Abstract
Background
Studies from multiple countries have shown that acute kidney injury (AKI) in hospitalized patients is associated with mortality and morbidity. There are no reliable data at present on the incidence and mortality of AKI episodes among hospitalized patients in Germany. The utility of administrative codings of AKI for the identification of AKI episodes is also unclear.
Methods
In an exploratory approach, we retrospectively analyzed all episodes of AKI over a period of 3.5 years (2014–2017) on the basis of routinely obtained serum creatinine measurements in 103 161 patients whose creatinine had been measured at least twice and who had been in the hospital for at least two days. We used the “Kidney Disease: Improving Global Outcomes” (KDIGO) criteria for AKI. In parallel, we assessed the administrative coding of discharge diagnoses of the same patients with codes from the International Classification of Diseases (ICD-10-GM).
Results
Among 185 760 hospitalizations, stage 1 AKI occurred in 25 417 cases (13.7%), stage 2 in 8503 cases (4.6%), and stage 3 in 5881 cases (3.1%). AKI cases were associated with length of hospital stay, renal morbidity, and overall mortality, and this association was stage-dependent. The in-hospital mortality was 5.1% for patients with stage 1 AKI, 13.7% for patients with stage 2 AKI, and 24.8% for patients with stage 3 AKI. An administrative coding for acute kidney injury (N17) was present in only 28.8% (11 481) of the AKI cases that were identified by creatinine criteria. Like the AKI cases overall, those that were identified by creatinine criteria but were not coded as AKI had significantly higher mortality, and this association was stage-dependent.
Conclusion
AKI episodes are common among hospitalized patients and are associated with considerable morbidity and mortality, yet they are inadequately documented and probably often escape the attention of the treating physicians.
Acute kidney injury (AKI) is a frequent (8% to 22% of all hospital patients) and relevant clinical event (1, 2). It is characterized by a rapid worsening of kidney function to varying degrees and is associated with a 1.4- to 15.4-fold increase in the risk of mortality (3– 6). Patients with AKI are also at considerable risk for the development or worsening of chronic kidney disease (7– 10).
AKI is currently defined and staged depending on changes in serum creatinine and urine excretion according to the KDIGO AKI guidelines (table 1) (10, 11). According to international guidelines, the diagnosis of AKI should prompt a number of steps. These include identifying and treating triggering factors, adjusting drug doses to impaired renal function, avoiding nephrotoxins, monitoring of hemodynamics as well as fluid and electrolyte balance, and nephrological follow-up. A recently published study showed that the systematic implementation of these measures was associated with an absolute reduction in the risk of mortality of 16.6% (12). The main precondition for the prompt implementation of these measures is early diagnosis of AKI. However, a number of studies indicate that AKI episodes remained undetected in clinical routine in the majority of cases (57% to 99%) (13– 15).
Table 1. Diagnosis and staging of acute kidney injury according to KDIGO (10)*.
| Stage | Creatinine increase | Urine excretion |
| 1 | ≥ 0.3 mg/dL within a maximum of 48 h, or | <0.5 ml/kg/h for ≥ 6 h |
| ≥ 1.5- to 1.9-fold increase in serum creatinine within 7 days (observed or assumed) | ||
| 2 | ≥ 2.0- to 2.9-fold increase in serum creatinine | <0.5 ml/kg/h for ≥ 12 h |
| 3 | ≥ 3.0-fold rise in serum creatinine or | <0.3 ml/kg/h for ≥ 24 h or |
| increase by at least 0.5 mg/dL to ≥ 4.0 mg/dL or renal replacement therapy | anuria ≥ 12 h |
*The current study used only creatinine criteria
KDIGO, Kidney Disease: Improving Global Outcomes
Epidemiological studies often identify AKI episodes from administrative hospital data (16– 18). However, the extent to which administrative data reflect the actual incidence of AKI is controversial. Studies conducted in the US suggest that administrative data identify only a proportion of clinical AKI episodes (13, 14, 19). The usefulness of administrative data to identify AKI episodes in the hospital sector in Germany is currently unclear. In line with case coding according to the ICD-10-GM, acute kidney injury is coded as N17. Since 2011, the German Society of Nephrology (Deutsche Gesellschaft für Nephrologie) has recommended coding acute kidney injury (N17) if a 50% increase in creatinine within 7 days or a =0.3-mg/dL rise in creatinine within 48 h is observed (20). It is unclear to what extent these recommendations are implemented in coding practice.
This study retrospectively analyzed the presence or absence of AKI episodes according to the KDIGO creatinine criteria over a 3.5-year period (2014–2017) on the basis of serial creatinine concentration measurements in clinical routine during all hospital stays. In addition, patient characteristics, comorbidities, AKI codings (i.e., with the N17 code), as well as fatalities were recorded on the basis of administrative data for all hospital stays. This made it possible to determine the incidence and outcomes of creatinine-based AKI episodes and to calculate the proportion of coded AKI episodes in relation to the estimated true incidence of AKI in the defined study population.
Methods
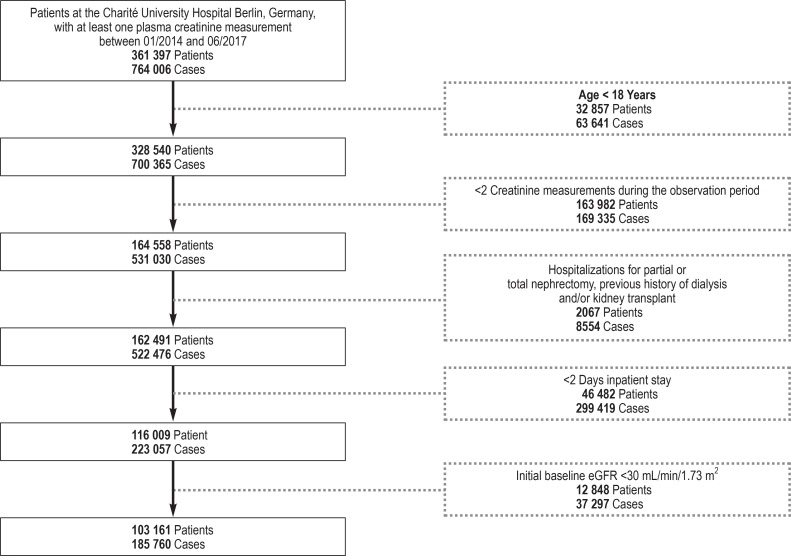
This explorative retrospective study included adult patients treated on an inpatient basis at the Charité University Hospital Berlin, Germany, between 1 January 2014 and 30 June 2017. The exclusion criteria are shown in the study flow chart (efigure).
eFigure.
Study flow chart: Each case represents a hospital stay; thus, a proportion of patients experienced several cases during the observation period.
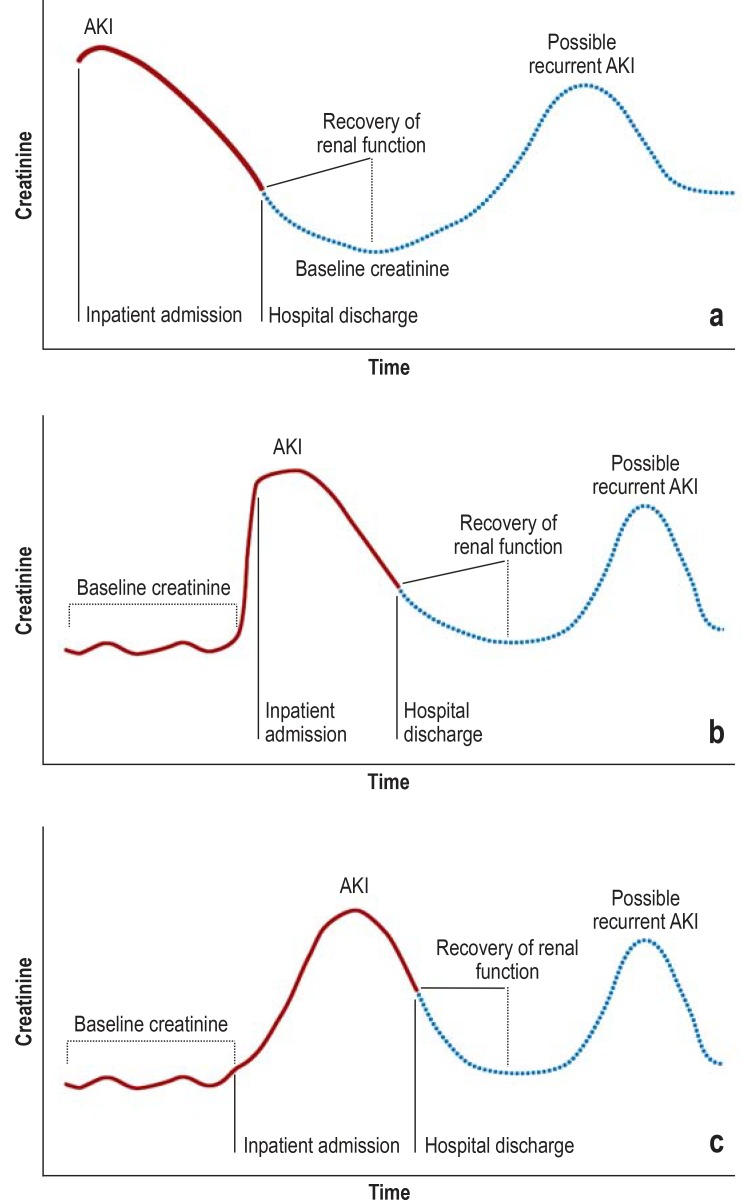
The definition and staging of acute kidney injury (AKI) was performed according to the KDIGO creatinine criteria (table 1) (11). Figure 1 provides a schematic representation of the various scenarios of AKI episodes and the associated determination of baseline creatinine (21, 22).
Figure 1.
Possible scenarios of AKI episodes:
a) Creatinine was already elevated at the time of inpatient admission, with no knowledge of previous creatinine levels. In the further course, renal function recovers and creatinine drops to the assumed baseline level.
b) Creatinine is already elevated at the time of inpatient admission. Baseline creatinine is known from previous hospital stays.
c) Creatinine is not elevated at the time of admission, but rises over the course of the inpatient stay. AKI, acute kidney injury
Mortality data were obtained from hospital data. As such, only fatalities that occurred at the Charité University Hospital Berlin were recorded. Descriptive analyses and Kaplan-Meier curves were stratified by AKI stage. Uni- and multivariate Cox regression analyses were performed to identify predictors of mortality. The study population, the extraction of administrative data, the definition of AKI stages, as well as the variables and statistical analyses, are described in detail in the eMethods Section.
Results
Incidence of creatinine-based AKI episodes, patient characteristics, and follow-up parameters
We analyzed the data on 103 161 adult patients treated on at least two consecutive days at the Charité University Hospital in Berlin during the period 2014–2017 (efigure). Including all available follow-up visits, the average observation period per patient was 248 days. AKI episodes and their staging were identified on the basis of serial creatinine measurements and using the KDIGO criteria. In a first step, we analyzed basic patient characteristics in relation to the maximum AKI stage observed. A total of 32 238 patients fulfilled the criteria for AKI at at least one point in time during the observation period (31.3% of the total population). AKI stage 1 (= 0.3 mg/dL or 1.5-fold increase in creatinine) was identified in 19 009 patients (18.4%), AKI stage 2 (= two-fold increase in creatinine) in 7499 patients (7.3%), and AKI stage 3 (= three-fold increase in creatine or a rise to = 4.0 mg/dL) was seen in 5730 patients (5.6%) (table 2). Patients with AKI had a higher average age compared to patients without AKI. There were more men than women among the AKI patients, whereas non-AKI patients were more evenly distributed in terms of sex. Patients with AKI had comorbidities such as diabetes mellitus, heart failure, coronary heart disease, and hypertension more frequently than did patients without AKI. Baseline estimated glomerular filtration rate (eGFR) was slightly lower in patients with AKI compared to patients without AKI. Recurrent AKI episodes were seen significantly more frequently in patients that had had stage-3 AKI at least once.
Table 2. Patient-related analysis: demography and comorbidities according to KDIGO stage of acute kidney injury.
| Basic characteristics |
No AKI (n = 70 923; 68.7%) |
AKI | ||
|
Stage 1 (n = 19 009; 18.4%) |
Stage 2 (n = 7499; 7.3%) |
Stage 3 (n = 5730; 5.6%) |
||
| Age (years), mean [95% CI] | 57.3 [57.1; 57.4] | 64.9 [64.6; 65.1] | 64.8 [64.4; 65.1] | 63.0 [62.6; 63.4] |
| Males, % [95% CI] | 50.6 [50.2; 50.9] | 56.0 [55.3; 56.7] | 53.3 [52.1; 54.4] | 57.0 [55.7; 58.3] |
| Comorbidities, % [95% CI] | ||||
| – Diabetes mellitus type 1 and 2 | 14.1 [13.8; 14.3] | 26.4 [25.8; 27.1] | 29.8 [28.8; 30.9] | 32.0 [30.8; 33.2] |
| – Diabetes mellitus type 2 | 12.6 [12.4; 12.9] | 21.8 [21.2; 22.4] | 21.8 [20.9; 22.8] | 21.8 [20.7; 22.9] |
| – Heart failure | 7.8 [7.6; 8.0] | 18.3 [17.7; 18.8] | 21.1 [20.2; 22.0] | 21.9 [20.9; 23.0) |
| – Coronary heart disease | 14.7 [14.5; 15.0] | 25.9 [25.3; 26.5] | 23.2 [22.3; 24.2] | 20.8 [19.7; 21.8] |
| – Hypertension | 41.8 [41.4; 42.1] | 57.8 [57.1; 58.5] | 55.5 [54.4; 56.6] | 54.7 [53.4; 56.0] |
| – Malignancy | 23.0 [22.7; 23.3] | 36.7 [36.0; 37.4] | 46.3 [45.2; 47.4] | 48.9 [47.6; 50.2] |
| Baseline eGFR (mL/min/1.73 m2) mean [95% CI] | 88.4 [88.2; 88.5] | 79.2 [78.8; 79.5] | 82.9 [82.2; 83.5] | 80.9 [80.1; 81.7] |
| Patients with >1 AKI episodes, % [95% CI] | – | 4.0 [3.8; 4.1] | 30.9 [29.9; 32.0] | 41.5 [40.2; 42.8] |
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; 95% CI, 95% confidence interval
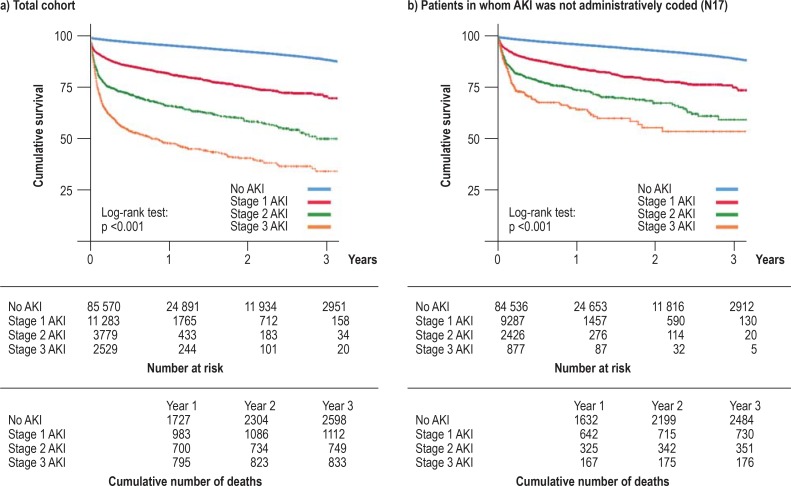
In a next step, we performed case-related analyses in which the 185 760 hospital cases of the patients under study were analyzed separately in order to identify primary and secondary diagnoses as well as procedures associated with AKI episodes (table 3). AKI episodes were observed in 21.4% of all hospital cases. It was found that cases associated with AKI more frequently had a primary diagnosis of acute coronary syndrome, acute respiratory disease, or cancer, and more frequently had sepsis as a primary or secondary diagnosis. In AKI cases, surgical procedures and mechanical ventilation were more frequently coded, with a clear association with the KDIGO stage. Renal replacement procedures were coded as stage-3 AKI in a substantial number of cases, as intermittent hemodialysis in 29.9%, and as continuous renal replacement procedures in 21.4%. With regard to short-term hospital outcomes, patients with AKI had significantly longer stays as well as increased hospital mortality (table 3). Patients with AKI also more often required renal replacement therapy at the time of hospital discharge. To analyze long-term outcomes, we investigated mortality data on a case-related basis for the available follow-up period (starting from the first documented hospital stay during the observation period). Kaplan-Meier survival curves showed progressively worse long-term survival from stage 1 to stage 3 (Figure 2a).
Table 3. Case-related analysis: primary and secondary diagnoses, procedures, and hospital outcomes according to KDIGO stage of AKI.
| Case characteristics |
No AKI (n = 145 959; 78.6%) |
AKI | ||
|
Stage 1 (n = 25 417; 13.7%) |
Stage 2 (n = 8503; 4.6%) |
Stage 3 (n = 5881; 3.1%) |
||
|
Documented AKIs, n, % [95% CI] Total period 2014–2017, including: |
1.3 [1.2; 1.4] | 4670 18.4 [17.9; 18.9] |
3054 35.9 [34.9; 37.0] |
3757 63.9 [62.6; 65.1] |
| 2014 | 0.9 [0.8; 1.0] | 13.4 [12.6; 14.2] | 29.5 [27.7; 31.4] | 60 [57.5; 62.4] |
| 2015 | 1.2 [1.1; 1.3] | 17.4 [16.5; 18.3] | 34.9 [33.0; 36.8] | 61.8 [59.4; 64.1] |
| 2016 | 1.6 [1.5; 1.7] | 21.7 [20.8; 22.7] | 40.3 [38.4; 42.2] | 67.7 [65.5; 69.9] |
| 2017 | 1.8 [1.6; 2.0] | 23.8 [22.4; 25.3] | 41.9 [39.1; 44.8] | 69.9 [66.8; 72.9] |
| Hospital primary diagnoses, % [95% CI] | ||||
| – Acute coronary syndrome | 1.6 [1.5; 1.7] | 4.1 [3.8; 4.3] | 3.7 [3.3; 4.1] | 2.4 [2.0; 2.8] |
| – Cornary heart disease | 3.8 [3.7; 3.9] | 3.3 [3.1; 3.5] | 1.6 [1.3; 1.9] | 0.8 [0.6; 1.1] |
| – Cerebrovascular disease | 3.3 [3.2; 3.4] | 3.8 [3.6; 4.1] | 3.8 [3.4; 4.2] | 2.4 [2.0; 2.8] |
| – Respiratory diseases | 4.0 [3.9; 4.1] | 5.5 [5.2; 5.8] | 7.3 [6.7; 7.8] | 7.9 [7.2; 8.6] |
| – Malignancies/hemato-oncological diseases | 23.0 [22.7; 23.3] | 28.0 [27.4; 28.5] | 30.9 [29.9; 31.9] | 27.3 [26.1; 28.4] |
| – Gastrointestinal diseases | 9.0 [8.8; 9.1] | 8.2 [7.9; 8.5] | 8.2 [7.7; 8.8] | 8.7 [8.0; 9.5] |
| – Liver disease | 1.1 [1.0; 1.1] | 1.7 [1.6; 1.9] | 2.7 [2.4; 3.1] | 5.2 [4.7; 5.8] |
| Sepsis, % [95% CI] | 0.12 [0.10; 0.14] | 1.9 [1.8; 2.1] | 9.4 [8.8; 10.0] | 24.8 [23.7; 25.9] |
| Surgical procedures, % [95% CI] | 39.6 [39.3; 39.8] | 47.5 [46.8; 48.1] | 60 [58.9; 61.0] | 63.7 [62.4; 64.9] |
| Mechanical ventilation, % [95% CI] | 1.2 [1.2; 1.3] | 4.0 [3.8; 4.3] | 10.3 [9.7; 11.0] | 15.8 [14.9; 16.8] |
| Renal replacement therapy, % [95% CI] | ||||
| – Intermittent hemodialysis | 0.05 [0.04; 0.07] | 1.3 [1.2; 1.5] | 7.1 [6.6; 7.7] | 29.9 [28.8; 31.1] |
| – Continuous renal replacement therapy | 0.02 [0.01; 0.03] | 0.9 [0.8; 1.1] | 5.6 [5.2; 6.1] | 21.4 [20.4; 22.5] |
| Total length of hospital stay (days), median (Q1; Q3) | 4.0 (3; 8) | 10 (6; 16) | 18 (10; 30) | 25 (12; 44) |
| Hospital mortality, % [95% CI] | 0.6 [0.5; 0.6] | 5.1 [4.9; 5.4] | 13.7 [12.9; 14.4] | 24.8 [23.7; 25.9] |
| Dialysis within 72 h of discharge, % [95% CI] | 0.04 [0.03; 0.05] | 0.8 [0.7; 0.9] | 2.7 [2.4; 3.1] | 11.7 [10.8; 12.5] |
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; 95% CI, 95% confidence interval
Figure 2.
Stage-dependent long-term survival following creatinine-based acute kidney injury (stages 1–3 according to KDIGO)
a) Stage-dependent Kaplan-Meier survival curves for the total population.
b) Stage-dependent Kaplan-Meier survival curves for the subgroup of patients whose administrative coding did not indicate acute kidney injury (N17) (N = 97 126 patients).
In each case, the number at risk for the following time interval is shown.
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes
Univariate Cox regression analysis confirmed the association between AKI and mortality. Likewise in the multivariate Cox regression model, a significant association remained between AKI and mortality when adjusted for age, male sex, comorbidities, baseline eGFR, sepsis, and mechanical ventilation (hazard ratio [HR] = 4.71; 95% confidence interval [CI]: [4.42; 5.00]) (table 4).
Table 4. Cox regression analysis for the prediction of mortality: univariate and multivariate regression analyses.
| Variables |
Univariate analyses HR [95% CI] |
Multivariate analysis* HR [95% CI] |
| Age, per year | 1.04 [1.03; 1.04] | 1.025 [1.023; 1.028] |
| Male sex | 1.30 [1.23; 1.37] | 1.13 [1.07; 1.19] |
| Comorbidities | ||
| – Diabetes mellitus | 2.38 [2.25; 2.52] | 1.20 [1.13; 1.27] |
| – Heart failure | 2.89 [2.72; 3.08] | 1.60 [1.49; 1.71] |
| – Malignancy | 2.87 [2.72; 3.03] | 2.74 [2.59; 2.89] |
| Baseline eGFR, per mL/min | 0.99 [0.98; 0.99] | 0.998 [0.997; 0.999] |
| Sepsis | 18.19 [16.83; 19.66] | 4.28 [3.91; 4.67] |
| Mechanical ventilation | 5.19 [4.73; 5.71] | 1.72 [1.55; 1.91] |
| Acute kidney injury | 7.63 [7.23; 8.06] | 4.42 [4.15; 4.70] |
*The multivariate model was adjusted for effects of the three Charité sites (campuses).
eGFR, estimated glomerular filtration rate; HR, hazard ratio; 95% CI, 95% confidence interval
In summary, associations with cardiovascular comorbidities, severe disease courses, unfavorable renal outcomes, as well as long-term and short-term mortality were seen depending on the KDIGO stage of AKI. It is of note that even stage 1 AKI is associated with an increase in unfavorable outcomes.
Administrative coding of acute kidney injury
We investigated the presence or absence of administrative coding with N17 (ICD-10-GM) among discharge diagnoses (N = 185 760 cases) on a case-related basis. The overall analysis showed that only 18.4% (N = 4670) of KDIGO stage-1 AKI episodes (according to serial creatinine analysis) were appropriately coded. With increasing severity of AKI the proportion of appropriately coded cases increased to 35.9% (AKI stage 2; N=3054) and 63.9% (AKI Stage 3; N=3757), respectively (table 3). We repeated these analyses separately for the individual years between 2014 and 2017 and found a gradual overall increase in the frequency of coding of AKI (table 3). Thus, for example, coding as stage-1 AKI increased from 13.4% in 2014 to 23.8% in 2017. This may reflect a rise in the implementation of coding recommendations.
In summary, these data yield clear evidence of administrative undercoding of AKI, which is evident even at higher stages of AKI. In order to investigate whether coding is consciously or unconsciously performed only in cases of more clinically relevant AKI episodes, we analyzed whether non-coded AKI episodes are also associated with increased long-term mortality and whether there was a link between AKI stage and mortality in those patients whose AKI episodes were not coded (N = 97 126) (Figure 2b). Also in this subgroup, a clear association was found between KDIGO stage of AKI and long-term mortality. This observation suggests that even clinically relevant episodes of AKI fall through the net of administrative coding.
Discussion
AKI: an underestimated risk factor
Early identification of comorbidities and risk factors is of crucial importance in hospitalized patients in order to:
Optimize care
Minimize the risks of inpatient treatment
Deploy resources efficiently
Optimize the long-term prognosis.
Acute kidney failure, even if severe, was long considered as largely “harmless” and reversible as long as the patient survived the conditions under which it developed. No particular importance was attributed to non-“dialysis-dependent” renal function impairment. Studies conducted over the last 15 years have refuted this view and shown that even comparatively mild temporary renal function decline is associated with an unfavorable prognosis; for example, a rise in creatinine of 0.3–0.4 mg/dL was associated with a 1.7-fold increase in the risk of mortality (3).
The present study underlines the importance of AKI as a risk factor in hospitalized patients on the basis of our retrospective analysis of a 3.5-year period at the Charité University Hospital in Berlin. Almost a third of all inpatients treated for at least 2 days—and more than a fifth of all hospital stays—during this period were associated with episodes of AKI. The fact that altogether less than 30% of all cases of AKI were administratively coded (18%–64% depending on the stage of AKI) suggests that the clinical recognition of AKI is still inadequate.
International comparison
The stage-dependent association observed here between AKI episodes and short- and long-term hospital mortality confirms US studies (3, 5, 23, 24). As in international studies (3, 5, 25), we also observed a pronounced stage-dependent association between AKI and increasing length of hospital stay and dialysis dependence at the time of discharge.
Furthermore, our detailed analysis of primary and secondary diagnoses and procedures confirmed previously described links between AKI episodes and:
In summary, these comparisons show that AKI represents a remarkably consistent risk factor across disciplines and irrespective of the sometimes significant differences in inpatient hospital care in different healthcare systems.
Insufficient AKI coding
Our study found clear evidence of undercoding among the almost 40 000 cases involving creatinine-based AKI, particularly in stage-1 and -2 AKI. This underdocumentation likely points to a failure to identify all AKI episodes in clinical routine. However, since our analysis was based purely on administrative data and not on patient records, this lack of administrative AKI coding is not necessarily due to a failure on the physician‘s part to make the diagnosis. However, coding is generally carried out by skilled documentation personnel using medical reports, suggesting that a significant proportion of AKI episodes do indeed remain unrecognized. This is particularly relevant given that there was also a clear link between AKI episodes and mortality in the subgroup of AKI cases that were not administratively documented.
Grams et al. also reported that administrative billing codes have low sensitivity (<20%) in terms of the identification of patients with creatinine-based AKI in a US population (19). A retrospective cohort study conducted at a university hospital in the US demonstrated that only 43% of patients with AKI (defined as a doubling of creatinine) had this correspondingly noted in their medical records (14). Interestingly, Wilson et al.‘s study showed in an unadjusted analysis that formal documentation of AKI was associated with higher mortality, a phenomenon that was also observed in our cohort (Figure 2a, 2b). An unlikely explanation for this observation would be that recognized AKI episodes were associated with clinically counterproductive interventions that led to higher mortality. The far more likely explanation is that non-coded AKI episodes are associated with lower case severity and lower mortality. In fact, Wilson et al. showed that the association was inversed after adjustment for disease severity, whereupon non-documented AKI was linked to increased mortality. Studies in other countries also found non-recognition rates of AKI of over 70% (15), with evidence that the time of AKI recognition was likewise associated with hospital mortality.
The potential for automated analysis of routine clinical data
Our investigation is based on the use of an algorithm to identify AKI episodes using creatinine measurements taken in the routine clinical setting. Other studies have previously used similar AKI algorithms (9, 33– 36). The majority of these algorithms are designed to identify emerging AKI on the basis of rising creatinine levels. In order to perform a comprehensive analysis of AKI episodes, we developed an expanded algorithm that additionally identifies falls in serum creatinine levels over the course of a hospital stay, thereby indicating resolution of an AKI episode.
Taking AKI as an example, the discrepancy between documented and non-documented creatinine-based AKI episodes suggests that a comparatively simple, automated “digital” evaluation can yield important additional information. At the same time, this evaluation demonstrates the potential of using stored medical data to identify disease characteristics, risk associations, and treatment practice. Merely by combining the analysis of a single laboratory parameter (creatinine concentration) from one database with clinical data and coding data (primary diagnoses, secondary diagnoses, and procedures) from another administrative database, we were able to make a comprehensive status description.
Strengths and weaknesses of the study
Study strengths include the size of the cohort, which, by means of an objective database query, led to the identification of more than 39 000 AKI episodes in approximately 186 000 cases over a period of 3.5 years. Added to that is the fact that all creatinine values were measured in a central laboratory, which is particularly relevant given the possible variation in methods between laboratories. Another strength is the fact that both the emergence as well as the resolution of AKI episodes were recorded using a specially designed algorithm.
Weaknesses include the retrospective nature of the analysis, meaning that unidentified confounders might have affected the results. Furthermore, AKI episodes were recorded exclusively on the basis of creatinine criteria determined in the context of clinical routine. Other AKI criteria such as urine excretion or initiation of renal replacement therapy were not taken into account. Since AKI episodes are due to changes in creatinine levels, the analysis was restricted to patients with at least two creatinine measurements. This methodological approach may have resulted in a certain selection bias in favor of more severely ill patients, meaning that the AKI incidence in the entire hospital population may be overestimated. On the other hand, it remains unclear how many additional AKI episodes occurred but were not recorded since creatinine measurements were not taken. This could lead to an underestimation of the actual number of AKI episodes. A further limitation lies in the fact that the analyses on patient survival were based purely on hospital mortality data at the Charité University Hospital Berlin; deaths in the outpatient setting or in other hospitals were not recorded. Furthermore, irrespective of the multivariate analysis, the statistical links observed do not necessarily imply causality. Since this was a monocentric analysis, it is also possible that the observations made here cannot be extrapolated to other centers and hospitals with different treatment mandates.
Supplementary Material
eMethods section
Study population
This retrospective explorative study was conducted at the Charité University Hospital Berlin, Germany, with the approval of the institutional ethics committee (EA2/221/18). The study cohort included all adult patients (= 18 years) that underwent treatment at the Charité during the period from 1 January 2014 to 30 June 2017. The study period 2014–2017 was chosen in order to cover a period in which the 2012 KDIGO definition of AKI was already being used and, at the same time, was feasible in terms of data collection. The prerequisite for inclusion was at least two creatinine measurements (usually in plasma) and a hospital stay per case of at least 2 days. Exclusion criteria included a previous history of stage-G5 kidney disease, as defined by the coded secondary diagnosis (N18.5; Z49 according to the ICD-10-GM) or a coded (German) OPS code for peritoneal dialysis (8–857; on the assumption that peritoneal dialysis is only rarely or never used in acute kidney failure). In addition, all patients with coded OPS codes (5–553, 5–554, and 5–555) for partial or total nephrectomy, kidney transplantation, as well as with a previously estimated glomerular filtration rate (baseline eGFR) < 30 mL/min/1.73 m2 (calculated according to the Chronic Kidney Disease Epidemiology Collaboration [CKD-EP] equation (21) leaving ethnicity aside) were excluded (see eFigure for the study flow chart).
AKI definition and staging
The definition and staging of acute kidney injury (AKI) was performed according to the KDIGO creatinine criteria (table 1) (11).
Determination of AKI stages based on serial creatinine measurements
AKI stages were determined according to the above-mentioned criteria by a = 0.3-mg/dL rise in creatinine within 48 h (for KDIGO stage 1) or by the ratio of maximum creatinine during a hospital stay compared to baseline creatinine (for KDIGO stages 1–3). In the case of discordant results in the determination of AKI stage, the highest stage calculated was always the stage taken into account. Baseline creatinine was defined as follows: the lowest value for reference value 1 (RV1) and 2 (RV2), whereby RV1 is the lowest creatinine value within 7 days prior to the maximum AKI-defining creatinine value and RV2 the mean creatinine value between 8 and 365 days prior to the maximum AKI-defining creatinine value (22). In cases where neither RV1 or RV2 could be determined, the lowest creatinine value during the relevant hospital stay was defined as the baseline creatinine value (given that no renal replacement therapy had been documented for the case; otherwise, the lowest creatinine value from the subsequent hospital stay was used, if no renal replacement therapy had been performed).
A number of scenarios relating to AKI diagnosis and the respective determination of baseline creatinine are shown in Figure 1. Baseline eGFR was determined according to the CKD-EPI creatinine equation (21) from baseline creatinine, age, and patient sex (without regard to ethnicity).
Extraction of administrative data
Administrative data were extracted using the German hospital information system [Krankenhausinformationssystem (KIS), i.s.h.med (Cerner Deutschland GmbH/SAP Deutschland SE & Co KG)]. Administrative coding of acute kidney injury was evaluated in line with the ICD-10-GM by the presence of one of the codes N17.0–N17.9 in the discharge diagnosis for the relevant hospital stay. Demographic variables for the cohort included age and sex. Other extracted data comprised case-related primary diagnoses, secondary diagnoses, and procedures (from discharge coding based on ICD-10-GM), as well as outcome data (length of hospital stay, hospital mortality, dialysis within 72 h prior to discharge). Relevant pre-existing diseases were taken from the discharge diagnoses coded for the first hospital case. Changes in comorbidity status over time were not taken into account. The administrative coding of pre-existing disease and primary diagnoses was extracted from discharge diagnoses for the respective hospital stay according to the ICD-10-GM from the presence of one of the codes E10–E14.91 for diabetes mellitus, I50–I50.9 for heart failure, I20–I25.9 for coronary heart disease, I10–I15.91 for hypertension, C00–C96.9 for malignancies, I60–I69.8 for cerebrovascular diseases, J00–J99.8 for respiratory diseases, K00–K93.8 for gastrointestinal diseases, and A02.1, A22.7, A24.1, A26.7, A32.7, A39.1–A39.4, A40.x, A41.x, A42.7, A48, B37.7, B44.7, I33, B00.7, R57.2–R57.9, T80.2, T82.7, T83.5, T84.5–T84.7, T85.71–T85.72 for sepsis.
The administrative coding of procedures was extracted from the OPS codes. Renal replacement therapy was identified by the presence of one of the codes 8-853–8-856, 8-854.6–8-854.8, 8-855.7–8-855.9, a surgical procedure by the presence of one of the codes 5.01–5.99, and mechanical ventilation by the presence of one of the codes 8-71–8-719 in the discharge documentation for the respective hospital stay.
Definition of variables, estimators, and analyses
The available data were analyzed in a patient-related (patient characteristics) and in a case-related (diagnostic features) approach. Each hospital stay was analyzed from the time of admission to the time of discharge. Patients‘ hospital cases were analyzed separately in order to evaluate primary and secondary diagnoses as well as procedures associated with AKI episodes. Estimates for the crude 3-year incidence of AKI in the study population were made using the creatinine values determined for actual AKI episodes. The coding level of AKI was determined in relation to the estimated true incidence of AKI in the defined study population from the number of AKI cases documented in routine hospital data. The number of fatalities was determined from routine data at the Charité University Hospital; fatalities following discharge were not recorded. Hospital mortality for the study population was estimated on the basis of these data.
Statistical analysis
All analyses were performed using SPSS version 23 statistical software (SPSS Inc., IBM, Armonk, USA). Depending on the scale of measurement of the characteristic, mean values and standard deviations or absolute and relative frequencies were calculated. As an indication of the precision of the estimators, 95% confidence intervals (95% CI) were calculated for all mean values and proportions shown. Due to the extremely large sample size and the explorative nature of the study, significance tests were not performed for the group comparisons, with the exception of the log-rank test.
Time-to-event data starting from the first hospital stay were shown using Kaplan-Meier plots and group differences were performed using the log-rank test.
Univariate and multivariate Cox regression analyses were carried out to investigate the association between acute kidney injury as well as other predictors and patient death. In order to investigate the associations between AKI status, covariates, and the study endpoint (patient death), the patient-related baseline characteristics, case-related analyses, and survival analyses are presented stratified for AKI status were shown and AKI status was included in the multivariate regression analysis. An additional multivariate adjustment was made for the variables age, sex, Charité site (campus 1–3), comorbidities, malignancy, sepsis, baseline eGFR, and mechanical ventilation in order to control for possible confounding.
Due to the definition of an undiagnosed AKI episode, the inclusion criteria defined for this study included at least two creatinine measurements, meaning the completeness of the creatinine values was already provided by the inclusion criteria.
The data on administrative codes and demography also showed a very high degree of completeness. The chosen approach to evaluation essentially complied with a complete-case analysis. Due to the extremely high level of data completeness, a possible bias due to the effects of a missing-at-random situation can also be considered very low with regard to the risk estimators in the regression model; as such, we did not expect that an approach using multiple imputation would be able to significantly minimize any possible slight bias of the estimators. Missing values in the data on Charité location (campus) were coded in an extra category in order to include them in the univariate and multivariate regression analyses and to check whether there was “informative missingness.”
Key Messages.
The study was a retrospective investigation of the data on 103 161 patients that were hospitalized for at least 2 days between 2014 and 2017 at the Charité University Hospital Berlin, Germany.
Episodes of acute kidney injury (AKI) according to creatinine-based KDIGO criteria occurred during more than 20% of all hospital stays.
AKI episodes were closely associated with short-term and long-term mortality, with a gradual increase in mortality from AKI stage 1 to AKI stage 3 being observed.
Administrative coding of AKI using the code N17 covered only a small percentage of AKI episodes.
Significant undercoding was found for all three stages (<20% coding in AKI stage1, 60%–70% coding in AKI stage 3), indicating that there is inadequate clinical perception of this entity.
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests
The authors state that there are no conflicts of interest.
References
- 1.Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis. 2017;69:18–28. doi: 10.1053/j.ajkd.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Soroko SH, Paganini EP, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- 5.Liangos O, Wald R, O‘Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 7.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67:742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. Am J Kidney Dis. 2013;61:686–688. doi: 10.1053/j.ajkd.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease. Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012:1–138. [Google Scholar]
- 12.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 14.Wilson FP, Bansal AD, Jasti SK, et al. The impact of documentation of severe acute kidney injury on mortality. Clin Nephrol. 2013;80:417–425. doi: 10.5414/CN108072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 16.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95:20–28. doi: 10.1016/j.athoracsur.2012.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 18.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9:682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsche Gesellschaft für Nephrologie. Aktualisierte Stellungnahme zur Kodierung von Nierenerkrankungen (AKI, CKD) www.dgfn.eu/kommission-drg-details/dgfn-aktualisierte-stellungnahme-zur-kodierung-von-nierenerkrankungen-aki-ckd-22.html (last accessed on 15 March 2019) [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawhney S. Automated alerts for acute kidney injury warrant caution. BMJ. 2015;350 doi: 10.1136/bmj.h19. [DOI] [PubMed] [Google Scholar]
- 23.See EJ, Jayasinghe K, Glassford N, et al. Longterm risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;951:60–72. doi: 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang KV, Sileanu FE, Clermont G, et al. Modality of RRT and recovery of kidney function after AKI in patients surviving to hospital discharge. Clin J Am Soc Nephrol. 2016;11:30–38. doi: 10.2215/CJN.01290215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liborio AB, Leite TT, Neves FM, Teles F, Bezerra CT. AKI complications in critically ill patients: association with mortality rates and RRT. Clin J Am Soc Nephrol. 2015;10:21–28. doi: 10.2215/CJN.04750514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65:870–877. doi: 10.1053/j.ajkd.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perazella MA, Rosner MH. Acute kidney injury in patients with cancer. Oncology (Williston Park) 2018;32:351–359. [PubMed] [Google Scholar]
- 30.Chen N, Chen X, Ding X, Teng J. Analysis of the high incidence of acute kidney injury associated with acute-on-chronic liver failure. Hepatol Int. 2018;12:262–268. doi: 10.1007/s12072-018-9866-x. [DOI] [PubMed] [Google Scholar]
- 31.Gessolo Lins PR, Carvalho Padilha WS, Magalhaes Giradin Pimentel CF, Costa Batista M, Teixeira de Gois AF. Risk factors, mortality and acute kidney injury outcomes in cirrhotic patients in the emergency department. BMC Nephrol. 2018;19 doi: 10.1186/s12882-018-1061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O‘Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42:521–530. doi: 10.1007/s00134-015-4157-7. [DOI] [PubMed] [Google Scholar]
- 33.Broce JC, Price LL, Liangos O, Uhlig K, Jaber BL. Hospital-acquired acute kidney injury: an analysis of nadir-to-peak serum creatinine increments stratified by baseline estimated GFR. Clin J Am Soc Nephrol. 2011;6:1556–1565. doi: 10.2215/CJN.08470910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Baek SH, Ahn S, et al. Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: a quality improvement study. Am J Kidney Dis. 2018;71:9–19. doi: 10.1053/j.ajkd.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Porter CJ, Juurlink I, Bisset LH, Bavakunji R, Mehta RL, Devonald MA. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant. 2014;29:1888–1893. doi: 10.1093/ndt/gfu082. [DOI] [PubMed] [Google Scholar]
- 36.Siew ED, Parr SK, Abdel-Kader K, et al. Predictors of recurrent AKI. J Am Soc Nephrol. 2016;27:1190–1200. doi: 10.1681/ASN.2014121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods section
Study population
This retrospective explorative study was conducted at the Charité University Hospital Berlin, Germany, with the approval of the institutional ethics committee (EA2/221/18). The study cohort included all adult patients (= 18 years) that underwent treatment at the Charité during the period from 1 January 2014 to 30 June 2017. The study period 2014–2017 was chosen in order to cover a period in which the 2012 KDIGO definition of AKI was already being used and, at the same time, was feasible in terms of data collection. The prerequisite for inclusion was at least two creatinine measurements (usually in plasma) and a hospital stay per case of at least 2 days. Exclusion criteria included a previous history of stage-G5 kidney disease, as defined by the coded secondary diagnosis (N18.5; Z49 according to the ICD-10-GM) or a coded (German) OPS code for peritoneal dialysis (8–857; on the assumption that peritoneal dialysis is only rarely or never used in acute kidney failure). In addition, all patients with coded OPS codes (5–553, 5–554, and 5–555) for partial or total nephrectomy, kidney transplantation, as well as with a previously estimated glomerular filtration rate (baseline eGFR) < 30 mL/min/1.73 m2 (calculated according to the Chronic Kidney Disease Epidemiology Collaboration [CKD-EP] equation (21) leaving ethnicity aside) were excluded (see eFigure for the study flow chart).
AKI definition and staging
The definition and staging of acute kidney injury (AKI) was performed according to the KDIGO creatinine criteria (table 1) (11).
Determination of AKI stages based on serial creatinine measurements
AKI stages were determined according to the above-mentioned criteria by a = 0.3-mg/dL rise in creatinine within 48 h (for KDIGO stage 1) or by the ratio of maximum creatinine during a hospital stay compared to baseline creatinine (for KDIGO stages 1–3). In the case of discordant results in the determination of AKI stage, the highest stage calculated was always the stage taken into account. Baseline creatinine was defined as follows: the lowest value for reference value 1 (RV1) and 2 (RV2), whereby RV1 is the lowest creatinine value within 7 days prior to the maximum AKI-defining creatinine value and RV2 the mean creatinine value between 8 and 365 days prior to the maximum AKI-defining creatinine value (22). In cases where neither RV1 or RV2 could be determined, the lowest creatinine value during the relevant hospital stay was defined as the baseline creatinine value (given that no renal replacement therapy had been documented for the case; otherwise, the lowest creatinine value from the subsequent hospital stay was used, if no renal replacement therapy had been performed).
A number of scenarios relating to AKI diagnosis and the respective determination of baseline creatinine are shown in Figure 1. Baseline eGFR was determined according to the CKD-EPI creatinine equation (21) from baseline creatinine, age, and patient sex (without regard to ethnicity).
Extraction of administrative data
Administrative data were extracted using the German hospital information system [Krankenhausinformationssystem (KIS), i.s.h.med (Cerner Deutschland GmbH/SAP Deutschland SE & Co KG)]. Administrative coding of acute kidney injury was evaluated in line with the ICD-10-GM by the presence of one of the codes N17.0–N17.9 in the discharge diagnosis for the relevant hospital stay. Demographic variables for the cohort included age and sex. Other extracted data comprised case-related primary diagnoses, secondary diagnoses, and procedures (from discharge coding based on ICD-10-GM), as well as outcome data (length of hospital stay, hospital mortality, dialysis within 72 h prior to discharge). Relevant pre-existing diseases were taken from the discharge diagnoses coded for the first hospital case. Changes in comorbidity status over time were not taken into account. The administrative coding of pre-existing disease and primary diagnoses was extracted from discharge diagnoses for the respective hospital stay according to the ICD-10-GM from the presence of one of the codes E10–E14.91 for diabetes mellitus, I50–I50.9 for heart failure, I20–I25.9 for coronary heart disease, I10–I15.91 for hypertension, C00–C96.9 for malignancies, I60–I69.8 for cerebrovascular diseases, J00–J99.8 for respiratory diseases, K00–K93.8 for gastrointestinal diseases, and A02.1, A22.7, A24.1, A26.7, A32.7, A39.1–A39.4, A40.x, A41.x, A42.7, A48, B37.7, B44.7, I33, B00.7, R57.2–R57.9, T80.2, T82.7, T83.5, T84.5–T84.7, T85.71–T85.72 for sepsis.
The administrative coding of procedures was extracted from the OPS codes. Renal replacement therapy was identified by the presence of one of the codes 8-853–8-856, 8-854.6–8-854.8, 8-855.7–8-855.9, a surgical procedure by the presence of one of the codes 5.01–5.99, and mechanical ventilation by the presence of one of the codes 8-71–8-719 in the discharge documentation for the respective hospital stay.
Definition of variables, estimators, and analyses
The available data were analyzed in a patient-related (patient characteristics) and in a case-related (diagnostic features) approach. Each hospital stay was analyzed from the time of admission to the time of discharge. Patients‘ hospital cases were analyzed separately in order to evaluate primary and secondary diagnoses as well as procedures associated with AKI episodes. Estimates for the crude 3-year incidence of AKI in the study population were made using the creatinine values determined for actual AKI episodes. The coding level of AKI was determined in relation to the estimated true incidence of AKI in the defined study population from the number of AKI cases documented in routine hospital data. The number of fatalities was determined from routine data at the Charité University Hospital; fatalities following discharge were not recorded. Hospital mortality for the study population was estimated on the basis of these data.
Statistical analysis
All analyses were performed using SPSS version 23 statistical software (SPSS Inc., IBM, Armonk, USA). Depending on the scale of measurement of the characteristic, mean values and standard deviations or absolute and relative frequencies were calculated. As an indication of the precision of the estimators, 95% confidence intervals (95% CI) were calculated for all mean values and proportions shown. Due to the extremely large sample size and the explorative nature of the study, significance tests were not performed for the group comparisons, with the exception of the log-rank test.
Time-to-event data starting from the first hospital stay were shown using Kaplan-Meier plots and group differences were performed using the log-rank test.
Univariate and multivariate Cox regression analyses were carried out to investigate the association between acute kidney injury as well as other predictors and patient death. In order to investigate the associations between AKI status, covariates, and the study endpoint (patient death), the patient-related baseline characteristics, case-related analyses, and survival analyses are presented stratified for AKI status were shown and AKI status was included in the multivariate regression analysis. An additional multivariate adjustment was made for the variables age, sex, Charité site (campus 1–3), comorbidities, malignancy, sepsis, baseline eGFR, and mechanical ventilation in order to control for possible confounding.
Due to the definition of an undiagnosed AKI episode, the inclusion criteria defined for this study included at least two creatinine measurements, meaning the completeness of the creatinine values was already provided by the inclusion criteria.
The data on administrative codes and demography also showed a very high degree of completeness. The chosen approach to evaluation essentially complied with a complete-case analysis. Due to the extremely high level of data completeness, a possible bias due to the effects of a missing-at-random situation can also be considered very low with regard to the risk estimators in the regression model; as such, we did not expect that an approach using multiple imputation would be able to significantly minimize any possible slight bias of the estimators. Missing values in the data on Charité location (campus) were coded in an extra category in order to include them in the univariate and multivariate regression analyses and to check whether there was “informative missingness.”