Abstract
The genus Periploca belongs to the family Apocynaceae, which is composed of approximately ten species of plants according to incomplete statistics. Most of these plants serve as folk medicines with a long history, especially Periploca sepium and Periploca forrestii. The botanical classifications, chemical constituents, biological activities and toxicities of the genus Periploca were summarized in the literature from 1897 to early 2019. Though the botanical classification of this genus is controversial, these species are well-known to be rich sources of diverse and complex natural products—above all, cardiac steroids and C21 pregnane steroids with special structures and obvious pharmacological activities. The various crude extracts and 314 isolated metabolites from this genus have attracted much attention in intensive biological studies, indicating that they are equipped with cardiotonic, anti-inflammatory, immunosuppressive, antitumor, antimicrobial, antioxidant, insecticidal and other properties. It is noteworthy that some cardiac glycosides showed hepatotoxicity and cardiotoxicity at certain doses. Therefore, in view of the medical and agricultural value of the genus Periploca, in-depth investigations of the pharmacology in vivo, the mechanisms of biological actions, and the pharmacokinetics of the active ingredients should be carried out in the future. Moreover, in order to ensure the safety of clinical medication, the potential toxicities of cardiac glycosides or other compounds should also be paid attention. This systematic review provides an important reference base for applied research on pharmaceuticals and pesticides from this genus.
Keywords: genus Periploca, phytochemistry, biological activities, classification, toxicology, review
1. Introduction
The genus Periploca belongs to the family Apocynaceae [1] and involves approximately ten species which are diffusely distributed in temperate Asia, southern Europe and tropical Africa [2]. Many species of this genus have been historically used as folk medicines in China, especially Periploca sepium and Periploca forrestii. The root bark of P. sepium, known as “Xiangjiapi” or “Bei-wujiapi,” is utilized as a traditional Chines medicine (TCM) for the treatment of rheumatoid arthritis and bone and muscle pain, which is recorded in the Chinese Pharmacopoeia (2015 version). The processing of Cortex Periplocae requires removing impurities, cleaning, slicing, and drying. The dried Cortex Periplocae (3~6 g) and other medicinal materials are decocted in water and taken for internal use [3]. The stems or the whole plant of P. forrestii, which is a well-known herb known as “Heiguteng,” are widely used by the Miao nationality in China to treat many diseases, including rheumatic arthritis, traumatic injury, stomachache, dyspepsia, and amenorrhea. The usage of P. forrestii is usually decocted in water or soaked in wine for internal use, or it is smashed for external application [4]. The title genus is abundant with an array of attractive metabolites, such as steroids, oligosaccharides, terpenoids, phenylpropanoids, and flavonoids. These novel and complex metabolites and various crude extracts from the genus Periploca have attracted increasing attention to intensive biological studies, indicating that they possess significant cardiotonic, anti-inflammatory, immunosuppressive, antitumor, antimicrobial, antioxidant, insecticidal and other properties. Some cardenolide-type steroids exhibit obvious cardiotonic and antitumor effects at a certain dose. Their strong immunosuppression and insecticidal potentials provide new development prospects for pregnane glycosides. In addition, cardiac steroids were also found to have latent toxicity. In the 2000s, several researchers provided reviews of the chemical constituents and biological activities of the genus Periploca [5,6]. However, these concise reviews were expressed in Chinese. Additionally, a large amount of scientific research results on this genus has emerged in recent years. To make researchers know elaborate information on the chemical and biological activities of the genus Periploca and to make a modest contribution to the rational application of this genus, a systematic review of the genus Periploca is imperative. We covered topics including the botanical classification, phytochemistry, biological activities and toxicology of the genus Periploca through a literature survey from 1897 to early 2019. The outline of this paper and some representative plants of the genus Periploca are shown in Figure 1.
Figure 1.
The outline and some representative plants of the genus Periploca. (Left: The fruits of P. forrestii; Centre: The flowers of P. sepium; Right: The stems of P. forrestii. The center and right pictures were downloaded from Plant Photo Bank of China, PPBC.).
2. Botanical Classification of the Genus Periploca
In 1895, Schumann was the first to classify the P. species. Browicz defined the genus Periploca and recognized eleven species in 1966. According to Flora of China (1995) [2], the genus Periploca comprises about ten species, and five species are distributed in China. Additionally, in 1997, Venter [7] suggested correcting the nomenclature of Periploca to provide diagnostic characteristics for the genus and to improve classification. However, fourteen species belonging to genus Periploca were recorded by Venter, not including P. forrestii, which largely grows in southern China. Moreover, several Chinese scholars are interested in the taxonomy of this genus. Periploca omeiensis Z. Y. Zhu, which is merely distributed in Emei Mountain, China, was identified as a new species by Zhu in 1991 [8]. Furthermore, another new species, named Periploca chrysantha D. S. Yao, X. C. Chen et J. W. Ren, was found in Gansu, China in 2002 and is similar to P. sepium, with a difference in the corolla [9]. However, these two new species have not been included in the flora at home and abroad to date. To our best knowledge, the botanical classification of Periploca is inconsistent at home and abroad, and the flora has not been updated in time. We did not take into account the disputes over the taxonomy of the genus Periploca and summarized all the mentioned plants of this genus which have been studied for phytochemistry and biological activities. The names and references of these thirteen studied species are shown in Table 1.
Table 1.
Thirteen studied species of the genus Periploca.
| Name | Ref. |
|---|---|
| P. sepium Bunge | [2,7] |
| P. forrestii Schlechter | [2] |
| P. graeca L | [7] |
| P. calophylla (Wight) Falconer | [2,7] |
| P. aphylla Decne | [7] |
| P. nigrescens Afz. | [7] |
| P. linearifolia, Quart.-Dill. and A. Rich | [7] |
| P. laevigata Ait | [7] |
| P. hydaspidis Falc | [7] |
| P. angustifolia Labill | [7] |
| P. somaliensis Browicz | [10] |
| P. omeiensis Z. Y. Zhu | [8] |
| P. chrysantha D. S. Yao, X. C. Chen et J. W. Ren | [9] |
3. Chemical Constituents from the Genus Periploca
Phytochemical investigations of Periploca species revealed predominant metabolites, such as steroids, carbohydrates, terpenoids, phenylpropanoids, flavonoids, quinones, and aromatics. To date, three hundred and fourteen compounds (1–314) have been isolated and identified from the plants of genus Periploca.
3.1. Steroids
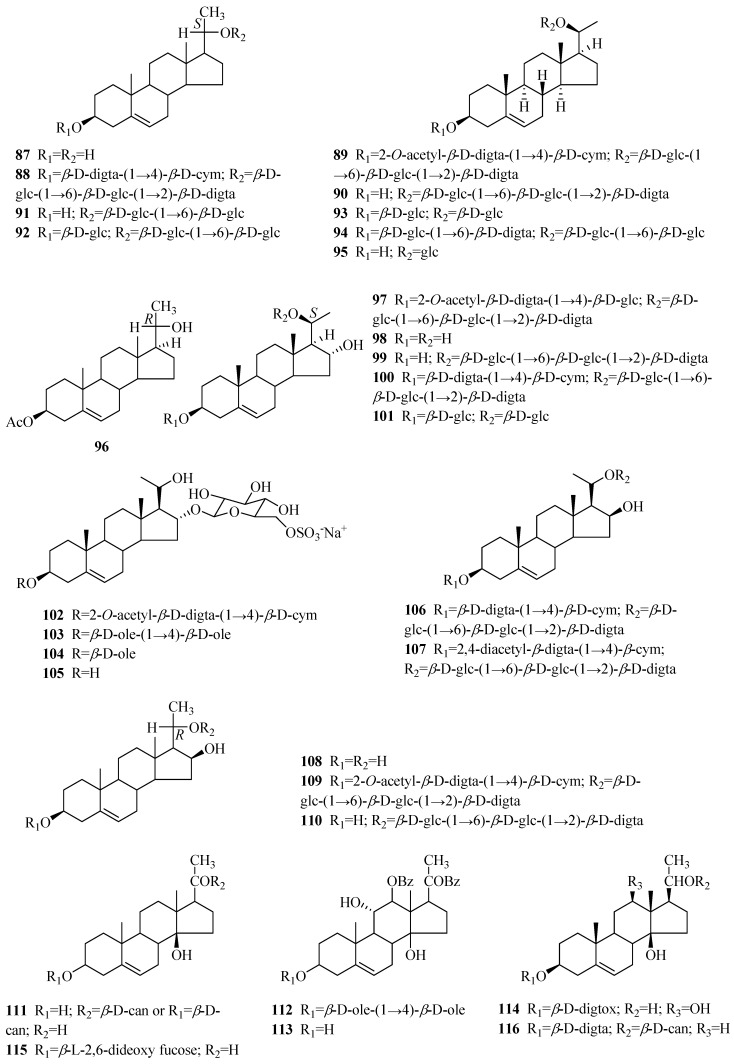
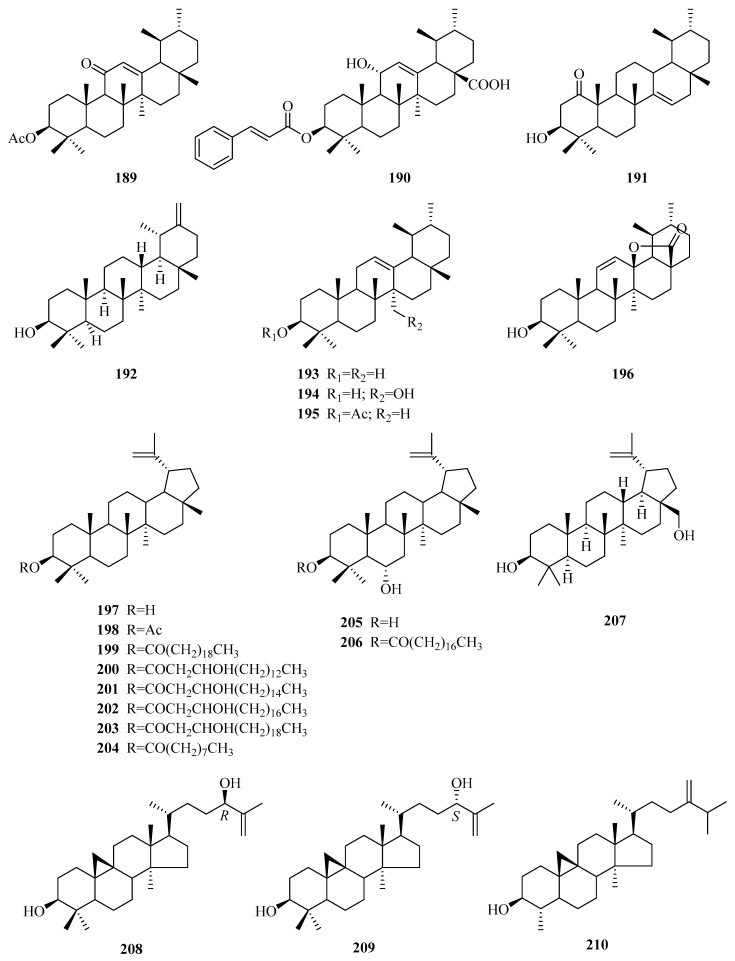
One hundred and forty-two steroids (1–142) were isolated from Periploca species, including forty-six cardenolides (1–46), ninety-two C21 pregnane-type steroids (47–138), and four phytosterols (139–142). Among them, approximately 80% of the C21 pregnane-type steroids come from P. sepium, while P. forrestii is a rich source of cardenolides. Their structures, names, corresponding sources, parts of plants and references are illuminated in Table 2 and Figure 2, Figure 3 and Figure 4.
Table 2.
Steroids from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| Cardenolide-type steroids | ||||
| 1 | periplocin |
P. graeca
P. omeiensis P. calophylla P. forrestii P. sepium |
bark whole plant stems whole plant cortex |
[11] [8] [12] [13] [14] |
| 2 | periplogenin |
P. graeca
P. calophylla P. forrestii P. sepium |
bark, stems stems whole plant cortex |
[11,15] [12] [13] [16] |
| 3 | periplocymarin |
P. graeca
P. sepium P. forrestii |
stalks and bark cortex whole plant |
[15,17] [14] [13] |
| 4 | biondianoside A | P. graeca | stems | [15] |
| 5 | periplogenin 3-O-β-d-glucopyranoside | P. graeca | stems | [15] |
| 6 | periplogenin 3-[O-β-glucopyranosyl-(1→4)-β-sarmentopyranoside] | P. sepium | root bark | [18] |
| 7 | periplogenin 3-O-β-d-glucopyranosyl-(1→4)-O-β-D-digitoxopyranoside | P. forrestii | stems | [19,20] |
| 8 | periplogenin 3-O-β-d-glucopyranosyl-β-digitaloside | P. forrestii | stems | [20] |
| 9 | periplogenin 3-O-β-d-digitoxopyranoside | P. forrestii | stems | [19] |
| 10 | 7β-hydroxy-periplogenin | P. forrestii | stems | [19] |
| 11 | 7-hydroxyl-periplogenin 3-O-β-d-digitoxopyranoside | P. forrestii | stems | [20] |
| 12 | 7-hydroxyl-periplogenin 3-O-β-d-cymaropyranoside | P. forrestii | stems | [20] |
| 13 | 7-hydroxyl-periplogenin 3-O-β-d-glucopyranosyl-β-d-digitoxopyranoside | P. forrestii | stems | [20] |
| 14 | 8β-hydroxy-periplogenin | P. forrestii | rhizome, stems | [19,21] |
| 15 | 8-hydroxyl-periplogenin 3-O-β-d-digitoxopyranoside | P. forrestii | stems | [20] |
| 16 | periforoside G | P. forrestii | stems | [19] |
| 17 | periforoside H | P. forrestii | stems | [19] |
| 18 | 7,8-dihydroxyl-periplogenin 3-O-β-d-cymaropyranoside | P. forrestii | stems | [20] |
| 19 | periforgenin C | P. forrestii | stems | [19] |
| 20 | periforoside F | P. forrestii | stems | [19] |
| 21 | 7,8-epoxy-periplogenin 3-O-β-d-glucopyranosyl-β-d-cymaropyranoside | P. forrestii | stems | [20] |
| 22 | periploforgeside A | P. forrestii | unknown | [22] |
| 23 | periploforgeside B | P. forrestii | unknown | [22] |
| 24 | echubioside | P. graeca | stems | [15] |
| 25 | xysmalogenin | P. sepium | root bark | [23] |
| 26 | 3β,5β-dihydroxy-14-en-card-20(22)-enolide | P. forrestii | stems | [19] |
| 27 | periforoside E | P. forrestii | stems | [19] |
| 28 | strophanthidin | P. nigrescens | branches | [24,25] |
| 29 | strophanthidol | P. nigrescens | branches | [24] |
| 30 | strophanthidin-β-d-glucoside | P. nigrescens | branches | [26] |
| 31 | strophadogenin | P. nigrescens | branches | [25] |
| 32 | convallatoxin | P. nigrescens | branches | [25] |
| 33 | 16β-acetoxystrophanthidin | P. nigrescens | branches | [27] |
| 34 | 3-O-rhamnosyl-16β-acetoxystrophanthidin | P. nigrescens | branches | [27] |
| 35 | 16-dehydrostrophanthidin | P. nigrescens | branches | [27] |
| 36 | 16-dehydrostrophanthidol | P. nigrescens | branches | [27] |
| 37 | 3-O-digitoxosyl-16-dehydrostrophanthidin | P. nigrescens | branches | [27] |
| 38 | alloperiplogenin 3-O-β-d-glucopyranosyl-(1→4)-β-d-cymaropyranoside | P. graeca | stems | [15] |
| 39 | alloperiplogenin | P. graeca | stems | [15] |
| 40 | biondianoside B | P. graeca | stems | [15] |
| 41 | periforgenin A | P. forrestii | rhizome, stems | [19] |
| 42 | periforoside I | P. forrestii | rhizome, stems | [19,28] |
| 43 | periforgenin A 3-O-β-cymaropyranoside | P. forrestii | roots | [29] |
| 44 | periforgenin A-3-O-β-digitoxopyranoside | P. forrestii | stems | [30] |
| 45 | periforoside D | P. forrestii | stems | [19] |
| 46 | periforgenin 3-O-β-d-glucopyranosyl-β-d-glucopyranosyl-β-d-cymaropyranoside | P. forrestii | stems | [20] |
| C21 pregnane-type steroids | ||||
| 47 | periploside A (periplocoside E) |
P. sepium
P. calophylla |
root bark stems |
[31,32,33,34,35] [12] |
| 48 | periploside B | P. sepium | root bark | [32] |
| 49 | periploside C (periplocoside A) | P. sepium | root bark | [32,33,34,35,36] |
| 50 | periploside D (periplocoside D) | P. sepium | root bark | [31,33,34,35] |
| 51 | periploside E (periperoxide A) | P. sepium | root bark | [33,34,35] |
| 52 | periploside F (periplocoside F) | P. sepium | root bark | [33,34,35,37] |
| 53 | periploside G (periperoxide B) | P. forrestii | root bark | [33,34,35] |
| 54 | periploside H (periperoxide E) | P. forrestii | root bark | [33,34,35] |
| 55 | periploside I (periperoxide C) | P. forrestii | root bark | [33,34,35] |
| 56 | periploside J (periplocoside J) | P. sepium | root bark | [34,35,37] |
| 57 | periploside K (periplocoside K) | P. sepium | root bark | [34,35,37] |
| 58 | periploside L (periperoxide D) | P. forrestii | root bark | [33,34,35] |
| 59 | periploside M (periplocoside B) | P. sepium | root bark | [34,35,36] |
| 60 | periploside N (periplocoside C) | P. sepium | root bark | [34,35,36] |
| 61 | periploside O | P. sepium | root bark | [38] |
| 62 | periploside P | P. sepium | root bark | [38] |
| 63 | periploside Q | P. sepium | root bark | [38] |
| 64 | periploside R | P. sepium | root bark | [38] |
| 65 | periploside S | P. sepium | root bark | [38] |
| 66 | periploside T | P. sepium | root bark | [38] |
| 67 | periploside U | P. sepium | root bark | [38] |
| 68 | periploside V | P. sepium | root bark | [38] |
| 69 | 3-O-formyl-periploside A | P. sepium | root bark | [38] |
| 70 | periploside W | P. chrysantha | root bark | [39] |
| 71 | periploside X | P. chrysantha | root bark | [39] |
| 72 | periploside Y | P. chrysantha | root bark | [39] |
| 73 | 3-O-formyl-periploside F | P. chrysantha | root bark | [39] |
| 74 | Δ5-pregnene-3β,17α,20(S)-triol | P. sepium | root bark | [31,40] |
| 75 | periplocogenin | P. sepium | root bark | [41] |
| 76 | periplocoside L |
P. sepium
P. forrestii |
root bark rhizome |
[31] [42] |
| 77 | periplocoside M periploside B |
P. sepium
P. forrestii P. calophylla P. sepium |
root bark stems stems root bark |
[31,43] [30] [12] [44] |
| 78 | periplocoside N |
P. sepium
P. forrestii |
root bark root bark |
[31] [45] |
| 79 | periplocoside O | P. sepium | root bark | [37] |
| 80 | periplocoside X | P. sepium | roots | [46] |
| 81 | periplocoside P | P. sepium | root bark | [47] |
| 82 | periplocoside NW | P. sepium | root bark | [48] |
| 83 | (3β,20S)-pregn-5-ene-3,17,20-triol 20-[O-β-glucopyranosyl-(1→6)-O-glucopyranosyl-(1→4)-β-canaropyranoside] | P. sepium | root bark | [18] |
| 84 | periseoside A | P. sepium | root bark | [40] |
| 85 | periseoside B | P. sepium | root bark | [40] |
| 86 | perisepiumoside I | P. sepium | root bark | [43] |
| 87 | Δ5-pregnene-3β,20(S)-diol | P. sepium | root bark | [44,49] |
| 88 | Δ5-pregnene-3β,20(S)-diol 3-O-[β-d-digitalopyranosyl (1→4)β-d-cymaropyranoside] 20-O-[β-d-glucopyranosyl (l→6)-β-d-glucopyranosyl (1→2)-β-d-digitalopyranoside] |
P. sepium
P. graeca |
root bark small branches |
[49] [50] |
| 89 | Δ5-pregnene-3β,20(S)-diol 3-O-[2-O-acetyl-β-d-digitalopyranosyl (1→4)-β-d-cymaropyranoside] 20-O-[β-d-glucopyranosyl (l→6)-β-d-glucopyranosyl (l→2)-β-d-digitalopyranoside] |
P. sepium | root bark | [49] |
| 90 | plocoside A | P. sepium | root bark | [49,51] |
| 91 | biondianoside C | P. sepium | root bark | [52] |
| 92 | biondianoside D | P. sepium | root bark | [52] |
| 93 | periseoside C | P. sepium | root bark | [40] |
| 94 | periseoside D | P. sepium | root bark | [40] |
| 95 | 5-pregnen-3β,20β-diol glucoside | P. sepium | root bark | [43] |
| 96 | Δ5-pregnene-3β,20(R)-diol 3-O-monoacetate | P. sepium | root bark | [31] |
| 97 | glycoside H2 |
P. sepium
P. graeca |
cortex, root bark small branches |
[40,49,53] [50] |
| 98 | Δ5-pregnene-3β,16α,20(S)-triol | P. sepium | root bark | [16,49] |
| 99 | Δ5-pregnene-3β,16α,20(S)-triol 20-O-β-d-glucopyranosyl (1→6)-β-d-glucopyranosyl (l→2)-β-d-digitalopyranoside | P. sepium | root bark | [49,52] |
| 100 | plocoside B | P. sepium | root bark | [40,51] |
| 101 | periseoside E | P. sepium | root bark | [40] |
| 102 | 16α-[(6-O-sulfo-β-d-glucopyranosyl)oxy] pregn-5-en-20-ol-3β-yl O-(2-O-acetyl-β-d-digitalopyranosyl)-(1→4)-β-d-cymaropyranoside | P. graeca | small branches | [50] |
| 103 | 16α-[(6-O-sulfo-β-d-glucopyranosyl) oxy] pregn-5-en-20-ol-3β-yl O-β-d-oleandropyranosyl-(1→4)-β-d-oleandropyranoside |
P. graeca | small branches | [50] |
| 104 | 16α-[(6-O-sulfo-β-d-glucopyranosyl)oxy] pregn-5-en-20-ol-3β-yl O-β-d-oleandropyranoside | P. graeca | small branches | [50] |
| 105 | 16α-[(6-O-sulfo-β-D-glucopyranosyl)oxy] pregn-5-ene-3β,20-diol | P. graeca | small branches | [50] |
| 106 | 20-O-[(β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl-(1→2)-β-d-digitalopyranosyl)oxy] pregn-5-en-16β-ol-3β-yl O-β-d-digitalopyranosyl-(1→4)-β-d-cymaropyranoside | P. graeca | small branches | [50] |
| 107 | 3β,16β,20-trihydroxy-pregn-5-en-3-O-2,4-diacetyl-β-digitalopyranosyl-(1→4)-O-β-cymaropyranosyl-20-O-β-glucopyranosyl-(1→6)-O-β-glucopyranosyl-(1→2)-O-β-digitalopyranoside | P. forrestii | roots | [29] |
| 108 | Δ5-pregnene-3β,16β,20(R)-triol | P. sepium | root bark | [49] |
| 109 | Δ5-pregnene-3β,16β,20(R)-triol 3-O-[2-O-acetyl-β-d-digitalopyranosyl (1→4)-β-d-cymaropyranoside] 20-O-[β-d-glucopyranosyl (l→6)-β-d-glucopyranosyl (l→2)-β-d-digitalopyranoside] |
P. sepium
P. graeca |
root bark small branches |
[49] [50] |
| 110 | Δ5-pregnene-3β,16β,20(R)-triol 20-O-β-d-glucopyranosyl (l→6)-β-d-glucopyranosyl (l→2)-β-d-digitalopyranoside | P. sepium | root bark | [49] |
| 111 | calocin | P. calophylla | twigs | [54] |
| 112 | plocin | P. calophylla | twigs | [55] |
| 113 | plocigenin | P. calophylla | twigs | [55] |
| 114 | locin | P. calophylla | twigs | [56] |
| 115 | calocinin | P. calophylla | twigs | [57] |
| 116 | calogenin 3-O-β-d-digitalopyranoside-20-O-β-d-canaropyranoside | P. graeca | small branches | [50] |
| 117 | (3β,14β)-3,14-dihydroxy-21-methoxypregn-5-en-20-one | P. sepium | root bark | [44] |
| 118 | perisepiumosides E | P. sepium | root bark | [58] |
| 119 | (3β,14β,17α)-3,14,17-trihydroxy-21-methoxypregn-5-en-20-one 3-[O-β-oleandropyranosyl-(1→4)-O-β-cymaropyranosyl-(1→4)-β-cymaropyranoside] | P. sepium | root bark | [18] |
| 120 | (3β,14β,17α)-3,14,17-trihydroxy-21-methoxypregn-5-en-20-one | P. sepium | root bark | [18] |
| 121 | perisepiumoside G | P. sepium | root bark | [43] |
| 122 | perisepiumoside H | P. sepium | root bark | [43] |
| 123 | calogenin | P. calophylla | unknown | [59] |
| 124 | plocinine | P. calophylla | twigs | [60] |
| 125 | 21-O-methyl-5-pregnene-3β,14β,17β,20,21-pentaol | P. sepium | root bark | [23] |
| 126 | perisepiumosides A | P. sepium | root bark | [58] |
| 127 | perisepiumosides B | P. sepium | root bark | [58] |
| 128 | perisepiumoside F | P. sepium | root bark | [43] |
| 129 | (3β,14β,17β)-3,14,17-trihydroxy-21-methoxypregn-5-en-20-one | P. sepium | root bark | [23] |
| 130 | perisepiumosides C | P. sepium | root bark | [58] |
| 131 | perisepiumosides D | P. sepium | root bark | [58] |
| 132 | 21-O-methyl-Δ5-pregnene-3β,14β,17β,21-tetraol-20-one-3-O-β-d-oleandropyranosyl(1→4)-β-d-cymaropyranosyl-(1→4)-β-d-cymaropyranosyl (periplocoside P) | P. sepium | root bark | [61] |
| 133 | 21-O-methyl-5,14-pregndiene-3β,17β,20,21-tetraol | P. sepium | root bark | [23] |
| 134 | Δ5,16-pregnadiene-3β,20α-diol diacetate | P. sepium | root bark | [62] |
| 135 | neridienone A | P. sepium | root bark | [41] |
| 136 | 5α-pregn-6-ene-3β,17α,20(S)-triol-20-O-β-d-digtoxopyranoside | P. forrestii | stems | [63] |
| 137 | teikagenin-3-O-β-d-dignitalosyl-20-O-β-d-canaroside | P. forrestii | stems | [63] |
| 138 | 3-O-β-d-digitalosyl-3β,17α,20α-tihydroxy-5α-pregn-6-ene | P. forrestii | stems | [63] |
| Phytosterol-type steroids | ||||
| 139 | β-sitosterol |
P. omeiensis
P. calophylla P. laevigata P. forrestii P. aphylla P. linearifolia |
whole plant root bark roots stems whole plant stem bark |
[8] [64] [65] [66] [67] [68] |
| 140 | β-daucosterol |
P. omeiensis
P. calophylla P. forrestii P. sepium P. laevigata P. aphylla |
whole plant root bark root bark root bark fruit bark above ground part |
[8] [64] [21] [62] [69] [70] |
| 141 | (6′-O-palmitoyl)-sitosterol-3-O-β-d-glucoside | P. calophylla | root bark | [71] |
| 142 | stigmasterol | P. aphylla | unknown | [72] |
Figure 2.
Cardenolide-type steroids from the genus Periploca.
Figure 3.
C21 pregnane-type steroids from the genus Periploca.
Figure 4.
Phytosterol-type steroids from the genus Periploca.
3.1.1. Cardenolides
The chemical investigation of this genus led to the discovery of cardenolides. According to their structural skeletons, forty-six cardenolides (1–46) are classified into two categories, namely periplogenin-type cardenolides (1–40) and periforgenin A-type cardenolides (41–46). Among them, there are fourteen aglycones, sixteen monosaccharide glycosides, fourteen disaccharide glycosides, and two trisaccharide glycosides. It is worth noting that all glycosides possess a sugar chain only at the C-3 position. Additionally, every disaccharide glycoside contains a deoxysugar as the inner sugar and a moiety of glucose as the outer sugar, with a linkage mode of 1→4, while the sugar unit of monosaccharide glycosides is a deoxygenated or an oxygenated sugar.
The isolation and identification of periplocin (1), the first cardenolide with digitoxin-like efficacy, was reported by Lehmann in 1897 [11]. Periplocin (1) was contained in the bark of P. graeca found in the southwest Caucasus. Upon heating the glucoside periplocin with dilute sulphuric acid, periplogenin (2) [11] was obtained. In 1939, Stoll and co-workers discovered that the enzymatic hydrolysis of periplocin with strophanthobiase produced periplocymarin (3) [17]. Later, compounds 1–3 were also repeatedly isolated from other species of genus Periploca [8,12,13,14,16]. Compared with periplocin, compounds 4–9 [15,18,19,20] have the same structural characteristics in their aglycones, with a difference in the sugar chains at C-3. Yu and co-workers [19,20] identified a series of new cardiac glycosides by online analysis or analysis after separation from the stems of P. forrestii. During this work, compounds 10–17 were found to have a hydroxyl group in the β configuration at C-7 or C-8, while a new compound (18) was found with two hydroxyl groups in the β configuration at C-7 and C-8. In addition, three new 7,8-β-epoxy cardiac glycosides (19–21) were designated. It is worth mentioning that a substitution at C-7 and C-8 led to a decrease of cytotoxic activity. Two new cardiac glycosides with 8,14-β-epoxy groups were isolated and identified to be periploforgeside A and periploforgeside B (22,23) [22]. Echubioside (24) [15], without the 5-β-hydroxyl group, has a logPo/w value (3.6) similar to that of estradiol (3.3), as determined in the same set of experiments. Compounds 25–27 are characterized by a double bond at position Δ5,6, Δ14,15 or Δ7,8 [19]. In 1954, the chemical investigation of P. nigrescens resulted in strophanthidin (28) and strophanthidol (29), with both containing an oxygenic substituent at C-19 [24]. Later, in 1957 and 1965, eight analogues (30–37) were isolated from the same species [25,26]. Compounds 35–37 are reduced by a pair of hydrogen atoms to form a double bond at C-16 and C-17 compared with 28 and 29. Compounds 38–40 are characteristic of 17α-cardenolides [15].
The study of pharmacologically active ingredients from P. forrestii led to the discovery of periforgenin A and periforoside I (41,42), displaying a 15(14→8)abeo-(8S)-14-ketone-card skeleton characterized by a ketone at C-14 and a transformed C/D ring [28]. The structure of periforoside I was supported by X-ray diffraction in 1990. Four newly found cardiac glycosides (43–46) [19,20,29,30] have different structural characteristics in the saccharide chains. The six distinct periforgenin A-type cardiac glycosides were only found in P. forrestii, which is the difference between P. forrestii and other plants of the genus.
3.1.2. C21 Pregnane-Type Steroids
According to the structural characteristics of the ninety-two C21 pregnane-type steroids (47–138, Table 2, Figure 3), a simple conclusion can be summarized. Up to now, all C21 steroids from Periploca species have possessed a typical pregnane unit with four unbroken rings. Most exist in the form of glycosides, with the sugar chains linked to C-3 or C-20. These saccharide chains are composed of oxygenated sugars, deoxygenated sugars, and their derivatives. Some oxygen-containing groups, such as hydroxy and methoxy groups, are mostly present at the C-3, C-14, C-16, C-17, C-20 and C-21 positions of the aglycone. Additionally, the absolute configuration at C-20 of most compounds (47–81,87–92, 97–101,126–128) has been deduced to be S.
From 1967 to 1995, some Japanese researchers made great contributions to the chemical investigation of P. sepium. They isolated and identified many compounds with novel features, especially a series of pregnane glycosides. In 1987 and 1988, Itokawa and co-workers [31,36,37,41] discovered periplocosides A–K, all containing a peroxy function, from the antitumor fraction of the CHCl3 extract from the root bark of P. sepium. During the same period, periplosides A–C were isolated by Hikino and co-workers [32]. Additionally, periplosides A and C are equipped with an orthoester group. Later, in 2008, to study the antirheumatoid arthritis effect of the genus Periploca, Zhao’s group determined perperoxides A–E [33]. From further work performed by Zhao’s group, it is worth noting that the peroxy function of the sugar chains of the above thirteen pregnane glycosides (periplocosides A–F, J, K and perperoxides A–E) was revised to an orthoester group according to 2D NMR spectroscopic analysis, chemical transformation, and X-ray diffraction analysis. Hence, the names of the fourteen pregnane glycosides were assigned as periplosides A–N (47–60) in 2011. The relative configurations at the spiro-quaternary carbons of the periplosides were revised according to the single-crystal X-ray structure of periploside F (52) [34,35]. With further research, in 2017, the absolute configuration of periploside C (49) was successfully established by single-crystal X-ray diffraction analysis. In addition, nine new spiro-orthoester group-containing pregnane-type steroidal glycosides, periplosides O–V, along with 3-O-formyl-periploside A (61–69), were isolated from the root bark of P. sepium [38]. To explore more about the structure-immunosuppressive activity relationship of spiroorthoester group-containing pregnanes, Zhao and co-workers performed a chemical analysis of P. chrysantha. Four new periplosides were obtained, and they were named periplosides W–Y and 3-O-formyl-periploside F (70–73) [39].
In addition to the abovementioned twenty-seven periplosides, there are some pregnane glycosides (74–86) which have three oxygen-containing substituents in the β configuration at C-3 and C-17 and in the S configuration at C-20. In 1988, Δ5-pregnene-3β,17α,20(S)-triol (74) was found from the acid hydrolysates of periplocosides D, E, L–N (periplocosides L–N, 76–78) [31]. However, Δ5-pregnene-3β,17α,20α-triol is an analogue of 74 without information on the absolute configuration at C-20 [73,74]. In 1987, Itokawa’s group [36] isolated a new compound S-2A, named periplocogenin (75). It is confusing that periplocoside M (77) [43] and periploside B (48) [44], which have two different names, have an identical chemical structure. Periplocoside N (78) has the same relative configuration as that of glycoside E [75] discovered in 1972. Periplocoside O (79) was isolated along with periplocosides J, K and F [37]. Periplocoside X (80) was confirmed to contain a 3,7-dioxy-heptulose group [46] according to its NMR spectroscopic data and by referring to periplocoside A. In Wu and co-workers’ search for insecticidal compounds from P. supium, they discovered two new glycosides, namely periplocoside P (81) and periplocoside NW (82) [47,48]. Compounds 83–86 are all newly found pregnane glycosides in recent ten years [18,40,43] which are different from 47–82 in terms of the length and constitution of the saccharide chain at C-3 or C-20.
Compounds 87–96 merely have two oxygenated patterns at C-3 and C-20. In 1988, Takeya and co-workers reported that compounds 88 and 89, which were hydrolyzed with acid to yield the same aglycone (87) [49], were two new pregnane glycosides from the antitumor fraction of P. sepium. Additionally, compounds 88 and 89 both yielded 90 upon partial acid hydrolysis. Glycosides H1 and K, isolated in 1972 and 1969, respectively [14,76], have identical relative configurations to those of compounds 89 and 90. Additionally, glycoside K is the first gregnane-type glycoside whose sugar chain links to the hydroxyl group, rather than C-3 of the aglycone. The biondianosides C,D (91,92), firstly isolated from Biondia hemsleyana (Warb.) Tsiang belonging to the same family [77], were also obtained from the genus Periploca in 2009 [52]. Periseosides C and D (93,94) are two new compounds [40]. Compound 96 [31] was established to have an R configuration at the C-20 position, which distinguishes it from compounds 87–95.
Compounds 97–106 are characterized by the inclusion of two hydroxyl groups at C-16 and C-20 of the aglycones, and the configuration of C-16 was deduced to be α. Glycoside H2 (97) was found by Sakuma and co-workers [53] in 1980, and it showed significant potentiation of the effect of nerve growth factor. The hydrolysis of compound 97 yielded glycoside 98. Due to a hydroxyl group at the D-ring, plocoside B (100) was found to exhibit higher differentiation-inducing activity [51]. Periseoside E [40] (101), along with periseosides A–D, was obtained in 2011. The study of the small branches of P. graeca collected at Marina di Vecchiano, Italy resulted in five new pregnane glycosides (102–106). It is worth mentioning that 102–105 are characterized by a 6-sulfated-β-d-glucopyranosyl unit at C-16, and the occurrence of sulfated glycosides as pregnane derivatives is an unusual finding [50]. Compound 106 bears a hydroxyl group in the β configuration at C-16. Compound 107 [29] is similar to 106, with the difference between them being in the structures of the oligosaccharide moieties. Compounds 108–110 are equipped with the R configuration at C-20 and a β-oriented hydroxyl group at C-16.
In the 1980s, chemical investigations of P. calophylla led to some pregnane glycosides with a hydroxyl group at C-14 (111–115). Unfortunately, the position of the sugar moiety in calocin (111) was not confirmed based on spectroscopic analysis in 1982 [54]. A new pregnane ester diglycoside and a new pregnane ester aglycone (112,113) [55]—each characterized by two hydroxyl groups that are benzoylated at C-11 and C-12—both contain the structure of 12,20-di-O-dibenzoyl drevogenin-d. Locin (114) [56] displays two β-oriented hydroxyl groups at C-12 and C-14. Calocinin (115) [57] has a special 2,6-dideoxy-l-fucose sugar unit, which was determined by spectroscopic analysis and a chemical reaction. Twenty years later, a new pregnane glycoside (116) with a β-oriented hydroxyl group at C-14 was discovered from the small branches of P. graeca [50].
Six C21 steroidal glycosides (117–122) have the chemical framework of 21-methoxypregnane-20-one and feature a β configuration hydroxyl group at C-14, five of which are new compounds. Ye and co-workers [44] found that compound 117 was biogenetically connected to (3β,5β,14β)-3,14,21-trihydroxypregnan-20-one, which is a precursor of cardenolides. In addition, the presence of compound 117 could indicate the pregnane pathway for the biosynthesis of cardenolides in P. sepium. Perisepiumoside E (118) [58], a glycoside of 117, was isolated in 2008. Two years later, a new pregnane glycoside (119) with an α-oriented hydroxyl group at C-17, together with its known pregnane genin (120), was obtained [18]. In comparison with compound 120, the pregnane glycosides 121 and 122 [43] contain disparate saccharide chains.
It is noteworthy that the carbon side-chain at C-17 of the ten distinct compounds (123–132) is α-oriented. Interestingly, these compounds all possess a β-oriented hydroxyl group at C-14, and eight are new compounds. An aglycon calogenin (123) [59] was established as containing a C-17 hydroxyethyl chain in α configuration. However, it is puzzling that the β configuration at C-17 was assigned to calogenin 3-O-β-d-digitalopyranoside-20-O-β-d-canaropyranoside (116) [50]. Plocinine (124) [60] is characterized by two cinnamoyl groups assigned to the methine protons at C-12 and C-20 of ornogenin. Compounds 125–128 are a series of 21-methoxypregnanes with a hydroxyl group in the β configuration at C-17. Similar to compounds 117–122, the pregnane glycosides 129–132 are also 21-methoxypregnane-20-one derivatives, but the remarkable difference is in the configurations at C-17. Furthermore, compounds 129 and 132 are stereoisomers at C-17 of 120 and 119, respectively. What is concerning is that compound 81 [47] was assigned the trivial name of periplocoside P in 2014, whereas 132 [61] was given the same name in 2015.
The vast majority of C21 steroid constituents from genus Periploca have one unsaturated double bond at C-5. Nevertheless, compounds 133–138, in particular, have double bonds at other positions, such as C-14, C-16 and C-6. In 1968, compound 134 [62] was obtained as a dehydrated product of Δ5-pregnene-3β,17α,20α-triol diacetate. Neridienone A (135) has three olefinic bonds [41]. In a search for compounds with antioxidant and melanogenesis-inhibitory abilities from P. forrestii, three known pregnane glycosides (136–138) [63] bearing an ethylene bond at C-6 were discovered.
3.1.3. Phytosterols
Four usual phytosterols (139–142, Table 2, Figure 4) were obtained from the genus Periploca.
3.2. Carbohydrates
A total of twenty-six carbohydrates have been isolated from the genus Periploca and mainly came from P. sepium and P. forrestii, eighteen of which are new compounds. Compounds 143–145 are monosaccharides, and compounds 146–168 are oligosaccharides. Carbohydrates can be considered characteristic components of Periploca species because most of them contain one or more 2,6-dideoxysugars or/and 6-deoxysugars and their derivatives. What is noticeable is that the sugar sequences of these oligosaccharides are ruled by the regularity in cardiac- and pregnane-type glycosides of the Apocynaceae (Asclepiadaceae) plants [78].
The oligosaccharides (147,148) were hydrolysis products of glycosides from P. sepium. Similarly, upon partial acid hydrolysis, compound 88 yielded 149, a deacetylation product of 147. Compounds 150–153 were isolated and elucidated as four new-type oligosaccharides in 1977, which were the first examples of oligosaccharides composed of 2,6-dideoxyaldonic lactone and 2,6-dideoxysugars [78]. Thirty years later, Zhao’s group [33] engaged in the discovery of new immunosuppressive compounds from traditional Chinese medicine, which resulted in five oligosaccharides being obtained. Among them, three were new compounds, named perisaccharides A–C (154–156). In their ongoing chemical investigation of P. forrestii, four new oligosaccharides (157–160) [29], characterized by diverse sugar units and an oleandro-1,5-lactone, were elucidated. In 2010, Ye and co-workers [79] reported five new oligosaccharides, perisesaccharides A–E (161–165), and assigned their absolute configurations. Intriguingly, they put forward that the conformation of the oleandronic acid δ-lactone should be confirmed as “boat,” rather than the “chair” assessment in previous literatures, on the basis of a combination of X-ray diffraction analysis, a modified Mosher’s method, CD measurements and acid hydrolysis. Additionally, the “boat” conformation of oleandronic acid δ-lactone was reported for the first time. Perisesaccharide F (166) [80], as a new natural product, was isolated from P. sepium, while another new oligosaccharide (167) [81] was discovered from P. calophylla. The structures, names, corresponding sources, parts of plants and references of these compounds are summarized in Table 3 and Figure 5. A small number of polysaccharides isolated from this genus are not listed here.
Table 3.
Carbohydrates from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 143 | cymarose | P. calophylla | twigs | [55] |
| 144 | β-d-glucopyranose | P. laevigata | roots | [65] |
| 145 | α-d-glucopyranose | P. laevigata | roots | [65] |
| 146 | sucrose | P. sepium | stems | [82] |
| 147 | 4-O-(2-O-acetyl-β-d-digitalopyranosyl)-d-cymaropyranose | P. sepium | cortex, root bark | [49,83] |
| 148 | methyl 4-O-(2-O-acetyl-β-d-digitalopyranosyl)-β-d-cymaropyranoside | P. sepium | cortex | [83] |
| 149 | methyl β-d-digitalopyranosyl(1→4)-β-d-cymaropyranoside | P. sepium | root bark | [49] |
| 150 | oligosaccharide C1 | P. sepium | cortex | [78] |
| 151 | oligosaccharide D2 | P. sepium | cortex, root bark | [33,78] |
| 152 | oligosaccharide F1 | P. sepium | cortex | [78] |
| 153 | oligosaccharide F2 | P. sepium | cortex, root bark | [33,78] |
| 154 | perisaccharide A | P. sepium | root bark | [33] |
| 155 | perisaccharide B |
P. sepium
P. calophylla |
root bark stems |
[33,43] [81] |
| 156 | perisaccharide C | P. sepium | root bark | [33] |
| 157 | perifosaccharide A | P. forrestii | roots | [29] |
| 158 | perifosaccharide B | P. forrestii | roots | [29] |
| 159 | perifosaccharide C | P. forrestii | roots | [29] |
| 160 | perifosaccharide D | P. forrestii | roots | [29] |
| 161 | perisesaccharide A | P. sepium | root bark | [79] |
| 162 | perisesaccharide B | P. sepium | root bark | [43,79] |
| 163 | perisesaccharide C | P. sepium | root bark | [43,79] |
| 164 | perisesaccharide D | P. sepium | root bark | [79] |
| 165 | perisesaccharide E | P. sepium | root bark | [79] |
| 166 | perisesaccharide F | P. sepium | root bark | [80] |
| 167 | 4-O-acetyl-β-cymaropyranosyl(1→4)-O-β-d-cymaropyranosyl(1→4)-O-β-d-canaropyranosyl(1→4)-O-β-d-cymaropyranosy(l→4)-O-oleandronic acid-δ-lactone | P. calophylla | stems | [81] |
| 168 | 2-O-acetyl-β-D-digitalopyranosyl-(1→4)-β-d-cymaropyranosyl- (1→4)-β-d-cymaropyranosyl-(1→4)-β-d-cymaropyranosyl- (1→4)-β-d-oleandronic-δ-lactone |
P. sepium | root bark | [43] |
Figure 5.
Carbohydrates from the genus Periploca.
3.3. Terpenoids
There are forty-two terpenoids (169–210) composed of triterpenoids and three other types of terpenoids (169–171). According to their structural characteristics, these triterpenoids (172–210) are divided into four sub-types, namely oleanane-type triterpenes, ursane-type triterpenes, lupane-type triterpenes and cycloartane-type triterpenes. Their structures, names, corresponding sources, parts of plants and references are illuminated in Table 4 and Figure 6.
Table 4.
Terpenoids from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 169 | loliolide | P. forrestii | whole plant | [73] |
| 170 | periplocadiol | P. laevigata | roots | [65] |
| 171 | 2α,6α-dihydroxy-5-[(E)-3′-hydroxy-3′-methyl-1′-butenyl]-6-methyl-4-cyclohexen-3-one | P. aphylla | whole plant | [84] |
| 172 | oleanolic acid |
P. forrestii
P. sepium P. laevigata P. aphylla |
unknown leaves roots above ground part |
[85] [86] [65] [70] |
| 173 | hederagenin |
P. omeiensis
P. forrestii |
whole plant whole plant |
[8] [13] |
| 174 | 2α,3β,5,24-tetrahydroxy-olean-12-en-28-oic acid | P. forrestii | stems | [66] |
| 175 | masilinic acid |
P. laevigata
P. aphylla |
fruit bark above ground part |
[69] [70] |
| 176 | arjunolic acid | P. laevigata | latex | [87] |
| 177 | 12α-hydroxy-δ-lactone of oleanolic acid | P. laevigata | latex | [87] |
| 178 | 11α,12α-Epoxy-3β-hydroxy-olean-13β,28-olide | P. somaliensis | fruits | [10] |
| 179 | 3-O-acety loleanolic acid | P. forrestii | stems | [88] |
| 180 | 3β-hydroxy-11,13(18)-diene-olean-28-oicacid | P. forrestii | unknown | [85] |
| 181 | β-amyrin |
P. calophylla
P. laevigata P. forrestii P. sepium P. linearifolia |
twigs roots unknown stem bark stem bark |
[89] [65] [90] [91] [68] |
| 182 | β-amyrin acetate |
P. forrestii
P. sepium |
unknown root bark, stem bark |
[90] [92,93] |
| 183 | P1 | P. calophylla | twigs | [89] |
| 184 | P2 | P. calophylla | twigs | [89] |
| 185 | P3 | P. calophylla | twigs | [89] |
| 186 | ursolic acid |
P. omeiensis
P. calophylla P. forrestii P. somaliensis P. aphylla P. nigrescens |
whole plant rhizome rhizome fruits unknown leaves |
[8] [64] [21] [10] [72] [94] |
| 187 | 2α,3β-dihydroxy ursolic acid |
P. forrestii
P. calophylla P. aphylla |
stems stems unknown |
[88] [64] [72] |
| 188 | asiatic acid |
P. laevigata
P. calophylla P. forrestii P. aphylla |
roots stems stems unknown |
[65] [12] [30] [72] |
| 189 | 12-ursen-3β-acetyl-11-one | P. forrestii | unknown | [85] |
| 190 | jacoumaric acid | P. forrestii | stems | [88] |
| 191 | 14-ursen-3-ol-1-one | P. forrestii | stems | [88] |
| 192 | taraxasterol | P. forrestii | stems | [88] |
| 193 | α-amyrin |
P. forrestii
P. sepium P. laevigata |
unknown root bark roots |
[90] [92] [65] |
| 194 | 27-hydroxy-α-amyrin |
P. omeiensis
P. forrestii |
whole plant unknown |
[8] [90] |
| 195 | α-amyrin acetate |
P. calophylla
P. forrestii P. sepium |
twigs unknown root bark |
[89] [90] [95] |
| 196 | 3β-hydroxy-urs-11-en-13β,28-olide | P. somaliensis | fruits | [10] |
| 197 | lupeol |
P. laevigata
P. aphylla |
roots, latex above ground part |
[65,87] [70] |
| 198 | lupeol acetate |
P. calophylla
P. laevigata P. sepium P. aphylla |
rhizome latex cortex above ground part |
[64] [87] [95] [70] |
| 199 | lupeol arachidate | P. laevigata | latex | [87] |
| 200 | procrim a | P. laevigata | latex | [87] |
| 201 | procrim b |
P. laevigata
P. linearifolia |
latex stem bark |
[87] [68] |
| 202 | laevigatin I | P. laevigata | latex | [87] |
| 203 | laevigatin II | P. laevigata | latex | [87] |
| 204 | lupeol-20(29)-en-3-nonadecanoate | P. forrestii | roots | [96] |
| 205 | 3β,6α-dihydroxylup-20(29)-ene | P. aphylla | stems | [67,72] |
| 206 | 6α-hydroxylup-20(29)-en-3β-octadecanoate | P. aphylla | stems | [67,72] |
| 207 | betuline | P. aphylla | unknown | [72] |
| 208 | (24R)-9,19-cycloart-25-ene-3β,24-diol | P. sepium | root bark | [92] |
| 209 | (24S)-9,19-cycloart-25-ene-3β,24-diol | P. sepium | root bark | [92] |
| 210 | cycloeucalenol | P. sepium | root bark | [92] |
Figure 6.
Terpenoids from the genus Periploca.
Loliolide (169) [73] is a monoterpene lactone contained in P. forrestii. Periplocadiol (170) is a new elemane-type sesquitone [70]. A new norterpenoid (171) [84] with a Δ4-3-one unit was found in the whole plant of P. aphylla.
Compounds 172–185 are a series of oleanane-type triterpenes. In 1971, oleanolic acid (172), together with maslinic acid (175), was first isolated from the genus Periploca [70]. Two known triterpenes (177,178) [10,87] characterized by a 13,28-lactone ring were discovered from P. laevigata and P. somaliensis, respectively. Additionally, three new triterpenoid acids of the oleanane series, named P1, P2, P3 (183–185), were isolated from the twigs of P. calophylla, without definite chemical structures [89]. Eleven ursane-type triterpenes (186–196) were found in the species of genus Periploca. Ursolic acid (186) [94] was acquired in 1987 from P. nigrescens. Moreover, these triterpenes also illuminated a new triterpene (196), which also has a 13,28-lactone ring [94]. Compounds 197–207 are triterpenes of the lupane type, and seven (197–203) of them were obtained from the latex of P. laevigata collected in Tunisia [87]. In addition, compounds 199–204 have the characteristic of a long-chain alkanonic ester or a β-hydroxy fatty acid ester at C-3. The structures of two new triterpenes (205,206) from P. aphylla resembled each other very closely, but the remarkable difference was in the substituent at C-3. Due to the difference, compound 205 exhibited strong inhibition of α-glucosidase type VI and a temperate antibacterial activity, while 206 was inactive or weak [67]. There are three cycloartane-type triterpenes (208–210) [92] with a hydroxyl group in the β configuration at C-3 which were isolated from this genus for the first time.
3.4. Phenylpropanoids
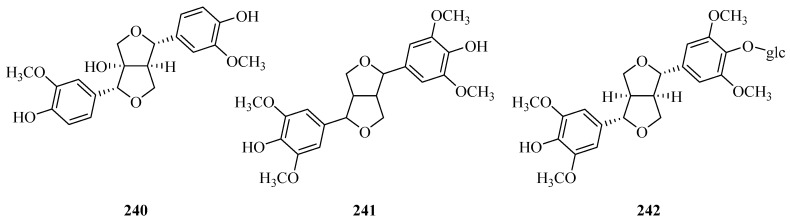
To date, thirty-two phenylpropanoids (211–242) have been reported, and they are divided into three categories: Simple phenylpropanoids, coumarins, and lignans. Their structures, names, corresponding sources, parts of plants and references are illuminated in Table 5 and Figure 7. Compounds 211–228 are simple phenylpropanoid compounds, among which compounds 219–228 [74] are a series of caffeoylquinic acids which were isolated from the hydrosoluble fraction of P. forrestii for the first time in 2017. Scopoletin and cleomiscosins A and B (229–231) are the only three coumarins from Periploca species [66,97]. In addition, there are eleven lignans (232–242). (+)-lyoniresinol (232) is an arylnaphthalene-type lignin [84], while tortoside B (235) is a tetrahydrofurane-type lignan [98]. Two new tetrahydrofurane-type lignins (236,237), along with four known lignans (233,234,238,239), were determined from P. forrestii in 2017 [63]. In addition, the remaining three compounds 240–242 appear as furoferran-type lignins [8,73,99].
Table 5.
Phenylpropanoids from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 211 | sinapic acid | P. calophylla | rhizome | [71] |
| 212 | sinapate glucose-1-ester | P. calophylla | rhizome | [99,100] |
| 213 | E-p-hydroxy-cinnamic acid | P. forrestii | stems | [88] |
| 214 | caffeic acid | P. forrestii | stems | [88] |
| 215 | ethyl caffeate |
P. forrestii
P. sepium |
stems root bark |
[101] [98] |
| 216 | (E)-1-(2,4-dihydroxy phenyl)-ethyl acrylate | P. forrestii | stems | [101] |
| 217 | trans-3,4-methylenedioxy cinnamyl alcohol | P. forrestii | whole plant | [73] |
| 218 | 6′-O-(E)-feruloyl sucrose | P. forrestii | whole plant | [73] |
| 219 | 3-O-caffeoylquinic acid |
P. forrestii
P. sepium |
roots and stems roots |
[74] [102] |
| 220 | 4-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 221 | 5-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 222 | 3-O-caffeoylquinic acid methyl ester | P. forrestii | roots and stems | [74] |
| 223 | 4-O-caffeoylquinic acid methyl ester | P. forrestii | roots and stems | [74] |
| 224 | 5-O-caffeoylquinic acid methyl ester | P. forrestii | roots and stems | [74] |
| 225 | 1,3-di-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 226 | 3,4-di-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 227 | 3,5-di-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 228 | 4,5-di-O-caffeoylquinic acid | P. forrestii | roots and stems | [74] |
| 229 | scopoletin |
P. forrestii
P. sepium P. nigrescens |
whole plant cortex and seedlings bark, leaves, seeds |
[73,85] [103] [97] |
| 230 | cleomiscosin A |
P. forrestii
P. calophylla |
root bark, stems rhizome |
[45,66] [71] |
| 231 | cleomiscosin B | P. forrestii | stems | [66] |
| 232 | (+)-lyoniresinol | P. aphylla | whole plant | [84] |
| 233 | (+)-lyoniresinol-3α-O-β-d-glucopyranoside | P. forrestii | stems | [63] |
| 234 | (-)-lyoniresinol-3α-O-β-d-glucopyranoside | P. forrestii | stems | [63] |
| 235 | tortoside B | P. sepium | root bark | [98,104] |
| 236 | (-)-gentioluteol-9-O-β-d-glucoside | P. forrestii | stems | [63] |
| 237 | (-)-berchemol 9-O-β-d-apiofuranosyl-(1→6)-O-β-d-glucopyranoside | P. forrestii | stems | [63] |
| 238 | (7S,8R)-dihydrodehydrodiconiferyl alcohol 9-O-β-d-apiofuranosyl-(1→6)- O-β-d-glucopyranoside |
P. forrestii | stems | [63] |
| 239 | 7S,7′S,8R,8′R-lcariol A2-9-O-β-d-glucopyranoside | P. forrestii | stems | [63] |
| 240 | (+)-1-hydroxypinoresinol |
P. omeiensis
P. forrestii |
whole plant whole plant |
[8] [13] |
| 241 | syringaresinol | P. forrestii | whole plant | [13,73] |
| 242 | (+)-syringaresinol-4′-O-β-d-monoglucoside |
P. forrestii
P. calophylla |
whole plant rhizome |
[73] [99,105] |
Figure 7.
Phenylpropanoids from the genus Periploca.
3.5. Flavonoids
A great variety of flavonoids (243–273, Table 6, Figure 8) have been isolated and identified from this genus, and most were obtained in the past fifteen years. Familiar flavonols, such as kaempferol, quercetin and their derivatives (243–256), are also found in Periploca species. Compounds 257–260 are four simple flavones, while 261 and 262 are two common flavanones. There are three usual chalcones and isoflavones (263–265). The genus Periploca is also characterized by containing flavanes (266–272) [63,84]. Moreover, (−)-maackiain (273) is a pterocarpan [45].
Table 6.
Flavonoids from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 243 | kaempferol | P. forrestii | unknown | [85] |
| 244 | kaempferol-3-O-α-d-arabinopyranoside | P. calophylla | rhizome | [99] |
| 245 | kaempferol-3-O-β-d-glucopyranoside |
P. calophylla
P. laevigata P. graeca |
rhizome aerial parts bark, leaves, and seeds |
[99] [106] [97] |
| 246 | kaempferol-3-O-β-d-galactopyranoside | P. forrestii | stems | [107] |
| 247 | kaempferol-3-O-β-l-arabinopyranoside |
P. forrestii
P. laevigata |
stems aerial parts |
[107] [106] |
| 248 | rutin |
P. laevigata
P. aphylla P. graeca |
aerial parts aerial parts bark, leaves, and seeds |
[106] [108] [97] |
| 249 | quercetin-3-O-β-d-glucopyranoside |
P. forrestii
P. sepium P. laevigata P. aphylla P. graeca |
stems leaves aerial parts aerial parts bark, leaves, and seeds |
[107] [86] [106] [108] [97] |
| 250 | quercetin |
P. forrestii
P. sepium |
unknown leaves |
[85] [86] |
| 251 | quercetin-3-O-β-l-arabinopyranoside | P. forrestii | stems | [107] |
| 252 | 6′-Methyl ester of quercetin-3-O-β-d-glucuronide | P. sepium | leaves | [86] |
| 253 | quercetin-3-O-α-l-rhamnopyranoside | P. aphylla | aerial parts | [108] |
| 254 | quercetin-3-O-α-l-arabinopyranoside | P. forrestii | unknown | [109] |
| 255 | baohuoside I | P. sepium | cortex | [110] |
| 256 | quercetin-7-O-β-d-glucopyranoside | P. forrestii | unknown | [109] |
| 257 | apigenin | P. nigrescens | leaves | [94] |
| 258 | isorhoifolin | P. nigrescens | leaves | [94] |
| 259 | wogonin | P. forrestii | rhizome | [42] |
| 260 | negletein | P. forrestii | rhizome | [42] |
| 261 | liquiritigenin | P. forrestii | root bark | [45] |
| 262 | 3′,4′,5,7-tetrahydroxy-flavanone-2(S)-3′-O-β-d-glucopyranoside | P. calophylla | rhizome | [99] |
| 263 | isoliquiritigenin | P. forrestii | root bark | [45] |
| 264 | daidzein | P. forrestii | root bark | [45] |
| 265 | formononetin | P. forrestii | root bark | [45] |
| 266 | (−)-epicatechin | P. forrestii | stems | [63] |
| 267 | cinchonain Ia | P. forrestii | stems | [63] |
| 268 | cinchonain Ib | P. forrestii | stems | [63] |
| 269 | proanthocyanidin A2 | P. forrestii | stems | [63,88] |
| 270 | aesculitannin B | P. forrestii | stems | [63] |
| 271 | catechin-(3′→O→3′’’)-afzelechin | P. aphylla | whole plant | [84] |
| 272 | epicatechin-(3′→O→7′’)-epiafzelechi | P. aphylla | whole plant | [84] |
| 273 | (−)-maackiain | P. forrestii | root bark | [45] |
Figure 8.
Flavonoids from the genus Periploca.
3.6. Quinones
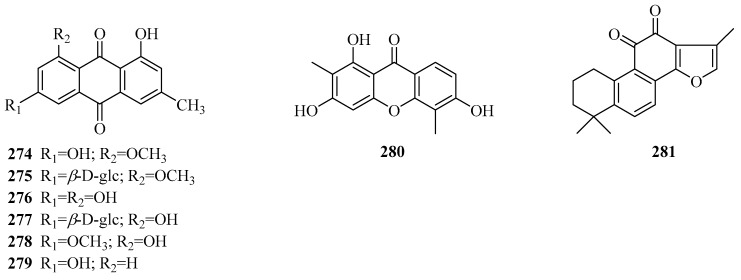
A total of eight quinone compounds (Table 7, Figure 9) has been found in the genus Periploca, and seven of them were derived from P. forrestii. Compounds 274–279 are six emodin-type anthraquinones. An anthrone (280) was reported for the first time from P. aphylla [84]. Additionally, tanshinone IIA (281) [111] possesses a diterpenoid quinone skeleton, which was discovered from the portion of P. forrestii with anti-inflammatory activity.
Table 7.
Quinones from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 274 | physcion |
P. calophylla
P. forrestii |
rhizome rhizome, root bark |
[64] [45,112] |
| 275 | physcion-8-O-β-d-glucopyranoside | P. forrestii | stems | [107] |
| 276 | emodin | P. forrestii | stems | [90] |
| 277 | emodin-8-O-β-d-glucopyranoside | P. forrestii | stems | [107] |
| 278 | emodin-8-methyl ether | P. forrestii | stems | [66] |
| 279 | chrysophanol | P. forrestii | root bark | [45] |
| 280 | 1,3,6-trihydroxy-2,5-dimethoxyxanthone | P. aphylla | whole plant | [84] |
| 281 | tanshinone IIA | P. forrestii | unknown | [111] |
Figure 9.
Quinones from the genus Periploca.
3.7. Aromatics
Twelve aromatic acids and their derivatives 282–293 (Table 8, Figure 10) were discovered from the genus Periploca. These carboxylic acid groups could be esterified with alkanols or form ester glycosides with sugar moieties. Aromatics 294–301 are eight aromatic aldehydes and their derivatives. 4-methoxy salicylaldehyde (294) was first reported in the bark of P. graeca in 1935 [113]. Compound 301 is a new diphenylmethane [44]. Compounds 302 and 303 are two aromatic ether compounds, and a new naphthalene derivative named periplocain A (304) [108] was discovered in 2016.
Table 8.
Aromatics from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 282 | vanillic acid |
P. calophylla
P. forrestii |
rhizome unknown |
[64] [66] |
| 283 | protocatechuic acid | P. forrestii | root bark | [45] |
| 284 | salicylic acid |
P. calophylla
P. forrestii |
rhizome roots and stems |
[71] [74] |
| 285 | 4-methoxysalicylic acid | P. sepium | root bark | [92] |
| 286 | syringic acid | P. forrestii | stems, roots and stems | [74,90] |
| 287 | p-hydroxybenzoic acid | P. forrestii | roots and stems | [74] |
| 288 | 2,4-dihydroxybenzoic acid methyl ester | P. forrestii | whole plant | [13] |
| 289 | 2-ethylhexyl benzoate | P. aphylla | aerial parts | [108] |
| 290 | o-phthalic acid bis(2-ethylnonyl) ester | P. aphylla | whole plant | [84] |
| 291 | erigeside C | P. calophylla | rhizome | [99] |
| 292 | 4′-hydroxy-3′-methoxy-phenol-β-d-[6-O-9-(4′’-hydroxy-3′’,5′’-dimethoxy benzoate)] glucopyranoside | P. forrestii | stems | [63] |
| 293 | 4-hydroxy-2-methoxyphenyl 6′-O-syringoyl-β-d-glucopyranoside | P. forrestii | stems | [63] |
| 294 | 4-methoxy salicylaldehyde |
P. graeca
P. sepium |
bark root bark |
[113] [16,114] |
| 295 | 4-methoxybenzaldehyde-2-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside] | P. sepium | root bark | [115] |
| 296 | 4-hydroxy-3,5-dimethoxy benzaldehyde | P. calophylla | rhizome | [64] |
| 297 | 4-hydroxy-3-methoxyl benzaldehyde |
P. calophylla
P. sepium P. forrestii |
rhizome root bark whole plant |
[71] [92] [73] |
| 298 | 4-methoxy salicylaldehyde lactoside | P. sepium | root bark | [93] |
| 299 | isovanillin |
P. sepium
P. forrestii |
root bark rhizome |
[92] [42] |
| 300 | protocatechuic aldehyde | P. forrestii | roots and stems | [74] |
| 301 | 2-hydroxy-5-(2-hydroxy-4-methoxybenzyl)-4-methoxybenzaldehyde | P. sepium | root bark | [44] |
| 302 | 3-propyl anisole | P. forrestii | rhizome | [116] |
| 303 | 2,6-dimethoxy-4-hydroxyphenol-1-O-β-d-glucoside | P. calophylla | rhizome | [116] |
| 304 | periplocain A | P. aphylla | aerial parts | [108] |
Figure 10.
Aromatics from the genus Periploca.
3.8. Others
Two ceramide compounds 305 and 306 were obtained from the trichloromethane fraction of the 80% alcohol extract of P. forrestii for the first time in 2014 [116]. There are six aliphatic compounds (308–313) and two stereoisomeric fatty esters (312,313) [63]. Obaculactone (314) is a lactone [80]. Their structures, names, corresponding sources, parts of plants and references are showed in Table 9 and Figure 11. Additionally, it should be noted that some researchers have reported that the plants of genus Periploca are rich in volatile oils, whereas the volatile oil components detected online are not listed in detail in this paper.
Table 9.
Other compounds from the genus Periploca.
| No. | Compounds | Sources | Parts of Plants | Ref. |
|---|---|---|---|---|
| 305 | 1-O-β-d-glucopyranosyl-(2S,3S,4R,10E)-2-[(2R)-2-hydroxytetracosanoylamino]-10-octadecene-3,4-diol | P. forrestii | whole plant | [116] |
| 306 | (2S,3S,4R,10E)-2-[(2R)-2-hydroxytetracosanoylamino]-10-octadecene-1,3,4-triol | P. forrestii | whole plant | [116] |
| 307 | N-(4-ethoxyphenyl)-acetamide | P. forrestii | rhizome | [112] |
| 308 | malonic acid | P. forrestii | whole plant | [13] |
| 309 | palmitic acid | P. forrestii | unknown | [85] |
| 310 | n-heptadecane | P. forrestii | unknown | [85] |
| 311 | 1-triacontanol | P. calophylla | rhizome | [71] |
| 312 | methyl (9S,12S,13S)-9,12,13-trihydroxy-10E-octadecenoate | P. forrestii | stems | [63] |
| 313 | methyl (9S,12R,13S)-9,12,13-trihydroxy-10E-octadecenoate | P. forrestii | stems | [63] |
| 314 | obaculactone | P. sepium | root bark | [80] |
Figure 11.
Other compounds from the genus Periploca.
4. Biological Activities
The extracts of the genus Periploca have been investigated for a series of biological activities since the 1920s. It has been found that they show diverse and valuable activities, including cardiotonic, anti-inflammatory, immunosuppressive, antitumor, antimicrobial, antioxidant, insecticidal and other properties. The biological activities of the genus Periploca, primarily interrelated species and active components are illustrated in Table 10.
Table 10.
Biological activities of the genus Periploca, primarily interrelated species and active components.
| Biological Activities | Interrelated Species 1 | Primarily Active Components |
|---|---|---|
| cardiotonic effect | b, c, d | periplocin |
| anti-inflammatory and immunosuppressive effects | a | periplosides; periplocin; several triterpenoids |
| antitumor ability | a, b, d, e | periplocin; periplocymarin; several pregnane glycosides; several oligosaccharides; baohuoside I; lupeol acetate |
| antimicrobial and antioxidant abilities | a, b, e, f | 4-methoxy salicylaldehyde; several lignins; several flavanes; several polysaccharides |
| insecticidal activity | a | 4-methoxysalicylaldehyde; periplosides |
| other abilities | a, b | several pregnane glycosides; several cardenolides; several triterpenes |
1 a: P. sepium; b: P. forrestii; c: P. calophylla; d: P. graeca; e: P. laevigata; f: P. angustifolia.
4.1. Cardiotonic Effect
As early as the 1920s, periplocin (1) was detected to show digitalis-like cardiotonic capacity. Periplocin enhanced the tonus and the contractility of the cardiac muscle, as well as the tonus of the arterial muscle, whereas it led to cardiac irregularity and systolic arrest of the rat heart at excessive doses [117]. Additionally, a 10% infusion gave rise to a decrease of the frequency of the heartbeat and an increase of the tonus, arterial pressure and diuresis [118]. Through its effects on frog and rabbit hearts in situ and isolated guinea pig hearts, the total glycosides fraction extracted from the fresh stem bark of P. forrestii displayed that its cardiotonic property was analogous to that of g-strophanthin, and the average lethal dose in pigeons was 5.9 ± 1.0 mg/kg. Concerning the changes of rabbit electrocardiograms, both compounds produced the phenomena of prolonged R-R and P-R intervals, a shortened QRS interval and a flattened T wave [119]. The ethanol extract of P. calophylla, with an average lethal dose in pigeons of 31.86 ± 1.62 mg/kg, was also proven to be equipped with cardiotonic function via experiments on frog and rabbit hearts in situ, isolated guinea pig hearts, and through electrocardiograms in cats [120]. Wang’s group discovered that periplocin could improve the left ventricular structure and function in rats with chronic heart failure at a dose of 8 mg/kg [121]. They inferred that the underlying mechanism of periplocin against heart failure might involve its ability to increase the expression of sarco endoplasmic reticulum Ca2+-ATPase mRNA, decrease the expression of phospholamban mRNA and improve the value of PLB/SERCA in chronic heart failure-model rats [122] The proliferative activity of periplocin was verified in mouse cardiac microvascular endothelial cells by Gao and co-workers [123]. Compared with periplocin (1), periplogenin (2) and xysmalogenin (25) both showed positive inotropic and negative chronotropic effects on isolated rat hearts, but their activities were weaker and failed more quickly [124].
4.2. Anti-Inflammatory and Immunosuppressive Effects
Pharmacological tests determined that the ethanol extracts, volatile oils and water extracts mainly containing polysaccharides of P. forrestii all had anti-acute inflammation properties [125,126,127]. The ethanol extract of P. forrestii displayed anti-inflammatory activity which was connected to reducing the protein expression of inducible nitric oxide synthase and cyclooxygenase-2 (COX-2); to the production of inflammation factors, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, nitric oxide and prostaglandin E2 (PGE2); and, mainly, to the suppression of lipopolysaccharide-mediated stimulation of the nuclear factor-κB (NF-κB) and mitogen-activated protein kinases signaling pathways [128]. Additionally, the extract of P. laevigata was also able to ameliorate carrageenan-induced edema and acetic acid-induced abdominal writhing, in addition to enhancing the latency in a hot plate test [129]. In 1997, three triterpenes from P. sepium, named α-amyrin (193), α-amyrin acetate (195), and β-amyrin acetate (182), were shown to have anti-inflammatory effects in animal experiments [130]. At a dose of 20 μg/mL, Periplocin (1) could distinctly inhibit histamine release from mast cells cultured in vitro and from mast cells of sensitized rats. As such, periplocin was considered to be one of the effective anti-inflammatory ingredients of P. sepium by Gu’s group [131]. It is worth noting that periplocoside A (49) showed a sufficiently preventative effect on concanavaline A-induced hepatitis by inhibiting natural killer T-derived inflammatory cytokine production, which suggested that periplocoside A possesses therapeutic potential for the treatment of human autoimmune-related hepatitis [132].
In 2005, periplocoside E (47) was first found to specifically and markedly inhibit the activation of extracellular signal-regulated kinase and Jun N-terminal kinase, while the activation of p38 was not influenced by T cells stimulated with anti-CD3, showing its potency for the treatment of T cell-mediated disorders [133]. In addition, its immunosuppressive efficacy was confirmed again by the effect of treatment with periploside A (47) on a positive selection of thymocytes from C57BL/6 mice in vitro [134]. After finding that the crude extract of P. sepium and periplocoside E had the ability to inhibit the proliferation of T cells, Zhao’s group carried out a systematic chemical investigation and searched for immunosuppressive compounds, making vital contributions to this domain. Compared with the immunosuppressants rapamycin (IC50 0.19 μM) and cyclosporin A (IC50 0.27 μM), nine pregnane glycosides (47,49–55,58) characterized by an orthoester group displayed significant inhibitory activities against the proliferation of T lymphocytes in vitro, with IC50 values ranging from 0.29 to 1.97 μM. However, other pregnane glycosides without the orthoester group showed no apparent inhibitory activity [33]. Twelve spiro-orthoester group-containing periplosides (49,59–69) were evaluated for their inhibitory activities. Among them, periploside C (49), the most abundant spiro-orthoester moiety-bearing component in the root bark of P. sepium, exhibited the best selective index (SI) value at 82.5. More notable is that the length and constitution of the saccharide chain in this compound class have a crucial impact on both the suppressive activity and the SI value. Nevertheless, the substitution of a formyl group in replacement of the 4,6-dideoxy-3-O-methyl-Δ3-2-hexosulose function at the C-3 position of the aglycone might not affect this activity in periplosides [38]. Additionally, Zhao’s group demonstrated the potential mechanism of the immunosuppressive ability of periplocoside A (periploside C, 49). Though inhibiting IL-17 production and suppressing the differentiation of Th17 cells in vitro, the oral administration of periplocoside A ameliorated experimental autoimmune encephalomyelitis [135]. The other four compounds of the same type (70–73) were equipped with notable inhibitory activities against the proliferation of B and T lymphocytes, and advantageous selective index values comparable to those of cyclosporin A, which perfectly supplemented the immunosuppressive potency of the periplosides [39].
In 2006, lupane acetate (198) was found to induce the differentiation and maturation of dendritic cells, upregulate cytokines production, and enhance the immune activity of dendritic cells in vitro [136]. The ability of lupane acetate to increase the immune response of lymphocytes and macrophages from human peripheral blood might be associated with its ability to kill carcinoma cells [137]. Additionally, periplocin (1) was also discovered to protect the immune organs of tumor-bearing mice, obviously enhance the lymphocyte proliferation in mice spleens, and stimulate the production of TNF-α, IL-2 and IL-12, which indicated that it had immunoregulatory effects [138]. The polysaccharide (HGT-5A) from P. forrestii showed immunosuppressive effects on the cellular immune response and that its four ingredients played a major role [139]. However, three oligosaccharides (154–156) displayed no activity against the proliferation of T lymphocytes in vitro [33]. In 2017, by in vitro experimentation, suppressing hyaluronidase, microwave extraction combined ultrasonic pretreatment of flavonoids from P. forrestii were found to produce resistance to allergies with an IC50 value of 1.033 mg/mL [140].
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease, and extracts of P. forrestii and P. sepium showed favorable anti-arthritis efficacies. In 2004, the aqueous extract of P. sepium was found to inhibit the growth and IL-6 production of human rheumatoid arthritis-derived fibroblast-like cells in a dose-dependent manner [141]. The water-soluble components of P. forrestii could suppress the inflammatory response in collagen-induced arthritic rats through regulating the expression of the TAK-1, TAB-1, NF-κB p65, MCP-1 and CXCL-1 proteins [142]. Huang’s group [143] revealed that a 60% ethanol extract of P. forrestii possessed activity against RA capacity. They inferred that the potential mechanism might be associated with the regulation of immune organ function and the levels of pro-inflammatory cytokines containing IL-1β, IL-6 and TNF-α. Liang and co-workers [144] drew a conclusion that the effect of a 50% ethanol extract of P. forrestii on RA was concerned not only with restraining the proliferation of rheumatoid arthritis synovial fibroblasts but also with the downregulation of the expression of COX-2 and PGE2. Through further investigation, Feng’s group [145] provided new insight into the underlying mechanism of the therapeutic action in collagen-induced arthritis (CIA). By inhibiting the activation of Src kinase and the nuclear translocation of NF-κB in rats, the ethanol extract of P. forrestii was shown to be a potential candidate for the treatment of patients with RA. The anti-arthritis effect of P. forrestii was discovered to be positively correlated with the contents of total flavonoids and total saponins, and the influence of the total saponins was greater than that of the total flavonoids [146]. Sun’s group [147] verified that P. forrestii saponin and periplocin (1) performed prophylactic therapeutic action against autoimmune arthritis by controlling the systemic autoimmune responses and local inflammation and bone destruction of the joints. Additionally, they deduced that the protective mechanism of P. forrestii saponin toward CIA had something to do with regulatory actions on proinflammatory factors, as well as on the crosstalk between NF-κB and c-Fos/AP-1 in vivo and in vitro [148]. Another recent biological study of the cardenolide-rich and caffeoylquinic acid-rich fractions of P. forrestii demonstrated that the inhibitory activities toward the NF-κB and MAPK signaling pathways might be involved in the antiarthritic effect based on a set of experimental results [149].
4.3. Antitumor Ability
Abundant experiments concerning the antitumor activities of P. sepium were carried out by Shan and co-workers. Above all, Shan’s group explored the role of periplocin (1) in affecting human cancer cell lines in vitro from 2005 to 2017. They found that periplocin expressed a noteworthy inhibitory effect on diverse cancer cell lines—including hepatocellular carcinoma cells, colon carcinoma cells, lung carcinoma cells, and breast carcinoma cells—basically though blocking the cell cycle and inducing apoptosis [150,151,152,153]. Furthermore, the potential antitumor mechanisms of periplocin were inferred by Shan’s group. Periplocin was detected to suppress the proliferation and induce apoptosis of the human hepatocarcinoma cell line SMMC-7721 via restraining Stat3 signal transduction [150]. By the downregulation of the Wnt/β-catenin signaling pathway, periplocin (0.5 μg/mL) markedly inhibited proliferation of the human colon carcinoma cell line SW840 [151]. The probable mechanism by which periplocin induced apoptosis in the human lung cancer cell line A549 was related to the downregulation of the survivin mRNA and protein [152]. In addition, periplocin had an effect on breast carcinoma MCF-7 cells with the IC50 value of 4.88 ± 0.16 ng/mL, which was associated with the increased expression of the mRNA and protein of gene p21WAF1/CIP1 [153]. Additionally, periplocin (1) also displayed the ability to suppress the growth of human (A549) and mouse (LL/2) lung cancer cells in vitro and in vivo by blocking the protein kinase B/extracellular signal regulated kinase signal pathway [154]. In 2013, Pan’s group deduced that periplocin (1) sensitized TNF-related apoptosis-inducing ligand-resistant human hepatocellular carcinoma cells via inducing the expression of the death receptors 4 and fas-associated death domain and the downregulation several inhibitors of apoptosis, which resulted in the activation of caspases 3, 8, and 9 and led to cell apoptosis [155]. Moreover, due to the inducing of DNA double strand breaks and death-receptor mediated apoptosis in liposarcoma cells, periplocin (1) was considered to be a promising lead compound for the development of new sarcoma therapeutics by Bauer’s group [156].
Additionally, periforoside E (27) showed higher cytotoxicity toward the A549 line than toward other lines. Notable is that 3β,5β-dihydroxy-14-en-card-20(22)-enolide (26) and periforoside D (45) were both inactive in terms of cytotoxic activity, indicating that the 14-hydroxy group and the C/D ring junction were responsible for this effect [19]. Moreover, the three 17α-cardiotonic steroids (38-40) showed apparently higher IC50 values (5.4 and 7.3 μM) in vitro in the hormone-independent prostate cancer cell line PC3 than those of 17β-isomers. Additionally, in the 17α-cardenolides, the presence of sugar units of these cardenolides had nothing to do with the IC50 values. However, a fascinating series of 17β-cardiotonic steroids (1,3–5,24), with a 14β hydroxyl group and at least one sugar molecule, demonstrated strong antiproliferative effects with IC50 values between 18 and 50 nM [15]. Four cardenolides (1–3,38) were tested in the human monocytic leukemia U937 and androgen-independent prostate adenocarcinoma PC3 cell lines. Periplocin (1) and periplocymarin (3) showed more effective activity toward tumor cells due to the activation of apoptotic pathways in PC3 cells and in U937 cells due to the induction of cell cycle impairment [157]. More interesting is that periplocymarin (3) was unlikely to encounter drug–drug interactions with P-glycoprotein and cytochrome P450s, suggesting that it should be taken forward for further investigations in drug development [158].
In addition to cardenolides, other types of compounds have been examined for their cytotoxic activities. Periseosides C (93) showed moderate inhibitory activity against the ConA-stimulated splenocyte proliferation in vitro [40]. Two C21 pregnane-type steroids (52,77) and four known oligosaccharides (168,155,162,163) were tested for their cytotoxicities against the A-549 and HepG2 human cancer cell lines with IC50 values ranging from 0.61 to 7.68 μM by Wang’s group [43]. Interestingly, compound 168, a derivative of 163 with the acetyl group at C-2 of the β-D-digitalopyranosyl moiety, exhibited moderate activities, while 163 and others were not active, which implied that the presence of an acetyl group might be related to the cytotoxic activity. Besides, Shan’s group discovered that baohuoside-I (255) restrained the proliferation of and arrested Eca109 human esophageal squamous carcinoma cell cycles, which might be associated with the downregulation of Cyclin B1 mRNA expression [159]. As the research continued, they reported that baohuoside-I (255), by inhibiting β-catenin-dependent signaling pathways, significantly inhibited the proliferation of the cells in a time- and dose-dependent manner with the IC50 value of 24.8 μg/mL at 48 h and induced apoptosis of Eca109 cells in vitro and in vivo [160]. Coincidentally, lupeal acetate (198) was also found to reduce the incidence of N-nitrosomethylbenzylamine-induced esophageal tumors from 93.3% to 33.3% in rats after twenty-five weeks of treatment, which might have occurred through the activation of glycogen synthase kinase-3β expression and the inhibition of β-catenin and c-myc expression [161].
To search for potent compounds against carcinoma disease, various extracts of the genus Periploca have been studied continually. In the 1980s, Japanese scholars were the first to discover that the methanol extract of P. sepium was equipped with significant anticancer activity toward sarcoma 180 ascites in mice with ascites cancer [36,41]. The ethanol extract of P. sepium had effects on the proliferation and apoptosis of human esophageal carcinoma cells, and this action might be related to the upregulation of Rb protein expression and downregulation of the expression of apoptosis suppressor genes and the induction of apoptosis [162,163]. Additionally, the ethanol extract of P. sepium was suspended in water and extracted by ethyl acetate, and the ethyl acetate extract was capable of inducing apoptosis in human esophageal carcinoma TE-13 cells because of the downregulation of cyclin-dependent kinase 4 expression [164]. Analogously, the ethanol extract of P. forrestii stems was suspended in water and extracted sequentially with four different polar solvents. Among them, ethyl acetate extract emerged as having the strongest inhibitory property against the proliferation of K562 tumor cells, with the maximum inhibitory of 80. 71% at 25 mg/L, from which periplocin was isolated [165]. In 1995, Umehara and co-workers [51] suspended the methanol extract of P. sepium in water and extracted it with ethyl acetate. The water layer displayed differentiation-inducing activity against mouse myeloid leukemia cells. Later, the effect of the water extract of P. sepium on the differentiation of K562 tumor cells was detected, which showed that this extract could kill K562 cells but could not induce differentiation [166]. The water extract of P. sepium also inhibited the growth of gastric carcinoma BGC-823 cells and induced apoptosis, both of which which was associated with the downregulation of the expression of B-cell lymphoma-2 and surviving genes and proteins and to the upregulation of the expression of bax genes and proteins [167].
In brief, the complex mixtures and some pure chemical components from the plants of genus Periploca have presented remarkable cytotoxicity activities toward about ten different carcinoma cells, while a large proportion of investigations have focused on cytotoxic assays in vitro. For the sake of discovering antitumor lead compounds, there are still many studies worth further development.
4.4. Antimicrobial and Antioxidant Abilities
Triterpenes from this genus have been known to exhibit antibacterial properties since 2000. 3β,6α-dihydroxylup-20(29)-ene (205) showed moderate activity against Staphylococcus pyogenes [67]. Based on the antibacterial activities of oleanolic acid (172), maslinic acid acetate, β-amyrin (181) and their derivatives, Jegham’s group [168] speculated the antibacterial structure-activity relationship of triterpenes. The free β-hydroxyl group at C-3 and the β-oriented carboxyl function at C-17 were closely associated with the antibacterial activities. Interestingly, the amine linked to the triterpenoid skeleton contributed to the activity against Pseudomonas aeruginosa, one of the bacteria most resistant to antibiotics and antiseptics.
The petroleum ether extract with the highest content of palmitic acid (309) from P. forrestii was detected to have antimicrobial properties [169]. Due to containing relatively high proportions of aldehydes and oxygenated monoterpenes, the essential oil from P. laevigata root bark was equipped with significant antibacterial and antifungal activities [170]. Larhsini and co-workers [171] found that the essential oil from P. laevigata leaves showed antimicrobial and antioxidant activities and had a good synergistic effect in association with conventional antibiotics. The root bark essential oil of P. sepium and 4-methoxysalicylaldehyde (294), a main component of the oil (78.8% of the total), were both elaborated to possess antimicrobial properties and moderate antioxidant activities, which indicated that the major portion of these antimicrobial and antioxidant activities was due to the presence of the aldehyde in the oil [172]. In addition, the activities of 4-methoxysalicylaldehyde (294) isolated from P. sepium oil and its six derivatives against six foodborne bacteria were evaluated by Lee’s group [173]. Compound 294 (MIC 30.1–67.3 μg/mL) and 4-hydroxysalicylaldehyde (MIC 41.1–61.5 μg/mL) both inhibited the growth of the six foodborne bacteria with finer effects than did the other derivatives.
The antioxidant capacity of phenols is intimately related to their content and the number of hydroxyl groups. The methanol extract with the highest contents of phenolics and flavonoids from P. laevigata displayed the greatest antioxidant properties, followed by the water and ethyl acetate extracts [174]. Intriguingly, six lignins (236,237) were evaluated for their antioxidant abilities, and they showed no or minor antioxidant activities. Feng’s group illustrated the probable structure-activity relationship. The more original hydroxyl groups of phenols are replaced by methoxyl groups, the lower the antioxidant activity is [63]. They also detected that flavanes (266–268,270) which exerted high radical-scavenging activities with IC50 values ranging from 14.61 to 28.80 μM. Additionally, aesculitannin B (270), a flavane trimer, was able to shrink α-melanocyte-stimulating hormone-induced melanogenesis by inhibiting the expression of microphthalmia-associated transcription factor and tyrosinase in a dose-dependent manner, owing to the multiple phenolic hydroxy groups [63]. Bisflavan-3-ols 271 and 272 from the ethyl acetate fraction of P. aphylla displayed evident inhibitory potential against the enzyme lipoxygenase with IC50 values of 19.7 and 13.5 μM. Malik and co-workers speculated that the stereochemistry of 272 was probably more conducive to the inhibition of lipoxygenase enzyme than that of 271 [84].
In the most recent three years, polysaccharides acquired from this genus have also been detected to show antioxidant and antibacterial capacities. Three crude Cortex Periplocae polysaccharides (CPPs 1–3) from the root bark of P. sepium presented evident antioxidant activities in four assays in vitro [175]. A novel polysaccharide (PLP1) from P. laevigata root bark displayed antioxidant activity in a dose-dependent manner and showed strong antibacterial activity against several Gram (+) and Gram (−) strains. Hence, it was shown to be a promising source for polysaccharides in the medical and food industries [176]. The polysaccharides isolated from P. angustifolia (PAPS) were shown to decrease the rates of lipid peroxidation and protein glycation in vitro. Furthermore, PAPS could defend human embryonic kidney cell HEK293 against oxidative stress and renal tissue against cadmium toxicity [177].
Furthermore, the antioxidant capacity of PAPS was demonstrated by the hepatoprotective potency toward cadmium chloride (CdCl2)-induced toxicity in rats, and it could ameliorate the alteration of liver tissue [178]. Athmouni’s group [179] testified that the methanolic extract of Periploca angustifolia leaves also possessed antioxidant ability and exerted salutary effects in the treatment of Cd-induced hepatotoxicity. The methanol extract of P. hydaspidis, which is a less-studied species found in Pakistan, had the ability to adjust the levels of various parameters provoked by CCl4 toxicity in a dose-dependent manner due to the presence of antioxidant and anti-inflammatory constituents in the methanol extract [180]. What is striking is that ZnO nanostructures with tremendous potential against multidrug-resistant E. coli, S. marcescens and E. cloacae were synthesized through reduction by P. aphylla aqueous extract without the utilization of any acid or base. In this manner, the aqueous extract of P. aphylla was proven to be a valid chelating agent [181].
4.5. Insecticidal Activity
Since 2004, extracts from the plants of genus Periploca have been found to possess favorable insecticidal activities and promising agricultural value. The ethanol extract from the root bark of P. sepium had a significant repellent effect against the oviposition of imported cabbage worms [182]. Additionally, the benzene fraction from that ethanol extract showed strong antifeeding and poisoning effects on Plutella xylostella larvae [183]. The methanol extract of P. aphylla showed activity against Leishmania infantum (IC50 6.0 μg/mL, SI 4.0) [184]. The volatile oil from the root bark of P. sepium showed forceful contact-killing activity against Myzus avenae, and its main component was 4-methoxysalicylaldehyde (294), which also displayed antinematodal activity against Bursaphelenchus xylophilus at a minimum effective dose of 200 μg/ball [114,185]. In 2012, it was also proven that the dominating component in the oil (294) was active against Drosophila melanogaster L. and the maize weevil (Sitophilus zeamais) with the LD50 values of 1.47 and 6.99 mg/adult, respectively [186]. In addition, the acaricidal ability of 4-methoxysalicylaldehyde (294) was greater than that of synthetic acaricides [187].
Zeng’s group discovered periplocoside X (80), which induced severe time-dependent cytotoxicity in the midgut epithelial cells of the red imported fire ant and inhibited amylase activity (Solenopsis invicta). Therefore, the ant insect midgut was deduced to be a target of periplocoside X [188]. It is noteworthy that, in addition to periplocoside X (80), there are some other periplocosides capable of impacting the digestive system of insects. Hu’s group has made vital contributions to the discovery of these periplocosides and has clarified the underlying mechanism. Periplocoside NW (82) [48,189,190] displayed potent stomach toxicity against some insect pests, including third-instar larvae of P. xylostella, Mythimna separata larva and Pieris rapae. The membrane system in insect midgut epithelial cells was the initial action site of periplocoside NW against insects. In 2012, periplocoside D (50) and F (52) were both detected by bioassay tests, and they showed stomach toxicity activities against third-instar larvae of M. separata with the LC50 values of 0.39 and 0.34 mg/mL, and against third-instar larvae of P. xyllostella with the LC50 values of 1.21 and 1.39 mg/mL, respectively [191]. The insecticidal activities against the third-instar larvae of Pseudaletia separata and P. xylostella of periplocoside P (81) were detected by an insecticidal activity bioassay.
To explore the insecticidal mechanisms of these compounds, some studies were implemented by Hu’s group. Periplocoside P caused toxicity toward M. separate though activating the weakly alkaline trypsin-like protease [192] and inhibiting the activity of cytochrome P450 in the midgut. Additionally, glutathione-S-transferase might be involved in insect detoxification [193]. Through their in-depth research exploring new pesticides and their action mechanisms against insects, Hu’s group found that the apical and basolateral membrane voltage (Va) rapidly decreased in the presence of periplocoside P in a time- and dose-dependent manner, similar to the effects of Cry1Ab. They speculated that periplocoside P adjusted the transport mechanisms at the apical membrane of the midgut epithelial cells by inhibiting the V-type H+ ATPase [194]. In addition, the V-type ATPase A subunit in the midgut epithelium might be regarded as the target binding site of periplocosides, which provided preliminary evidence for the mode of action of periplocosides [195]. Furthermore, aminopeptidases N and N3 of the midgut epithelium cells of M. separata larvae were extrapolated as potential targets of periplocoside E (47) by affinity chromatography [196].
4.6. Other Abilities
As early as the 1980s, glycosides K, H1 and H2 from P. sepium showed the potentiation of NGF-mediated nerve fiber outgrowth in organ cultures of chicken embryonic dorsal roots and sympathetic ganglia, and glycoside H2 (97) had the best effect [53]. Later, in 2012, the alcohol extract of P. forrestii led to a good reparation of nerve injury in Pristionchus pacificus caused by thermal stimulation in the concentration range of 2–16 mg/mL, while obvious toxicity was also detected when the concentration was increased [197]. Intriguingly, the sedative and potent analgesic effects of the methanolic extract of P. aphylla at the dose of 500 mg/kg were shown in a series of animal experiments [198].
P. forrestii, a folk medicine used to cure wounds, is also equipped with wound-healing activity. Zhang’s group [199] evaluated its ability through the proliferation of fibroblasts, migration and collagen production using L929 cells. The 65% ethanol eluate mainly composed of cardiac glycosides was the most effective fraction, suggesting that the cardiac glycosides may be the potential active ingredients in wound healing. In further research, periplocin (1) demonstrated its ability to significantly boost proliferation and migration and stimulate collagen production in fibroblast L929 cells. Its mechanism of promoting wound healing was intensely associated with the activation of the Src/ERK and PI3K/Akt pathways mediated by Na/K-ATPase [200]. Moreover, Abdel-Monem and co-workers [10] reported that ursolic acid played an important role in the protective effect against CCl4-induced injury on human hepatoma cell line (Huh7). Additionally, the antiacetylcholinesterase activities of triterpenes 199 and 198 with esterification of the alcohol function at C-3 were lower than that of lupeol (197). Ben Jannet’s group speculated that the triterpenic skeleton and the free secondary alcohol function at C-3 could be responsible for the inhibition ability [87]. In addition to the abovementioned abilities, both lipoxygenase inhibitory activity [84] and antimalarial activity [201] of this genus were detected but without further studies on the mechanisms of these actions.
5. Toxicology
Excessive or prolonged use of P. sepium leads to toxic effects, mainly consisting of cardiotoxicity and hepatorenal toxicity. Acute toxicity tests in mice and long-term toxicity tests in rats showed that the toxicity of ethanol extract from the root bark of P. sepium was greater than that of the water extract [202,203].
The water extract from the root bark of P. sepium was dried into powder, in which the content of periplocin was 21.91 mg/g. After the water extract was injected intraperitoneally into guinea pigs, their electrocardiograms showed obvious changes, and the incidence of abnormal electrocardiograms was proportional to the dose [204]. Sun’s group found that within seven days after oral administration, the water extract (0.78–15.0 g/kg) and alcohol extract (0.78–6.0 g/kg) from the root bark of P. sepium both caused obvious hepatotoxic damage in mice, which was represented by increased levels of alanine transaminase, aspartate transaminase, alkaline phosphatese and total bilirubin and a decreased content of albumin, as well as increases of the ratio of the liver to kidneys to different degrees [205]. The pathway of liver injury was inferred to be related to lipid peroxidation induced by oxidative stress [206]. Furthermore, periplocin (1) was revealed to have toxic effects at a certain dose. Deng and co-workers [207] detected that the maximum tolerable dose for the oral administration of periplocin in mice was 103 mg/kg. Periplocin was injected intraperitoneally into mice, resulting in an LD50 value of 15.20 mg/kg. In addition, periplocin at 0.39 mg/kg brought about electrocardiogram abnormalities in half of the tested guinea pigs. Noteworthy is that the content of periplocin had a tight correlation with the acute toxicity test results of the water extract from the root bark of P. sepium, while the content of 4-methoxysalicylaldehyde (294), a quality control index of Cortex Periplocae in the Chinese Pharmacopoeia (2015 version), did not directly affect the results of acute toxicity [208].
It was demonstrated that the oral bioavailability of periplocin (1) in rats was low [209]. Gao’s group [210] studied periplocin in the field of pharmacokinetics. After a single oral administration of periplocin at 50 mg/kg to rats, it was found that periplocin could not be detected at any time point, while the mean plasma profiles of its two metabolites periplocymarin (3) and periplogenin (2) were regular. Intriguingly, two metabolites participated in the pharmacokinetic process, and the main constituent in the blood was periplocymarin. In addition, periplocin could reach its peak concentration quickly after oral administration, whereas the two metabolites reached maximum concentrations more than 4.83 h after administration. It is worth noting that the tissue distributions of periplocin and two metabolites were found to include the heart, liver, spleen, lung and kidneys, but a small amount of chemical constituents were distributed in the brain [211].
To date, some toxicity studies on cardiac glycosides from the genus Peripioca have been carried out, but toxicity tests on other compounds are absent. To improve the safety of clinical therapy, comprehensive and in-depth investigations of the pharmacology and toxicity of plants of the genus Peripioca are necessary.
6. Conclusions
In this review, the botanical classification, chemical constituents, biological activities and toxicology studies of Periploca species were systematically summarized. There are differences in the classification of this genus at home and abroad, and some new species have not yet been included in flora. Currently, 314 diverse metabolites have been isolated from the genus Periploca, and are they are composed of steroids, carbohydrates, terpenoids, phenylpropanoids, flavonoids, quinones, aromatics, and so on. Steroids obtained from the genus Periploca account for the largest proportion. Moreover, these steroids, mainly composed of C21 pregnane and cardiotonic steroids, should be considered the characteristic compounds of this genus. Oligosaccharides, which have a high rate of new compounds (78.3%) featuring multifarious deoxysugar and non-deoxysugar, strictly conform to the regularity of the saccharide chains in the cardenolides and C21 pregnane glycosides of this genus. Hence, these oligosaccharides could also be regarded as representative compounds of this genus. Furthermore, Periploca species are rich in aromatic compounds and volatile oils, especially P. sepium. However, only 4-methoxy salicylaldehyde is assigned as the index component for the quality evaluation of P. sepium root bark in the Chinese Pharmacopoeia (2015 Version), which requires its content to be not less than 0.20% [3]. Other components characterized by a high content, favorable pharmacological activity or potential toxicity should perhaps also be used as legal quality evaluation indicators.
In regard to biological activities, the genus Periploca has received increasing attention over the last fifteen years. The relationships between various activities and their main related plant sources and active ingredients are concisely concluded. It is worth noting that periplocin is a research hotspot and has been demonstrated to possess various activities, such as cardiotonic, antitumor, immunosuppressive and wound-healing effects. However, periplocin has hepatotoxicity and cardiotoxicity at certain doses, suggesting that further pharmacological, toxicological and pharmacokinetics studies on this active compound are necessary. In addition, a series of periplosides with fascinating immunosuppressive and insecticidal properties should be another research focus. In view of periplosides’ available immunosuppressive effect in vitro, detailed animal experiments in vivo and the structure-activity relationships of the periplosides should also be studied to identify new immunosuppressants for the treatment of RA. More importantly, periplosides may be promising insecticidal alternatives, which is of great value to agriculture. Thus far, most experiments of biological activities of the genus Periploca have been conducted in vitro. Taking into account the important medical and agricultural value of this genus, intensive studies on the bioactivity-guided chemical isolation, pharmacological activities in vivo, and underlying mechanisms of the biological activities of this genus should be further performed.
Acknowledgments
We thank Qin Tian from Guizhou University for kindly providing the photograph of the fruit of P. forrestii. Thanks to Haifen Bu from Yancheng Teachers’ University for editing the pictures.
Author Contributions
M.H. classified the pharmacological literatures, wrote the original manuscript and revised the review. S.S. classified the chemical constituents, drafted the chemical formulas, wrote the original manuscript and managed the references. C.L. and Y.R. revised the review critically for important intellectual content.
Funding
This work was supported by the Construction Program of Biology First-Class Discipline in Guizhou (No. GNYL2017009), the Guizhou Science & Technology Foundation (No. QKHJ20132095) and the Doctor Foundation of Guizhou University (No. GDRJ2010018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stevens P.F. Angiosperm Phylogeny Website. [(accessed on 15 July 2019)];2017 Available online: http://www.mobot.org/MOBOT/research/APweb/
- 2.Flora of China Editorial Committee . Flora of China. Volume 16. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MO, USA: 1995. pp. 189–196. [Google Scholar]
- 3.National Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. Beijing Chemical Industry Press; Beijing, China: 2015. pp. 257–258. [Google Scholar]
- 4.Editorial Board of Chinese Materia Medica, State Administration of Traditional Chinese Medicine . Miao Medicine Volume of Chinese Materia Medica. Volume Miao Medicine. Guizhou science and technology press; Guiyang, China: 2005. pp. 526–527. [Google Scholar]
- 5.Zhang Y.H., Wang F.P. Recent adcances of chemical constituents of Periploca plants. Nat. Prod. Res. Dev. 2003;15:157–161. [Google Scholar]
- 6.Luo J.R., Fu Q.J. Advances of research of Periploca Plants. J. Dali Univ. 2006;5:54–58. [Google Scholar]
- 7.Venter H.J.T. A revision of Periploca (Periplocaceae) S. Afr. J. Bot. 1997;63:123–128. doi: 10.1016/S0254-6299(15)30723-7. [DOI] [Google Scholar]
- 8.Zhang Y.H., Chen D.L., Wang F.P. Studies on chemical constituents of Periploca omeiensis. Nat. Prod. Res. Dev. 2006;18:772–774. [Google Scholar]
- 9.Yao D.S., Chen X.C., Ren J.W. A new species of Periploca from Gansu province. Bull. Bot. Res. 2002;22:259–260. [Google Scholar]
- 10.Azza R.A.M., Zeinab A.K., Ashraf B.A.N., Essam A.S. A new triterpene and protective effect of Periploca somaliensis Browicz fruits against CCl4-induced injury on human hepatoma cell line (Huh7) Nat. Prod. Res. 2015;29:423–429. doi: 10.1080/14786419.2014.950960. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann E. Active principles contained in the bark of periploca graeca. Arch. Der. Pharm. 1897;235:157–176. doi: 10.1002/ardp.18972350113. [DOI] [Google Scholar]
- 12.Tan X.H., Zhang Y.H., Zhou L., Tan M.C., Sun Q.Y., Hao X.J. Study on chemical constituents of Periploca calophylla. China Pharm. (ChongqingChina) 2010;21:4463–4465. [Google Scholar]
- 13.Zhang Y.H., Yang Y.T., Chen D.L., Wang F.P. Chemical constituents from Periploca forestti. Chin. Tradit. Herb. Drugs. 2006;37:345–347. [Google Scholar]
- 14.Sakuma S., Ishizone H., Kawanishi S., Shoji J. On the structure of glycoside G and K of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1969;17:2183–2185. doi: 10.1248/cpb.17.2183. [DOI] [PubMed] [Google Scholar]
- 15.Spera D., Siciliano T., Tommasi N., Braca A., Vessières A. Antiproliferative cardenolides from Periploca graeca. Planta Med. 2007;73:384–387. doi: 10.1055/s-2007-967133. [DOI] [PubMed] [Google Scholar]
- 16.Shoji J., Kawanishi S., Sakuma S., Shibata S. Studies on the chemical constituents of Chinese drug Wujiapi. Chem. Pharm. Bull. (Tokyo) 1967;15:720–723. doi: 10.1248/cpb.15.720. [DOI] [PubMed] [Google Scholar]
- 17.Stoll A., Renz J. Cardiac glucosides. XV. Periplocin, the true cardiac glucoside of Periploca graeca. Helv. Chim. Acta. 1939;22:1193–1208. doi: 10.1002/hlca.193902201153. [DOI] [Google Scholar]
- 18.Deng Y.R., Wei Y.P., Yin F., Yang H., Wang Y. A new cardenolide and two new pregnane glycosides from the root barks of Periploca sepium. Helv. Chim. Acta. 2010;93:1602–1609. doi: 10.1002/hlca.200900320. [DOI] [Google Scholar]
- 19.Li Y., Liu Y.B., Yu S.S., Chen X.G., Wu X.F., Ma S.G., Qu J., Hu Y.C., Liu J., Lv H.N. Cytotoxic cardenolides from the stems of Periploca forrestii. Steroids. 2012;77:375–381. doi: 10.1016/j.steroids.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Wu X.F., Li J.B., Wang Y.H., Yu S.S., Lv H.N., Qu J., Abliz Z., Liu J., Liu Y.Y., et al. Identification of cardiac glycosides in fractions from Periploca forrestii by high-performance liquid chromatography/diode-array detection/electrospray ionization multi-stage tandem mass spectrometry and liquid chromatography/nuclear magnetic resonance. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010;878:381–390. doi: 10.1016/j.jchromb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y.J., Mu Q.Z. The chemical constituents of Periploca forrestii. Acta Bot. Yunnanica. 1989;11:465–470. [Google Scholar]
- 22.Zhang Y.H., Chen D.L., Wang F.P. Two novel cardiac glycosides from Periploca forestti. Chin. J. Org. Chem. 2006;26:329–332. [Google Scholar]
- 23.Xu J.P., Takeya K., Itokawa H. Pregnanes and cardenolides from Periploca sepium. Phytochemistry. 1990;29:344–346. doi: 10.1016/0031-9422(90)89071-G. [DOI] [Google Scholar]
- 24.Schenker E., Hunger A., Reichstein T. Glycosides and aglycons. CXXXIV. The glycosides of Periploca nigrescens Afzel. Helv. Chim. Acta. 1954;37:1004–1036. doi: 10.1002/hlca.19540370408. [DOI] [Google Scholar]
- 25.Berthold R., Wehrli W., Reichstein T. Die cardenolide von Parquetina nigrescens (Afzel) Bullock. Helv. Chim. Acta. 1965;48:1634–1658. doi: 10.1002/hlca.19650480725. [DOI] [Google Scholar]
- 26.Mauli R., Tamm C. Glycosides and aglycons. CLXXI. The glycosides of Periploca nigrescens. Helv. Chim. Acta. 1957;40:299–305. doi: 10.1002/hlca.19570400208. [DOI] [Google Scholar]
- 27.Berthold R., Wehrli W., Reichstein T. Die cardenolide von Parquetina nigrescens (Afzel) Bullock) 3. Mitt.) Die konstitution 5 neuer cardenolide (16-Dehydrostrophanthidin und verwandte substanzen) Helv. Chim. Acta. 1965;48:1659–1665. doi: 10.1002/hlca.19650480726. [DOI] [Google Scholar]
- 28.Hu Y.J., Mu Q.Z., Zheng Q.T., He C.H. New cardenolides of Periploca forrestii. Acta Chim. Sin. (Chin. Ed.) 1990;43:714–719. [Google Scholar]
- 29.Feng J.Q., Zhao W.M. Complete 1H and 13C NMR assignments of four new oligosaccharides and two new glycosides from Periploca forrestii. Magn. Reson. Chem. 2009;47:701–705. doi: 10.1002/mrc.2440. [DOI] [PubMed] [Google Scholar]
- 30.Xu R., Du J., Deng L.L., Yang F.M., Zhang J.X., Wang D.P., Zhang Y.H. A new cardiac glycoside from Periploca forrestii. China J. Chin. Mater. Med. 2012;37:2286–2288. [PubMed] [Google Scholar]
- 31.Itokawa H., Xu J.P., Takeya K. Studies on chemical constituents of antitumor fraction from Periploca sepium. IV. Structures of new pregnane glycosides, Periplocosides D, E, L and M. Chem. Pharm. Bull. (Tokyo) 1988;36:2084–2089. doi: 10.1248/cpb.36.2084. [DOI] [PubMed] [Google Scholar]
- 32.Oshima Y., Hirota T., Hikino H. Periplosides A, B and C, steroidal glycosides of Periploca sepium root barks. Heterocycles. 1987;26:2093–2098. [Google Scholar]
- 33.Feng J.Q., Zhang R.J., Zhou Y., Chen Z.H., Tang W., Liu Q.F., Zuo J.P., Zhao W.M. Immunosuppressive pregnane glycosides from Periploca sepium and Periploca forrestii. Phytochemistry. 2008;69:2716–2723. doi: 10.1016/j.phytochem.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Wang L.Y., Chen Z.H., Zhou Y., Tang W., Zuo J.P., Zhao W.M. Structural revision of periplocosides and periperoxides, natural immunosuppressive agents from the genus Periploca. Phytochemistry. 2011;72:2230–2236. doi: 10.1016/j.phytochem.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Wang L.Y., Chen Z.H., Zhou Y., Tang W., Zuo J.P., Zhao W.M. Corrigendum to “Structural revision of periplocosides and periperoxides, natural immunosuppressive agents from the genus Periploca” [Phytochemistry 72/17 (2011) 2230-2236] Phytochemistry. 2013;95:445. doi: 10.1016/j.phytochem.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Itokawa H., Xu J.P., Takeya K., Watanabe K., Shoji J. Studies on chemical constituents of antitumor fraction from Periploca sepium. II. Structures of new pregnane glycosides, Periplocosides A, B and C. Chem. Pharm. Bull. (Tokyo) 1988;36:982–987. doi: 10.1248/cpb.36.982. [DOI] [PubMed] [Google Scholar]
- 37.Itokawa H., Xu J.P., Takeya K. Studies on chemical constituents of antitumor fraction from Periploca sepium. V. Structures of new pregnane glycosides, Periplocosides J, K, F and O. Chem. Pharm. Bull. (Tokyo) 1988;36:4441–4446. doi: 10.1248/cpb.36.4441. [DOI] [PubMed] [Google Scholar]
- 38.Wang L.Y., Qin J.J., Chen Z.H., Zhou Y., Tang W., Zuo J.P., Zhao W.M. Absolute configuration of Periplosides C and F and isolation of minor spiro-orthoester group-containing pregnane-type steroidal glycosides from Periploca sepium and their T-Lymphocyte proliferation inhibitory activities. J. Nat. Prod. 2017;80:1102–1109. doi: 10.1021/acs.jnatprod.7b00017. [DOI] [PubMed] [Google Scholar]
- 39.Qin J.J., Lin Z.M., Xu Y.S., Ren J.W., Zuo J.P., Zhao W.M. Spiroorthoester group-containing pregnane glycosides from the root barks of Periploca chrysantha and their inhibitory activities against the proliferation of B and T lymphocytes. Bioorg. Med. Chem. Lett. 2018;28:330–333. doi: 10.1016/j.bmcl.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 40.Wang L., Yin Z.Q., Zhang Q.W., Zhang X.Q., Zhang D.M., Liu K., Li Y.L., Yao X.S., Ye W.C. Five new C21 steroidal glycosides from Periploca sepium. Steroids. 2011;76:238–243. doi: 10.1016/j.steroids.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Itokawa H., Xu J.P., Takeya K. Studies on chemical constituents of antitumor fraction from Periploca sepium Bge. I. Chem. Pharm. Bull. (Tokyo) 1987;35:4524–4529. doi: 10.1248/cpb.35.4524. [DOI] [PubMed] [Google Scholar]
- 42.Zhang N.L., Liu X., Shen X.C., Cai J.Z., Hu Y.J., Qiu S.X. Chemical Constituents from Periploca forrestii. J. Chin. Med. Mater. 2016;39:329–330. [PubMed] [Google Scholar]
- 43.Gu X.Y., Wu Z.W., Wang L., Li F., Chen B., Yu K., Wang M.K. C21 steroidal glycosides and oligosaccharides from the root bark of Periploca sepium. Fitoterapia. 2017;118:6–12. doi: 10.1016/j.fitote.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Wang L., Yin Z.Q., Shen W.B., Zhang Q.W., Ye W.C. A new pregnane and a new diphenylmethane from the root barks of Periploca sepium. Helv. Chim. Acta. 2007;8:1581–1585. doi: 10.1002/hlca.200790165. [DOI] [Google Scholar]
- 45.Zhang N.L., Cai J.Z., Huang R.M., Jie H.Y., Yun X., Qiu S.X. Chemical constituents from Periploca forestti. Chin. Tradit. Herb. Drugs. 2011;42:1909–1912. [Google Scholar]
- 46.Li Y., Zeng X.N., Wang W.Z., Luo C.H., Yan Q., Tian M. Chemical constituents from the roots of Periploca sepium with insecticidal activity. J. Asian Nat. Prod. Res. 2012;14:811–816. doi: 10.1080/10286020.2012.691880. [DOI] [PubMed] [Google Scholar]
- 47.Shi B.J., Zhang J.W., Gao L., Chen C.C., Ji Z.Q., Hu Z.N., Wu W.J. A new pregnane glycoside from the root barks of Periploca sepium. Chem. Nat. Compd. 2014;49:1043–1047. doi: 10.1007/s10600-014-0819-x. [DOI] [Google Scholar]
- 48.Feng M.X., Zhao J., Zhang J.W., Hu Z.N., Wu W.J. Fluorescence localization and comparative ultrastructural study of periplocoside NW from Periploca sepium Bunge in the midgut of the oriental amyworm, Mythimna separata Walker (Lepidoptera: Noctuidae) Toxins. 2014;6:1575–1585. doi: 10.3390/toxins6051575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itokawa H., Xu J.P., Takeya K. Pregnane glycosides from an antitumour fraction of Periploca sepium. Phytochemistry. 1988;27:1173–1179. doi: 10.1016/0031-9422(88)80297-8. [DOI] [Google Scholar]
- 50.Siciliano T., Bader A., De Tommasi N., Morelli I., Braca A. Sulfated pregnane glycosides from Periploca graeca. J. Nat. Prod. 2005;68:1164–1168. doi: 10.1021/np050031d. [DOI] [PubMed] [Google Scholar]
- 51.Umehara K., Sumii N., Satoh H., Miyase T., Kuroyanagi M., Ueno A. Studies on differentiation inducers. V. Steroid glycosides from Periplocae Radicis Cortex. Chem. Pharm. Bull. (Tokyo) 1995;43:1565–1568. doi: 10.1248/cpb.43.1565. [DOI] [PubMed] [Google Scholar]
- 52.Yin Z.Q., Wang L., Zhang X.Q., Ye W.C., Zhang J. Steroids from the roots of Periploca sepium. Chin. Pharm. J. (BeijingChina) 2009;44:968–971. [Google Scholar]
- 53.Sakuma S., Kawanishi S., Shoji J. Constituents of the Chinese crude drug “Wujiapi.” IX. Structure of glycoside H2, a patentiator of NGF-mediated nerve fiber outgrowth. Chem. Pharm. Bull. (Tokyo) 1980;28:163–168. doi: 10.1248/cpb.28.163. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava O.P., Khare A., Khare M.P. Structure of calocin. J. Nat. Prod. 1982;45:211–215. doi: 10.1021/np50020a018. [DOI] [Google Scholar]
- 55.Deepak D., Khare M.P., Khare A. A pregnane ester glycoside from Periploca calophylla. Phytochemistry. 1985;24:1037–1039. doi: 10.1016/S0031-9422(00)83178-7. [DOI] [Google Scholar]
- 56.Deepak D., Khare M.P., Khare A. A pregnane ester diglycoside from Periploca calophylla. Indian J. Chem. B. 1986;25B:44–45. [Google Scholar]
- 57.Sethi A., Deepak D., Khare M.P., Khare A. A novel pregnane glycoside from Periploca calophylla. J. Nat. Prod. 1988;51:787–790. doi: 10.1021/np50058a025. [DOI] [Google Scholar]
- 58.Feng J.Q., Zhao W.M. Five new pregnane glycosides from the root barks of Periploca sepium. Helv. Chim. Acta. 2008;91:1798–1805. doi: 10.1002/hlca.200890192. [DOI] [Google Scholar]
- 59.Khare N.K., Deepak D., Khare A. Absolute configuration of calogenin and its 20-keto derivative. Indian J. Chem. B. 2008;47B:1744–1747. [Google Scholar]
- 60.Deepak D., Khare M.P., Khare A. A pregnane ester diglycoside from Periploca calophylla. Phytochemistry. 1985;24:3015–3017. doi: 10.1016/0031-9422(85)80047-9. [DOI] [Google Scholar]
- 61.Liu Y., Ou Y.Y., Wang Z.Q., Qiao L., Li S., Zhao S.H., Liu M.Y. A new C21 steroidal saponins from Periplocae Cortex. China J. Chin. Mater. Med. 2015;40:455–457. [PubMed] [Google Scholar]
- 62.Sakuma S., Kawanishi S., Shoji J., Shibata S. Constituents of Chinese crude drug “Wujiapi.” I. Studies on the aglycones of steroidal glycosides of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1968;16:326–331. doi: 10.1248/cpb.16.326. [DOI] [PubMed] [Google Scholar]
- 63.Sun C.L., Chen L., Xu J., Qu W., Guan L., Liu W.Y., Akihisa T., Feng F., Zhang J. Melanogenesis-inhibitory and antioxidant activities of phenolics from Periploca forrestii. Chem. Biodivers. 2017;14:e1700083. doi: 10.1002/cbdv.201700083. [DOI] [PubMed] [Google Scholar]
- 64.Guo H.L., Zhou J.Y. Study on the chemical constituents of Periploca calophylla. Chin. Pharm. J. (BeijingChina) 2003;38:497–499. [Google Scholar]
- 65.Askri M., Mighri Z. Medicinal plants of Tunisia. The structure of Periplocadiol, a new elenmane-type sesquiterpene isolated from the roots of Periploca Laevigata. J. Nat. Prod. 1989;52:792–796. doi: 10.1021/np50064a021. [DOI] [Google Scholar]
- 66.Xu R., Zhang Y.H., Zhao Y.T. Study on chemical constituents of Periploca forrestii Schlecht. Chin. Pharm. J. (BeijingChina) 2011;46:823–826. [Google Scholar]
- 67.Mustafa G., Anis E., Ahmed S., Anis I., Ahmed H., Malik A., Shahzad-ul-Hassan S., Choudhary M.I. Lupene-type triterpenes from Periploca aphylla. J. Nat. Prod. 2000;63:881–883. doi: 10.1021/np990426v. [DOI] [PubMed] [Google Scholar]
- 68.Teresa A., Leah N., Cornelius W., Omollo N.I. Triterpenoids from the stem bark of Periploca linearifolia (Asclepiadaceae) J. Kenya Chem. Soc. 2011;6:1–8. [Google Scholar]
- 69.Hichri F., Hammouda O., Ben Jannet H., Mighri Z., Abreu P.J.M. Triterpenoids from the fruit bark of Periploca laevigata growing in Tunisia. J. Soc. Chim. Tunis. 2002;4:1565–1569. [Google Scholar]
- 70.Mitsuhashi H., Tomimoto K. Components of Periploca aphylla. Constituents of Asclepiadaceae plants. Shoyakugaku Zasshi. 1971;25:7–10. [Google Scholar]
- 71.Guo H.L., Zhou J.Y. Study on the chemical constituents of Periploca calophylla (II) Chin. Tradit. Herb. Drugs. 2005;36:350–351. [Google Scholar]
- 72.Rehman A.U., Riaz N., Ahmad H., Ahmad Z., Malik A. Phytochemical studies on Periploca aphylla. J. Chem. Soc. Pak. 2003;25:257–259. [Google Scholar]
- 73.Li Y., Liu Y.B., Yu S.S. Chemical constituents of Periploca forrestii. China J. Chin. Mater. Med. 2013;38:1536–1538. [PubMed] [Google Scholar]
- 74.Zhao S., Zhang B., Xiong D.D., Zou H., Ma X., Sun J., Lu Y., Zheng L., Li Y.J. Chemical constituents from Periploca Radix. Chin. Tradit. Herb. Drugs. 2017;48:1513–1518. [Google Scholar]
- 75.Ishizone H., Sakuma S., Kawanishi S., Shoji J. Constituents of Chinese crude drug “Wujiapi.” VII. On the structure of glycoside E of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1972;20:2402–2406. doi: 10.1248/cpb.20.2402. [DOI] [PubMed] [Google Scholar]
- 76.Kawanishi S., Sakuma S., Shoji J. Constituents of Chinese crude drug “Wujiapi.” V. On the structure of glycoside H1 of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1972;20:469–475. doi: 10.1248/cpb.20.469. [DOI] [Google Scholar]
- 77.Tan X.G., Zhang X.R., Peng S.L., Liao X., Ding L.S. Chemical constituents from the roots of Biondia Hemsleyana. Chem. J. Chin. Univ. 2003;24:436–441. [Google Scholar]
- 78.Kawanishi S., Kasai R., Sakuma S., Shoji J. Constituents of Chinese crude drug “Wujiapi.” VIII. On the structures of new oligosaccharides C1, D2, F1, and F2 of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1977;25:2055–2060. doi: 10.1248/cpb.25.2055. [DOI] [Google Scholar]
- 79.Wang L., Yin Z.Q., Wang Y., Zhang X.Q., Li Y.L., Ye W.C. Perisesaccharides A-E, new oligosaccharides from the root barks of Periploca sepium. Planta Med. 2010;76:909–915. doi: 10.1055/s-0029-1240842. [DOI] [PubMed] [Google Scholar]
- 80.Bi Z.M., Li J., Zhou J.J., Liu L.J. Chemical constituents in the root barks of Periploca sepium. Pharm. Clin. Res. 2011;19:521–522. [Google Scholar]
- 81.Long X.H., Xu R., Zhang Y.H., Tan X.H., Sun Q.Y. A new oligosaccharide from Periploca calophylla. China J. Chin. Mater. Med. 2012;37:226–229. [PubMed] [Google Scholar]
- 82.Yu B., Ma Y.M., Kong Y., Shi Q.H. A study on chemical constituents of the stem of Periploca sepium. J. Northwest. Univ. 2005;20:145–146. [Google Scholar]
- 83.Kawanishi S., Sakuma S., Okino H., Shoji J. Constituents of Chinese crude drug “Wujiapi.” IV. On the structure of a new acetylbiose from steroidal glycosides of Bei-Wujiapi. Chem. Pharm. Bull. (Tokyo) 1972;20:93–96. doi: 10.1248/cpb.20.93. [DOI] [Google Scholar]
- 84.Aziz-ur-Rehman, Malik A., Riaz N., Nawaz H.R., Ahmad H., Nawaz S.A., Choudhary M.I. Lipoxygenase inhibitory constituents from Periploca aphylla. J. Nat. Prod. 2004;67:1450–1454. doi: 10.1021/np030494o. [DOI] [PubMed] [Google Scholar]
- 85.Gang X.H., Zhou X., Zhao C., Gong X.J., Chen H.G. Chemical constituents of Periploca forrestii Schltr. Chin. Tradit. Herb. Drugs. 2009;40:708–710. [PubMed] [Google Scholar]
- 86.Ma Y.M., Wang P., Chen L., Feng C.L. Chemical composition of the Periploca sepium. Chem. Nat. Compd. 2010;46:464–465. [Google Scholar]
- 87.Ben nejma A., Besbes M., Guérineau V., Touboul D., Ben jannet H., Hamza M.H.A. Isolation and structure elucidation of acetylcholinesterase lipophilic lupeol derivatives inhibitors from the latex of the Tunisian Periploca laevigata. Arab. J. Chem. 2017;10:S2767–S2772. doi: 10.1016/j.arabjc.2013.10.026. [DOI] [Google Scholar]
- 88.Gang X.H., Zhou X., Chen H.G., Gong X.J., Zhao C. Chemical constituents of Periploca forrestii. China J. Chin. Mater. Med. 2009;34:3225–3228. [PubMed] [Google Scholar]
- 89.Srivastava O.P., Khare A., Khare M.P. Triterpenoids of Periploca calophylla. J. Nat. Prod. 1983;46:458–461. doi: 10.1021/np50028a003. [DOI] [Google Scholar]
- 90.Gang X.H., Zhou X., Chen H.G., Gong X.J., Zhang J.X., Wang D.P. Chemical constituents of Periploca forrestii Schltr. J. Trop. Subtrop. Bot. 2009;17:160–163. [Google Scholar]
- 91.Ma Y.M., Shi Q.H., Kong Y. Chemical constituents of stem bark from Periploca sepium. Chem. Nat. Compd. 2007;43:497–498. doi: 10.1007/s10600-007-0175-1. [DOI] [Google Scholar]
- 92.Wang L., Yin Z.Q., Zhang L.H., Ye W.C., Zhang X.Q., Shen W.B., Zhao S.X. Chemical constituents from root barks of Periploca sepium. China J. Chin. Mater. Med. 2007;32:1300–1302. [PubMed] [Google Scholar]
- 93.Shi Q.H., Ma Y.M. Structural analysis on four compounds in root bark from Periploca sepium. J. Northwest. Univ. 2007;22:132–135. [Google Scholar]
- 94.Ogundaini A.O., Okafor E.O. Isorhoifolin, a flavonoid glycoside from Periploca nigrescens leaves. Planta Med. 1987;53:391. doi: 10.1055/s-2006-962750. [DOI] [PubMed] [Google Scholar]
- 95.Chen S.H., Yang J.S., Ren F.Z., Liu G.S., Zhang J., San B.E., Li N. Study on anti-tumor active components of Periploca sepium. Chin. Tradit. Herb. Drugs. 2006;37:519–520. [Google Scholar]
- 96.Wang J., Wang C.F., Chen J.X., Du J., Qiu D.W., Qiu M.H. Chemical constituents of Periploca forrestii and their cytotoxicity activity. China J. Chin. Mater. Med. 2009;34:3214–3216. [PubMed] [Google Scholar]
- 97.Komissarenko N.F., Bagirov R.B. Chemical study of Periploca graeca. Izv Akad Nauk Azerb Ssr. Biol. 1969:122–127. [Google Scholar]
- 98.Li J.N., Zhao L.Y., Yu J., Gao Y., Deng Y.R. Chemical constituents of the root barks of Periploca sepium. Chin. Tradit. Pat. Med. 2010;32:1552–1556. [Google Scholar]
- 99.Guo H.L., Zhou J.Y. Isolation and structure elucidation of glycosides in n-butanol extracts from rhizome of Periploca calophylla. China J. Chin. Mater. Med. 2005;30:44–46. [PubMed] [Google Scholar]
- 100.Ishimaru K., Sudo H., Satake M., Shimomura K. Phenyl glucosides grom a hairy root culture of Swertia japonica. Phytochemistry. 1990;29:3823–3825. doi: 10.1016/0031-9422(90)85340-L. [DOI] [Google Scholar]
- 101.Wang F.H., Yu J.P. Studies on chemical components of Periploca forrestii Schltr. J. Mt. Agric. Biol. 2008;27:560–562. [Google Scholar]
- 102.Guan Y.L., Yang R.Y., Xie W.X. Study on the content of the chlorogenic ccid in water extract of Cortex periplocae at the different times by UV. North. Hortic. 2011;35:177–178. [Google Scholar]
- 103.Komissarenko N.F., Khvorost P.P., Ivanov V.D. Coumarins and cardenolides from Periploca sepium. Chem. Nat. Compound. 1983;19:98–99. doi: 10.1007/BF00579977. [DOI] [Google Scholar]
- 104.Wang C.Z., Jia Z.J. Lignan, phenylpropanoid and iridoid glycosides from Pedicularis torta. Phytochemistry. 1997;45:159–166. doi: 10.1016/S0031-9422(96)00770-4. [DOI] [Google Scholar]
- 105.Deyama T., Ikawa T., Nishibe S. The constituents of Eucommia ulmoides Oliv. II. Isolation and structures of three new lignan glycosides. Chem. Pharm. Bull. (Tokyo) 1985;33:3651–3657. doi: 10.1248/cpb.33.3651. [DOI] [Google Scholar]
- 106.Mezhoud S., Aissaoui H., Derbre S., Mekkiou R., Richaume P., Benayache S., Benayache F. Flavonoid glycosides from Periploca laevigata (Asclepiadaceae) from Algeria. Pharma. Chem. 2016;8:129–131. [Google Scholar]
- 107.Zhou X., Gang X.H., Gong X.J., Zhao C., Chen H.G. Glycosides from Periploca forrestii. China J. Chin. Mater. Med. 2010;35:610–612. doi: 10.4268/cjcmm20100515. [DOI] [PubMed] [Google Scholar]
- 108.Al Musayeib N.M., Al-Massarani S.M., Amina M., El Dib R.A., Mohamed G.A., Ibrahim S.R.M. Periplocain A, a new naphthalene derivative from Periploca aphylla growing in Saudi Arabia. Helv. Chim. Acta. 2016;99:466–468. doi: 10.1002/hlca.201500524. [DOI] [Google Scholar]
- 109.Chen H.G., Liang Q., Zhou X., Wang X.Y. Preparative separation of the flavonoid fractions from Periploca forrestii Schltr. ethanol extracts using macroporous resin combined with HPLC analysis and evaluation of their biological activities. J. Sep. Sci. 2019;42:650–661. doi: 10.1002/jssc.201800422. [DOI] [PubMed] [Google Scholar]
- 110.Wang L.F., Shan B.E., Ren F.Z., Shan T.Q., Zhao L.M., Liu L.H. Inhibitory effect of Baohuoside I from Cortex Periplocae on esophageal carcinoma cells and its transplantable models in nude mice. Canc. Res. Prev. Treat. 2009;36:635–638. [Google Scholar]
- 111.Wang Y., Sun L., Qiao S.Y. A diterpenoid quinone from Periploca forrestii. China J. Chin. Mater. Med. 2010;35:1648. [PubMed] [Google Scholar]
- 112.Zhu X.T., Liu Y., Yu J.P. Separation and identification of chemical compounds in Periploca forrestii Schltr. J. Mt. Agric. Biol. 2008;27:186–188. [Google Scholar]
- 113.Solacolu T., Mavrodin A., Herrmann G. The odoriferant principle of the plant Periploca graeca. J. Pharm. Chim. 1935;22:546–548. [Google Scholar]
- 114.Shi J.X., Yamashita T., Todo A., Nitoda T., Izumi M., Baba N., Nakajima S. Repellent from traditional chinese medicine, Periploca sepium Bunge. Z. Nat. C. 2007;62:821–825. doi: 10.1515/znc-2007-11-1208. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J. Ph.D. Thesis. Hebei Medical University; Shijiazhuang, China: 2006. Antitumor and immunoregulatory effects of active components from Cortex Periplocae. [Google Scholar]
- 116.Zhao C., Gang X.H., Gong X.J., Chen H.G., Zhou X. Ceramides of Periploca forrestii. Chin. J. Exp. Tradit. Med. Formulae. 2014;20:83–85. [Google Scholar]
- 117.MacKeith M.H. Pharmacological properties of Periplocin. J. Pharm. 1926;27:449–466. [Google Scholar]
- 118.Levi A., Vaccari D. The use of the “greek” Periploca in heart diseases. Rev. Sud-Am. Endocrinol. Immunol. Quim. 1932;15:610. [Google Scholar]
- 119.Teng S.H., Wang T.E., Wang M., Ho K., Mo Y. Cardiotonic action of a glucosidal preparation obtained from Periploca forrestii. Acta Pharm. Sin. 1964;11:75–79. [PubMed] [Google Scholar]
- 120.Deng S.X., Wang D.C., He G.B. Cardiotonic action of Periploca calophylla (Wight.) Falconer. Acta Pharm. Sin. 1980;15:245–247. [PubMed] [Google Scholar]
- 121.Ma L., Ji Y.S., Han J., Li F., Wang Y. Effect of periplocin on left ventricular structure and function in chronic heart failure rats by color doppler-echocardiography. J. Tianjin Univ. Tradit. Chin. Med. (2006-) 2008;27:81–83. [Google Scholar]
- 122.Ma L., Wang Y. Effect of Periplocin on expression of myocardial PLB and SERCA mRNA in rats with chronic heart failure. Jiangsu J. Tradit. Chin. Med. (2002-) 2009;41:71–72. [Google Scholar]
- 123.Wang X.Y., Gao X.M., Liu H., Zhang H., Liu Y., Jiang M., Hu L.M., Zhang B.L. Gene expression profiling of the proliferative effect of periplocin on mouse cardiac microvascular endothelial cells. Chin. J. Integr. Med. 2010;16:33–40. doi: 10.1007/s11655-010-0033-z. [DOI] [PubMed] [Google Scholar]
- 124.Sun D., Zhang J., Chen J.T., Zhou K., Deng Y.R. Comparison of the effect of cardiac glycosides in Periplocae Cortex on isolated heart in rats. Chin. Arch. Tradit. Chin. Med. 2011;29:2633–2635. [Google Scholar]
- 125.Li J.J., Dai Y.L., Yang Y.M., Li C.X., Wang Z.G., Luo Y.H., Liu X.B. Experimental study on the anti-inflammatory and analgesic effects of Periploca forrestii Schlechter. Yunnan J. Tradit. Chin. Med. 2008;29:35–36. [Google Scholar]
- 126.Wu R.R., Zhang X.R., Sun L., Qi C.H. Anti-inflammatory and immun modulation activities from active fraction of Periploca forrestii Schltr. Her. Med. 2010;29:1425–1428. [Google Scholar]
- 127.Song G.B., Xi G.P., Yu J.P. Anti-inflammatory effects and mechanism of emulsion of volatile oil from Periploca forrestii. Chin. J. Exp. Tradit. Med. Formulae. 2012;18:252–255. [Google Scholar]
- 128.Dong L., Zhang Y., Wang X., Dong Y.X., Zheng L., Li Y.J., Ni J.M. In vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii Schltr. Chin. J. Integr. Med. 2017;23:528–534. doi: 10.1007/s11655-017-2803-3. [DOI] [PubMed] [Google Scholar]
- 129.Tlili N., Tir M., Feriani A., Yahia Y., Allagui M.S., Saadaoui E., El Cafsi M., Nasri N. Potential health advantages of Periploca laevigata: Preliminary phytochemical analysis and evaluation of in vitro antioxidant capacity and assessment of hepatoprotective, anti-inflammatory and analgesic effects. J. Funct. Foods. 2018;48:234–242. doi: 10.1016/j.jff.2018.06.028. [DOI] [Google Scholar]
- 130.Wang B.X. Pharmacology of Modern Chinese Medicine. Tianjin Science and Technology Press; Tianjing, China: 1997. p. 436. [Google Scholar]
- 131.Gu W., Zhao L.J., Zhao A.G. Effect of periplogenin on mast cell degranulation and histamine release. China Pharm. (ChongqingChina) 2008;19:166–168. [Google Scholar]
- 132.Wan J., Zhu Y.N., Feng J.Q., Chen H.J., Zhang R.J., Ni J., Chen Z.H., Hou L.F., Liu Q.F., Zhang J., et al. Periplocoside A, a pregnane glycoside from Periploca sepium Bge, prevents Concanavalin A-induced mice hepatitis through inhibiting NKT-derived inflammatory cytokine productions. Int. Immunopharmacol. 2008;8:1248–1256. doi: 10.1016/j.intimp.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Zhu Y.N., Zhao W.M., Yang Y.F., Liu Q.F., Zhou Y., Tian J., Ni J., Fu Y.F., Zhong X.G., Tang W. Periplocoside E, and effective compound from Periploca sepium Bge, inhibited T cell activation in vitro and in vivo. J. Pharm. Exp. 2005;316:662–669. doi: 10.1124/jpet.105.093732. [DOI] [PubMed] [Google Scholar]
- 134.Deng H.F., Yang H., Wang D.N., Zeng B.H., Shi S.H., Yu H., Li Y.Z., Xu Z.Y., Zhong X.G. Effects of an immunosuppressive active component of Periplocae Cortex (Periploca sepium Bge.) on positive selection of thymocytes in vitro. J. Tradit. Chin. Med Sci. 2018;5:161–167. doi: 10.1016/j.jtcms.2018.03.002. [DOI] [Google Scholar]
- 135.Zhang J., Ni J., Chen Z.H., Li X., Zhang R.J., Tang W., Zhao W.M., Yang Y.F., Zuo J.P. Periplocoside A prevents experimental autoimmune encephalomyelitis by suppressing IL-17 production and inhibits differentiation of Th17 cells. Acta Pharm. Sin. 2009;30:1144–1152. doi: 10.1038/aps.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J., Shan B.E., Zhang C., Zhao R.N., Li Q.L., Liu J.H., Li Q.M., Li R.Q. Effects on differentiation and maturation of dendritic cells by lupane acetate of Cortex Periplocae. Chin. J. Cell. Mol. Immunol. 2006;22:26–32. [PubMed] [Google Scholar]
- 137.Shan B.E., Zhao L.M., Ai J., He L.X., Chen S.H., Ding C.Y. Effect of Cortex Periplocae lupane acetate on immunoregulation of human peripheral blood lymphocytes. Chin. Tradit. Herb. Drugs. 2008;39:1035–1039. [Google Scholar]
- 138.Zhang J., Shan B.E., Li Q.M., Zhang C., Zhao R.N., Ai J. Immunoregulatory effects of Periplocin from Cortex Periplocae in tumor-bearing mice. Chin. J. Cell. Mol. Immunol. 2009;25:887–890. [PubMed] [Google Scholar]
- 139.Zhang L.L., Xiao Z.Y., Ma Y., Qi C.H., Zhou W.X., Zhang Y.X., Qiao S.Y., Sun L. Cellular immunosuppression effect of polysaccharide HGT-5A from Periploca forrestii Schlechter and its possible active components. Chin. J. Pharm. Toxicol. 2011;25:538–542. [Google Scholar]
- 140.Liang Q., Chen H.G., Zhou X., Deng Q.F., Hu E.M., Zhao C., Gong X.J. Optimized microwave-assistant extraction combined ultrasonic pretreatment of flavonoids from Periploca forrestii Schltr. and evaluation of its anti-allergic activity. Electrophoresis. 2017;38:1113–1121. doi: 10.1002/elps.201600515. [DOI] [PubMed] [Google Scholar]
- 141.Tokiwa T., Harada K., Matsumura T., Tukiyama T. Oriental medicinal herb, Periploca sepium, extract inhibits growth and IL-6 production of human synovial fibroblast-like cells. Biol. Pharm. Bull. 2004;27:1691–1693. doi: 10.1248/bpb.27.1691. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y., Liu M., Li Y.F., Wang B.J., Liang J. The mechanism of Periploca forrestii by regulating the inflammatory response on CIA rat model. J. Chin. Med. Mater. 2018;42:713–716. [Google Scholar]
- 143.Huang M.J., Luo C.L., Guo G., Qiu D.W., Wang W.Q. Effects and molecular mechanism of Periploca forrestii against rheumatoid arthritis. Chin. J. Exp. Tradit. Med. Formulae. 2011;17:174–177. [Google Scholar]
- 144.Liang J., Ma W.K., Liu Z.Q., An Y., Yao X.M., Tang Z.Y. Effects of Periploca forrestii ethanol extract (PFE) on proliferation, COX-2 expression, and PG2 expression of rheumatoid arthritis synovial fibroblasts from patients. Lishizhen Med. Mater. Med. Res. 2015;26:24–27. [Google Scholar]
- 145.Chen L., Li J.S., Ke X., Qu W., Zhang J., Feng F., Liu W. The therapeutic effects of Periploca forrestii Schltr. Stem extracts on collagen-induced arthritis by inhibiting the activation of Src/NF-kappaB signaling pathway in rats. J. Ethnopharmacol. 2017;202:12–19. doi: 10.1016/j.jep.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Li H.M., Zhao X.L., Li X.Y., Li Q.L., Li J., Xiong Y., Zhang H. Studies on the effective components of Periploca forrestii for anti-rheumatoid arthritis. J. Chin. Med. Mater. 2016;39:649–651. [Google Scholar]
- 147.Liu Y.Q., Li M.H., He Q.H., Yang X.P., Ruan F., Sun G.C. Periploca forrestii saponin ameliorates murine CFA-induced arthritis by suppressing cytokine production. Mediat. Inflamm. 2016;2016:7941684. doi: 10.1155/2016/7941684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bao C.M., Liu Y.Q., Sun X., Xing C.C., Zeng L.T., Sun G.C. Periploca forrestii saponin ameliorates CIA via suppressing proinflammatory cytokines and nuclear factor kappa-B pathways. PLoS ONE. 2017;12:e0176672. doi: 10.1371/journal.pone.0176672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu T., Wang X., He Y.L., Wang Y., Dong L., Ma X., Zheng L., Liu C.H., Wang G.C., Zheng J., et al. In vivo and in vitro anti-arthritic effects of cardenolide-rich and caffeoylquinic acid-rich fractions of Periploca forrestii. Molecules. 2018;23:1988. doi: 10.3390/molecules23081988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L.J., Lu G., Zhang Y.J., Liu G.S., Shan B.E. Periplocin of Cortex Periplocae inhibits Stat3 signaling and induces apoptosis in human hepatocellular carcinoma cell line SMMC-7721. Acta Acad. Med. Mil. Tert. 2008;30:1448–1451. [Google Scholar]
- 151.Du Y.Y., Liu X., Shan B.E. Periplocin extracted from Cortex Periplocae induces apoptosis of SW480 cells through inhibiting the Wnt/β-catenin signaling pathway. Chin. J. Cancer (1982–2010) 2009;28:456–460. [PubMed] [Google Scholar]
- 152.Zhang J., Zhang C., Yang G., Shan B.E., Liu J.H. Effects of Periplocin from Cortex Periplocae on apoptosis of human lung cancer A549 cells and expression of survivin. Her. Med. 2015;34:705–710. [Google Scholar]
- 153.Zhang J., Yang G., Shan B.E., Zhang C. Effects of periplocin from Cortex Periplocae on cell cycle of MCF-7 cells and expression of p21WAFI/CIPI. Canc. Res. Prev. Treat. 2010;37:864–868. [Google Scholar]
- 154.Lu Z.J., Zhou Y., Song Q., Qin Z., Zhang H., Zhou Y.J., Gou L.T., Yang J.L., Luo F. Periplocin inhibits growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways. Cell. Physiol. Biochem. 2010;26:609–618. doi: 10.1159/000322328. [DOI] [PubMed] [Google Scholar]
- 155.Cheng C.F., Lu I.H., Tseng H.W., Sun C.Y., Lin L.T., Kuo Z.K., Pan I.H., Ko C.H. Antitumor effect of Periplocin in Trail-resistant human hepatocellular carcinoma cells through downregulation of IAPs. Evid. Based Complement. Altern. Med. 2013;2013:958025. doi: 10.1155/2013/958025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lohberger B., Wagner S., Wohlmuther J., Kaltenegger H., Stuendl N., Leithner A., Rinner B., Kunert O., Bauer R., Kretschmer N. Periplocin, the most anti-proliferative constituent of Periploca sepium, specifically kills liposarcoma cells by death receptor mediated apoptosis. Phytomedicine. 2018;51:162–170. doi: 10.1016/j.phymed.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 157.Bloise E., Braca A., De Tommasi N., Belisario M.A. Pro-apoptotic and cytostatic activity of naturally occurring cardenolides. Cancer Chemother. Pharm. 2009;64:793–802. doi: 10.1007/s00280-009-0929-5. [DOI] [PubMed] [Google Scholar]
- 158.Martey O.N.K., He X., Xing H.Y., Deng F.C., Feng S., Li C., Shi X.Y. Periplocymarin is a potential natural compound for drug development: Highly permeable with absence of P-glycoprotein efflux and cytochrome P450 inhibitions. Biopharm. Drug Dispos. 2014;35:195–206. doi: 10.1002/bdd.1884. [DOI] [PubMed] [Google Scholar]
- 159.Liu X.X., Liu H.Z., Chen J.H., Chen Y.M., Shan B.E. Effect of Baohuoside-I on proliferat ion and cell cycle of human esophageal carcinoma cell Eca-109. Chin. Tradit. Herb. Drugs. 2009;40:1590–1593. [Google Scholar]
- 160.Wang L.F., Lu A., Liu X.X., Sang M.X., Shan B.E., Meng F.R., Cao Q., Ji X. The flavonoid Baohuoside-I inhibits cell growth and downregulates survivin and cyclin D1 expression in esophageal carcinoma via β-catenin-dependent signaling. Oncol. Rep. 2011;26:1149–1156. doi: 10.3892/or.2011.1400. [DOI] [PubMed] [Google Scholar]
- 161.Wang L.F., Lu A., Meng F.R., Cao Q., Shan B.E. Inhibitory effects of lupeal acetate of Cortex periplocae on N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Oncol. Lett. 2012;4:231–236. doi: 10.3892/ol.2012.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shan B.E., Zhao L.M., He L.X., Di H.Q., Ren F.Z. Effects of Periplocin on proliferation of human esophageal carcinoma cell TE-13 and its relation with Rb expression. Carcinog. Teratog. Mutagen. 2008;20:342–345. [Google Scholar]
- 163.Zhao L.M., Ai J., Zhang J., Han L.N., Liu L.H., Wang L., Shan B.E. Periplocin (a sort of ethanol from Cortexperiplocae) induces apoptosis of esophageal carcinoma cells by influencing expression of related genes. Tumor. 2009;29:1025–1030. [Google Scholar]
- 164.Shan X.H., Shan X.L., Shan B.E., Chen Y.M., Ren F.Z., Liu X.X. Ethylacetate extract from Cortex periplocae induced apoptosis of human esophageal carcinoma cellline TE-13. Tumor. 2010;30:6–10. [Google Scholar]
- 165.Liang Y., Liao Y., Zhang H., Liu G., Zhang X.Y., Huang C.P. Anti-tumor activity of extract from Periploca forrestii. Chin. J. Exp. Tradit. Med. Formulae. 2011;17:119–122. [Google Scholar]
- 166.Li J.X., Shan B.E. The study of Cortex Periplocae extract on the differentiation of K562 cells. Carcinog. Teratog. Mutagen. 2008;20:449–450, 455. [Google Scholar]
- 167.Shan B.E., Li J.X., Zhang J. Inductive ef fect of Cortex Periplocae extract on apoptosis of human gastric cancer cells BGC-823. Chin. Tradit. Herb. Drugs. 2005;36:1184–1188. [Google Scholar]
- 168.Hichri F., Jannet H.B., Cheriaa J., Jegham S., Mighri Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003;6:473–483. doi: 10.1016/S1631-0748(03)00066-3. [DOI] [Google Scholar]
- 169.Song G.B., Xi G.P., Yu J.P., Ji Y. Chemical constituents of petroleum ether extracts from Periploca forrestii and its antimicrobial effects. Lishizhen Med. Mater. Med. Res. 2013;24:1085–1087. [Google Scholar]
- 170.Hajji M., Masmoudi O., Souissi N., Triki Y., Kammoun S., Nasri M. Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem. 2010;121:724–731. doi: 10.1016/j.foodchem.2010.01.021. [DOI] [Google Scholar]
- 171.Ait Dra L., Ait Sidi Brahim M., Boualy B., Aghraz A., Barakate M., Oubaassine S., Markouk M., Larhsini M. Chemical composition, antioxidant and evidence antimicrobial synergistic effects of Periploca laevigata essential oil with conventional antibiotics. Ind. Crop. Prod. 2017;109:746–752. doi: 10.1016/j.indcrop.2017.09.028. [DOI] [Google Scholar]
- 172.Wang J.H., Liu H., Zhao J.L., Gao H.F., Zhou L.A., Liu Z.L., Chen Y.Q., Sui P. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component. Molecules. 2010;15:5807–5817. doi: 10.3390/molecules15085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jeong E.Y., Lee M.J., Kang M.S., Lee H.S. Antimicrobial agents of 4-methoxysalicylaldehyde isolated from Periploca sepium oil against foodborne bacteria: Structure-activity relationship. Appl. Biol. Chem. 2018;61:397–402. doi: 10.1007/s13765-018-0373-5. [DOI] [Google Scholar]
- 174.Mohamed H., Ons M., Yosra E.-T., Rayda S., Neji G., Moncef N. Chemical composition and antioxidant and radical-scavenging activities of Periploca laevigata root bark extracts. J. Sci. Food Agric. 2009;89:897–905. doi: 10.1002/jsfa.3532. [DOI] [Google Scholar]
- 175.Wang X.L., Zhang Y.F., Liu Z.K., Zhao M.Q., Liu P.F. Purification, characterization, and antioxidant activity of polysaccharides isolated from Cortex Periplocae. Molecules. 2017;22:1866. doi: 10.3390/molecules22111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hajji M., Hamdi M., Sellimi S., Ksouda G., Laouer H., Li S., Nasri M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohyd. Polym. 2019;206:380–388. doi: 10.1016/j.carbpol.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 177.Athmouni K., Belhaj D., Chawech R., Jarraya R., El Feki A., Ayadi H. Characterization of polysaccharides isolated from Periploca angustifolia and its antioxidant activity and renoprotective potential against cadmium induced toxicity in HEK293 cells and rat kidney. Int. J. Biol. Macromol. 2019;125:730–742. doi: 10.1016/j.ijbiomac.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 178.Athmouni K., Belhaj D., El Feki A., Ayadi H. Optimization, antioxidant properties and GC-MS analysis of Periploca angustifolia polysaccharides and chelation therapy on cadmium-induced toxicity in human HepG2 cells line and rat liver. Int. J. Biol. Macromol. 2018;108:853–862. doi: 10.1016/j.ijbiomac.2017.10.175. [DOI] [PubMed] [Google Scholar]
- 179.Athmouni K., Belhaj D., Mkadmini Hammi K., El Feki A., Ayadi H. Phenolic compounds analysis, antioxidant, and hepatoprotective effects of Periploca angustifolia extract on cadmium-induced oxidative damage in HepG2 cell line and rats. Arch. Physiol. Biochem. 2018;124:261–274. doi: 10.1080/13813455.2017.1395890. [DOI] [PubMed] [Google Scholar]
- 180.Ali S., Khan M.R., Shah S.A., Batool R., Maryam S., Majid M., Zahra Z. Protective aptitude of Periploca hydaspidis Falc against CCl4 induced hepatotoxicity in experimental rats. Biomed. Pharm. 2018;105:1117–1132. doi: 10.1016/j.biopha.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 181.Abbas F., Maqbool Q., Nazar M., Jabeen N., Hussain S.Z., Anwaar S., Mehmood N., Sheikh M.S., Hussain T., Iftikhar S. Green synthesised zinc oxide nanostructures through Periploca aphylla extract shows tremendous antibacterial potential against multidrug resistant pathogens. Iet Nanobiotechnol. 2017;11:935–941. doi: 10.1049/iet-nbt.2016.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu J.S., Qiao X.W., Wang J., Qin S. Effects of the crude extracts from Periploca sepium Bunge on the bioactivity of imported cabbage worm Pieris rapae. Chin. J. Eco-Agric. 2006;14:185–188. [Google Scholar]
- 183.Zhu J.S., Qiao X.W., Wang J., Qin S. Study on antifeedant and insecticidal activities of extracts and fractions from Periploca sepium Bunge against Plutella xylostella (L.) Chin. J. Pestic. Sci. 2004;6:48–52. [Google Scholar]
- 184.Al-Musayeib N.M., Mothana R.A., Al-Massarani S., Matheeussen A., Cos P., Maes L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules. 2012;17:11379–11390. doi: 10.3390/molecules171011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shi Q.H., Ma Y.M., Qin H.Q. Chemical components and insecticidal activity of essential oil in Periploca sepium root bark to Schizaphis graminum. Acta Bot. Boreali-Occident. Sin. 2006;26:620–623. [Google Scholar]
- 186.Chua S.S., Jiang G.H., Liu W.L., Liu Z.L. Insecticidal activity of the root bark essential oil of Periploca sepium Bunge and its main component. Nat. Prod. Res. 2012;26:926–932. doi: 10.1080/14786419.2010.534991. [DOI] [PubMed] [Google Scholar]
- 187.Jeong E., Cho K., Lee H. Food protective effects of Periploca sepium oil and its active component against stored food mites. J. Food Prot. 2012;75:118–122. doi: 10.4315/0362-028X.JFP-11-315. [DOI] [PubMed] [Google Scholar]
- 188.Li Y., Zeng X.N. Effects of periplocoside X on midgut cells and digestive enzymes activity of the soldiers of red imported fire ant. Ecotoxicol. Env. Saf. 2013;93:1–6. doi: 10.1016/j.ecoenv.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 189.Zhao Y.C., Shi B.J., Hu Z.N. The insecticidal activity of Periplocoside NW. Chin. Bull. Entomol. 2008;45:950–952. [Google Scholar]
- 190.Feng M.X., Shi B.J., Zhao Y.C., Hu Z.N., Wu W.J., Wu W. Histopathological effects and immunolocalization of Periplocoside NW from Periploca sepium Bunge on the midgut epithelium of Mythimna separata Walker larvae. Pestic Biochem. Physiol. 2014;115:67–72. doi: 10.1016/j.pestbp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 191.Shi B.J., Gao L.T., Ji Z.Q., Zhang J.W., Wu W.J., Hu Z.N. Isolation of the insecticidal ingredients from Periploca sepium. Chin. J. Pestic. Sci. 2012;14:103–106. [Google Scholar]
- 192.Chen C.C., Gao L.T., Shi B.U., Wen W.D., Hu Z.N., Wu W.J. Effects of Periplocosides P and E from Periploca sepium on the proteinase activities in the midgut of larvae of Mythimna separata and Agrotis ypsilon (Lepidoptera: Noctuidae) Acta Entomol. Sin. 2012;55:412–419. [Google Scholar]
- 193.Wang Y.Y., Hu Z.N. Effects of Periplocin P and E from Periploca sepium on activities of three detoxification enzymes in midgut of Mythimna separata and Agrotis ypsilon (Lepidoptera: Noctuidae) Acta Agric. Boreali-Occident. Sin. 2016;25:939–944. [Google Scholar]
- 194.Wang Y.Y., Qi Z.J., Qi M., Hu Z.N., Wu W.J. Effects of Periplocoside P from Periploca sepium on the midgut transmembrane potential of Mythimna separata Larvae. Sci. Rep. 2016;6:36982. doi: 10.1038/srep36982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feng M.X., Li Y.K., Chen X.T., Wei Q.S., Wu W.J., Hu Z.N. Comparative proteomic analysis of the effect of Periplocoside P from Periploca sepium on brush border membrane vesicles in midgut epithelium of Mythimna separata Larvae. Toxins. 2018;10:7. doi: 10.3390/toxins10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Feng M.X., He Z.Y., Wang Y.Y., Yan X.F., Zhang J.W., Hu Z.N., Wu W.J. Isolation of the binding protein of Periplocoside E from BBMVs in midgut of the oriental amyworm Mythimna separata walker (lepidoptera: Noctuidae) through affinity chromatography. Toxins. 2016;8:139. doi: 10.3390/toxins8050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang G.Y., Gong L.L., Dang R.M., Lu Y.J., Yang Z.C. Experimental study on the treatment of nerve injury caused by Pristionchus pacificus by Periploca forrestii Schltr. Lishizhen Med. Mater. Med. Res. 2012;23:2758–2759. [Google Scholar]
- 198.Jabbar A., Muhammad S., Razaque G., Qadir A., Younis M., Ahmad N., Baloch I., Mustafa G. Studies on neuropharmacological and analgesic effects of Periploca aphylla extract in mice. Pure Appl. Biol. 2016;5:1207–1215. doi: 10.19045/bspab.2016.50145. [DOI] [Google Scholar]
- 199.Chen L., Li J.S., Ke X., Sun C.L., Huang X.X., Jiang P., Feng F., Liu W.Y., Zhang J. Chemical profiling and the potential active constituents responsible for wound healing in Periploca forrestii Schltr. J. Ethnopharmacol. 2018;224:230–241. doi: 10.1016/j.jep.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 200.Chen L., Jiang P., Li J.S., Xie Z.J., Xu Y.H., Qu W., Feng F., Liu W.Y. Periplocin promotes wound healing through the activation of Src/ERK and PI3K/Akt pathways mediated by Na/K-ATPase. Phytomedicine. 2019;57:72–83. doi: 10.1016/j.phymed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 201.Belay W.Y., Endale Gurmu A., Wubneh Z.B. Antimalarial activity of stem bark of Periploca linearifolia during early and established plasmodium infection in mice. Evid. Based Complement. Altern. Med. 2018;2018:4169397. doi: 10.1155/2018/4169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sun R., Bao Z.Y., Huang W., Lv L.L., Zhang Z.P., Ren H.Y. Experimental comparison study on mice’s acute toxicity of different composition in Cortex Periplocae. Chin. J. Pharm. 2010;7:579–584. [Google Scholar]
- 203.Huang W., Zhang Y., Qian X.L., Bao Z.Y., Sun R. Experimental study on chronic toxicity in rats caused by different components of Cortex Periplocae radicis. Chin. J. Pharm. 2012;9:9–15. [Google Scholar]
- 204.Chen J.T., Sun D., Bi B., Zhao C.F., Zhou K. The changed characteristics of electrocardiogram in guinea pigs’ poisoned by Cortex Periploca. Lishizhen Med. Mater. Med. Res. 2010;21:1094–1096. [Google Scholar]
- 205.Bao Z.Y., Huang W., Zhang Y.N., Sun R. Experimental study on the “dose-time-toxicity” relationship of hepatotoxicity induced by different components from Cortex Periplocae in mice. Chin. J. Pharm. 2012;9:16–19. [Google Scholar]
- 206.Sun R., Huang W., Bao Z.Y., Zhang Y.N. The research of oxidative damage mechanism in hepatical toxical injury caused by different components from Cortex Periplocae in mice. Chin. J. Pharm. 2012;9:23–25. [Google Scholar]
- 207.Sun D., Zhang J., Chen J.T., Bi B., Zhou K., Deng Y.R. Toxicity of periplocin in single administration. J. Health Toxicol. 2010;24:461–463. [Google Scholar]
- 208.Xu X., MA Z.H., Sun D., Chen J.T., Zhou K., Liu Y. Study on correlation of the content of periplocin with acute toxicity of Cortex Periplocae. Chin. J. Pharm. 2015;12:261–263. [Google Scholar]
- 209.Yi L.X., Bi K.S., Chen X.Y., Zhang Q.F., Che S., Liu W.T., Lu D., Chen X.H. Determination and pharmacokinetics of periplocin in rat plasma by LC-MS. Biomed. Chromatogr. 2010;24:1089–1093. doi: 10.1002/bmc.1409. [DOI] [PubMed] [Google Scholar]
- 210.He J., Bo F., Tu Y.R., Azietaku J.T., Dou T., Ouyang H.Z., Chang Y.X., Liu H., Gao X.M. A validated LC-MS/MS assay for the simultaneous determination of periplocin and its two metabolites, periplocymarin and periplogenin in rat plasma: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2015;114:292–295. doi: 10.1016/j.jpba.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 211.Liu H.M., Zhang D.D., Tang Z.D., Sun M., Azietaku J.T., Ouyang H.Z., Chang Y.X., Wang M., He J., Gao X.M. Tissue distribution study of periplocin and its two metabolites in rats by a validated LC-MS/MS method. Biomed. Chromatogr. 2018;32:e4302. doi: 10.1002/bmc.4302. [DOI] [PubMed] [Google Scholar]