Abstract
Hypothesis:
The presence of bony inner ear malformations may associate with a number of anatomical abnormalities affecting the middle ear structures.
Background
Inner ear malformations associate with varying degrees of hearing loss, and frequently require cochlear implantation for hearing rehabilitation. Therefore, the abnormalities affecting the middle- and inner-ear structures may increase the risk of surgical complications.
Methods:
We examined 38 human temporal bones from donors with bony inner ear malformations. Using light microscopy, we analyzed the presence of abnormalities in the structures of the middle ear.
Results:
Our collection comprises of 38 specimens with inner-ear malformations (cochlear aplasia, n=3; cochlear hypoplasia, n=30; incomplete partition, n=3; isolated vestibular malformation, n=2). The anatomy of the middle ear was abnormal in most temporal bones with cochlear aplasia, cochlear hypoplasia, and incomplete partition type I (40%-100%). Some of those abnormalities (hypoplastic or obliterated mastoid, 55.2%; aplastic or obliterated round window, 71.0%; aberrant course of the facial nerve, 36.8%) may hinder the access to the round window using the conventional facial recess approach for cochlear implantation. The cochlear nerve and associated bony structures (internal auditory canal and bony canal for cochlear nerve) were normal in 71.0% of all temporal bones with inner ear malformations.
Conclusion:
Each different type of malformation may create specific surgical challenges to surgeons. Comprehensive preoperative imaging is imaging is fundamental towards the surgical success of cochlear implants in patients with malformations. Alternatives to circumvent those middle- and inner-ear abnormalities and potential complications are further discussed.
Keywords: Congenital abnormalities, syndrome, temporal bone, inner ear, cochlea
Introduction
Inner ear malformations are responsible for 20% of all types of congenital sensorineural hearing loss.1 For years, the term “Mondini dysplasia” and “Michel aplasia” were used in reference to all types of bony cochlear malformation detected by imaging tests.2 Only in 1987, Jackler et al.2 proposed a classification system based in imaging findings, and also hypothesized potential embryogenic and developmental causes for each type of malformation; their classification was later modified by Marangos,3 and Sennaroglu et al.4-6
Several studies reported histopathologic features of cochlear malformations in the past.5-9 Considering that inner ear malformations are frequent indications for cochlear implantation, an in-depth understanding of the anatomic, morphologic and functional aspects of each specific type of malformation is necessary to increase the chances of optimal functional outcomes.1,5,10 Therefore, the aim of this study is to describe the histopathologic features of inner ear malformations, and identify potential pitfalls for cochlear implantation surgery.
Methods
From the human temporal bone (HTB) archive of the Otopathology laboratory at the University of Minnesota (USA), we selected specimens from donors with inner ear malformations. Specimens were harvested during autopsy, processed using a standard protocol,11 and then serially sectioned at a thickness of 20μm. Every 10th section was stained with hematoxylin and eosin and mounted on a glass slide. The Institutional Review Board of the University of Minnesota (0206M26181) approved this study.
Inner ear malformations were classified using descriptions of Sennaroglu et al.4-6 (Suppl. file 1) Initially, we used a light microscope to scrutinize the midmodiolar sections of every HTB specimen from the collection. Abnormalities in the modiolus were classified according to Sennaroglu5 in: (I) normal; (II) shortened; (III) enlarged scala vestibuli; (IV) superior modiolar defect; (V) partial modiolar defect; (VI) subtotal modiolar defect; and (VII) absence of the modiolus.5 Later, we analyzed (in all available stained HTB sections) anatomy and morphologic aspects of the middle and inner ear. We excluded specimens in which analysis of the middle ear or inner ear was impossible due to removal or processing artifacts.
Results
Of the 2240 HTBs initially scrutinized, 38 (1.7%) had inner ear malformations. Those bones were from 20 different donors (bilateral malformations, n=18; unilateral malformation, n=2), and among the 18 donors with bilateral malformation, 14 (77.7%) had similar types of malformations bilaterally.
Among the 38 HTBs, 19 (50.0%) were from donors who had syndromes (trisomy of 21, n=7; CHARGE, n=4; 1st and 2nd branchial arch syndrome, n=2; C/D chromosome translocation, n=2; trisomy of 13, n=4), 4 (10.5%) were from patients without any syndromes, stigma or malformations, 10 (26.3%) were from donors with malformations but no syndromic diagnosis, and no clinical history was found in the charts of 4 (10.5%). In our collection, we found cases of cochlear aplasia (3 HTBs, 7.8%), cochlear hypoplasia (30 HTBs, 78.9%), incomplete partition (3 HTBs, 7.8%); in addition, we found 2 HTBs (5.2%) from 1 donor with vestibular malformations in isolation (Table 1). We did not find cases of complete labyrinthine aplasia, rudimentary otocyst, common cavity, cochlear hypoplasia types 1 and 4, and incomplete partition type III in our collection.
Table 1:
Characteristics of the middle ear cleft and mastoid of temporal bones with inner ear malformations
| MF | Middle ear | Mastoid | Malleus/incus | Stapes | Facial nerve | Oval window |
Round window |
|---|---|---|---|---|---|---|---|
|
Cochlear aplasia (n=3) |
N/A (100%) | Hypodeveloped (100%) | N/A (100%) | N/A (100%) | Hypoplastic (100%); aberrant course (100%) | Aplastic (100%) | Aplastic (100%) |
|
CH-2 (n=5) |
Otitis media (60%); hypoplastic (20%); normal (20%) | Hypodeveloped (100%) | Dysplastic (40%); normal (20%); artifacts (40%) | Dysplastic (60%); aplastic (20%); normal (20%) | Aberrant course (80%); dehiscent (20%); N/A (20%) | Normal (60%); aplastic (40%) | Aplastic (20%); Obliterated by overhanging bone (20%); obliterated by granulation tissue (20%); normal (40%) |
|
CH-3 (n=25) |
Mesenchymal remnants (44%); Otitis media (40%); hypoplastic (16%); persistent stapedial artery (8%); retracted TM (4%); Normal (28%) | Hypodeveloped (56%); mucosal hyperplasia or granulation tissue (24%); mesenchymal remnants (16%); normal (12%); N/A (8.0%) | Dysplastic (12%); aplastic (4%); normal (84%) | Dysplastic (40%); normal (60%) | Aberrant course (24%); dehiscent (16%); hypoplastic (16%); large fallopian canal (12%); normal (40%); artifacts (8%); N/A (4%) | Aplastic (24%); fixed (4%); dysplastic (4%); normal (68%) | Hypoplastic (44%); overhanging bone (44%); obliterated by granulation or mesenchymal tissue (16%); aplastic (12%); obliteration by aberrant facial nerve course (4%); normal (20%) |
|
IP-I
(n=1) |
Hypoplastic (100%) | N/A (100%) | Dysplastic (100%) | Dysplastic (100%) | Aberrant course (100%) | Dysplastic (100%) | Aplastic (100%) |
|
IP-II (n=2) |
Normal (100%) | Normal (100%) | Normal (100%) | Normal (100%) | Normal (100%) | Normal (100%) | Normal (100%) |
Legends: MF, Malformation; CH, cochlear hypoplasia; IP, incomplete partition; N/A, not available in the available sections (may be due to aplasia or removal artifact).
Cochlear aplasia
Our collection has 3 HTBs (2 donors) with cochlear aplasia. Two HTBs were from a 3-month old infant with multiple malformations (low-set pinnae, microtia and bilateral atresia of the external auditory canal, tracheoesophageal fistula, cleft palate, hypoplasia of mandible, asymmetry of the tongue, heart defects, undescended testicles, facial abnormalities), and the remaining specimen was from an anencephalic 3-day-old girl. We did not find any information regarding hearing tests in their clinical charts. In all three specimens, neural structures were aplastic (Table 2). Structures corresponding to the vestibular compartments were present in all 3 bones. In the anterior aspect of the otic capsule, the cochlea is completely absent, and the space which it should be occupying was filled by mesenchymal tissue.
Table 2:
Analysis of the inner ear structures of temporal bones from donors with inner ear malformations
| MF | Modiolus | Cochlear nerve |
Internal auditory canal |
Bony Canal CN |
Vestibule | SCCs | VA |
|---|---|---|---|---|---|---|---|
| Cochlear aplasia | Aplastic (100%) | Hypoplastic (66%); aplastic (34%) | Hypoplastic (100%) | N/A | Dilated (33%), hypoplastic (66%) | Dilated (33%), hypoplastic (66%) | Aplastic (100%) |
| CH-2 | VI (80%), V (20%) | Normal (60%), hypoplastic (40%) | Normal (60%), hypoplastic (40%) | Normal (80%), hypoplastic (20%) | Dilated (80%), hypoplastic (20%) | Dilated (40%), Hypoplastic (40%), aplastic (20%) | Hypoplastic (20%), aplastic (40%), enlarged (20%), normal (20%) |
| CH-3 | II (76%), V (24%) | Hypoplastic (16%); Normal (86%) | Hypoplastic (8%); Normal (92%) | Hypoplastic (8%); Normal (92%) | Dilated (28%); hypoplastic (28%); normal (44%) | Dilated (60%); aplastic (20%); hypoplastic (8%); normal (12%) | Aplastic (24%); hypoplastic (20%); dilated (16%); Enlarged (12%); normal (28%) |
| IP-I | VII (100%) | Hypoplastic (100%) | Hypoplastic (100%) | Hypoplastic (100%) | Dilated (100%) | Dilated (100%) | Normal (100%) |
| IP-II | III (100%) | Normal (100%) | Normal (100%) | Normal (100%) | Dilated (100%) | Dilated (100%) | Severely enlarged (100%) |
Legends: MF, Malformation; CH, cochlear hypoplasia; IP, incomplete partition; CN, cochlear nerve; SCC, semicircular canals; VA, vestibular aqueduct.
Cochlear hypoplasia type 2 (CH2)
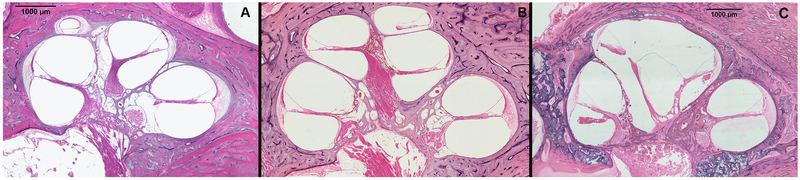
Our collection houses 5 HTBs from 3 donors with CH2 (Fig. 1, Tables 1 and 2). Of those HTBs, 1 (20%) was from a donor with trisomy of 13, 1 (20%) was from a donor with anencephaly, and 1 (20%) was from a donor with CHARGE syndrome. Most frequent middle ear finding was the presence of otitis media (60%) (Table 1). Four of the 5 HTBs at least one abnormal middle ear structure. (Fig. 2, Table 1). The mastoid and the middle ear were small and obliterated in all specimens. The course of the facial nerve was also abnormal in most of the HTBs (80%), possibly due to the smaller dimensions of the cochlea: facial nerve was dislocated anteriorly at the level of geniculate ganglion. Furthermore, it reached the mastoid (at the level of facial recess) in a more anterior position, which, in turn, narrowed the space between the facial nerve and chorda tympani.
Fig. 1:
Three human temporal bone specimens, one from a donor with cochlear hypoplasia type 3 (A), one from a donor without inner ear malformations (B), and one from a donor with cochlear hypoplasia type 2 (C) (Hematoxylin & Eosin, 400x). In (A) (CH3), the internal and external structure of the cochlea is similar to normal (B), except for its smaller size. In (C) (CH2), the size of the cochlea is smaller, and the upper part is cystic due to absence of interscalar septum. The modiolus is partially defective, as only its lower part is present.
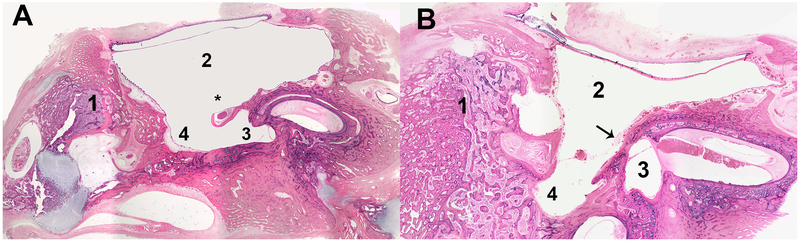
Fig. 2:
Two representative human temporal bone samples from donors with cochlear hypoplasia type 3 (A) and type 2 (B). Both specimens had a very hypodeveloped mastoid, filled by bone and bone marrow. In (A), the round window niche is partially obliterated by the facial nerve, which has an aberrant course in the middle ear. In B, the round window niche is obliterated by bone. Legend: (1) = Mastoid; (2) = middle ear; (3) = round window niche; (4) = sinus tympani; (*) facial nerve; (arrow) = bony wall blocking the round window niche.
External architecture of the cochlea was very similar in all HTBs: the basal turn was similar to normal, and the upper turns were smaller-sized, cystic, and round-shaped (Fig. 1). The modiolus was partially defective (VI, n=4; V, n=1) in all specimens, and one had a small communication between the basal turn of the cochlea and the internal auditory canal.
The vestibule was frequently dilated, while semicircular canals were mostly dysplastic (Table 2). Analysis of the neural structures revealed that the internal auditory canal and the vestibulocochlear nerve were hypoplastic in 2 (40%) of the specimens (Table 2).
Cochlear hypoplasia type 3 (CH3)
Our collection comprises 25 HTBs from 15 donors who had CH3 (Fig. 1). Nine of those donors (15 HTBs) had syndromes: 4 (7 HTBs) had Down’s syndrome, 1 (2 HTBs) had 1st and 2nd branchial syndrome, 1 (2 HTBs) had translocation of C/D chromosomes and partial trisomy of C, 2 (3 HTBs) had CHARGE syndrome, and 1 had trisomy of 13. Most frequent otologic malformations were low-set pinnae (5 donors) aplastic or hypoplastic external auditory canal (2 donors), microtia (5 donors); 5 donors had no otologic malformations and no chart information was found for 5. Clinical information regarding hearing/speech was available for 13 (52.0%) of the 25 HTBs – among those, most donors (61.5%) had (as reported) normal hearing. Hearing tests were available for 5 HTBs (3 donors): they were normal in 2 ears of 1 donor who also had normal hearing, and it was abnormal in the remaining 3 ears (profound mixed hearing loss, n=1; moderate conductive hearing loss; n=2).
Histologic examination of the middle ear cavity showed a number of abnormalities, including the presence of mesenchymal tissue remnants (44%) and otitis media (40%) (Table 1). The mastoid was hypodeveloped or partially obliterated in the majority of analyzed specimens (56%) (Table 1). Oval window and/or stapes were abnormal in 10 specimens (40%), and we also found a number of abnormalities affecting the round window of 20 (80%) HTBs (Table 1). In 1 HTB, the round window was partially obliterated by the facial nerve (Fig. 2).
Except for the smaller dimensions and decreased number of turns, the external architecture of the cochlea in all specimens with CH3 was normal (Fig. 3). We found a great variation in the internal architecture: some HTBs had very hypoplastic upper turns, while in others they were similar to of a normal cochlea (Fig.1). The modiolus was normal but shortened in most HTBs (76%), and 24% had partial defects. The vestibular architecture varied greatly among ears with CH3, ranging from aplastic to hypoplastic (Table 2). In 2 specimens (8.0%), we observed a large communication between the saccule and utricle, which were fused into a single structure. Neural elements of the cochlea and associated bony structures were mostly normal among the HTBs with CH3 (Table 2).
Fig. 3:
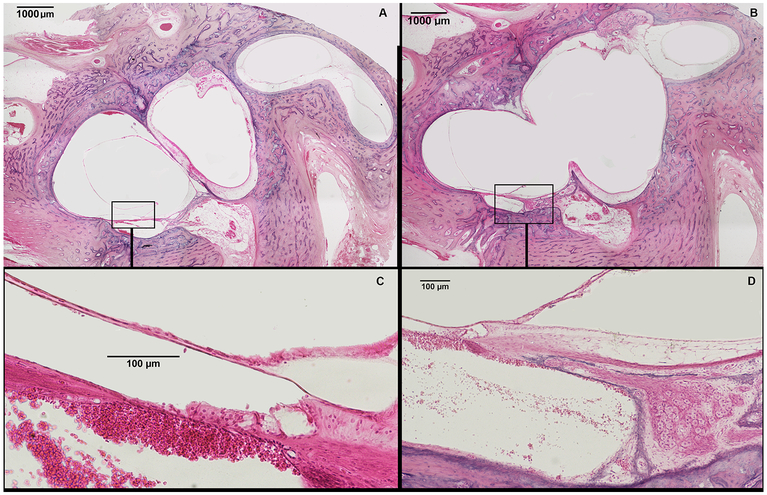
Representative human temporal bone slides showing two different levels of the inner ear from a donor with incomplete partition type I. (A) shows a cystic cochlea, devoid of any internal arquitecture except for a rudimentary organ of Corti (squared area); vestibule and semicircular canals are dilated. (B) shows a wide communication between the base of the cochlea and the vestibule. (C) shows squared area of figure A in higher magnification – a rudimentary organ of Corti is observed. (D) depicts squared area of figure B in higher magnification, showing normal spiral ganglion cells and spiral ganglion nerves supplying the rudimentary organ of Corti.
Incomplete partition type I (IP-I)
We found one HTB with cochlear abnormalities that are characteristic of IP-I. The donor was a 3-day-old female (born at full term) with anencephaly; in the contralateral ear, she had cochlear aplasia. The middle ear was severely dysplastic (Table 1), and the cochlea had an ovoid shape (Fig. 3). Internally, the modiolus was severely hypoplastic (grade VII) (Fig. 3), with areas of defects between base of the cochlea with the IAC. The In the posterior-inferior cochlear wall, rudimentary organ of Corti was present (Fig. 3). However, no sensorial structure or internal architecture was seen beyond that point. In the Rosenthal’s canal, we found some spiral ganglion cells supplying the organ of Corti (Fig. 3); in addition, scattered cochlear nerve fibers were present in the posterior cochlear wall. In its superior portion, the cochlea is separated from the vestibule by a very thin bone wall; in the inferior part, a large communication between the cochlea and the vestibule was observed (Fig. 3). Vestibule and semicircular canals were dilated, saccular and utricular membranes were hydropic, and vestibular aqueduct was normal. The vestibulocochlear nerve, internal auditory canal, and bony canal of the cochlear nerve were very hypoplastic (Table 2).
Incomplete partition type II (IP-II) (Mondini dysplasia)
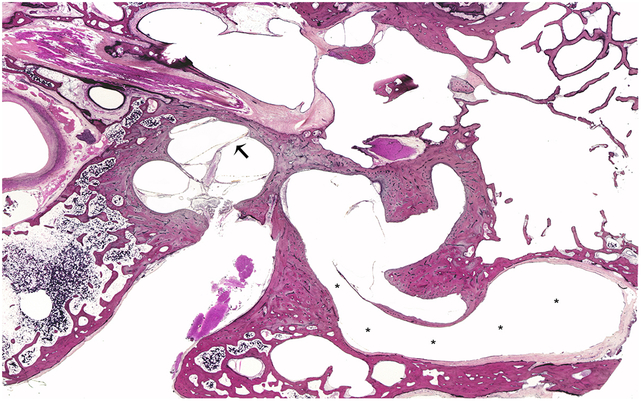
We found 2 HTBs which fit the characteristics of IP-II malformation, both from a 90 years old woman who died of pulmonary embolism. She had “normal hearing” before the age of 4 and developed adequate speech, but reported having progressive hearing loss, and became completely deaf by the age of 6. Histopathologic examination showed that the middle ear was normal in both specimens. Internally, the modiolus was fully formed in both HTBs (grade III), but the interscalar septum in the basal turn was bulged upwards, suggesting scala vestibuli hydrops (Fig. 4). The bony architecture of the vestibule and semicircular canals were dilated, and vestibular aqueducts were severely enlarged and patent in both specimens (Fig. 4). Internal auditory canal and bony cochlear nerve canal were normal in both specimens, but we observed severe loss of nerve fibers and spiral ganglion cells in the Rosenthal’s canal.
Fig. 4:
A representative human temporal bone specimen from a donor with incomplete partition type II. There are signs of scala vestibuli hydrops (arrow), represented by the interscalar septum bulged upwards in the vestibular face of the cochlea. The vestibular aqueduct is severely enlarged (*).
Isolated vestibular malformation
In our collection, we found 2 HTBs (1 donor) with isolated vestibular malformation. Those HTBs were from a 7-month old female with a true in-vivo mosaicism of trisomy of 13. Clinically, she had a constellation of facial malformations (microcephaly, anophthalmia, and low-set ears). A brainstem evoked response audiometry performed in life revealed bilateral severe-to-profound hearing loss, with a conductive component; tympanogram revealed B-type curves. Histopathologic examination of the specimens showed that the middle ears and mastoids were normal, except for the presence of chronic otitis media. The cochlea was structurally normal in both HTBs, but analysis of the internal cochlear architecture was not possible due to bilateral removal artifacts. The vestibular architecture was very abnormal bilaterally: vestibules were dilated, semicircular canals were hypoplastic, and vestibular aqueducts were enlarged bilaterally. Interestingly, in both ears, structures of the pars superior were severely hypoplastic, while pars inferior (saccule and cochlea) were similar to normal. In spite of the bilateral profound hearing loss, the vestibulocochlear nerves, internal auditory canals, and bony canals for the cochlear nerve were normal.
Discussion
The anatomical abnormalities found in the middle and inner ears of patients with inner ear malformations can result in a variety of surgical difficulties, increasing risks of both surgical complications and poor functional outcomes.1,12 The most frequent abnormalities reported in previous studies are aberrant course of the facial nerve, unusual anatomy of the promontory and round window niche, hypoplasia of the middle ear, and hypopneumatization or complete bony obliteration of the mastoid.5,7,13 In the inner ear, different degrees of modiolar defects have been observed, ranging from partial to complete defects – in the latter case, cochleostomy may lead to severe, profuse cerebrospinal gusher. The internal auditory canal, bony canal of the cochlear nerve, and the vestibulocochlear/cochlear nerve may be hypoplastic or aplastic.14 Therefore, preoperative computed tomography and magnetic resonance imaging exams, in addition to adequate hearing evaluation, are essential tools to anticipate those potential surgical pitfalls and increase the likelihood of good functional results.1,14
In our study, clinical data from the donors revealed that the majority of patients who had inner ear malformations were syndromic or had several other associated malformations. Those results are consistent with the literature: it has been demonstrated that inner ear malformations in isolation are uncommon.5 Among our donors, malformations of the external ear (microtia, low-set pinnae and atresia of the external auditory canal) were also frequent (58.3%) among our donors.
Analysis of the middle ear and mastoid revealed that those structures (middle ear cleft, mastoid, ossicles, facial nerve, and oval/round windows) were all normal in only 2 HTBs (1 donor). Some of those abnormalities (hypodevelopement or obliteration of the mastoid, aberrant course of the facial nerve, aplasia of the round window, and hypoplastic promontory) may hinder or completely prevent access to the round window or cochleostomy site. To circumvent those abnormalities, surgical alternatives have been proposed: Sennaroglu and Bajin4 suggested an additional transmeatal approach with or without a labyrinthotomy, aiming to better expose the promontory and cochleostomy site. Other alternatives proposed were the posterior suprameatal,15 the Veria,16 and the middle fossa approaches.17,18 However, some of those approaches are challenging even for experienced surgeons, and associate with high risks of complications such as facial nerve injury even in patients without malformations. In patients with inner ear (and other) malformations, identification of the correct surgical landmarks may be even more difficult.19
The most frequent type of inner ear malformation in our collection was CH3. Results of hearing tests varied greatly among donors with CH3, ranging from normal hearing to profound hearing loss – similar findings were also reported in clinical studies.4,20 Given the high frequency of abnormalities affecting ossicular chain and oval/round windows, it seems that most of those donors had at least some degree of conductive hearing loss in life.5,20 In the rare instances that a conductive deficit secondary to stapes fixation is the predominant component of the hearing loss, those patients may benefit of stapedectomy4,20. It is noteworthy that previous studies have linked those malformations of the footplate and/or oval window with an increased risk of meningitis.4,21-23 Among our donors, 2 (4 HTBs) had a history of recurrent meningitis, and both had dysplastic footplates in both ears. Our findings also demonstrate that, in CH3, the internal architecture of the cochlea is favorable to cochlear implantation in cases of severe or profound hearing loss: neural structures were normal in over 85% of the HTBs, and no other major architectural problems.1,4,20 We did not find defects in the modiolar base in any of the HTBs with CH3, suggesting that risk of gusher is not as typical as in other types of malformations.5 However, other potential surgical pitfalls were identified: the mastoid was small, hypoplastic, and obliterated in over 50% of HTBs with CH3, and the course of the facial nerve was abnormal in 25% of the times. In one of the HTBs, the facial nerve completely blocked the opening of the round window niche.24 The middle ear cleft was also frequently hypoplastic, and considering that the promontory may be flattened and round/oval windows may be aplastic, finding the right spot to perform a safe cochleostomy may constitute a challenging task. Therefore, an isolated facial recess approach may not provide adequate visualization of the round window niche or cochleostomy site.1
Among our HTB donors with CH2, hearing tests ranged from mild conductive (1 donor) to profound hearing loss (1 donor). Clinical studies from other centers showed that although hearing loss secondary to CH2 may range from mild to severe, over 80% have severe-to-profound hearing loss requiring cochlear or brainstem implantation.20 Similarly to our HTBs with CH3, most of the HTBs with CH2 (60%) did show normal neural structures (cochlear nerve, internal auditory canal and bony canal for the cochlear nerve were within normal limits). Therefore, it seems that CH2 is favorable for cochlear implantation surgery.1,14,25 Yet, we identified that those donors would have a conceivable risk of gusher, in view of the high frequency of partial or complete modiolar defects observed in our HTBs with CH2.26 The mastoid and the middle ear were also hypodeveloped in all HTBs, and the course of the facial nerve was also abnormal in 80% of the bones. Considering those middle ear abnormalities, our observations suggest that the conventional facial recess approach for cochlear implantation in those patients would bear high chances of facial nerve injury.5,20,27,28 Therefore, our results agree with previous observations showing that comprehensive preoperative imaging is fundamental towards the surgical success of cochlear implants in patients with CH2.5,20,27,28
Our only HTB with IP-I had very hypoplastic middle ear, and a cochlea with severely deformed external and internal architecture. The footplate was very dysplastic, similarly to what was observed in previous reports.4,5 Due to the frequent oval window abnormalities, IP-I is associated with an increased risk of meningitis.4,5,23 Neural structures (internal auditory canal and vestibulococlear nerve) were markedly abnormal as well – considering that this donor had a cochlear aplasia in the contralateral ear, a profound, bilateral hearing loss would be expected.29 In this HTB, we found nerve fibers in the inferior part of the cochlea, mostly along the lateral wall. Therefore, to ensure optimal stimulation of those fibers, straight electrodes with contacts on both surfaces or complete rings should be preferred.5 Considering that this type of malformation is frequently associated with varying degrees of modiolar defects, surgeon must be prepared for potentially severe gusher 1,5,26
Regarding our HTBs with cochlear aplasia, those donors would certainly not benefit from cochlear implantation, and if hearing restoration was needed, an auditory brainstem implant may be considered.1,5,10
Conclusion
Based on our results, the most important anatomic structures to be studied pre-operatively are (1) mastoid (growth, obliteration); (2) middle ear (maturity, obliteration); (3) facial nerve (aberrant course, dehiscence); (4) round window (obliterated, hypoplastic); (5) modiolar defects (potential risk of gusher); and (6) size of the internal auditory canal and cochlear nerve (assessment of cochlear nerve status). Histopathologic observation of those patients with CH2, CH3, IPI and IPII would support maximum benefit from cochlear implantation as a hearing restoration strategy.
Acknowledgements:
We would like to thank Lori L. Gulbranson for proofreading the final version of this manuscript. The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the NIH (UG3NS107688), the International Hearing Foundation, the 5M Lions International, the Starkey Foundation, and the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES). The authors have no financial relationships, or conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Funding sources: RCM received a scholarship from the the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)” (Finance Code 001).
Footnotes
Conflicts of interest: None to declare
References
- 1.Sennaroglu L Cochlear implantation in inner ear malformations--a review article. Cochlear Implants Int. 2010;11:4–41. doi: 10.1002/cii.416 [DOI] [PubMed] [Google Scholar]
- 2.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope. 1987;97:2–14. [DOI] [PubMed] [Google Scholar]
- 3.Marangos N Dysplasien des Innenohres und inneren Gehörganges. HNO. 2002;50:866–881. [DOI] [PubMed] [Google Scholar]
- 4.Sennaroğlu L, Bajin MD. Classification and Current Management of Inner Ear Malformations. Balk Med J. 2017;34:397–411. doi: 10.4274/balkanmedj.2017.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sennaroglu L Histopathology of inner ear malformations: Do we have enough evidence to explain pathophysiology? Cochlear Implants Int. 2016;17:3–20. doi: 10.1179/1754762815Y.0000000016 [DOI] [PubMed] [Google Scholar]
- 6.Sennaroglu L, Saatci I. A new classification for cochleovestibular malformations. Laryngoscope. 2002;112:2230–2241. doi: 10.1097/00005537-200212000-00019 [DOI] [PubMed] [Google Scholar]
- 7.Paparella MM. Mondini’s deafness. A review of histopathology. Ann Otol Rhinol Laryngol Suppl. 1980;89:1–10. [DOI] [PubMed] [Google Scholar]
- 8.Paparella MM, ElFiky FM. Mondini’s Deafness. Arch Otolaryngol. 1972;95:134–140. doi: 10.1001/archotol.1972.00770080222009 [DOI] [PubMed] [Google Scholar]
- 9.Kaya S, Hızlı Ö, Kaya FK, Monsanto RD, Paparella MM, Cureoglu S. Peripheral vestibular pathology in Mondini dysplasia. Laryngoscope. 2017;127:206–209. doi: 10.1002/lary.25995 [DOI] [PubMed] [Google Scholar]
- 10.Monsanto R da C, Bittencourt AG, Neto NJB, et al. Auditory Brainstem Implants in Children: Results Based on a Review of the Literature. Int J Adv Otol 2014;10:284–290. [Google Scholar]
- 11.Monsanto R da C, Schachern P, Paparella MM, Cureoglu S, Penido N de O. Progression of changes in the sensorial elements of the cochlear and peripheral vestibular systems: The otitis media continuum. Hear Res. 2017;351:2–10. doi: 10.1016/j.heares.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colletti L, Colletti G, Mandalà M, Colletti V. The Therapeutic Dilemma of Cochlear Nerve Deficiency: Cochlear or Brainstem Implantation? Otolaryngol Head Neck Surg. 2014;151:308–314. doi: 10.1177/0194599814531913 [DOI] [PubMed] [Google Scholar]
- 13.Bartel-Friedrich S, Wulke C. Classification and diagnosis of ear malformations. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2008;6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3199848/. Accessed November 26, 2018. [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir E, Bajin MD, Atay G, Mocan BÖ, Sennaroğlu L. Bony cochlear nerve canal and internal auditory canal measures predict cochlear nerve status. J Laryngol Otol. 2017;131:676–683. doi: 10.1017/S0022215117001141 [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg J, Baumgartner W, Migirov L, Dagan T, Hildesheimer M. The suprameatal approach: an alternative surgical approach to cochlear implantation. Otol Neurotol 2004;25:41–44; discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 16.Kiratzidis T, Arnold W, Iliades T. Veria operation updated. I. The trans-canal wall cochlear implantation. ORL J Otorhinolaryngol Relat Spec. 2002;64:406–412. doi: 10.1159/000067578 [DOI] [PubMed] [Google Scholar]
- 17.Bittencourt AG, Tsuji RK, Ratto Tempestini JP, Jacomo AL, Bento RF, de Brito R. Cochlear implantation through the middle cranial fossa: a novel approach to access the basal turn of the cochlea. Braz J Otorhinolaryngol. 2013;79:158–162. doi: 10.5935/1808-8694.20130028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colletti V, Fiorino FG, Saccetto L, Giarbini N, Carner M. Improved auditory performance of cochlear implant patients using the middle fossa approach. Audiology. 1999;38:225–234. [DOI] [PubMed] [Google Scholar]
- 19.de Brito R Bittencourt AG, Tsuji RK, Magnan J, Bento RF Cochlear implantation through the middle fossa: an anatomic study for a novel technique. Acta Otolaryngol (Stockh). 2013;133:905–909. doi: 10.3109/00016489.2013.795291 [DOI] [PubMed] [Google Scholar]
- 20.Cinar BC, Batuk MO, Tahir E, Sennaroglu G, Sennaroglu L. Audiologic and radiologic findings in cochlear hypoplasia. Auris Nasus Larynx. 2017;44:655–663. doi: 10.1016/j.anl.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 21.Kimitsuki T, Inamitsu M, Komune S, Komiyama S. Congenital malformation of the inner ear associated with recurrent meningitis. Eur Arch Otorhinolaryngol. 1999;256 Suppl 1:S11–14. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni GB, Roopa S, Madhu N, et al. Cystic cochleovestibular anomaly presenting with congenital deafness and recurrent bacterial meningitis in childhood. Ann Indian Acad Neurol. 2013;16:272–275. doi: 10.4103/0972-2327.112496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham JM, Phelps PD, Michaels L. Congenital malformations of the ear and cochlear implantation in children: review and temporal bone report of common cavity. J Laryngol Otol Suppl. 2000;25:1–14. [DOI] [PubMed] [Google Scholar]
- 24.de Marques LHS, Martins DV, Juares GL, Lorenzetti FTM, Monsanto R da C. Otologic manifestations of Larsen syndrome. Int J Pediatr Otorhinolaryngol. 2017;101:223–229. doi: 10.1016/j.ijporl.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 25.Freeman SR, Sennaroglu L. Management of Cochlear Nerve Hypoplasia and Aplasia. Adv Hear Rehabil. 2018;81:81–92. doi: 10.1159/000485542 [DOI] [PubMed] [Google Scholar]
- 26.Cabbarzade C, Sennaroglu L, Süslü N. CSF gusher in cochlear implantation: The risk of missing CT evidence of a cochlear base defect in the presence of otherwise normal cochlear anatomy. Cochlear Implants Int. 2015;16:233–236. doi: 10.1179/1754762813Y.0000000048 [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Majhi BN, Yadav KK, Agrawal SP, Singh R. Radiological Anatomy of Inner Ear Malformation in Hearing Impaired Children and it’s Correlation with Hearing Loss: A Hospital Based Observational Study. Indian J Otolaryngol Head Neck Surg 2018;70:278–283. doi: 10.1007/s12070-017-1238-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svrakic M Rare case of bilateral aural atresia and cochlear dysplasia: when cochlear implantation is not the answer. Cochlear Implants Int 2018;19:234–238. doi: 10.1080/14670100.2018.1438767 [DOI] [PubMed] [Google Scholar]
- 29.Özbal Batuk M, Çınar BÇ, Özgen B, Sennaroğlu G, Sennaroğlu L. Audiological and Radiological Characteristics in Incomplete Partition Malformations. J Int Adv Otol 2017;13:233–238. doi: 10.5152/iao.2017.3030 [DOI] [PubMed] [Google Scholar]