Abstract
Intestinal tract development is a coordinated process involving signaling among the progenitors and developing cells from all three germ layers. Development of endoderm-derived intestinal epithelium has been shown to depend on epigenetic modifications, but whether that is also the case for intestinal tract cell types from other germ layers remains unclear. We found that functional loss of a DNA methylation machinery component, ubiquitin-like protein containing PHD and RING finger domains 1 (uhrf1), leads to reduced numbers of ectoderm-derived enteric neurons and severe disruption of mesoderm-derived intestinal smooth muscle. Genetic chimeras revealed that Uhrf1 functions both cell-autonomously in enteric neuron precursors and cell-non-autonomously in surrounding intestinal cells, consistent with what is known about signaling interactions between these cell types that promote one another’s development. Uhrf1 recruits the DNA methyltransferase Dnmt1 to unmethylated DNA during replication. Dnmt1 is also expressed in enteric neurons and smooth muscle progenitors. dnmt1 mutants have fewer enteric neurons and disrupted intestinal smooth muscle compared to wildtypes. Because dnmt1;uhrf1 double mutants have a similar phenotype to dnmt1 and uhrf1 single mutants, Dnmt1 and Uhrf1 must function together during enteric neuron and intestinal muscle development. This work shows that genes controlling epigenetic modifications are important to coordinate intestinal tract development, provides the first demonstration that these genes influence development of the ENS, and advances uhrf1 and dnmt1 as potential new Hirschsprung disease candidates.
Keywords: Enteric nervous system, DNA methylation, intestinal smooth muscle, intestinal epithelium, intestinal development, Hirschsprung disease
Introduction
Proper organ function requires coordinated development of multiple cell types over an appropriate temporal window. For example, development of a functioning intestinal tract involves coordination among intestinal epithelial cells derived from endoderm, muscle cells derived from mesoderm, and enteric neurons and glia derived from ectoderm. Cells from each of the three germ layers initially migrate and then proliferate, prior to their differentiation, which occurs essentially contemporaneously (Ganz, 2018; Ganz et al., 2016; Hao et al., 2016; Olden et al., 2008; Wallace et al., 2005a). Endoderm and mesoderm progenitor cells initially co-mingle (Gays et al., 2017; Warga and Nusslein-Volhard, 1999; Zorn and Wells, 2009), whereas the ectoderm that generates the neural crest cells that give rise to the enteric nervous system (ENS) is positioned far from the nascent endoderm and mesoderm. Thus, these ENS precursors must migrate for a significant distance to reach the developing intestinal tract before they can begin to differentiate and innervate it (Ganz, 2018; Lake and Heuckeroth, 2013). Signaling among these distinct cell types, or their progenitor cells, is critical for coordinated intestinal development. The intestinal epithelium influences development and differentiation of intestinal smooth muscle precursors (ISMPs) and intestinal smooth muscle cells (ISMCs), as well as development and differentiation of enteric precursor cells (EPCs) and the ENS (Fu et al., 2004; Korzh et al., 2011; Pietsch et al., 2006; Reichenbach et al., 2008; Sukegawa et al., 2000). The lateral plate mesoderm from which ISMPs and ISMCs arise influences intestinal epithelial development and EPC migration and differentiation (Fu et al., 2004; Graham et al., 2017; Hao et al., 2016; Mwizerwa et al., 2011; Natarajan et al., 2002; Puzan et al., 2018; Reichenbach et al., 2008; Sukegawa et al., 2000). Similarly, the ENS regulates intestinal epithelial development and integrity (Neunlist et al., 2007; Neunlist et al., 2013; Puzan et al., 2018). Thus, there is interdependence of intestinal cell type development through signaling between progenitor cells of intestinal epithelium, smooth muscle, and ENS. Mutations in genes involved in development of any of these cell types can have profound and often deleterious consequences (Brosens et al., 2016; Goldstein et al., 2016; Wallace et al., 2005b; Yamamoto and Oda, 2015). For example, mutations in genes that regulate ENS development can result in disorders, such as Hirschsprung Disease (HSCR), in which the distal intestine is uninnervated and dysmotile (Heuckeroth, 2018).
A number of studies have revealed the importance of epigenetic modulation during development and functioning of the endodermal and mesodermal cell types that contribute to the intestinal tract (Elliott and Kaestner, 2015; Elliott et al., 2015; Jorgensen et al., 2018). For example, epigenetic modulation has been shown to be important for integrity of the intestinal epithelium (Marjoram et al., 2015) and proper development of intestinal smooth muscle (Jorgensen et al., 2018). Epigenetic modification via DNA methylation is a key regulator of the differential gene expression that underlies the ability of cells to develop distinct fates. DNA methylation patterns are established by the de novo DNA methyl transferases (Dnmt) 3a and 3b and maintained by Dnmt1 (Robertson and Wolffe, 2000). DNA methylation regulates intestinal epithelium formation by controlling the balance between cell proliferation and differentiation during development (Elliott and Kaestner, 2015; Marjoram et al., 2015; Sheaffer et al., 2014). Dnmt1 has also been shown to have an essential role in regulating intestinal smooth muscle differentiation, integrity, and survival (Jorgensen et al., 2018). DNA methylation has further been linked to ENS development because EPCs have decreased Dnmt expression in HSCR patients compared to controls and some HSCR patients have presumed pathogenic missense mutations in Dnmt3b (Torroglosa et al., 2014). Zebrafish mutants of histone deacetlyase 1 (hdacl), another epigenetic modifier gene, have fewer enteric neurons in addition to other neural crest defects (Ignatius et al., 2013). However, how epigenetic modifications, such as DNA methylation, affect ENS development remains poorly understood and it is also unclear how DNA methylation coordinates proper temporal development of the distinct cell types that coalesce to form the intestinal tract. Here, we investigate the role of epigenetic modulation on coordinated development of the intestinal tract by focusing on development of ectodermal derivatives that form the ENS and mesodermal derivatives that form the intestinal smooth muscle.
Dnmt proteins require partners to effect DNA methylation. For example, Dnmt1 is recruited by the modular protein Ubiquitin-like protein containing PHD and RING finger domains 1 (Uhrf1) to unmethylated DNA to maintain DNA methylation patterns after replication (Bestor, 2000; Bostick et al., 2007; Ooi and Bestor, 2008; Sharif et al., 2007). Zebrafish has been instrumental in elucidating the role of Uhrf1, as mouse mutants die early in development (Bostick et al., 2007; Muto et al., 2002; Sharif et al., 2007). Zebrafish uhrf1 mutants show compromised intestinal barrier function resulting from disruption of the intestinal epithelium (Marjoram et al., 2015). They also show intestinal inflammation reminiscent of inflammatory bowel disease (Marjoram et al., 2015). However, little is known about how Uhrf1 influences ENS or intestinal smooth muscle cell development.
In this study, we examine the role of Uhrf1 and Dnmt1 in the coordination of intestinal development. To this end we analyze their effects on development of the ENS and intestinal muscle using a mutant uhrf1 allele (uhrf1b1115, hereafter referred to as uhrf1−/− ) that we isolated in a zebrafish forward genetic screen for mutants with changes in enteric neuron number (Kuhlman and Eisen, 2007). As previously reported, uhrf1 mutants exhibit significant disruption of intestinal epithelial morphology (Marjoram et al., 2015). We demonstrate that they also exhibit severe disruption of intestinal smooth muscle and a variable reduction in enteric neuron number. This disruption of both EPCs and smooth muscle cells results in displacement of enteric neurons via both cell-autonomous and cell-non-autonomous mechanisms. We show that both uhrf1 and dnmt1 are expressed in EPCs and surrounding intestinal cell types, including intestinal smooth muscle. Consistent with the known interactions between Uhrf1 and Dnmt1, zebrafish dnmt1 single mutants exhibit similar ENS and intestinal muscle phenotypes to uhrf1 mutants. Our double mutant analysis demonstrated that Uhrf1 and Dnmt1 function together to regulate enteric neuron and intestinal smooth muscle development. This work provides evidence that genes controlling epigenetic modifications play an important role in coordination of intestinal development.
Materials and Methods
Zebrafish husbandry
All zebrafish experiments were performed following protocols approved by the University of Oregon Institutional Animal Care and Use Committee. The uhrf1b1115 and dnmt1s904 mutations were propagated by mating heterozygous carriers to AB wildtypes. Homozygous uhrf1b1115 and dnmt1s904 single mutants were obtained by mating heterozygous carriers. Complementation testing was performed by mating adult zebrafish heterozygous for uhrf1b1115 and uhrf1hl272 (Sadler et al., 2007). Mutant larvae were first identified visually by smaller eyes and jaws and subsequently confirmed by fixing and staining with the pan-neuronal marker anti-Elavl and analyzing neuron numbers in the intestine. For some experiments, uhrf1b1115 and dnmt1 mutant carriers were outcrossed to Tg(phox2b:EGFP)w37 (Nechiporuk et al., 2007) and their progeny were grown up and screened for single and double mutant carriers that were subsequently crossed to visualize EPCs and neurons in living animals. uhrf1b1115 and dnmt1s904 single mutants had indistinguishable phenotypes. uhrf1b1115 and dnmt1s904 double mutants were generated by crossing heterozygotes to obtain a double mutant line.
Genotyping was performed on genomic DNA extracted from adult tails or heads obtained from larvae processed through immunohistochemistry. The dnmt1s904 mutation removes a BslI restriction site. A 230 bp PCR fragment containing the mutated site was amplified with forward 5’-TCTGTATCTTGTTTGCTCTGCTC-3’ and reverse 5’-CTCACAGACACCACACCGT-3’ primers. Digestion of the PCR product with BslI yielded two fragments in wildtype (185 and 45 bp) and did not cut the mutant PCR product. The uhrf1 mutation creates a Haelll restriction site. A 294 bp PCR fragment was amplified with forward 5’-AAGGGACGCCGAAGAAGAT-3’ and reverse 5’GACGTTGTGTTGGCACTCTG-3’ primers. Digestion with Haelll generated three fragments in wildtypes (177, 94 and 23 bp) and four fragments (128, 94, 49 and 23 bp) in the presence of the mutation. Double mutants were genotyped using both assays.
RAD-tag genotyping
Previously we performed a forward-genetic screen to uncover regulators of neural crest development (Kuhlman and Eisen, 2007). One of the mutants identified, b1115, displayed a severe enteric neuron phenotype [Fig. 1A,B, (Kuhlman and Eisen, 2007)]. Using Restriction site Associated DNA (RAD) sequencing of genomic DNA (Baird et al., 2008), we mapped the b1115 genetic lesion to a 2.2 Mb region on chromosome 22. Phenotypic mutant and wildtype fish from a single-pair cross were identified by phox2b:EGFP transgene expression in the ENS at 5 days post fertilization (dpf) and five mutant or five wildtype individuals were pooled for sequencing. Genomic DNA was purified from the cross parents, from nine wildtype larval pools, and from 34 b1115 mutant larval pools. RAD-tag libraries were prepared as described (Amores et al., 2011; Baird et al., 2008; Miller et al., 2007) and sequenced on an Illumina HiSeq 2000 to obtain 100-nucleotide single-end reads. We used Stacks version 1.19 (http://catchenlab.life.illinois.edu/stacks/) to organize reads into loci and to identify polymorphisms (Catchen et al., 2013; Catchen et al., 2011). Illumina sequences were quality filtered with the process_radtags program of Stacks (Catchen et al., 2013; Catchen et al., 2011) and aligned to the zebrafish genome (v. Zv9) (Howe et al., 2013) using GSNAP (Wu and Nacu, 2010). We ran pstacks with default parameters on the parent sequences, and with the parameters--bound_high .001 and--alpha .001 on pooled samples. The Stacks catalog was built from the parent sequences and sample stacks were matched to the catalog with sstacks. We excluded all loci with indels and more than two alleles in a single locus, and used the program SNPstats to calculate a G-test statistic comparing genotypes in mutants and wildtype pools (Hohenlohe et al., 2010). Sequences have been deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under the accession number SRP118720.
Figure 1. The zebrafish b1115 mutation has a non-synonymous single nucleotide polymorphism in an ultra-conserved amino acid of the RING domain of uhrfl.
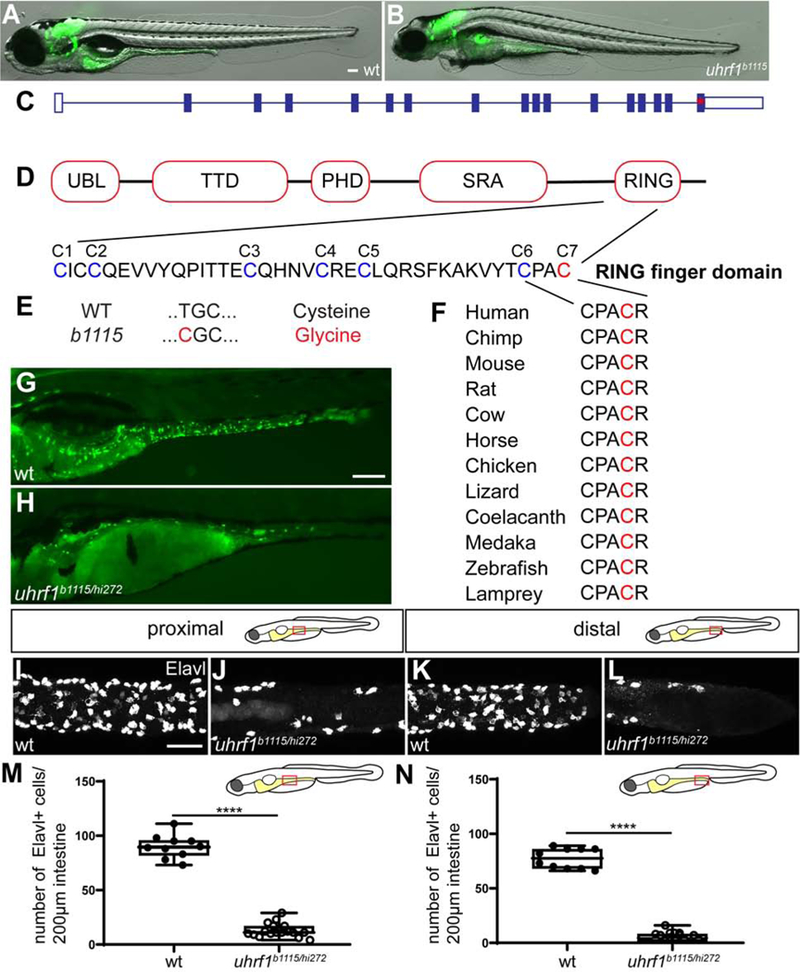
(A-B) Live images of 5 dpf wildtype (A) and uhrf1b1115 mutant (B) larvae show that mutants have smaller eyes and heart edema compared to wildtype siblings. (C) Exon 16 of uhrf1 contains the mutation (red asterisk) in the b1115 allele. (D) Schematic of important protein domains of uhrf1 with a close-up of the seven conserved cysteines (Tauber and Fischle, 2015). (E) A non-synonymous single nucleotide polymorphism (T=>C) mutates the conserved cysteine 7 (red) to a Glycine. (F) Cysteine 7 (red) is conserved among phylogenetically diverse vertebrate species. (G) Complementation cross between the b1115 allele and the hi272 allele of uhrf1 shows failure to complement in 5 dpf larvae as demonstrated by fewer phox2b:EGFP enteric neurons (green; H) compared to wildtype (green; G). In both proximal (J) and distal (L) intestine, uhrf1b1115/hi72 mutants have fewer enteric neurons than wildtype siblings at 5 dpf (proximal I; distal K) as quantified in M and N. (M,N) Quantification of Elavl positive cells per 200μm of proximal or distal intestine in wildtype or uhrf1b1115/hi212 mutant [wildtype n=10 (proximal), n=10 (distal); uhrf1b1115/hi272 n=19 (proximal), n=18 (distal)]. UBL, ubiquitin like; TTD, tandem tudor domain; PHD, plant homeodomain; SRA, SET and RING associated; PBR, polybasic region; RING, really interesting and new gene. A,B,G,H: side-views of whole-mount zebrafish larvae at 5 dpf. I-L: Confocal images of dissected intestines at 5 dpf. Scale bar = 100μm in A,B,E,F; Scale bar = 50μm in I-L. Unpaired t-test: **** = p < 0.0001.
Analysis of genes within the interval implicated uhrf1 as a candidate due to the similarities in phenotype between uhrf1b1115 and the previously described transgene insertion allele uhrf1hi272 (Amsterdam and Hopkins, 2004; Sadler et al., 2007). Crossing the b1115 allele to the hi272 allele resulted in non-complementation (Fig. 1G, H) indicating that b1115 is a mutated allele of uhrf1. We quantified the number of ENS neurons in our complementation cross and found that the Elavl+ cells were significantly reduced in uhrf1b1115/hi272 individuals compared to wildtype siblings in both proximal and distal intestine (Fig. 1I–N).To learn the site of the genetic lesion, we generated cDNA from pools of wildtype and mutant larvae, amplified and sequenced the uhrf1 gene in each pool and performed sequence comparisons. We identified a single base pair change resulting in a cysteine-to-glycine change in amino acid 744, which lies in the zinc finger RING domain of the protein (Fig. 1C-E). Sequence analysis of vertebrate species in the conservation track of the human-centric 100-way species alignment in the UCSC Genome Browser (https://genome.ucsc.edu/; representative species shown in Fig. 1F) revealed that the affected amino acid is invariably conserved in all vertebrates in the alignment and that the affected cysteine (C7) is an essential part of the RING finger domain involved in binding of one of the zinc ions that are critical for the function of the RING finger domain (Fig. 1F) (Borden and Freemont, 1996).
Immunohistochemistry
Antibody staining for Elavl (1:10,000, Thermo Fisher Scientific, Eugene, OR, catalog number A-21271), GFP (1:1000, Thermo Fisher Scientific, Eugene, OR, A11122, catalog number A11120, nNOS (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, catalog number sc-1025), 5-HT (1:10,000, Immunostar, Hudson, WI, catalog number 20080), Smooth muscle myosin (1:100, Alfa Aesar, catalog number J64817 BT-562), and Desmin (1:100, Sigma-Aldrich, catalog number D8281) was performed at 5 dpf as previously described (Uyttebroek et al., 2010). Immunostaining for Dnmt1 (1–250, Santa Cruz Biotechnology, Santa Cruz, CA, catalog number sc-20701) was performed on transverse cryosections of 48 hpf embryos expressing phox2b:EGFP. Staining with this antibody required a 5 minute incubation in 3% H2O2/0.5%o potassium hydroxide prior to standard staining methods. Antigens were visualized with standard fluorophore-labeled antibodies for rabbit IgG (1:1,000, Thermo Fisher Scientific, Eugene, OR, catalog number A-11008 or A-11071) and mouse IgG (1:1,000, Thermo Fisher Scientific, Eugene, OR, catalog number A-11001 or A-11030 ). DAPI staining was accomplished using ProLong Diamond Antifade Mountant with DAPI (Therm Fisher Scientific; catalog number p36966).
Hematoxylin and Eosin staining
Embryos were fixed in 4% paraformaldehyde and processed for paraffin sectioning and Hematoxylin and Eosin staining as previously described (Cheesman et al., 2011).
In situ hybridization
Embryos were fixed and processed for in situ hybridization as previously described (Seredick et al., 2012). Following in situ hybridization, embryos were embedded in agar, frozen, cryosectioned (Beattie and Eisen, 1997), and imaged on a confocal microscope as described below.
ENS Transplantation
ENS transplantation was performed as previously described (Rolig et al., 2017). Briefly, donor embryos were labeled by injection of 5% tetramethylrhodamine dextran (3000 MW) at the 1–2 cell stage and reared until the next manipulation in filter-sterilized embryo medium (EM). Embryos at the 12–14 somite stage were mounted in agar, a small hole dissected in the skin, and cells transplanted as previously described (Eisen, 1991). At 5 dpf, hosts and donors were fixed in 4% paraformaldehyde in 1× PBS for 3 h at room temperature and then washed 3 × 10 min in 1× PBS. We performed immunohistochemistry with anti-Elavl and anti-GFP (see immunohistochemistry section) to reveal both host and donor-derived enteric neurons.
Image acquisition
Confocal images were acquired on a Zeiss Pascal confocal microscope using a 40X water immersion objective and AIM or ZEN software (Carl Zeiss Microscopy, LLC, Thornwood, New York, USA) or a Leica SP8 confocal microscope using a 40X water immersion objective or a 63X oil immersion objective, 405-diode, white light lasers and LASX software, or a Leica TCSSPE confocal microscope using a 40X oil immersion objective and LASX software. Low magnification images were acquired using a Leica MZ205 FA fluorescent stereomicroscope and LAS software. Images were processed and analyzed using Photoshop CC (Version 19.1.1, Adobe Systems, Inc., San Jose, CA, USA) and FiJi software to adjust contrast.
Imaging analysis
Following immunohistochemistry, intestines were dissected and mounted in PBS between two coverslips. For cell quantification, projections along the y-axis were generated from confocal stacks in ZEISS LSM Image browser and used for manual cell counts and colocalization analysis between Elavl and nNOS or Elavl and 5-HT. Counts were performed in proximal intestine, defined as extending 200 μM from the caudal end of the intestinal bulb, and in the distal intestine, defined as extending 200 μM proximal to the anus. Alternatively, confocal stacks were loaded into Imaris (Version 9.2.1), appropriate regions of interest were selected, estimated cell diameter was set to 5.38 mm, spot selection was checked for appropriate threshold levels, and cell counts were manually verified. To determine significant differences, we performed an unpaired t-test using GraphPad Prism 8.01. For multiple comparisons, we performed One-way Anova followed by Tukey’s post-hoc test using GraphPad Prism 8.01.
Results
Zebrafish uhrfl mutants have fewer enteric neurons
uhrf1b1115 was originally isolated from a forward genetic screen for mutations affecting ENS development (Kuhlman and Eisen, 2007) based on a decrease in the number of ENS neurons in larvae. The line has been maintained for over a decade by outcrossing to AB. The uhrf1b1115 mutation was identified using RAD-mapping, as described in the Methods and confirmed by non-complementation with another allele of uhrf1 (hi272) that has been shown to have strongly reduced CG DNA methylation (Fig. 1G-N, Table 1) (Feng et al., 2010; Sadler et al., 2007). Larvae show the smaller eye phenotype that has previously been described for 5 dpf uhrf1 mutants (Fig. 1A,B) (Tittle et al., 2011). There are several additional uhrf1 mutant alleles that have also been demonstrated to decrease DNA methylation (Marjoram et al., 2015; Tittle et al., 2011).
Table 1.
Complementation scoring of uhrf1b1115 heterozygous carriers crossed to uhrf1hi272 heterozygous carriers shows that b1115 and hi272 are both mutant alleles of uhrf1.
| Pair # | Female | Male | Wildtype | b1115/hi272 | Total | % Mutants |
|---|---|---|---|---|---|---|
| 1 | hi272 | b1115 | 90 | 40 | 130 | 31 |
| 2 | hi272 | b1115 | 88 | 24 | 112 | 21 |
| 3 | hi272 | b1115 | 81 | 41 | 122 | 34 |
| 4 | hi272 | b1115 | 137 | 38 | 175 | 22 |
| 5 | hi272 | b1115 | 100 | 33 | 133 | 25 |
| 6 | b1115 | hi272 | 75 | 24 | 99 | 24 |
| 7 | b1115 | hi272 | 112 | 46 | 158 | 29 |
| 8 | b1115 | hi272 | 126 | 48 | 174 | 28 |
| 9 | b1115 | hi272 | 16 | 7 | 23 | 30 |
| 10 | b1115 | hi272 | 42 | 13 | 55 | 24 |
| 11 | hi272 | b1115 | 12 | 3 | 15 | 20 |
| 12 | hi272 | b1115 | 13 | 4 | 17 | 24 |
| Total | 892 | 321 | 1213 | 26 |
To learn about the role of Uhrf1 in ENS development, we examined five time points spanning different stages of enteric progenitor cells (EPC) migration and ENS neuron specification using the phox2b:EGFP transgenic line that expresses in enteric neurons and their precursors (Taylor et al., 2016). At 30–32 hours post fertilization (hpf), phox2b:EGFP+ EPCs enter the rostral intestine and migrate caudally, reaching the distal end at around 66 hpf. ENS neurogenesis starts around 48 hpf rostrally and continues caudally until a functional ENS has formed at 5 dpf (Olden et al., 2008; Shepherd et al., 2004). At 48 and 57 hpf, EPC migration in uhrf1 mutant embryos is indistinguishable from wildtype siblings (data not shown). At 72 and 96 hpf, fewer phox2b:EGFP+ cells are present in uhrf1 mutants compared to their wildtype siblings (Fig. 2), but the ENS phenotype is variable in uhrf1 mutants. At 5 dpf, the reduction in phox2b:GFP+ ENS neurons is significant and robust (Fig. 3, 4). To investigate the ENS phenotype in more detail at 5 dpf, we examined the number of ENS neurons in proximal and distal intestine using immunohistochemistry with the pan-neuronal marker Elavl (Fig. 4). At this stage, there are two main neuronal subtypes, those expressing neuronal nitric oxide synthase (nNOS) and those expressing serotonin (5-HT) that together comprise about 80% of ENS neurons (Uyttebroek et al., 2010). A change in the number of ENS neurons could reflect a change in either one or both of these subtypes. We counted the overall number of neurons using an antibody to Elavl which is expressed in all ENS neurons (Kuhlman and Eisen, 2007), as well as antibodies to these two neurotransmitters (Uyttebroek et al., 2010). We found that the overall number of ENS neurons was significantly reduced in both proximal and distal intestine, as a result of reductions in both subtypes, indicating that uhrf1 does not differentially affect specification of these neuronal subtypes (Fig. 4). Notably, ENS neurons were essentially absent from the distal-most 200 μm of the intestine (Fig. 3D; Fig. 4D,H,I-K). This phenotype is not due to a developmental delay, as mutant larvae die around 7 dpf still displaying the mutant ENS phenotype observed at 5 dpf.
Figure 2. The uhrf1 mutant ENS progenitor phenotype emerges around 72 hpf.

Whole-mount side views of wildtype (A, C) and uhrf1 mutants (B,D). uhfr1 mutants have fewer phox2b:EGFP positive cells (green) than wildtype siblings at 72 hpf (A,B) and 96 hpf (C,D). Scale bar = 100μm.
Figure 3. uhrf1 mutants have fewer neurons in both proximal and distal intestine.

Confocal images of dissected intestines of wildtype (A,C) and uhrf1 mutants (B,D). uhfr1 mutants have fewer phox2b:EGFP positive enteric neurons (green) than wildtype siblings at 5 dpf (A,C) in both proximal (B) and distal (D) intestine. Scale bar = 50μm.
Figure 4. Both of the major ENS neuronal subtypes are reduced in uhrf1 mutants.

Confocal images of dissected intestines from wildtypes (A,C,E,G) and uhrf1 mutants (B,D,F,H) stained for expression of the pan-neuronal marker Elavl, and either nNOS or 5-HT. In both proximal (B,F) and distal (D,H) intestine, uhrf1 mutants have fewer enteric neurons than wildtype siblings at 5 dpf (proximal A,E; distal C,G) as quantified in (I). Both nNOS and 5-HT expressing ENS neuronal subtypes show similar reductions at 5 dpf [Elavl (green), nNOS or 5-HT (magenta)] as quantified in (J,K). Arrows point to examples of nNOS or 5-HT positive neurons. (I) Quantification of Elavl positive cells per 200μm of proximal or distal intestine in wildtype (blue) or mutant (red) [wildtype n=30 (proximal), n=29 (distal); uhrf1- n=29 (proximal), n=30 (distal)]. (J-K) Quantification of Elavl and nNOS (J) and 5-HT (K) positive cells per 200μm proximal or distal intestine in wildtype (blue) and mutant (red) [nNOS: wildtype n=11 (proximal), n=15 (distal); uhrfl−/− n=17 (proximal), n=17 (distal); 5-HT: wildtype n=17 (proximal), n=14 (distal); uhrf1−/− n=15 (proximal), n=13 (distal)]. Asterisk in B indicates autofluorescent background common to uhrf1 mutant intestines. Scale bar = 50μm in A-H. Unpaired t-test: **** = p < 0.0001; ** = p = 0.01.
Enteric progenitors and surrounding intestinal cells express uhrf1 and dnmt1
As a first step toward elucidating the role of uhrf1 in the developing intestinal tract, we examined the uhrf1 expression pattern by in situ hybridization. Expression of uhrf1 has been documented in zebrafish embryos in developing endoderm (Sadler et al., 2007). Previous studies showed that Uhrf1 is required for normal development of the intestinal epithelium in zebrafish, but did not investigate its role in development of other intestinal cell types such as intestinal smooth muscle cells or ENS neurons (Marjoram et al., 2015). EPCs, endoderm, and developing mesoderm are in such close proximity during intestinal development (Wallace et al., 2005a) that previous studies did not differentiate the expression pattern of uhrf1 among these cell types. The transgene Tg(phox2b:EGFP) is expressed in migrating EPCs beginning around 30–32 hpf (Shepherd et al., 2004; Taylor et al., 2016), allowing identification of EPCs among surrounding intestinal cells. We tested several commercially available antibodies against Uhrf1 using a variety of antigen retrieval methods, but none of them showed specific staining at relevant developmental stages. Thus, we evaluated uhrf1 mRNA expression in embryos homozygous for the phox2b:EGFP transgene at 48 hpf and performed immunohistochemistry to detect GFP expression in EPCs (Fig. 5A). Similar to Sadler and colleagues (2007), we observed uhrf1 transcript in the tectum, retina, branchial arches, and developing endoderm (Fig. 5A and data not shown). We also observed uhrf1 expression in phox2b:EGFP-positive EPCs and surrounding intestinal cells including ISMPs, suggesting that uhrf1 plays a role in both ENS and intestinal smooth muscle development (Fig. 5A), as well as in intestinal epithelium development, as previously shown (Marjoram et al., 2015).
Figure 5. uhrf1 and Dnmt1 are expressed in enteric, epithelial, and smooth muscle progenitors during development.

Transverse sections of stained embryos. (A) At 48 hpf, uhrf1 (red) is expressed in phox2b:EGFP (green) positive EPCs and also in DAPI-positive (blue) ISMPs (asterisks) and epithelial progenitors (white arrow). Yellow arrows point to uhrf1 negative cells outside of the nascent intestine. White dashed line indicates intestinal epithelium (ie). Insets show enlargement of cell (white box), GFP, uhrf1, DAPI, and overlay from left to right. Note that the GFP staining appears speckled due to the RNA in situ hybridization procedure. (B) At 48 hpf, Dnmt1 (red) is expressed in phox2b:EGFP (green) positive EPCs and in DAPI-positive (blue) ISMPs (asterisk). -Insets show enlargements of outlined cell, GFP, Dnmt1, DAPI and overlay from left to right. Yellow arrow points to Dnmt1 negative cell outside of the intestine. Scale bar = 10μm in A-B.
Uhrf1 is necessary to recruit Dnmt1 to unmethylated DNA during replication (Bostick et al., 2007; Muto et al., 2002; Sharif et al., 2007) and zebrafish dnmt1s904 mutants exhibit hypomethylation (Anderson et al., 2009). Because Dnmtl is necessary to maintain intestinal epithelial progenitor cells (Elliott et al., 2015) and expression of Dnmtl has been documented in zebrafish embryos in developing endoderm (Liu et al., 2015; Rai et al., 2006), we wondered whether Dnmt1 protein is also expressed in EPCs and surrounding intestinal cell types. To address this question, we immunostained transverse sections of phox2b:EGFP-expressing embryos at 48 hpf with a Dnmt1 antibody. Our results identified Dnmt1-positive EPCs migrating within the Dnmt1-positive population of endodermal cells and ISMPs that prefigure the intestinal epithelium (Fig. 5B). We conclude that EPCs and ISMPs express both uhrf1 and dnmt1. We note, however, that there are also cells surrounding the nascent intestine that are negative for Dnmt1 or uhrf1 (Fig. 5).
Epigenetic modifiers Uhrf1 and Dnmt1 function together during intestinal tract development
The expression patterns of dnmt1 and uhrf1 showed that they are expressed in the same cells at the same time, suggesting that Uhrf1 and Dnmt1 function together during establishment of the intestinal tract. To test this hypothesis, we first analyzed ENS development in dnmt1 mutants at the same five time points at which we analyzed uhrf1 mutants (Fig. 6) and then we analyzed uhrf1;dnmt1 double mutants. dnmt1s904, the mutation we used for our studies, was isolated from an ENU mutagenesis screen for regulators of pancreas development. We have maintained this allele, a splice acceptor mutation resulting in a complete loss of Dnmt1 catalytic activity (Anderson et al., 2009), on an AB background carrying phox2b:EGFP. As in uhrf1 mutants, EPC migration in dnmt1 mutant embryos is indistinguishable from wildtype siblings at 48 and 57 hpf (data not shown). At 72 and 96 hpf, fewer phox2b:EGFP+ cells are present in dnmt1 mutants compared to their wildtype siblings (Fig. 6), but as in uhrf1 mutants the reduction in phox2b:GFP+ cells is strongest and most robust at 5 dpf (Fig. 7C, G). We then quantified the number of enteric neurons in dnmt1s904 and uhrf1 mutants at 5 dpf. Dnmt1 and uhrf1 mutant larvae both exhibited reductions in ENS neurons that were statistically the same, and significantly different from wildtype larvae (Fig. 7A-C, E-G). To further test interactions between Dnmt1 and Uhrf1, we crossed dnmt1;uhrf1 double heterozygotes into a Tg(phox2b:EGFP) line and examined enteric neuron number. These double mutants displayed the same ENS phenotype as uhrf1 and dnmt1 single mutants (Fig. 7D,H). Quantification revealed that enteric neuron number was the same in both single and double mutants and significantly different from wildtypes (Fig. 7I,J), consistent with the hypothesis that Uhrf1 and Dnmt1 act together during ENS development.
Figure 6. The dnmt1 mutant ENS progenitor phenotype emerges around 72 hpf.

Whole-mount side views of wildtype (A, C) and dnmt1 mutants (B,D). dnmt1 mutants have fewer phox2b:EGFP positive cells (green) than wildtype siblings at 72 hpf (A,B) and 96 hpf (C,D). Scale bar = 100μm.
Figure 7. uhrf1;dnmt1 double mutants show similar reduction in enteric neurons compared to uhrf1 and dnmt1 single mutants.

Confocal images of dissected 5 dpf intestines of wildtypes (A,E) and uhrf1 (B,F), dnmt1 (C,G) and uhrf1;dnmt1 (D,H) mutants. In both proximal and distal intestine, single and double mutants show a similar reduction in Elavl positive enteric neurons (white) compared to wildtype siblings. Quantification of Elavl positive cells per 200μm in proximal (I) or distal (J) intestine at 5 dpf in wildtype (wt), uhrfl−/−, dnmt1−/− and uhrf1;dnmt1 double mutants [wildtype n=7 (proximal), n=7 (distal); uhrf1−/− n=8 (proximal), n=8 (distal); dnmt1−/− n=9 (proximal), n=9 (distal); uhrf1−/−;dnmt1−/− n=9 (proximal), n=9 (distal)]. Larvae were genotyped as described in the Methods section. One-way Anova followed by Tukey’s post-hoc test, letters indicate P<0.0001. A-H: Scale bar = 50μm.
Homozygous uhrf1 mutants were previously shown to have disrupted intestinal epithelium development (Marjoram et al., 2015). We found that uhrf1 is expressed in ISMPs, prompting us to test whether uhrf1 mutants also show altered development of intestinal smooth muscle. We analyzed smooth muscle development in 5 dpf whole-mount uhrf1 mutants and found that both longitudinal and circumferential intestinal smooth muscle fibers were essentially absent (Fig. 8A-D). Cross-sections stained with neuronal and smooth muscle markers or with Hematoxylin and Eosin (HE) revealed severe disruption of both intestinal smooth muscle and ENS components (Fig. 8E-L). They also showed that, instead of being sandwiched between the longitudinal and circumferential smooth muscle cell layers, cell bodies of enteric neurons of uhrf1 mutants often appeared to be ‘floating’ at some distance from the intestinal epithelium (Fig. 6H, arrow). Although this aspect of the phenotype is subtle and difficult to quantify, many of these neurons had a more rounded appearance than those of wildtypes, consistent with not being located between two closely apposed muscle layers.
Figure 8. Disrupted smooth muscle development contributes to intestinal dysgenesis in uhrf1 mutants.

Confocal images of 5 dpf intestines labeled with smooth muscle myosin (SMM) and desmin (magenta) antibodies. Dissected intestines (A-D) of wildtype (A,C) and uhrf1 mutants (B,D). In both proximal (B) and distal (D) intestine, uhrf1 mutants essentially lack smooth muscle cells, as revealed by whole-mount antibody staining. Transverse sections of wildtype (E,G) and uhrf1 mutants (F,H) showing loss of intestinal smooth muscle in mutants. White arrows point to phox2b:EGFP (green) positive ENS neurons that are further removed from the intestinal epithelium in mutants (F,H) than in wildtypes (G). Brightfield images of transverse sections of 5 dpf wildtypes (I,K) and uhrf1 mutants (J,L) stained with hematoxylin and eosin show disrupted proximal and distal intestinal development in mutants compared to wildtypes. Increased shedding of cells in uhrf1 mutants is indicated by a yellow arrow and disrupted intestinal epithelium with a blue arrow. Higher magnification insets of boxed areas indicate disrupted intestinal epithelium morphology in uhrf1 mutants. ie = intestinal epithelium. Scale bar = 50μm in A-D, and 25μm in E-L.
Dnmt1 has an essential role in smooth muscle cell development in mouse and humans (Jorgensen et al., 2018). To test whether this role of Dnmt1 is conserved, we examined intestinal smooth muscle development in zebrafish dnmt1 mutants. We found that, like uhrf1 mutants, dnmt1 mutants had severely disrupted intestinal smooth muscle development (Fig. 9). In addition, enteric neurons of dnmt1 mutants shared the same subtle morphological phenotypes as those of uhrf1 mutants.
Figure 9. Disrupted smooth muscle development contributes to intestinal dysgenesis in dnmt1 mutants.

Confocal images of 5 dpf intestines labeled with smooth muscle myosin (SMM) and desmin (magenta) antibodies. Dissected intestines (A-D) of wildtype (A,C) and dnmt1 mutants (B,D). In both proximal (B) and distal (D) intestine, dnmt1 mutants essentially lack smooth muscle cells, as revealed by whole-mount antibody staining. Transverse sections of wildtype (E,G) and dnmt1 mutants (F,H) showing loss of intestinal smooth muscle in mutants. White arrow points to phox2b:EGFP (green) positive ENS neuron that is further removed from the intestinal epithelium in mutants (H) than in wildtypes (G). ie = intestinal epithelium. Scale bar = 50μm in A-D, and 25μm in E-H.
The experiments described in this section show that uhrf1 and dnmt1 mutants have indistinguishable intestinal smooth muscle and enteric neuron phenotypes. Previous work showed that these two mutants also have the same intestinal epithelial phenotypes (Marjoram et al., 2015). Together these studies support the conclusion that Uhrf1 and Dnmt1 function in the same pathway and together control normal establishment of the intestinal tract by regulating development of each of the constituent cell types.
Genetic chimeras reveal that uhrf1 functions both cell-autonomously and cell-non-autonomously in ENS development
EPCs, smooth muscle precursors, and intestinal epithelial precursors are intermingled during development and previous studies have shown that signals among these nascent intestinal constituents are critical for proper differentiation of each cell type (Fu et al., 2004; Graham et al., 2017; Hao et al., 2016; Korzh et al., 2011; Mwizerwa et al., 2011; Natarajan et al., 2002; Neunlist et al., 2007; Neunlist et al., 2013; Olden et al., 2008; Pietsch et al., 2006; Puzan et al., 2018; Reichenbach et al., 2008; Sukegawa et al., 2000). Cells of all three germ layers express uhrf1 during intestinal development, raising the possibility of cell-non-autonomous interactions. To learn whether loss of Uhrf1 disrupts interactions between cells derived from the different germ layers, we focused on EPCs in genetic chimeras in which the ENS was wildtype and the intestinal epithelium and muscle were mutant for uhrf1. We generated chimeras by transplanting vagal neural crest that generates the ENS from phox2b:EGFP-expressing wildtype donors into host embryos from an incross of heterozygous uhrf1 mutants. Thus, some hosts were wildtype, some were heterozygous, but phenotypically wildtype, and some were homozygous mutant (Fig. 10 A,B). In this experimental set-up, all GFP-positive enteric neurons were donor-derived. As predicted, wildtype EPCs transplanted into phenotypically wildtype hosts contributed to the ENS along the entire length of the intestinal tract (Fig. 10D). Notably, the GFP-positive enteric neurons are intermingled with unlabeled, host-derived enteric neurons. In the context of a uhrf1 mutant host environment, wildtype donor enteric neurons were distributed along most of the intestinal tract. Because there are so few enteric neurons in uhrf1 mutants, transplanted wildtype EPCs have the potential to proliferate more than they would in wildtypes, where they compete with the native EPCs (Rolig et al., 2017; Troll et al., 2018). Despite being present along most of the intestine, wildtype enteric neurons were specifically absent from the distal-most region of the intestine in uhrf1 mutants (Fig. 10E, asterisk, 5/5). This result is consistent with the idea that Uhrf1 function is necessary cell-non-autonomously for EPCs to migrate far enough caudally to populate the distal portion of the intestinal tract.
Figure 10. Genetic chimeras reveal that Uhrf1 functions cell-non-autonomously and cell-autonomously in ENS development.

(A) Transplantation experiment diagram. Wildtype or uhrf1 mutant donor embryos that carry the phox2b:EGFP transgene were injected with rhodamine dextran. Vagal neural crest cells were transplanted from donors into unlabeled wildtype or uhrf1 mutant hosts. The contribution of transplanted cells was evaluated at 5 dpf. (B) Possible results for transplantation experiments to test cell-non-autonomous function of uhrf1 in ENS development. (C) Possible results for transplantation experiments to test cell-autonomous function of uhrf1 in ENS development. (D) phox2b:EGFP positive (green) wildtype cells transplanted into wildtype hosts can populate the entire intestine. Note that the density of these cells is less than in non-transplanted animals because they share the territory with unlabeled ENS cells of the host. (E) phox2b:EGFP positive (green) wildtype cells transplanted into uhrf1 mutant hosts can expand, but are consistently excluded from distal intestine (outlined and labeled with asterisk). (F) phox2b:EGFP positive (green) uhrf1 mutant cells transplanted into wildtype hosts can migrate to distal intestine in rare cases (1/5), but the population of transplanted mutant cells that successfully integrate into the ENS is smaller than that of the wildtype host cells as revealed by Elavl staining (magenta). (D-F) Lateral views of whole-mount zebrafish larvae at 5 dpf. Scale bar = 100μm in D-F.
Because uhrf1 is expressed in EPCs, we also tested whether Uhrf1 function is required cell-autonomously. We performed the same transplantation as described above, but transplanted vagal neural crest from labeled donor embryos derived from a uhrf1 mutant incross carrying the phox2b:EGFP transgene into unlabeled wildtype hosts (Fig. 10A,C). As in the previous experiment, wildtype donor cells transplanted into wildtype hosts populated the entire length of the intestine (Fig. 10D). In contrast, in most cases mutant donor EPCs migrated only a short distance caudally after entering the wildtype host intestine (4/5). Even in the single case in which transplanted uhrf1 mutant cells reached the distal intestine (1/5), the mutant donor cells did not proliferate as much as wildtype precursors (Fig. 10F). This result provides evidence that Uhrf1 function is required cell-autonomously in EPCs for them to migrate and proliferate normally.
Discussion
This study provides evidence that disruption of DNA methylation factors interrupts the developmental trajectories of the three germ layers that contribute to the intestinal tract, thus preventing formation of a functional organ. Previous studies demonstrated that derivatives of these germ layers, intestinal epithelium, intestinal smooth muscle, and the ENS develop over a similar time frame. Importantly, cells derived from these germ layers provide signals that are critical for one another’s differentiation (Fig. 11) (Fu et al., 2004; Graham et al., 2017; Hao et al., 2016; Korzh et al., 2011; Mwizerwa et al., 2011; Natarajan et al., 2002; Neunlist et al., 2007; Neunlist et al., 2013; Olden et al., 2008; Pietsch et al., 2006; Puzan et al., 2018; Reichenbach et al., 2008; Sukegawa et al., 2000), making it difficult to discern which cell types require DNA methylation factor function for their differentiation. Mouse Uhrf1 mutants die early, before the intestine develops (Bostick et al., 2007; Muto et al., 2002; Sharif et al., 2007), and so mouse does not serve as a model in which to investigate the role of Uhrf1 in intestinal development. In contrast, zebrafish uhrf1 mutants survive to larval stages due to maternally provided Uhrf1 protein (Jacob et al., 2015), thus the role of uhrf1 in intestinal development can be investigated. Below we discuss the role of methylation factors in the context of intestinal disease and coordinated intestinal development.
Figure 11. Model of Uhrf1 and Dnmt1 function during ENS development.

Signaling is important for differentiation of intestinal cells derived from each of the germ layers. We showed that during early intestinal development, Uhrf1 and Dnmt1 have cell-autonomous functions in EPCs (green), but that EPC development is also affected cell-non-autonomously, probably as a result of Uhrf1 and Dnmt1 functions in surrounding intestinal epithelial cells (light pink) and smooth muscle cells (purple). Together these cell-autonomous and cell-non-autonomous Uhrf1 and Dnmt1 activities modulate ENS development. This diagram shows signaling from epithelial and muscle precursors to ENS precursors. Decreasing these signals in uhrf1 and dnmt1 mutants (lighter arrows) results in fewer smooth muscle and epithelial progenitors, and thus alters proliferation, migration, or differentiation of EPCs, potentially also contributing to EPC death. Although not shown here, it is also likely that cell-autonomous and cell-non-autonomous functions of these genes modulate intestinal epithelial and muscle cell development.
Dysregulation of Uhrf1 and Dnmt1 are linked to intestinal diseases
Epigenetic regulation of gene expression has been implicated in a variety of types of diseases that affect development of the intestinal tract (Anderson et al., 2009; Feng et al., 2010). For example, Uhrf1 mutations cause dysregulation of the intestinal epithelium in a zebrafish model of inflammatory bowel disease (IBD) (Marjoram et al., 2015). DNMT1 has been shown to be dysregulated in human Crohn’s disease, one type of IBD, (Jorgensen et al., 2018). Dnmt1 mutations engineered to be present solely in mouse intestinal smooth muscle cells cause muscle hypoproliferation and decreased intestinal peristalsis, phenotypes found in human diseases such as chronic intestinal pseudo-obstruction and megacystis-megacolon-intestinal hypoperistalsis syndrome (Jorgensen et al., 2018). DNA hypomethylation, due at least in some case to mutations in DNMT3, has also been linked to human HSCR and Waardenburg syndrome (Kim et al., 2011; Torroglosa et al., 2016; Torroglosa et al., 2014). In addition, epigenetic modulation of methylation is associated with other types of intestinal diseases, such as intestinal cancers (Gays et al., 2017).
In some cases, these intestinal diseases result from mutations that specifically affect cells in one of the germ layers that contributes to the developing intestinal tract. For example, HSCR results from failure of normal ENS development. Changes in maintenance of DNA methylation can alter gene expression patterns and several HSCR loci including RET, EDNRB, and HSCR candidate genes, such as PAX6, have been shown to be affected by altered DNA methylation of their promoters (Torroglosa et al., 2016). A single nucleotide polymorphism (SNP) in a conserved non-coding element suggested to be a RET enhancer is associated with susceptibility to HSCR (Emison et al., 2005; Griseri et al., 2005). Thus, SNPs in uhrf1 and dnmt1 enhancers driving expression in ENS cells could specifically affect ENS development, leading to HSCR-like phenotypes, while not directly affecting other intestinal cell types.
Although mutations in intestinal cells derived from a specific germ layer may cause disease, because of the intimate interactions among cells of these germ layers, mutations in more globally acting genes may have both cell-autonomous and cell-non-autonomous effects. For example, in the study by Marjoram and colleagues (2015), mutations in uhrf1 or dnmt1 resulted in hypoproliferation and excess shedding of epithelial cells into the intestinal lumen in their IBD model. However, these mutations were throughout the organism, including all cells of the intestinal tract, but contributions to the observed phenotype from intestinal smooth muscle or ENS progenitors were not examined. The ENS has been implicated in regulating intestinal barrier function (Neunlist et al., 2013; Sharkey, 2015), suggesting that the ENS phenotype observed in our study could be a contributing factor to compromised intestinal barrier function in uhrf1 mutants.
Uhrf1 and Dnmt1 promote coordinated intestinal development
Development of a functional intestine is the result of highly orchestrated processes (Fig. 11). Each of the contributing germ layers must be specified and component cells must segregate and migrate to their appropriate destinations (Ganz, 2018; Ganz et al., 2016; Hao et al., 2016; Wallace et al., 2005a). Endoderm reaches the midline early and forms a scaffold for the incoming mesoderm that migrates from the lateral plate (Gays et al., 2017). In contrast, ectoderm-derived neural crest cells first migrate ventrally to the nascent intestine and then migrate from rostral to caudal along the developing intestinal epithelium and mesoderm. All of these processes occur within a fairly narrow time window during which each cell type also proliferates, a process that has been best studied for the ENS (Shepherd and Eisen, 2011). In the ENS, migration and proliferation are intimately connected; decreased proliferation leads to decreased migration and vice versa (Landman et al., 2007; Nagy and Goldstein, 2017; Newgreen et al., 2017; Simpson et al., 2007). Uhrf1 and Dnmt1 mutations block the cell cycle, leading to fewer cells in the affected tissue or organ (Jacob et al., 2015), providing a potential mechanism for the decreased number of ENS neurons in these mutants. A similar mechanism may be at play in intestinal smooth muscle and intestinal epithelium, although this hypothesis remains to be tested.
Because each of the intestinal cell types independently fail to form properly in dnmt1 and uhrf1 mutants, signals produced by that cell type necessary for differentiation of the other two cell types decrease. Consistent with this idea, zebrafish mutants that exhibit decreased intestinal smooth muscle cell development also show ENS abnormalities (Wallace et al., 2005a). Mutations in genes involved in methylation can lead to premature differentiation (Sen et al., 2010), which would decrease the number of cells, leading to decreased migration and thus a diminution of signals important for adjacent cell types to differentiate. Premature differentiation and aberrant migration of EPCs, ISMPs, or intestinal epithelial progenitor cells would likely decrease inter-germ layer signaling. Thus, prematurely differentiating cells might receive and send incorrect signals to neighboring cells, leading to differentiated cells that become inappropriately specified. Another possibility is that progenitor or differentiated intestinal cells might die prematurely in these mutants, potentially because they are mis-specified. Such premature death would also lead to fewer cells providing diminished inter-germ layer signaling. Cell type specific knock-outs of uhrf1 and dnmt1, as well as time-lapse live imaging studies, would help distinguish between these different mechanisms.
Our transplantation experiments provide evidence that methylation factors act both cell-autonomously and cell-non-autonomously in the ENS. Given the expression patterns of the genes encoding these factors, this is not surprising. Thus, we suggest that this is also likely to be the case in intestinal smooth muscle and epithelium. This hypothesis can be tested by creating genetic chimeras for these cell populations or by cell type specific knock-outs.
Highlights.
-
–
Uhrf1 and dnmt1 mutants have disrupted enteric neurons and intestinal smooth muscle.
-
–
Uhrf1 functions cell-autonomously and cell-non-autonomously during ENS development.
-
–
Epigenetic modifiers Uhrf1 and Dnmt1 function together during intestinal development.
Acknowledgments
We thank Doug Turnbull and the University of Oregon Genomics and Cell Characterization Core Facility for their expertise in Illumina sequencing and bioinformatics, Kirsten Sadler Edepli and her laboratory for the uhrf1 plasmid, Poh Kheng Loi for histology, Adam Christensen, John Dowd, Markus Melancon, and the UO Zebrafish Facility staff for zebrafish husbandry. This work was supported by NIH P01 HD22486, NIH R01 OD11116, and by a research grant from the REACHirschsprung foundation to J.G.
Footnotes
Competing interests
No competing interests declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amores A, Catchen J, Ferrara A, Fontenot Q, Postlethwait JH, 2011. Genome evolution and meiotic maps by massively parallel DNA sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics 188, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins N, 2004. Retroviral-mediated insertional mutagenesis in zebrafish. Methods Cell Biol 77, 3–20. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PD, Shin D, Chi NC, Shin CH, Schlegel A, Halpern M, Stainier DY, 2009. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol 334, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA, 2008. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3, e3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie CE, Eisen JS, 1997. Notochord alters the permissiveness of myotome for pathfinding by an identified motoneuron in embryonic zebrafish. Development 124, 713–720. [DOI] [PubMed] [Google Scholar]
- Bestor TH, 2000. The DNA methyltransferases of mammals. Hum Mol Genet 9, 2395–2402. [DOI] [PubMed] [Google Scholar]
- Borden KL, Freemont PS, 1996. The RING finger domain: a recent example of a sequence-structure family. Curr Opin Struct Biol 6, 395–401. [DOI] [PubMed] [Google Scholar]
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE, 2007. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 317, 1760–1764. [DOI] [PubMed] [Google Scholar]
- Brosens E, Burns AJ, Brooks AS, Matera I, Borrego S, Ceccherini I, Tam PK, Garcia-Barcelo MM, Thapar N, Benninga MA, Hofstra RM, Alves MM, 2016. Genetics of enteric neuropathies. Dev Biol 417, 198–208. [DOI] [PubMed] [Google Scholar]
- Catchen J, Bassham S, Wilson T, Currey M, O’Brien C, Yeates Q, Cresko WA, 2013. The population structure and recent colonization history of Oregon threespine stickleback determined using restriction-site associated DNA-sequencing. Mol Ecol 22, 2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH, 2011. Stacks: building and genotyping Loci de novo from short-read sequences. G3 (Bethesda) 1, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K, 2011. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci U S A 108 Suppl 1, 4570–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, 1991. Determination of primary motoneuron identity in developing zebrafish embryos. Science 252, 569–572. [DOI] [PubMed] [Google Scholar]
- Elliott EN, Kaestner KH, 2015. Epigenetic regulation of the intestinal epithelium. Cell Mol Life Sci 72, 4139–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EN, Sheaffer KL, Schug J, Stappenbeck TS, Kaestner KH, 2015. Dnmt1 is essential to maintain progenitors in the perinatal intestinal epithelium. Development 142, 2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED, Chakravarti A, 2005. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434, 857–863. [DOI] [PubMed] [Google Scholar]
- Feng S, Cokus SJ, Zhang X, Chen PY, Bostick M, Goll MG, Hetzel J, Jain J, Strauss SH, Halpern ME, Ukomadu C, Sadler KC, Pradhan S, Pellegrini M, Jacobsen SE, 2010. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A 107, 8689–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Pachnis V, Tam PK, 2004. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol 166, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz J, 2018. Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system. Dev Dyn 247, 268–278. [DOI] [PubMed] [Google Scholar]
- Ganz J, Melancon E, Eisen JS, 2016. Zebrafish as a model for understanding enteric nervous system interactions in the developing intestinal tract. Methods Cell Biol 134, 139–164. [DOI] [PubMed] [Google Scholar]
- Gays D, Hess C, Camporeale A, Ala U, Provero P, Mosimann C, Santoro MM, 2017. An exclusive cellular and molecular network governs intestinal smooth muscle cell differentiation in vertebrates. Development 144, 464–478. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Thapar N, Karunaratne TB, De Giorgio R, 2016. Clinical aspects of neurointestinal disease: Pathophysiology, diagnosis, and treatment. Dev Biol 417, 217–228. [DOI] [PubMed] [Google Scholar]
- Graham HK, Maina I, Goldstein AM, Nagy N, 2017. Intestinal smooth muscle is required for patterning the enteric nervous system. J Anat 230, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griseri P, Bachetti T, Puppo F, Lantieri F, Ravazzolo R, Devoto M, Ceccherini I, 2005. A common haplotype at the 5’ end of the RET proto-oncogene, overrepresented in Hirschsprung patients, is associated with reduced gene expression. Hum Mutat 25, 189–195. [DOI] [PubMed] [Google Scholar]
- Hao MM, Foong JP, Bornstein JC, Li ZL, Vanden Berghe P, Boesmans W, 2016. Enteric nervous system assembly: Functional integration within the developing gut. Dev Biol 417, 168–181. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, 2018. Hirschsprung disease-integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 15, 152–167. [DOI] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA, 2010. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet 6, e1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL, 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MS, Unal Eroglu A, Malireddy S, Gallagher G, Nambiar RM, Henion PD, 2013. Distinct functional and temporal requirements for zebrafish Hdacl during neural crest-derived craniofacial and peripheral neuron development. PLoS One 8, e63218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob V, Chernyavskaya Y, Chen X, Tan PS, Kent B, Hoshida Y, Sadler KC, 2015. DNA hypomethylation induces a DNA replication-associated cell cycle arrest to block hepatic outgrowth in uhrf1 mutant zebrafish embryos. Development 142, 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen BG, Berent RM, Ha SE, Horiguchi K, Sasse KC, Becker LS, Ro S, 2018. DNA methylation, through DNMT1, has an essential role in the development of gastrointestinal smooth muscle cells and disease. Cell Death Dis 9, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang K, Ekram MB, Roh TY, Kim J, 2011. Aebp2 as an epigenetic regulator for neural crest cells. PLoS One 6, e25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzh S, Winata CL, Zheng W, Yang S, Yin A, Ingham P, Korzh V, Gong Z, 2011. The interaction of epithelial Ihha and mesenchymal Fgf10 in zebrafish esophageal and swimbladder development. Dev Biol 359, 262–276. [DOI] [PubMed] [Google Scholar]
- Kuhlman J, Eisen JS, 2007. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev Dyn 236, 118–127. [DOI] [PubMed] [Google Scholar]
- Lake JI, Heuckeroth RO, 2013. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 305, G1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman KA, Simpson MJ, Newgreen DF, 2007. Mathematical and experimental insights into the development of the enteric nervous system and Hirschsprung’s disease. Dev Growth Differ 49, 277–286. [DOI] [PubMed] [Google Scholar]
- Liu X, Jia X, Yuan H, Ma K, Chen Y, Jin Y, Deng M, Pan W, Chen S, Chen Z, de The H, Zon LI, Zhou Y, Zhou J, Zhu J, 2015. DNA methyltransferase 1 functions through C/ebpa to maintain hematopoietic stem and progenitor cells in zebrafish. J Hematol Oncol 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram L, Alvers A, Deerhake ME, Bagwell J, Mankiewicz J, Cocchiaro JL, Beerman RW, Willer J, Sumigray KD, Katsanis N, Tobin DM, Rawls JF, Goll MG, Bagnat M, 2015. Epigenetic control of intestinal barrier function and inflammation in zebrafish. Proc Natl Acad Sci U S A 112, 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Atwood TS, Eames BF, Eberhart JK, Yan YL, Postlethwait JH, Johnson EA, 2007. RAD marker microarrays enable rapid mapping of zebrafish mutations. Genome Biol 8, R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto M, Kanari Y, Kubo E, Takabe T, Kurihara T, Fujimori A, Tatsumi K, 2002. Targeted disruption of Np95 gene renders murine embryonic stem cells hypersensitive to DNA damaging agents and DNA replication blocks. J Biol Chem 277, 34549–34555. [DOI] [PubMed] [Google Scholar]
- Mwizerwa O, Das P, Nagy N, Akbareian SE, Mably JD, Goldstein AM, 2011. Gdnf is mitogenic, neurotrophic, and chemoattractive to enteric neural crest cells in the embryonic colon. Dev Dyn 240, 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Goldstein AM, 2017. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin Cell Dev Biol 66, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E, 2002. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development 129, 5151–5160. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW, 2007. Specification of epibranchial placodes in zebrafish. Development 134, 611–623. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F, Galmiche JP, 2007. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 292, G231–241. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M, 2013. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10, 90–100. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Zhang D, Cheeseman BL, Binder BJ, Landman KA, 2017. Differential Clonal Expansion in an Invading Cell Population: Clonal Advantage or Dumb Luck? Cells Tissues Organs 203, 105–113. [DOI] [PubMed] [Google Scholar]
- Olden T, Akhtar T, Beckman SA, Wallace KN, 2008. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis 46, 484–498. [DOI] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH, 2008. Cytosine methylation: remaining faithful. Curr Biol 18, R174–176. [DOI] [PubMed] [Google Scholar]
- Pietsch J, Delalande JM, Jakaitis B, Stensby JD, Dohle S, Talbot WS, Raible DW, Shepherd IT, 2006. lessen encodes a zebrafish trap100 required for enteric nervous system development. Development 133, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzan M, Hosic S, Ghio C, Koppes A, 2018. Enteric Nervous System Regulation of Intestinal Stem Cell Differentiation and Epithelial Monolayer Function. Sci Rep 8, 6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Nadauld LD, Chidester S, Manos EJ, James SR, Karpf AR, Cairns BR, Jones DA, 2006. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol Cell Biol 26, 7077–7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach B, Delalande JM, Kolmogorova E, Prier A, Nguyen T, Smith CM, Holzschuh J, Shepherd IT, 2008. Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish. Dev Biol 318, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Wolffe AP, 2000. DNA methylation in health and disease. Nat Rev Genet 1, 11–19. [DOI] [PubMed] [Google Scholar]
- Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K, 2017. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15, e2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler KC, Krahn KN, Gaur NA, Ukomadu C, 2007. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc Natl Acad Sci U S A 104, 1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA, 2010. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredick SD, Van Ryswyk L, Hutchinson SA, Eisen JS, 2012. Zebrafish Mnx proteins specify one motoneuron subtype and suppress acquisition of interneuron characteristics. Neural Dev 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T, Okamura K, Tajima S, Mitsuya K, Okano M, Koseki H, 2007. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmtl to methylated DNA. Nature 450, 908–912. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, 2015. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest 125, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, Schubeler D, Kaestner KH, 2014. DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28, 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd I, Eisen J, 2011. Development of the zebrafish enteric nervous system. Methods Cell Biol 101, 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd IT, Pietsch J, Elworthy S, Kelsh RN, Raible DW, 2004. Roles for GFRalpha1 receptors in zebrafish enteric nervous system development. Development 131, 241–249. [DOI] [PubMed] [Google Scholar]
- Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF, 2007. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol 302, 553–568. [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K, 2000. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 127, 1971–1980. [DOI] [PubMed] [Google Scholar]
- Tauber M, Fischle W, 2015. Conserved linker regions and their regulation determine multiple chromatin-binding modes of UHRF1. Nucleus 6, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Montagne WA, Eisen JS, Ganz J, 2016. Molecular fingerprinting delineates progenitor populations in the developing zebrafish enteric nervous system. Dev Dyn 245, 1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittle RK, Sze R, Ng A, Nuckels RJ, Swartz ME, Anderson RM, Bosch J, Stainier DY, Eberhart JK, Gross JM, 2011. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev Biol 350, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroglosa A, Alves MM, Fernandez RM, Antinolo G, Hofstra RM, Borrego S, 2016. Epigenetics in ENS development and Hirschsprung disease. Dev Biol 417, 209–216. [DOI] [PubMed] [Google Scholar]
- Torroglosa A, Enguix-Riego MV, Fernandez RM, Roman-Rodriguez FJ, Moya-Jimenez MJ, de Agustin JC, Antinolo G, Borrego S, 2014. Involvement of DNMT3B in the pathogenesis of Hirschsprung disease and its possible role as a regulator of neurogenesis in the human enteric nervous system. Genet Med 16, 703–710. [DOI] [PubMed] [Google Scholar]
- Troll JV, Hamilton MK, Abel ML, Ganz J, Bates JM, Stephens WZ, Melancon E, van der Vaart M, Meijer AH, Distel M, Eisen JS, Guillemin K, 2018. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, Van Nassauw L, 2010. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J Comp Neurol 518, 4419–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M, 2005a. Intestinal growth and differentiation in zebrafish. Mech Dev 122, 157–173. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Dolan AC, Seiler C, Smith EM, Yusuff S, Chaille-Arnold L, Judson B, Sierk R, Yengo C, Sweeney HL, Pack M, 2005b. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell 8, 717–726. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C, 1999. Origin and development of the zebrafish endoderm. Development 126, 827–838. [DOI] [PubMed] [Google Scholar]
- Wu TD, Nacu S, 2010. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Oda Y, 2015. Gastrointestinal stromal tumor: recent advances in pathology and genetics. Pathol Int 65, 9–18. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM, 2009. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol 25, 221–251. [DOI] [PMC free article] [PubMed] [Google Scholar]


