetes, n (%) | 72 (0.3) | 98(0.3) | 0.33 | .568 |
| History of hyperlipidemia, n (%) | 428 (1.9) | 694 (2.4) | 18.91 | <.001 |
| Antihypertensive treatment, n (%) | 1836 (8.0) | 1732 (6.1) | 74.54 | <.001 |
| Blood pressure classification, n (%) | 464.45 | <.001 | ||
| Normotension | 2948 (12.9) | 5336 (18.8) | ||
| Prehypertension | 12 738 (55.8) | 13 496 (47.4) | ||
| Hypertension | 7156 (31.3) | 9624 (33.8) | ||
Note: Short‐term BP Changes Group: participants with short‐term (2008.1‐2010.12) blood pressure changes, number (percentage) and mean (SD) represent characteristics in follow‐up 1 (2008.01‐07); Long‐term BP Changes Group: participants with long‐term (2004.1‐2010.12) blood pressure changes, number (percentage) and mean (SD) represent characteristics in baseline survey (2004.01‐2006.12).
Abbreviations: BP, blood pressure; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 2 shows the incidence of stroke, ischemic stroke and hemorrhagic stroke in short‐ and long‐term groups. During a median follow‐up period of 12.5 years, we identified 1014 (702 ischemic, 300 hemorrhagic and 13 uncategorized) and 1505 (1070 ischemic, 413 hemorrhagic and 23 uncategorized) strokes during the periods of short‐ and long‐term BP groups, respectively. At the overall person‐years of 202 085 in short‐term group and 251 587 in long‐term group, the overall incidence of stroke was respectively 5.02/1000 and 5.98/1000 person‐years, ischemic stroke 3.47/1000 and 4.25/1000 person‐years, and hemorrhagic stroke 1.48/1000 and 1.64/1000 person‐years. In terms of the overall trend, subjects who were able to maintain normotension or decrease their BP to normotension had a lower incidence rate in short‐ and long‐term groups. Meanwhile, we found that normotensive subjects in short‐term group presented higher incidence rate of the three outcomes than long‐term group, no matter if they remained normotensive or became prehypertensive or hypertensive. While the contrary happen when considering hypertensive subjects in long‐term vs short‐term group. Furthermore, after short‐ and long‐term BP changes, the incidence rate of all the other eight categories was higher than that for maintaining the normotension.
Figure 2.
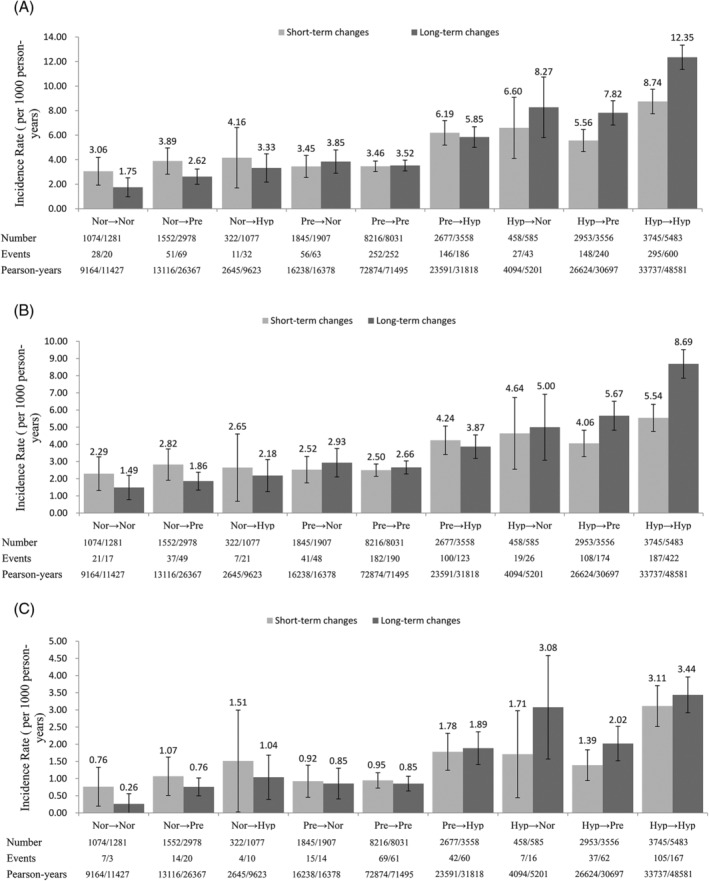
The incidence of stroke and its subtypes with short‐term and long‐term blood pressure changes. Stroke, A; ischemic stroke, B; hemorrhagic stroke, C; Nor, normotension; Pre, prehypertension; Hyp, hypertension. Error bars represent 95% CI
Table 2 shows HRs and 95% CI for the association between short‐ and long‐term BP changes and risk of stroke events using Cox proportional hazards models. Individuals who changed from a state of prehypertension to hypertension during a short‐term time had an increased risk of stroke (HR = 1.537, 95% CI: 1.248‐1.894), ischemic stroke (HR = 1.456, 95% CI: 1.134‐1.870) and hemorrhagic stroke (HR = 1.630, 95% CI: 1.099‐2.415), compared with subjects who maintained prehypertension. In contrast, individuals who changed from hypertension to prehypertension during a short‐term time was showed a protective effect against stroke (HR = 0.757, 95% CI: 0.619‐0.927) and hemorrhagic stroke (HR = 0.569, 95% CI: 0.388‐0.835) compared with subjects who maintained hypertension. Similarly, individuals who changed from prehypertension to hypertension during a long‐term time had an increased risk of stroke (HR = 1.291, 95% CI: 1.062‐1.569) and hemorrhagic stroke (HR = 1.818, 95% CI: 1.261‐2.623), compared with subjects who maintained prehypertension. However, individuals who changed from hypertension to prehypertension during a long‐term time showed a protective effect against stroke (HR = 0.825, 95% CI: 0.707‐0.963) and hemorrhagic stroke (HR = 0.777, 95% CI: 0.575‐0.949), compared with subjects who maintained hypertension. The results of the sensitivity analysis (excluding antihypertensive treatment) were similar to the whole population in short‐ and long‐term groups ( Table S1). In addition, β coefficients from Cox proportional hazards models were used to compare the effects of short‐ and long‐term BP changes on the risk of stroke and hemorrhagic stroke. For individuals whose BP changed from prehypertension to hypertension, short‐term changes were more strongly associated with increased risk of stroke than were long‐term changes on the basis of β coefficients (β = 0.430 vs 0.255, P < .001). Similarly, for individuals whose BP changed from hypertension to prehypertension, the β coefficient of short‐term BP changes was also higher compared to long‐term (β = −0.278 vs −0.193, P < .001). The opposite occurred in hemorrhagic stroke: for individuals whose BP changed from prehypertension to hypertension, long‐term changes were more strongly related to increased risk of hemorrhagic stroke compared to short‐term changes on the basis of β coefficients (β = 0.598 vs 0.488, P < .001). Conversely, for individuals whose BP changed from hypertension to prehypertension, the β coefficient of short‐term BP changes was higher compared to long‐term (β = −0.564 vs −0.253, P < .001).
Table 2.
Survival analysis of long‐ and short‐term changes in blood pressure on stroke eventsa
| >Changes in BP category | >Number | >Stroke | >Ischemic stroke | >Hemorrhagic stroke | |||||
|---|---|---|---|---|---|---|---|---|---|
| >Hazard ratio (95% CI) | >P‐ values | >β | >Hazard ratio (95% CI) | >P‐ values | >Hazard ratio (95% CI) | >P ‐values | >β | ||
| Short‐term BP changes group | |||||||||
| Normotension → normotension | 1074 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Normotension → prehypertension | 1552 | 1.238 (0.761,2.015) | 0.390 | … | 1.218 (0.687,2.160) | 0.500 | 1.323 (0.519,3.371) | 0.558 | … |
| Normotension →hHypertension | 322 | 1.223(0.595,2.513) | 0.584 | … | 1.044 (0.431,2.529) | 0.925 | 1.826 (0.519,6.430) | 0.348 | … |
| Prehypertension → prehypertension | 8216 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Prehypertension → normotension | 1845 | 1.240(0.924,1.666) | 0.152 | … | 1.291(0.914,1.824) | 0.147 | 1.167(0.661,2.059) | 0.595 | … |
| Prehypertension → hypertension | 2677 | 1.537 (1.248,1.894) | <0.001 | 0.430b | 1.456 (1.134,1.870) | 0.003 | 1.630 (1.099,2.415) | 0.015 | 0.488c |
| Hypertension → hypertension | 3745 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Hypertension → normotension | 458 | 1.077 (0.719,1.613) | 0.720 | … | 1.151 (0.709,1.868) | 0.570 | 0.887 (0.406,1.941) | 0.765 | … |
| Hypertension → prehypertension | 2953 | 0.757(0.619,0.927) | 0.007 | −0.2782 | 0.836(0.656,1.066) | 0.149 | 0.569(0.388,0.835) | 0.004 | −0.564c |
| Long‐term BP changes group | |||||||||
| Normotension → normotension | 1281 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Normotension → prehypertension | 2978 | 1.299 (0.779,2.164) | 0.316 | … | 1.118 (0.633,1.975) | 0.701 | 2.332 (0.678,8.018) | 0.179 | … |
| Normotension → hypertension | 1077 | 1.276 (0.714,2.279) | 0.411 | … | 1.038 (0.533,2.019) | 0.913 | 2.280 (0.602,8.638) | 0.225 | … |
| Prehypertension →prehypertension | 8031 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Prehypertension → normotension | 1907 | 1.381(1.043,1.828) | 0.024 | … | 1.390(1.008,1.917) | 0.045 | 1.291(0.716,2.327) | 0.396 | … |
| Prehypertension → hypertension | 3558 | 1.291 (1.062,1.569) | 0.010 | 0.255b | 1.114 (0.882,1.406) | 0.367 | 1.818 (1.261,2.623) | 0.001 | 0.598c |
| Hypertension → hypertension | 5483 | 1.000 (Ref.) | 1.000 (Ref.) | 1.000 (Ref.) | |||||
| Hypertension → normotension | 585 | 0.972 (0.709,1.331) | 0.857 | … | 0.840 (0.562,1.255) | 0.394 | 1.352 (0.800,2.285) | 0.261 | … |
| Hypertension → prehypertension | 3556 | 0.825 (0.707,0.963) | 0.015 | −0.193b | 0.846 (0.704,1.015) | 0.072 | 0.777 (0.575,0.949) | 0.040 | −0.253c |
Note: Short‐term blood pressure changes group, from the follow‐up 1 (2008.01‐2008.07) to the follow‐up 2 (2010.07‐2010.12); Long‐term blood pressure changes group, from the baseline surveys (2004.01–2006.12) to the follow‐up 2 (2010.07‐2010.12); Normotension, subjects with BP <120/80 mm Hg; prehypertension, subjects with BP of 120‐139/80‐89 mm Hg; hypertension, subjects with BP≥140/90 mmHg or antihypertensive treatment.
Abbreviations: BP, blood pressure; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Adjusted for variables in short‐ and long‐term groups, respectively: age, gender, ethnicity, SBP, DBP, BMI, education level, physical activity, current drinking, current smoking, family history of hypertension, history of diabetes, history of hyperlipidemia, antihypertensive treatment, the duration of hypertension.
β Coefficients different from 0 for short‐ and long‐term blood pressure changes groups in stroke: P < 0.05.
β Coefficients different from 0 for short‐ and long‐term blood pressure changes groups in hemorrhagic stroke: P < 0.05.
Bold values in Table 2 was considered statistically significant (P < .05).
Figure 3 shows the cumulative incidence risk of long‐ and short‐term BP changes to stroke. Regardless of short‐ or long‐term BP changes, individuals who were able to maintain or to decrease their BP values had a lower cumulative incidence of stroke. In contrast, those who had higher initial BP values or an increase in their BP values had a higher of cumulative incidence of stroke. The cumulative incidence of stroke for all other eight categories was higher than that for maintaining the normotension, and individuals who maintained the hypertension and normotension had the highest and lowest cumulative incidence, respectively.
Figure 3.
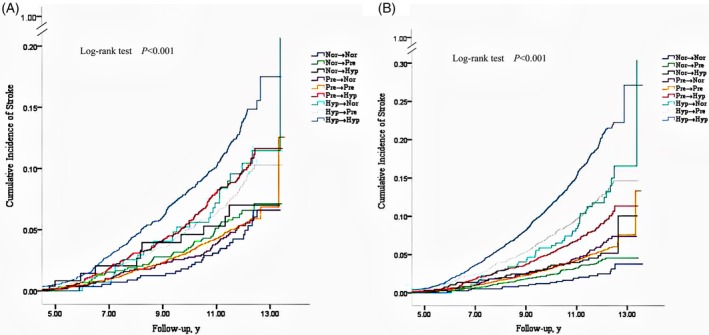
The cumulative incidence of stroke with short‐term and long‐term blood pressure changes. Short‐term blood pressure changes, A; long‐term blood pressure changes, B; Nor, normotension; Pre, prehypertension; Hyp, hypertension
4. DISCUSSION
We studied and compared the effects of BP changes over a short‐ and long‐term period on stroke and its subtypes in a prospective cohort study in Chinese rural adults, and found that the majority of individuals remained stable or experienced a slight increase in BP values over time. In general, significant positive associations were observed for rising BP and higher risk of stroke events, compared with individuals remaining stable or showing a decrease in BP values. In addition, compared to the same increase in BP levels over a long‐term period, the short‐term increase in BP had a greater impact on the risk of stroke events.
Previous studies have confirmed and reinforced the importance of BP change by different forms for stroke. Limited prospective studies in the form of longitudinal elevated or decreased BP trajectories at several time points have emphasized that increase in BP is a major risk factor for adverse outcomes of CVD (including stroke) in China or Western countries.9, 12, 13, 15, 24, 25 Another form of BP changes is the visit‐to‐visit blood pressure variability, and studies have found that higher variability of BP or SBP is a powerful predictor of cardiovascular events independently of mean SBP or DBP, which has been more common in previous studies.7, 11, 14, 26, 27, 28 A large longitudinal study of U.S. veterans found that greater long‐term visit‐to‐visit SBP variability was associated with higher risk of subsequent stroke, independent of mean SBP, which is consistent with our findings.7 Furthermore, our study considered baseline BP values and the effect of antihypertensive treatment. A significant proportion of individuals with hypertension with decreased BP showed a decreased risk of stroke. However, even if BP values were decreased, the incidence of stroke for individuals with hypertension or prehypertension was still higher than for those with normotension. The results suggest that a reduction in BP may not fully reverse the effect of sustained hypertension earlier in life, which may be because of physiological changes in the vasculature, such as arterial stiffening, fibrosis, and calcification.28 This finding was comparable to that in a previous study of 9845 individuals from the Atherosclerosis Risk in Communities (ARIC) study in the United States.9 Even so, lowering BP values below the guidelines of treatment is still the most direct and effective way to decrease incidence of stroke.27
In addition, with the hypertension as reference, individuals who changed from hypertension to prehypertension decreased the risk of stroke events in short‐ and long‐term groups. However, there was no statistical significance in the risk of stroke events in BP classifications of individuals who changed from hypertension to normotension compared with maintained hypertension. This is maybe a power issue (fewer people make the changing from hypertension to normotension), or it could also imply a J‐curve effect in lowering BP.29 Although some of the results in our subgroup analysis have no statistical significance, they cannot be ignored. We still cannot deny that antihypertensive treatment can reduce the risk of the stroke events. Our results may be by chance because of the small number of cases and insufficient statistical power.
Comparison of the effects of short‐ or long‐term changes in BP on stroke outcomes is the first study to date. Our results mainly found that short‐term BP changes had a greater impact on stroke going from prehypertension to hypertension or vice versa. A pair of opposite results were obtained with hemorrhagic stroke, where it appeared that short‐term BP changes had a greater impact going from hypertension to prehypertension and long‐term BP changes have a greater impact from prehypertension to hypertension. There is no clear mechanism to explain this difference. Compared to BP long‐term changes, short‐term changes in BP may make the compliance of large elastic arteries worse, resulting in more severe arterial stiffness30 and more frequent abnormal autoregulation,31 or may increase inflammatory markers, damage vascular endothelial cells and cause endothelial dysfunction.31, 32 Although we cannot be certain of the reasons for one result that long‐term BP changes had a greater impact on hemorrhagic stroke, it seems likely that the small number of hemorrhagic stroke events (only 43 and 61 events occurred in short‐term and long‐term groups) after 9 classifications might explain the discrepancy vs Further studies are needed to elucidate this issue.
Cumulative BP load is related to the risk of stroke in a later time period. In short‐ and long‐term BP change analyses, we found that initial BP level at a higher BP classification or more rapid increase in BP was significantly correlated with the cumulative incidence of stroke. Our results support previous findings of observational studies, which suggested that increase in BP is detrimental to health. A study of seven diverse US cohort studies estimated how BP changes affect the lifetime risk for CVD and found that increases in BP showed a positive relationship with remaining lifetime risk for CVD.10 Prevention efforts should continue to emphasize the importance of lowering BP and avoiding or delaying the incidence of hypertension to reduce the risk of stroke. In addition, our results also showed the necessity for dynamic analysis. Multiple longitudinal BP measurements to some extent prevent bias in assessing the risk of stroke incident in a single BP measurement, which provides a comprehensive explanation for the effects of BP changes on stroke incidence.
The strengths of our study include the relatively large sample size and long period of follow‐up period. Some limitations should also be considered in this study. First, our research was only based on a rural group of people in Northeast China, which limits the generalization of our results. More diverse populations are needed to confirm our results. Second, we did not have sufficient information on laboratory measurements, such as serum cholesterol, glucose, and inflammation biomarkers to control for other potential confounders. Third, it is also worth noting that grouping classifications of change in BP might have obscured some of the individual variability in temporal changes in BP and might have resulted in attenuated. Forth, the relatively small sample size in the BP changing groups might have been biased the results. More study population would be expected to predict the associations between BP changes and the risk of stroke. Fifth, information on the antihypertensive treatment of hypertensions in our study is limited. More information about hypertension population with or without antihypertensive treatment is needed to as the potential confounders to confirm our findings.
In conclusion, our study found that short‐ and long‐term changes in BP differentially affect the subsequent risk of stroke events independent of baseline BP levels and that elevated BP would increase the future risk of stroke and its subtypes. Furthermore, decreased BP can lower the risk of incidence but does not reverse the persistent negative effects of exposure to elevated BP. Even so, great attention should be paid to increasing the rate of hypertension control in rural areas of China.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
TABLE S1 Sensitivity analysis of long‐ and short‐term changes in blood pressure on stroke events after excluding antihypertensive treatment*
FIGURE S1 The study population inclusion and exclusion process
ACKNOWLEDGMENTS
This work was supported by funds from National Nature Science Foundation of China (No. 81773510) and National Key R&D Program of China (Grant Nos. 2017YFC1307600, 2018YFC1311600). All of the investigators and staff members were gratefully acknowledged. Thanks for all the enthusiastic participants. Especially, we thank Professor Yanan Ma for giving us guidance during the process of data collation and analysis.
Guo R, Xie Y, Zheng J, et al. Short‐term blood pressure changes have a more strong impact on stroke and its subtypes than long‐term blood pressure changes. Clin Cardiol. 2019;42:925–933. 10.1002/clc.23242
Funding information National Key R&D Program of China, Grant/Award Numbers: #2017YFC1307600, #2018YFC1311600; National Nature Science Foundation of China, Grant/Award Number: 81773510
Contributor Information
Yingxian Sun, Email: sunyingxian12@126.com.
Liqiang Zheng, Email: liqiangzheng@126.com.
REFERENCES
- 1. Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42:3651‐3654. [DOI] [PubMed] [Google Scholar]
- 2. Zhou M, Wang H, Zhu J, et al. Cause‐specific mortality for 240 causes in China during 1990‐2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387:251‐272. [DOI] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 4. Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513‐1518. [DOI] [PubMed] [Google Scholar]
- 5. Sun Z, Zheng L, Wei Y, et al. The prevalence of prehypertension and hypertension among rural adults in Liaoning province of China. Clin Cardiol. 2007;30:183‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng L, Li J, Sun Z, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in rural community of Liaoning Province, China. J Health Sci. 2007;53:209‐214. [Google Scholar]
- 7. Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of Systolic Blood Pressure Variability with Mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282‐1292. [DOI] [PubMed] [Google Scholar]
- 9. Petruski‐Ivleva N, Viera AJ, Shimbo D, et al. Longitudinal patterns of change in systolic blood pressure and incidence of cardiovascular disease: the atherosclerosis risk in communities study. Hypertension. 2016;67:1150‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd‐Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the cardiovascular lifetime risk pooling project. Circulation. 2012;125:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimbo D, Newman JD, Aragaki AK, et al. Association between annual visit‐to‐visit blood pressure variability and stroke in postmenopausal women: data from the Women's Health Initiative. Hypertension. 2012;60:625‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23‐year longitudinal Georgia stress and heart study. Hypertension. 2017;69:435‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan JH, Wang JB, Wang SM, Abnet CC, Qiao YL, Taylor PR. Longitudinal change in blood pressure is associated with cardiovascular disease mortality in a Chinese cohort. Heart. 2018;104:1764‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hastie CE, Jeemon P, Coleman H, et al. Long‐term and ultra long‐term blood pressure variability during follow‐up and mortality in 14,522 patients with hypertension. Hypertension. 2013;62:698‐705. [DOI] [PubMed] [Google Scholar]
- 15. Tielemans SM, Geleijnse JM, Menotti A, et al. Ten‐year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota business and professional men study and the Zutphen study. J Am Heart Assoc. 2015;4:e001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conen D, Chae CU, Guralnik JM, Glynn RJ. Influence of blood pressure and blood pressure change on the risk of congestive heart failure in the elderly. Swiss Med Wkly. 2010;140:202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sesso HD, Stampfer MJ, Rosner B, Gaziano JM, Hennekens CH. Two‐year changes in blood pressure and subsequent risk of cardiovascular disease in men. Circulation. 2000;102:307‐312. [DOI] [PubMed] [Google Scholar]
- 18. Zheng L, Sun Z, Zhang X, et al. Predictive value for the rural Chinese population of the Framingham hypertension risk model: results from Liaoning Province. Am J Hypertens. 2014;27:409‐414. [DOI] [PubMed] [Google Scholar]
- 19. Zheng L, Zhang Z, Sun Z, et al. The association between body mass index and incident hypertension in rural women in China. Eur J Clin Nutr. 2010;64:769‐775. [DOI] [PubMed] [Google Scholar]
- 20. O'Brien E, Petrie J, Littler W, et al. The British hypertension society protocol for the evaluation of automated and semi‐automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens. 1990;8:607‐619. [DOI] [PubMed] [Google Scholar]
- 21. Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC7report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 22. Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty‐one‐year observational study from the Sino‐MONICA‐Beijing project. Stroke. 2008;39:1668‐1674. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Srinivasan SR, Berenson GS. Path analysis of metabolic syndrome components in black versus white children, adolescents, and adults: the Bogalusa heart study. Ann Epidemiol. 2008;18:85‐91. [DOI] [PubMed] [Google Scholar]
- 24. Wu Z, Jin C, Vaidya A, et al. Longitudinal patterns of blood pressure, incident cardiovascular events, and all‐cause mortality in normotensive diabetic people. Hypertension. 2016;68:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UKcohorts. PLoS Med. 2011;8:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906‐915. [DOI] [PubMed] [Google Scholar]
- 27. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895‐905. [DOI] [PubMed] [Google Scholar]
- 28. Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing‐implications in hypertension. J Mol Cell Cardiol. 2015;83:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie X, Xu J, Gu H, et al. The J‐curve association between systolic blood pressure and clinical outcomes in ischemic stroke or TIA: the BOSS study. Sci Rep. 2017;7:14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit‐to‐visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens. 2011;5:184‐192. [DOI] [PubMed] [Google Scholar]
- 31. Ohkuma T, Woodward M, Jun M, et al. Prognostic value of variability in systolic blood pressure related to vascular events and premature death in type 2 diabetes mellitus: the ADVANCE‐ON study. Hypertension. 2017;70:461‐468. [DOI] [PubMed] [Google Scholar]
- 32. Kim KI, Lee JH, Chang HJ, et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J. 2008;72:293‐298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Sensitivity analysis of long‐ and short‐term changes in blood pressure on stroke events after excluding antihypertensive treatment*
FIGURE S1 The study population inclusion and exclusion process


