Abstract
Shenmai (SM) injection has been reported to attenuate ischemia-reperfusion (I/R) injury, but its effect on energy metabolism during I/R and the underlying mechanism remain unknown. To explore the protective mechanism of SM on ischemic cardiomyopathy, primary cardiomyocytes from SD rats were treated with SM, total saponins of Panax ginseng (TSPG), L-carnitine (LC) and trimetazidine (TMZ). Changes in glucose, free fatty acids (FFAs), pyruvic acid (PA), lactic acid (LA) and intracellular ATP capacity were observed with the appropriate assays. For each treatment group, the key enzymes and transporters of myocardial energy metabolism were detected and compared via Western blot. Furthermore, impairments after I/R were assessed by examining cardiomyocyte apoptosis and LDH and PK activity in the culture medium. Our results indicated that SM and TSPG markedly alleviated the decrease in key enzymes and transporters and the utilization of metabolic substrates following I/R, while SM prevented aberrant apoptosis and restored the depleted ATP resulting from I/R. Notably, the effects of SM were superior to those of its main components TSPG, LC and TMZ. Thus, the protective effect of SM in ischemic cardiomyopathy may be mediated by the upregulation of key enzymes and restoration of the depleted ATP content in the energy metabolism process.
Keywords: Shenmai, total saponins of Panax ginseng, ischemia-reperfusion, energy metabolism, cardiomyocytes
Introduction
Ischemic heart diseases, especially acute myocardial ischemia, have remained the leading cause of death in both developed and developing countries over the past quarter century. The prognosis of patients with acute myocardial infarction has dramatically improved with the development of highly successful approaches to restore blood flow to ischemic tissue by primary percutaneous coronary intervention and thrombolytic therapy. Paradoxically, while coronary reperfusion improves the prognosis of acute myocardial infarction patients, it also leads to myocardial reperfusion injury by extending myocardial damage within the ischemic period [1]. Therefore, intervention to alleviate reperfusion injury at the time of coronary recanalization is considered a promising strategy to further decrease infarct size and improve prognosis after myocardial infarction. Many studies have shown that myocardial energy deficiency, overexpression of metabolic enzymes and gene abnormalities can alter myocardial function and highly affect recovery. A previous study demonstrated that trimetazidine (TMZ) treatment significantly stimulates the cardiac AMP-activated protein kinase (AMPK) and extracellular signal-regulated kinase (ERK) signaling pathways. TMZ activates AMPK signaling and modulates substrate metabolism by shifting fatty acid oxidation (FAO) to glucose oxidation during reperfusion, leading to reduced oxidative stress in I/R hearts [2]. However, I/R injury is a process that is initiated in the ischemic phase due to hypoxia and exacerbated in the reperfusion phase, during which mitochondria, particularly ATPase, play a central role [3]. ATPase has been reported to respond to I/R by changing the expression of subunit protein(s) [4,5], leading to ATPase dysfunction. ATPase dysfunction results in ATP depletion, affecting all cellular energy-consuming processes, and is also implicated in the progression of apoptosis [6]. Thus, maintaining mitochondrial structure and function is expected to be a promising strategy for protection against cardiac I/R injury. Previous studies showed that the induction of cardiomyocyte hypoxia results in a significant downregulation of the expression of proteins related to energy metabolism and mitochondrial function, and the results confirmed that myocardial ischemia can induce decreases in ATP synthase [7]. In this study, we explored the mechanisms underlying the upregulation of key enzymes in metabolic pathways and the preservation of intracellular ATP capacity to reduce cell apoptosis and improve cardiac function following I/R, which may be important targets for the development of new treatment approaches for ischemic heart disease.
Shenmai (SM), which is used as a traditional Chinese medicine, is derived from a traditional decoction named Shenmai yin prescribed by the famous traditional Chinese medicine doctor Si-miao Sun in the Tang dynasty. SM, which consists of Panax ginseng and Ophiopogon japonicas [8], is usually used in China in complement with Western treatments for heart disease. SM has been approved for market use by the China Food and Drug Administration (CFDA) for the treatment of chronic corpulmonale heart failure since 1995. According to traditional Chinese medicine theory, SM benefits qi, nourishes yin, and replenishes bodily fluids. It is widely used for the treatment of qi-yin deficiency in shock, coronary heart disease, chronic pulmonary heart disease, viral myocarditis, and malignant diseases [9]. The relatively higher active components of SM are ginsenoside Rb1, Rb2, Rc, Rd, Re, Rg1, and Ophiopogon saponin D [10]. Recent clinical research has shown that SM plus routine drug therapy results in a significant improvement in the New York Heart Association (NYHA) functional classification [11]. Furthermore, many clinical trials have shown that SM benefits patients with chronic corpulmonale heart failure. A recent study [12] reported that SM improved the left ventricular ejection fraction (LVEF) in gerontal patients with chronic heart failure. SM is reportedly responsible for antioxidant activity when used to treat chronic heart failure [13], and the antioxidative effect of SM on myocardial injury and its ability to improve cardiac microcirculation by eradicating oxygen free radicals have been observed [14]. In addition, early studies have demonstrated that the effect of SM on intracellular Ca2+ homeostasis, especially on reducing phospholamban inhibition, has a myocardial protective effect on reperfusion after myocardial infarction [15]. Some studies report that the active components of SM ameliorate myocardial ischemia-reperfusion (I/R) injury in rats. Ginsenoside Rb3 significantly attenuated the changes in creatine kinase activity and lactate dehydrogenase (LDH) activity to ameliorate myocardial I/R injury in rats [16]. Ginsenoside Rb1 protects against I/R-induced myocardial injury via energy metabolism regulation mediated by the RhoA signaling pathway [17]. Total saponins protect against myocardial I/R injury through the AMPK pathway [18]. Although several active compounds of SM have cardioprotective effects, no scientific studies have investigated the mechanism of SM for the treatment of acute myocardial injury. Recent experiments have indicated that the hypoxia-induced impairment of energy metabolism and mitochondrial function can be attenuated by SM in primary cultured cardiac myocytes. The findings suggest that SM and its components can restore energy metabolism and mitochondrial function through the multitargeted activation or suppression of related protein levels [7]. However, whether the myocardial protection conferred by SM and total saponins of Panax ginseng (TSPG) during I/R function directly involves the selection, utilization and production of energy substrates and key regulatory enzymes in metabolic processes remains unclear.
In the present study, from the perspective of metabolomics, the selection and utilization of energy substrates in myocardial cells treated with SM, TSPG and TMZ were compared to determine the protective mechanism of SM and TSPG after I/R. Changes in the myocardial energy (substrate) transporter and key enzymes of myocardial energy metabolism were also systematically studied. Whether the traditional Chinese medicine compound is superior to monomer saponins in improving cardiac function according to the related energy metabolism pathway was also evaluated.
Materials and methods
Materials
SM was obtained from Chia Tai Qingchunbao Pharmaceutical Co., Ltd. (Hangzhou, China). TSPG (purity, >80%) was obtained from Shanghai Winherb Medical Science Co., Ltd. (Shanghai, China). Reference standards of ginsenosides Re, Rg1, and Rb1 (purity, >99%) were purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Acetonitrile of HPLC grade was obtained from Fisher Scientific (New Jersey, USA), and purified water was purchased from Robust Company (Hangzhou, China). TRIzol® reagent was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). The PrimeScript™ RT reagent kit and SYBR® Premix Ex Taq™ II were obtained from Takara Bio, Inc. (Otsu, Japan). Primers for the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). L-carnitine (LC) formulations were purchased from Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. (Rome, Italy). Trimetazidine was purchased from Servier Industry Laboratories (France). Dulbecco’s Modified Eagle’s Medium (DMEM) and trypsin solution (2.5 g/L trypsin solubilized in Hank’s balanced salt solution (HBSS) solution without Ca or Mg) were purchased from Gino Biopharmaceutical Technology Co., Ltd. (Hangzhou, China). Fetal bovine serum (FBS) and collagenase type II were purchased from Invitrogen Life Technologies (Grand Island, N.Y., USA). 5-Bromo-2’-deoxyuridine and the ADP/ATP ratio assay kit were obtained from Sigma-Aldrich Co. LLC (USA). The LDH, pyruvate (PA), glucose, lactic acid (LA) and free aliphatic acid content assay kits were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). Rabbit antibodies for mitochondrial uncoupling protein 3 (UCP3), fructose 6 phosphate kinase (PFKM), citrate synthetase, glucose transporter 4 (Glut4), carnitine palmitoyl transferase 1B (CPT1B), acyl-CoA dehydrogenase long chain (ACADL), acyl-CoA dehydrogenase medium chain (ACADM), cluster of differentiation 36 (CD36), isocitrate dehydrogenase, oxoglutarate dehydrogenase (OGDH), cardiac troponin I, pyruvate kinase (PK) assay kit, secondary antibody of donkey anti-sheep lgG H&L (HRP) and goat anti-rabbit lgG H&L (Alexa Fluor8) were purchased from Abcam (USA). Mouse anti-β-actin was purchased from Affinity Biosciences. The secondary antibodies horseradish peroxidase-labeled IgG (goat anti-rabbit and anti-mouse) and BeyoECL Plus were purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). The Annexin V/propidium iodide (PI) assay kit was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Other materials were acquired from Sigma (USA).
Animals
Neonatal Sprague-Dawley (SD) rats (within 24 hours of birth) were purchased from Zhejiang Academy of Medical Sciences (Zhejiang, China). The study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. In addition, the protocol utilized during the study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (Hangzhou, China).
Cardiomyocyte isolation and cell culture
Cardiomyocytes were isolated from the hearts of newborn SD rats and cultured as previously described with minor modifications [19]. To obtain myocardial cells, myocardial tissue was digested with 0.1% collagenase II (Invitrogen Life Technologies) and 0.125% trypsin solution (Gino Biopharmaceutical Technology Co., Ltd. Hangzhou, China) for 10 min at 37°C. After centrifugation, the supernatant was discarded, and the cell particles were resuspended in culture medium containing 10% FBS. These steps were repeated until the hearts were completely digested. Cells are placed in Petri dishes for 90 min to allow fibroblasts to adhere to the dish and to obtain purer cardiomyocytes. To inhibit the growth of other types of cells, myocardial cells were cultured in DMEM containing 10% FBS, 100 IU/ml 0.3% penicillin-streptomycin and 0.1 mM 5-bromo-2’-deoxyuridine (Invitrogen Life Technologies). After 48-72 hours of culture, subsequent myocardial cell experiments were carried out. Cardiomyocytes were randomly divided into the following six groups: normoxia (N), I/R, cotreatment with TMZ and I/R (I/R+TMZ), cotreatment with TSPG and I/R (I/R+TSPG), cotreatment with LC and I/R (I/R+LC) and cotreatment with SM and I/R (I/R+SM).
Hypoxia/reoxygenation treatment protocol
Cardiomyocytes were pretreated with SM (25 ml/L), TSPG (7.5 mg/L), TMZ (30 mol/L) or LC (25 ml/L) for 24 hours and then washed with HBSS containing 5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1 mM MgCl2 and 1.5 mM CaCl2 (pH 7.2). To simulate myocardial ischemia in vivo, sugar-free DMEM was added, and the cells were incubated at 37°C. Then, N2 (95%) and 5% CO2 were injected into the incubator to reduce the oxygen concentration to 1% and induce hypoxia for 6 hours. The cells were then cultured in DMEM+4.5 g/l glucose under normal oxygen for 12 hours to simulate the reoxygenation process.
Immunofluorescence staining
Immunocytochemistry was performed as previously described [20]. Briefly, cells were fixed, permeabilized, washed, blocked, and incubated with anti-cardiac troponin I antibodies overnight at 4°C. Cells were then washed and incubated with goat anti-rabbit IgG H&L (Alexa FluorR488) for 1 h at room temperature, and nuclei were stained for 10 min with DAPI (10 mg/ml; Sigma-Aldrich Co., St. Louis, MO). Stained cells were observed by fluorescence microscopy (Olympus, Tokyo, Japan). Image-Pro Plus version 6.0 software was used to quantify the fluorescence intensity of the cells.
Quantitative analysis of SM
The main SM components were quantitated by high-performance liquid chromatography (HPLC) fingerprint analysis in combination with pattern recognition techniques on an Agilent 1290 instrument (Agilent, America) as previously described [11,21,22] according to the Chinese Pharmacopoeia (2010) with slight modifications. All components were separated on a Diamond C18 column (4.6 mm × 250 mm, 5 μm) at a flow rate of 1.0 mL/min. The column temperature was 30°C, and the wavelength was set to 203 nm. The mobile phase comprised 0.1% phosphate in pure water (A) and acetonitrile (B), and a gradient elution was performed as follows: 10 min (90%, A), 25 min (80%, A), 60 min (60%, A), 90 min (45%, A), and 100 min (40-0%, A). All data were processed using Open LAB CDS Chemstation (Agilent, America).
ADP/ATP assay
The intercellular ATP and ADP contents were measured by a luciferin-luciferase assay (ADP/ATP Ratio Assay Kit, Sigma-Aldrich) as previously described with slight modifications [23,24]. In brief, according to the manufacturer’s instructions, 10 µl of cultured resuspended cells (103-104) were transferred to a 96-well plate. Then, 90 µl of ATP reagent and 5 µl of ADP reagent were added to each well of the plate. The ADP/ATP ratio was calculated using the formula from the assay kit.
LDH and PK measurements
After treatments, the culture medium and cells were collected to measure LDH and PK levels using commercial kit reagents according to the manufacturer’s instructions.
Annexin V/PI assay
Briefly, cardiac myocytes were collected, washed with phosphate buffer without Ca2+ and suspended in conjugate buffer. The cells were incubated at room temperature with Annexin V and PI (5 µM/L) for 15 min, and the cardiac myocytes were then analyzed on a flow cytometer (FC500MCL; Beckman Coulter, Brea, CA, USA).
Total mRNA isolation and quantitative RT-PCR analysis
Total mRNA was extracted from the cardiomyocytes using TRIzol reagent according to the manufacturer’s instructions. The total RNA concentration was measured at 260 nm. The PrimeScript RT reagent kit was used to reverse-transcribe the total RNA via RT-PCR. Gene expression was quantified with the CFX96 Touch™ Real-Time PCR Detection System (BIO-RAD, California, USA) using SYBR Premix Ex Taq II and gene-specific primers (Table 1). Individual 100 ng cDNA samples were amplified under the following cycle conditions: 95°C for 30 sec, followed by 40 cycles of 5 sec at 95°C and 34 sec at 60°C. The standard curve was generated with sequence-diluted cDNA synthesized from neonatal rat cardiac tissue (1.5-50 ng) to determine the efficiency of PCR, and similar results were obtained in all groups. The mRNA expression levels of CD36, CPT1B, ACADL, ACADM, UCP3, OGDH, PFKM, GLUT4, citrate synthetase and isocitrate dehydrogenase were evaluated relative to those of beta-actin and calculated by the standard curve and 2-ΔΔCt methods.
Table 1.
Primer sequences for RT-PCR analysis
| Primers | Sequences (5’-3’) | |
|---|---|---|
| CD36 | Forward | ATGGGCTGTGATCGGAACTG |
| Reverse | GTCTTCCCAATAAGCATGTCTCC | |
| CPT1B | Forward | GCACACCAGGCAGTAGCTTT |
| Reverse | CAGGAGTTGATTCCAGACAGGTA | |
| ACADL | Forward | TCTTTTCCTCGGAGCATGACA |
| Reverse | GACCTCTCTACTCACTTCTCCAG | |
| ACADM | Forward | AGGGTTTAGTTTTGAGTTGACGG |
| Reverse | CCCCGCTTTTGTCATATTCCG | |
| UCP3 | Forward | CTGCACCGCCAGATGAGTTT |
| Reverse | ATCATGGCTTGAAATCGGACC | |
| OGDH | Forward | AGGGCATATCAGATACGAGGG |
| Reverse | CTGTGGATGAGATAATGTCAGCG | |
| PFKM | Forward | TGTGGTCCGAGTTGGTATCTT |
| Reverse | GCACTTCCAATCACTGTGCC | |
| GLUT4 | Forward | GTGACTGGAACACTGGTCCTA |
| Reverse | CCAGCCACGTTGCATTGTAG | |
| Citrate synthetase | Forward | GGACAATTTTCCAACCAATCTGC |
| Reverse | TCGGTTCATTCCCTCTGCATA | |
| Isocitrate dehydrogenase | Forward | ATGCAAGGAGATGAAATGACACG |
| Reverse | GCATCACGATTCTCTATGCCTAA | |
| Beta-actin | Forward | GGAGATTACTGCCCTGGCTCCTA |
| Reverse | GACTCATCGTACTCCTGCTTGCTG |
Western blot analysis
Isolated cardiomyocytes were dissolved in a radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 10.5% sodium deoxycholate, 5% sodium dodecyl sulfate and 1 mM benzyl sulfonyl-enamel buffer. The total protein concentration was quantitatively determined using a BCA protein quantitative detection kit (Beyotime Biotechnology Co., Ltd. Shanghai, China). The protein was separated on 10-12% alkyl sulfate polyacrylamide. The protein was separated on twelve 10% alkyl sulfate polyacrylamide gels and then transferred to a polyvinylidene fluoride membrane. Nonspecific proteins were blocked in 5% skim milk incubated in Tris buffer containing 0.05% Tween-20 for 1 hour. The proteins were detected with the primary antibodies rabbit anti-CPT1B (1:1,000), rabbit anti-PFKM (1:1,000), rabbit anti-Glut4 (1:1,000), rabbit anti-Ucp3 (1:1,000), rabbit anti-ACADM (1:1,000), rabbit anti-ACADL (1:2,000), rabbit anti-citrate synthetase (1:1,000), rabbit anti-CD36 (1:1,000), rabbit anti-isocitrate dehydrogenase (1:1,000), rabbit anti-OGDH (1:1,000), goat anti-M-CK (1:1,0000) and anti-beta-actin (1:1,000) overnight at 4°C. The anti-rabbit IgG antibody labeled with horseradish peroxidase (1:1,000) was detected at room temperature for 1 hour, and the immunoreaction bands were observed by ECL-Plus reagent. Quantity One software 4.6.2 (Bio-Rad Laboratories, Inc., Berkeley, CA, USA) was used to analyze the signal intensity of each band, which was compared with that of the loading control beta-actin to calculate the corresponding protein level.
Metabolic substrate assay
After treatment, the culture medium was collected to measure the PA, glucose, LA and free aliphatic acid contents using commercial reagents according to the manufacturer’s instructions.
Statistical analysis
Data are presented as the means ± SD. One-way analysis of variance and Student’s t-tests were used to determine the statistical significance of differences between groups using SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Determination of primary cardiac cell purity
Primary cultures of neonatal rat cardiomyocytes have been widely used in studies of various cardiovascular diseases. To obtain primary cardiomyocytes with good activity and high purity, isolated cardiomyocytes were cultured for 72 hours. Cellular immunofluorescence detection [25] was used for identification. The fluorescence intensity of the cells was quantified, which revealed a cardiomyocyte purity greater than 95% (Figure 1).
Figure 1.
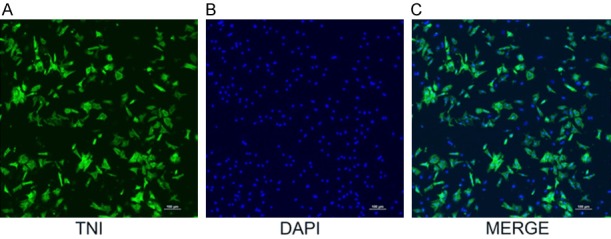
Observation of cardiomyocytes under confocal microscopy. Anti-labeled actin conjugated by second antibody is shown in yellowish green (B); nuclei are labeled by DAPI in blue (A). Combined fluorescence of the cytoplasm and nuclei of cardiac muscle cells (C). Cardiomyocyte purity was calculated by observing 5 random fields of view and counting 200 cells in each field (97.1±1.9)%.
Quantitation of SM by HPLC fingerprint analysis
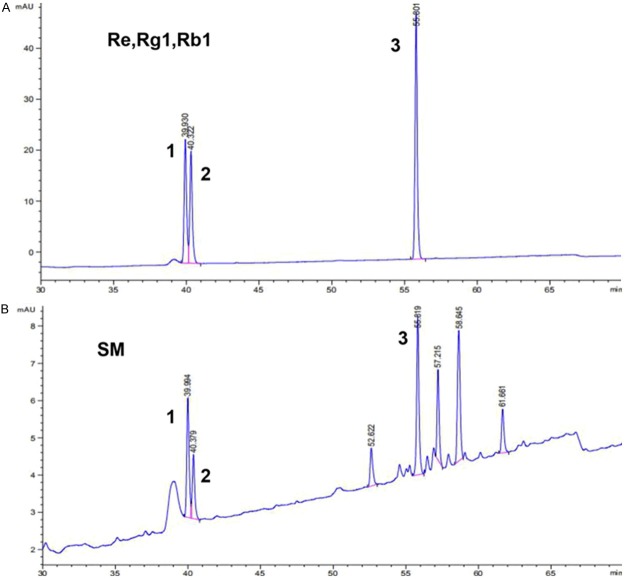
Original discrimination and quality control of SM was performed with HPLC fingerprint analysis to determine the content of TSPG in SM. The method was validated for parameters such as precision, repeatability and stability. As reported previously [26], the batch sample analyses and characteristic records of SM were maintained. According to the reference standards fingerprint, the peak areas of ginsenosides Re, Rg1, and Rb1 at the corresponding retention times (39.930 min, 40.322 min, 55.801 min, respectively) were used to calculate the total contents of Re, Rg1, and Rb1 in SM as 0.3 mg/ml. Ginsenosides Re, Rg1, and Rb1 were the major bioactive components of TSPG. The HPLC chromatogram of SM including ginsenosides as a standard is shown in Figure 2, and the SM fingerprint shows that the TSPG content in SM, represented by the total contents of Re, Rg1, and Rb1, was 0.3 mg/ml.
Figure 2.
HPLC fingerprint of Shenmai injection and ginsenosides Re, Rg1 and Rb1 as reference standards. A. Chromatogram of reference standards: 1 represents ginsenoside Re; 2 represents ginsenoside Rg1; 3 represents ginsenoside Rb1. B. Representative chromatogram of Shenmai injection samples: No. 1-3 represent the common peaks with reference standards Re, Rg1 and Rb1, respectively.
Effects of SM, LC, TSPG and TMZ on LDH and PK release levels following I/R injury
To evaluate the cytoprotection of SM, LC, TSPG and TMZ against I/R injury, rat cardiomyocytes were subjected to I/R, and the resulting LDH and PK release levels were compared with those in normoxic cells. As shown in (Figure 3A, 3B), the LDH and PK levels in the I/R group were higher than those in the control group (P<0.01), showing that SM, LC, TSPG and TMZ markedly reduced LDH and PK activity (Figure 3A, 3B). Furthermore, SM pretreatment significantly decreased the LDH and PK levels (P<0.01). During I/R of cardiomyocytes, the SM group exhibited more significant alterations in the release of LDH than the TSPG group (P<0.01, I/R+SM vs. I/R+TSPG). The PK release level was significantly decreased in the SM group compared with that in the TSPG group (P<0.05, I/R+SM vs. I/R+TSPG). Therefore, the protective effects of SM pretreatment were superior to those of TSPG (Figure 3).
Figure 3.
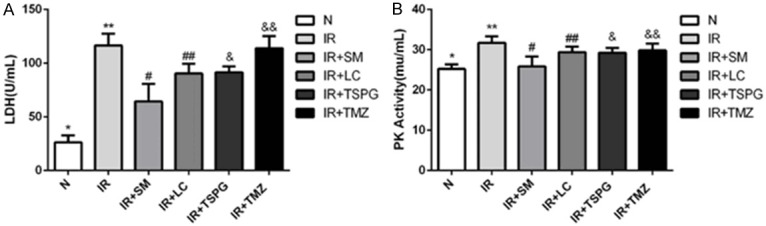
Injury of cardiomyocytes in different treatment groups was detected by LDH measurements and PK release levels. A. Release level of LDH in medium of different treatment groups. B. Release level of PK in medium of different treatment groups. The data are expressed as the mean ± SD. The experiments were repeated 10 times (n=10). A. **P<0.01 compared with the normal group; #P<0.01, ##P<0.01, &P<0.01, &&P<0.01 compared with the I/R group; #P<0.01 compared with the I/R+TSPG group. B. **P<0.01 compared with the normal group; #P<0.01, ##P<0.01, &P<0.01, &&P<0.05 compared with the I/R group; #P<0.01 compared with the I/R+TSPG group. N, normoxia.
Effects of SM, LC, TSPG and TMZ on the ATP content in cardiac myocytes following I/R injury
To investigate the effects of SM, LC, TSPG and TMZ on energy metabolism and mitochondrial function in myocardial cells after I/R injury, the intercellular ATP contents and ADP/ATP ratio were measured. As presented in Figure 4A, the ATP content in the I/R group was lower than that in the normal group, while the ADP/ATP ratio was higher in the I/R group than in the normal group (Figure 4B). SM, TSPG and TMZ markedly restored the ATP content in cardiomyocytes during I/R, and SM exerted the most significant effect among all the components analyzed (P<0.01). Furthermore, the intercellular ATP content in the SM group was significantly higher than that in the TSPG group after I/R (P<0.05, I/R+SM vs. I/R+TSPG). Treatment of I/R with TSPG (I/R+TPSG) or SM (I/R+SM) resulted in significant reductions in the ADP/ATP ratio, with SM treatment showing the most marked effect (P<0.01) (Figure 4).
Figure 4.
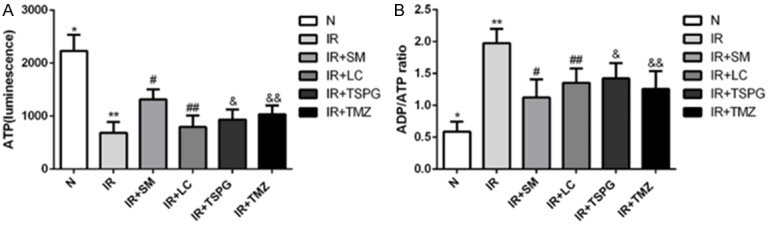
Production and utilization of energy in cardiomyocytes presented by intracellular ATP levels and ADP/ATP ratios in normoxic and I/R conditions following SM, LC, TSPG and TMZ treatment. A. The intracellular ATP levels of the different treatment groups. B. The ADP/ATP ratios of the different treatment groups. The data are expressed as the mean ± SD. The experiments were repeated 10 times (n=10). A. **P<0.01 compared with the normal group; #P<0.01, &P<0.05, &&P<0.01 compared with the I/R group; #P<0.01 compared with the I/R+TSPG group. B. **P<0.01 compared with the normal group; #P<0.01, ##P<0.01, &P<0.01, &&P<0.01 compared with the I/R group; #P<0.01 compared with the I/R+TSPG group. N, normoxia.
Effects of SM, LC, TSPG and TMZ on differential mRNA expression levels
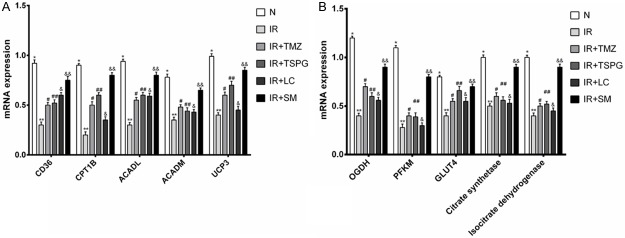
To explore the molecular mechanisms by which SM, LC, TSPG and TMZ protect against I/R injury, rat myocardial cells were subjected to I/R, and the resulting levels of mRNAs in the myocardial energy metabolism pathway were compared with those in normoxic cells by RT-PCR analysis. The expression levels of free fatty acid (FFA) metabolism- and glucose metabolism-related genes were significantly decreased after I/R injury (P<0.01) (Figure 5). Treatment of the I/R cells with SM (I/R+SM), TSPG (I/R+TSPG) and TMZ (I/R+TMZ) resulted in the significant upregulation of CD36, CPT1B, ACADL and UCP3 mRNA levels, with SM treatment showing the most marked effect (P<0.01). However, the mRNA level of ACADM was not significantly altered by treatment with TMZ, TSPG or LC (P>0.05) (Figure 5A). The I/R+SM group exhibited significantly increased mRNA levels of OGDH, PFKM, GLUT4, citrate synthetase and isocitrate dehydrogenase (P<0.01) (Figure 5B). However, the gene expression levels of PFKM, citrate synthetase and isocitrate dehydrogenase were not significantly affected by TSPG, LC or TMZ (P>0.05). Comparison of the OGDH and GLUT4 mRNA levels among the LC, TSPG and TMZ treatment groups showed significant increases following I/R treatment (P<0.05).
Figure 5.
Effects of SM, LC, TSPG and TMZ on CD36, CPT1B, ACADL, ACADM, UCP3, OGDH, PFKM, GLUT4, citrate synthetase and isocitrate dehydrogenase mRNA expression following I/R in cardiomyocytes. (A) Evaluation of FFA metabolism-related gene expression after I/R injury by RT-PCR analysis. (B) Evaluation of glucose metabolism-related gene expression after I/R injury by RT-PCR analysis. Beta-actin was used as the loading control. Data are expressed as the mean ± standard deviation (n=6). (A) mRNA levels of CD36, CPT1B, ACADL, ACADM and UCP3, **P<0.01 vs. N; &&P<0.01 vs. I/R. (A. CD36, CPT1B, ACADL, UCP3) ##P<0.05 vs. I/R; #P<0.05 vs. I/R. (A. CD36, ACADL) &P<0.05 vs. I/R. (A. ACADM) #P>0.05, ##P>0.05 and &P>0.05 vs. I/R. (B) mRNA levels of OGDH, PFKM, GLUT4, citrate synthetase and isocitrate dehydrogenase, **P<0.01 vs. N; &&P<0.01 vs. I/R. (B. OGDH, GLUT4) #P<0.05, ##P<0.05 and &P<0.05 vs. I/R. (B. OGDH, PFKM, citrate synthetase and isocitrate dehydrogenase) &&P<0.05 vs. I/R+TSPG; (B. PFKM, citrate synthetase and isocitrate dehydrogenase) ##P>0.05 vs. I/R. N, normoxia.
Effects of SM, LC, TSPG and TMZ on differentially expressed proteins in the fatty acid metabolic pathway
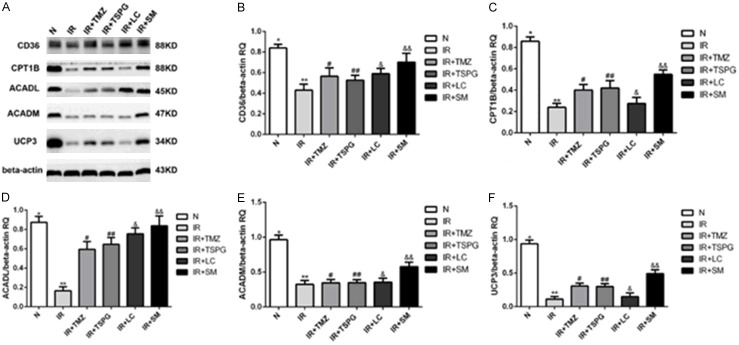
Western blots were performed to examine the levels of proteins related to the fatty acid metabolic pathway and mitochondrial function, such as CD36, CPT1B, ACADL, ACADM and UCP3, revealing that myocardial ischemia decreased the protein expression of CD36 (P<0.01), CPT1B (P<0.01), ACADL (P<0.01), ACADM (P<0.01), and UCP3 (P<0.01) (Figure 6). Following SM, TSPG or TMZ treatment, the protein expression levels of CD36, CPT1B, ACADL and UCP3 were significantly increased (P<0.05), while SM consistently exhibited a better efficacy than TSPG and TMZ. However, the treatment effect of LC was not significant (P>0.05), and the expression level of ACADM was not significantly altered by treatment with TMZ, TSPG or LC (P>0.05) (Figure 6).
Figure 6.
Expression of key enzymes and transporters in the fatty acid metabolism pathway of cardiomyocytes in the different treatment groups. A. The protein expression levels of CD36, CPT1B, ACADL, ACADM and UCP3 were evaluated by Western blot analysis; beta-actin served as the control. Quantification of protein bands with image analysis. B-F. The protein levels of CD36, CPT1B, ACADL, ACADM and UCP3 are shown as ratios relative to the loading control (beta-actin) and are presented as the mean ± SD. (n=6). The corresponding one-way ANOVA and LSD test results for each protein are shown. B-D, F. **P<0.01 compared the normal group; &&P<0.01 and ##P<0.05 compared with the I/R group. E. Protein levels of ACADM, **P<0.01 compared with the normal group, &&P<0.01 and ##P>0.05 compared with the I/R group. N, normoxia.
Effects of SM, LC, TSPG and TMZ on regulating protein expression in the glucose metabolic pathway during I/R
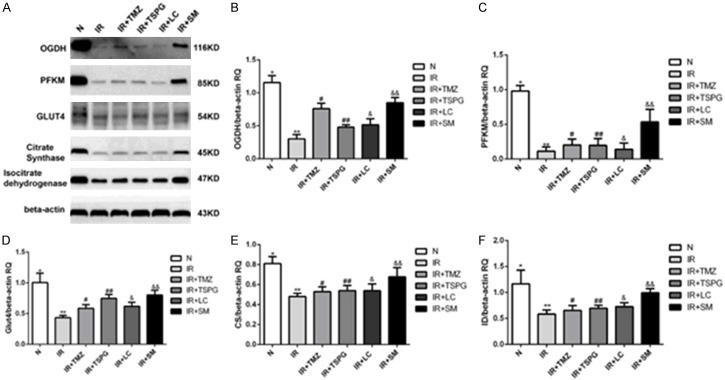
Myocardial cells during I/R and the expression levels of proteins in the glucose metabolic pathway indicated that SM, LC, TSPG and TMZ treatment affected the metabolic function of cardiomyocytes. Western blot was performed to validate specific proteins related to the glucose metabolic pathway. OGDH, citrate synthetase and isocitrate dehydrogenase are key enzymes of the tricarboxylic acid cycle, and PFKM and GLUT4 are two important proteins in glucose metabolism. After I/R treatment, the protein levels of OGDH, PFKM, GLUT4, citrate synthase and isocitrate dehydrogenase in the I/R group were significantly lower than those in the N group (all P<0.01). Furthermore, the I/R+SM group exhibited significantly increased levels of these proteins (P<0.01). However, protein regulation was not significantly affected by TSPG (Figure 7C, 7E, 7F) (P>0.05). Notably, the other two drug treatments (LC and TMZ) did not significantly alter PFKM, citrate synthetase or isocitrate dehydrogenase protein expression (P>0.05). Comparison of the OGDH and GLUT4 protein levels between the LC, TSPG and TMZ treatment groups revealed significant increases following I/R treatment (P<0.05). Thus, SM can improve energy metabolism by upregulating the expression of OGDH, PFKM, citrate synthetase, isocitrate dehydrogenase and GLUT4. Furthermore, SM exerts a more significant effect than its components (TSPG).
Figure 7.
Effects of SM, LC, TSPG and TMZ on the OGDH, PFKM, GLUT4, citrate synthetase and isocitrate dehydrogenase levels following I/R in cardiomyocytes; beta-actin was used as the loading control. (A) Western blot analysis of the OGDH, PFKM, GLUT4, citrate synthetase, isocitrate dehydrogenase and beta-actin levels. (B-F) Quantification of the (B) OGDH, (C) PFKM, (D) GLUT4, (E) citrate synthetase and (F) isocitrate dehydrogenase levels. Data are expressed as the mean ± standard deviation (n=6). (B-F) **P<0.01 vs. N; &&P<0.01 vs. I/R; (B, D) #P<0.05, ##P<0.05 and &P<0.05 vs. I/R; (B, C, E, F) &&P<0.05 vs. I/R+TSPG; (C, E, F) **P>0.05 vs. I/R+TSPG. N, normoxia.
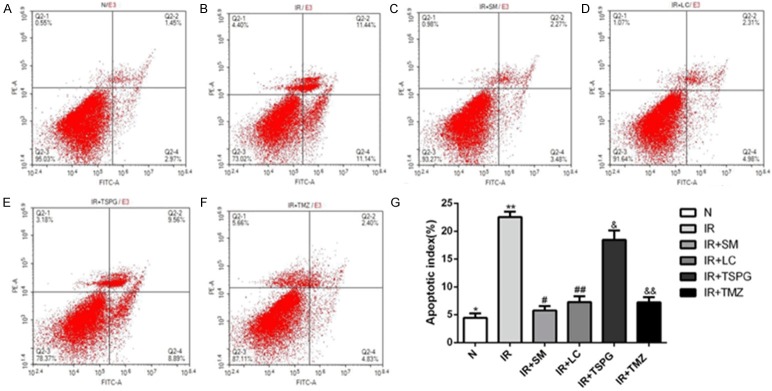
Effects of SM, LC, TSPG and TMZ on cardiomyocyte apoptosis following I/R injury
To observe the effects of SM, LC, TSPG and SM on apoptosis induced by I/R, flow flow cytometry was used to detect the apoptosis rate. As shown in Figure 8, I/R treatment significantly increased the apoptosis rate (P<0.01), while SM treatment reduced the apoptosis rate (P<0.01). The apoptosis rates of the I/R+LC and I/R+TMZ groups were decreased significantly (P<0.01), while that of the I/R+TSPG group was significantly but mildly reduced (P<0.05).
Figure 8.
Effects of SM, LC, TSPG and TMZ on I/R-induced cardiomyocyte apoptosis. A-F. Cardiomyocytes after I/R treatment with SM, LC, TSPG and TMZ were stained with Annexin V-FITC/PI and analyzed for apoptosis using a flow cytometer. Cardiomyocytes in regions Q4 and Q2 represent early- and late-apoptotic cardiomyocytes, respectively. G. The percentages of apoptotic cells were calculated and compared. Data are expressed as the mean ± standard deviation (n=3). **P<0.01 vs. N; #P<0.01 vs. I/R; &P<0.01 vs. I/R+SM; &P<0.05 vs. I/R. N, normoxia.
Effects of SM, LC, TSPG and TMZ on energy substrates in cardiomyocytes subjected to I/R
To further verify the effects of SM, LC, TSPG and TMZ on cardiomyocyte energy metabolism, we assessed the energy substrate consumption in culture medium under normal and I/R conditions in the absence or presence of SM, LC, TSPG and TMZ. FFAs and glucose are energy sources in the cardiomyocyte culture medium and can be used to evaluate the utilization of energy metabolic substrates. LA is the product of anaerobic glycolysis, while PA is an intermediate of the energy metabolic pathway. As shown in Figure 9, I/R markedly increased the FFA, glucose and LA contents in the culture medium compared with those in cultures grown under normal conditions (P<0.01) (Figure 9A-C); however, these increases were inhibited by SM, LC, TSPG and TMZ treatments. Notably, the I/R+SM group showed the greatest decrease in FFA and LA content following I/R treatment (P<0.01) (Figure 9A, 9C). In addition, the I/R+SM group showed a significant reduction in energy sources compared with that in the I/R group (P<0.01); conversely, the I/R+LC, TSPG and TMZ groups exhibited modest but nonsignificant decreases in glucose content compared with that in the I/R group (P>0.05) (Figure 9B). However, the levels of PA were not significantly different among the groups (P>0.05) (Figure 9D).
Figure 9.
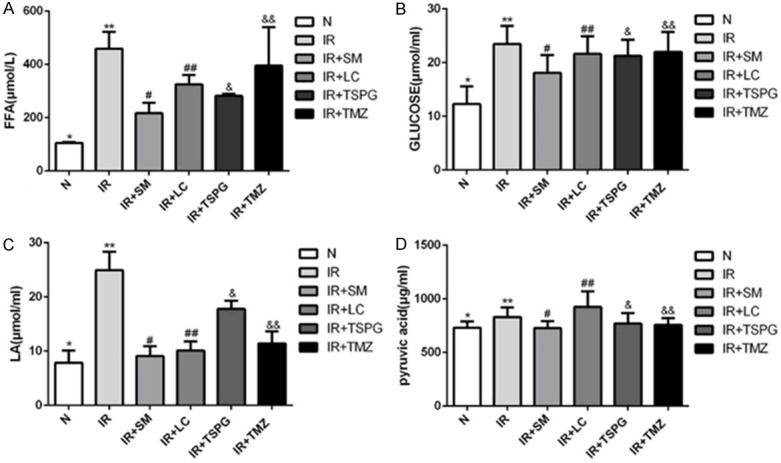
Effects of SM, LC, TSPG and TMZ on energy substrates in cardiomyocytes following I/R. A-D. Detection of the FFA, glucose, LA and PA levels in culture medium under normoxic or I/R conditions following SM, LC, TSPG and TMZ treatment. Data are expressed as the mean ± standard deviation (n=10). A. **P<0.01 vs. N; #P<0.01, ##P<0.01, &P<0.01, &&P<0.05 vs. I/R; **P<0.05 vs. I/R+TSPG. B. **P<0.01 vs. N; #P<0.01 vs. I/R; C. **P<0.01 vs. N; #P<0.01, ##P<0.01, &P<0.01, &&P<0.01 vs. I/R; **P<0.01 vs. I/R+TSPG. D. *P>0.05, #P>0.05, ##P>0.05, &P>0.05, &&P>0.05 vs. I/R; N, normoxia.
Discussion
In the present study, the protective mechanism of the myocardial metabolic pathway after myocardial I/R was studied. The purity of primary cardiomyocytes extracted for this I/R experimental study was more than 95%. In a previous study, the effects of SM and its bioactive components on highly purified primary cardiomyocytes following I/R were investigated [15]. SM, prepared from Panax ginseng and Ophiopogon japonicus, has been mass-produced as a patented drug based on national standards approved by the CFDA. To further elucidate the effects of SM on cardiomyocytes, the efficacies of four different drug treatments on reducing I/R-related complications were compared. HPLC fingerprint analysis showed that TSPG is the main bioactive ingredient of SM, and the content of TSPG in SM was 0.3 mg/ml. The results showed that 1 mL of SM contained 293.38±40.54 g of TSPG [27]. These components conformed to the national standards for SM injection approved by the CFDA [11]. In our pre-experiment, we evaluated the concentrations of SM, LC, TSPG and TMZ that yielded the optimal effects on protecting cardiac myocytes during I/R. According to the results, 25 ml/l SM was administered to cardiomyocytes in the present treatment regimen, which is equivalent to ~7.5 mg/l TSPG. The present study demonstrates that exposure to I/R resulted in significant alterations of a variety of functional indices for cardiomyocyte activity, including changes in key enzymes in the metabolic pathway within heart myocardial cells. In a previous study, the apoptosis and key mitochondrial pathway enzymes of cardiomyocytes were significantly altered following I/R, indicating that I/R injury was successful [28]. According to previous studies, pretreatment with TMZ protected cardiomyocytes from palmitate-induced mitochondrial fission and dysfunction, and the beneficial effects of TMZ on patients with different cardiovascular pathologies were shown to be related to the modulation of mitochondrial morphology and function [29]. LC is intrinsically involved in mitochondrial metabolism and function, as it plays key roles in FAO and energy metabolism. In addition to transporting FFAs across the inner mitochondrial membrane, LC modulates the FFA oxidation rate and is involved in the regulation of vital cellular functions, such as apoptosis [30].
LDH measurements and PK assays were both performed to evaluate myocardial damage after I/R. Cardiomyocytes contain marker enzymes such as LDH and PK, and I/R injury causes the cell membrane to become permeable or rupture, which results in leakage of these enzymes into the blood. Thus, the LDH and PK activities in serum reflect the degree of myocardial injury [16,31]. Our results showed that myocardial I/R significantly increased the LDH and PK activities. The SM, LC, TSPG and TMZ treatment groups exhibited decreased cell injury, and SM pretreatment conferred the most significant advantage. Notably, the effects of cytoprotection observed following I/R treatment were reversed by LC and TMZ treatment, which is consistent with the results of previous studies [32,33]. In accordance with previous studies that reported widespread disorder of myocardial structure and necrosis of cardiomyocytes following myocardial I/R [16,17], our apoptosis data demonstrated that cardiomyocytes were significantly damaged after I/R treatment. Furthermore, SM, LC, TSPG and TMZ reduced the damage to varying degrees, and SM provided the most obvious protection. Therefore, our research indicates that SM has protective effects on I/R-induced myocardial injury. In addition, the protective effect of SM on cardiomyocyte injury following I/R treatment is significantly superior to that of TSPG.
To further explore the protective effect of SM on cardiomyocytes subjected to I/R, cell membrane and cytoplasmic proteins in metabolic pathways, such as PFKM, Glut4 and CD36, were evaluated. PFKM is a key enzyme in glycolysis, catalytic fructose-6-phosphate formation and fructose-1, 6-diphosphate synthesis. Glut4 is a carrier protein embedded in the cell membrane that transports glucose, and CD36 is a membrane glycoprotein present in cardiomyocytes that is involved in the transmembrane transport of long-chain fatty acids (LCFAs). The expression of Glut4, CD36 and PFKM is closely related to the uptake and production of cardiomyocyte metabolic substrates [34-36]. It is therefore plausible that the observed reduction in Glut4, CD36 and PFKM expression may partially explain the decreased coefficients of FFA and glucose utilization in cardiomyocytes following I/R treatment. The present study demonstrated that SM, TMZ and TSPG were able to increase Glut4, CD36 and PFKM expression as determined by RT-PCR and Western blot analysis, and the effect of SM was most obvious. While SM, LC, TSPG and TMZ improved the utilization of FFA following I/R, only treatment with SM had an effect on the increased utilization of glucose. Thus, SM and TSPG protected all of these I/R-induced energy metabolism disorders in the myocardium as well as myocardial impairment by upregulating the proteins involved in the uptake of energy metabolic substrates.
Energy metabolism disorder and the resultant ATP deficiency arise in the myocardium after I/R injury due to many types of insults [37]. Pathway analysis showed that SM and its components might regulate mitochondrial function-related proteins in the ischemic myocardium. CPT1B mediates the transfer of LCFAs across the outer mitochondrial membrane and is a rate-limiting step in FAO [38]. UCP3 belongs to a superfamily of mitochondrial ion transporters, is expressed in the inner mitochondrial membrane of the heart, and modulates cardiac mCa1 single-channel activity in a ATP-dependent manner [39]. ACADL is a key enzyme involved in the FAO of LCFAs [40]. ACADM is a member of the acyl-CoA dehydrogenase (ACAD) family that comprises 9 known proteins and is involved in the oxidation of medium-chain fatty acids and amino acids [41]. The protein expression levels of CPT1B, UCP3, ACADL and ACADM are closely related to mitochondrial function during aerobic metabolic and energy production by FFAs. OGDH, citrate synthetase and isocitrate dehydrogenase are key enzymes of the tricarboxylic acid cycle that regulate aerobic metabolism and glucose production in mitochondria [42]. In this study, SM, LC, TSPG and TMZ administration relieved myocardial energy disorders after I/R challenge, which was manifested by increased CPT1B, ACADL and OGDH protein and mRNA expression levels. Furthermore, SM, LC, TSPG and TMZ attenuated the increase in the I/R-induced LA content, and the effects of SM treatment were the most significant. SM also elevated the expression of UCP3, ACADM, citrate synthetase and isocitrate dehydrogenase following I/R, while LC, TSPG and TMZ did not. Notably, the PA content after I/R was not altered by SM, LC, TSPG or TMZ treatment. This result is possibly attributable to the fact that PA is the end-product of glycolysis, a major substrate for oxidative metabolism, and a branching point for glucose, lactate, fatty acid and amino acid synthesis [43]. The multidirectional metabolic pathway resulted in no significant change in the PA content after I/R. These results suggest that the protective effects of SM and TSPG on mitochondrial metabolism are related to the upregulation of key enzymes in aerobic metabolism. Moreover, depletion of the intracellular ATP content is a crucial feature of ischemic processes. In the present study, we observed a significant decrease in the ATP content and key enzymes in cardiomyocytes after I/R, indicating that inhibition of the mitochondrial metabolic pathway and SM, LC, TSPG and TMZ treatment markedly increased the intracellular ATP content while reducing the ratio of ADP/ATP after the exposure of cardiomyocytes to I/R. These findings suggest that the cardioprotective mechanism of SM and TSPG is related to the restored production of ATP.
Conclusion
The findings of the present study suggest a protective role of SM and TSPG in I/R-induced energy metabolism disorders and cardiomyocyte apoptosis through the significant regulation of proteins involved in metabolic substrate transport, the glycolytic pathway and the mitochondrial aerobic metabolic process and improving the utilization rate of metabolic substrates. Furthermore, the results also showed that SM and TSPG could preserve the aerobic metabolic pathway and ATP contents in mitochondria following I/R treatment. Therefore, SM and TSPG are thought to improve energy metabolism by protecting myocardial cells via regulating energy metabolism-related enzymes, ATP content and mitochondrial function. Notably, the effects of SM are significantly superior to those of TSPG.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 81670447), the National Natural Science Foundation of Zhejiang Province (no. LY15H020006), the Zhejiang Province Key Subject of Medicine (Neurological Rehabilitation) and the Traditional Chinese Medicine Program of Zhejiang Provincial (no. 2017ZZ001). Li-hong Wang is sponsored by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Disclosure of conflict of interest
None.
References
- 1.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Chen JM, Huang H, Kuznicki M, Zheng S, Sun W, Quan N, Wang L, Yang H, Guo HM, Li J, Zhuang J, Zhu P. The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway. Metabolism. 2016;65:122–130. doi: 10.1016/j.metabol.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 4.He K, Yan L, Pan CS, Liu YY, Cui YC, Hu BH, Chang X, Li Q, Sun K, Mao XW, Fan JY, Han JY. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2014;307:H1764–1776. doi: 10.1152/ajpheart.00259.2014. [DOI] [PubMed] [Google Scholar]
- 5.Rouslin W, Broge CW, Grupp IL. ATP depletion and mitochondrial functional loss during ischemia in slow and fast heart-rate hearts. Am J Physiol. 1990;259:H1759–1766. doi: 10.1152/ajpheart.1990.259.6.H1759. [DOI] [PubMed] [Google Scholar]
- 6.Faccenda D, Campanella M. Molecular regulation of the mitochondrial F(1)F(o)-ATPsynthase: physiological and pathological significance of the inhibitory factor 1 (IF(1)) Int J Cell Biol. 2012;2012:367934. doi: 10.1155/2012/367934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhao Y, Jiang W, Zhao X, Fan G, Zhang H, Shen P, He J, Fan X. iTRAQ-based proteomic analysis reveals recovery of impaired mitochondrial function in ischemic myocardium by shenmai formula. J Proteome Res. 2018;17:794–803. doi: 10.1021/acs.jproteome.7b00450. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Xie Y, Liao X, Chai Y, Luo Y. Shenmai injection as an adjuvant treatment for chronic cor pulmonale heart failure: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern Med. 2015;15:418. doi: 10.1186/s12906-015-0939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong W, Hu Z. Clinical application of Shenmai injection. Her Med. 2000;19:181. [Google Scholar]
- 10.Yu J, Xin Y, Xuan Y. Research progress on material foundation of pharmacological effects of Shenmai injection. Her Med. 2013;32:497–499. [Google Scholar]
- 11.Shao X, Yang ZQ, Lee J, Jiang ZP, Ye XH, Luo LJ, Yang TL, Ye SL, Lu DF. A randomized, double-blind, multicenter, placebo-controlled clinical study on the efficacy and safety of Shenmai injection inpatients with chronic heart failure. Journal of Ethnopharmacology. 2016;186:136–142. doi: 10.1016/j.jep.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 12.Tang H, Chen ZB, Liang YB, M ZF. Effect of Shenmai injection on cardiac function in gerontal patients with chronic heart failure. HEART. 2012;98:E242. [Google Scholar]
- 13.Yu J, Xin YF, Gu LQ, Gao HY, Xia LJ, You ZQ, Xie F, Ma ZF, Wang Z, Xuan YX. One-month toxicokinetic study of SHENMAI injection in rats. J Ethnopharmacol. 2014;154:391–399. doi: 10.1016/j.jep.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Sodrul IMD, Wang C, Chen X, Du J, Sun H. Role of ginsenosides in reactive oxygen species-mediated anticancer therapy. Oncotarget. 2018;9:2931–2950. doi: 10.18632/oncotarget.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye LF, Zheng YR, Wang LH. Effects of Shenmai injection and its bioactive components following ischemia/reperfusion in cardiomyocytes. Exp Ther Med. 2015;10:1348–1354. doi: 10.3892/etm.2015.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Han B, Yu X, Qu S, Sui D. Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats. Pharm Biol. 2011;49:900–906. doi: 10.3109/13880209.2011.554845. [DOI] [PubMed] [Google Scholar]
- 17.Cui YC, Pan CS, Yan L, Li L, Hu BH, Chang X, Liu YY, Fan JY, Sun K, Li Q, Han JY. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci Rep. 2017;7:44579. doi: 10.1038/srep44579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Duan J, Wu X, Guo C, Yin Y, Zhu Y, Hu T, Wei G, Wen A, Xi M. Total saponins from Aralia taibaiensis protect against myocardial ischemia/reperfusion injury through AMPK pathway. Int J Mol Med. 2015;36:1538–1546. doi: 10.3892/ijmm.2015.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 20.Qi XF, Chen ZY, Xia JB, Zheng L, Zhao H, Pi LQ, Park KS, Kim SK, Lee KJ, Cai DQ. FoxO3a suppresses the senescence of cardiac microvascular endothelial cells by regulating the ROS-mediated cell cycle. J Mol Cell Cardiol. 2015;81:114–126. doi: 10.1016/j.yjmcc.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Lu XF, Bi KS, Zhao X, Chen XH. Authentication and distinction of Shenmai injection with HPLC fingerprint analysis assisted by pattern recognition techniques. J Pharm Anal. 2012;2:327–333. doi: 10.1016/j.jpha.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Li Y, Liu X, Ma Z, Michael S, Orgah JO, Fan G, Zhu Y. Mitochondrial dynamics modulation as a critical contribution for Shenmai injection in attenuating hypoxia/reoxygenation injury. J Ethnopharmacol. 2019;237:9–19. doi: 10.1016/j.jep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Dutta AK, Okada Y, Sabirov RZ. Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J Physiol. 2002;542:803–816. doi: 10.1113/jphysiol.2002.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou DC, Su YH, Jiang FQ, Xia JB, Wu HY, Chang ZS, Peng WT, Song GH, Park KS, Kim SK, Cai DQ, Zheng L, Qi XF. CpG oligodeoxynucleotide preconditioning improves cardiac function after myocardial infarction via modulation of energy metabolism and angiogenesis. J Cell Physiol. 2018;233:4245–4257. doi: 10.1002/jcp.26243. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Wang Y, Nie J, Fan X, Cheng Y. A network pharmacology approach to evaluating the efficacy of chinese medicine using genome-wide transcriptional expression data. Evid Based Complement Alternat Med. 2013;2013:915343. doi: 10.1155/2013/915343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao SP, Nie LX, Wang GL, Lin RC. Study on ginsenosides contents of Shenmai injection and its intermediates by UPLC. Yao Wu Fen Xi Za Zhi. 2014;7:1264–1268. [Google Scholar]
- 28.Zhao S, Lin Q, Li H, He Y, Fang X, Chen F, Chen C, Huang Z. Carbon monoxide releasing molecule2 attenuated ischemia/reperfusioninduced apoptosis in cardiomyocytes via a mitochondrial pathway. Mol Med Rep. 2014;9:754–762. doi: 10.3892/mmr.2013.1861. [DOI] [PubMed] [Google Scholar]
- 29.Kuzmicic J, Parra V, Verdejo HE, Lopez-Crisosto C, Chiong M, Garcia L, Jensen MD, Bernlohr DA, Castro PF, Lavandero S. Trimetazidine prevents palmitate-induced mitochondrial fission and dysfunction in cultured cardiomyocytes. Biochem Pharmacol. 2014;91:323–336. doi: 10.1016/j.bcp.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Marcovina SM, Sirtori C, Peracino A, Gheorghiade M, Borum P, Remuzzi G, Ardehali H. Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of L-carnitine. Transl Res. 2013;161:73–84. doi: 10.1016/j.trsl.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaft FR, Ban RW, Imfeld H. Serum pyruvate kinase in acute myocardial infarction. Am J Cardiol. 1970;26:143–150. doi: 10.1016/0002-9149(70)90772-1. [DOI] [PubMed] [Google Scholar]
- 32.Roussel J, Thireau J, Brenner C, Saint N, Scheuermann V, Lacampagne A, Le Guennec JY, Fauconnier J. Palmitoyl-carnitine increases RyR2 oxidation and sarcoplasmic reticulum Ca2+ leak in cardiomyocytes: Role of adenine nucleotide translocase. Biochim Biophys Acta. 2015;1852:749–758. doi: 10.1016/j.bbadis.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Y, Zhong P, He S, Zhang Y, Tang L, Ling Y, Fu S, Tang Y, Yang P, Luo T, Chen B, Chen A, Wang X. Trimetazidine protects cardiomyocytes against hypoxia/reoxygenation injury by promoting AMP-activated protein kinase-dependent autophagic flux. J Cardiovasc Pharmacol. 2017;69:389–397. doi: 10.1097/FJC.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 34.Ren W, Sun Y, Du K. Glut4 palmitoylation at Cys223 plays a critical role in Glut4 membrane trafficking. Biochem Biophys Res Commun. 2015;460:709–714. doi: 10.1016/j.bbrc.2015.03.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auranen M, Palmio J, Ylikallio E, Huovinen S, Paetau A, Sandell S, Haapasalo H, Viitaniemi K, Piirila P, Tyynismaa H, Udd B. PFKM gene defect and glycogen storage disease GSDVII with misleading enzyme histochemistry. Neurol Genet. 2015;1:e7. doi: 10.1212/NXG.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walters AM, Porter GA Jr, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 39.Motloch LJ, Gebing T, Reda S, Schwaiger A, Wolny M, Hoppe UC. UCP3 regulates single-channel activity of the cardiac mCa1. J Membr Biol. 2016;249:577–584. doi: 10.1007/s00232-016-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA, O’Brien WE, Wood PA. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A. 1998;95:15592–15597. doi: 10.1073/pnas.95.26.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JJ, Miura R. Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences. Eur J Biochem. 2004;271:483–493. doi: 10.1046/j.1432-1033.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 42.Board M, Humm S, Newsholme EA. Maximum activities of key enzymes of glycolysis, glutaminolysis, pentose phosphate pathway and tricarboxylic acid cycle in normal, neoplastic and suppressed cells. Biochem J. 1990;265:503–509. doi: 10.1042/bj2650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCommis KS, Finck BN. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J. 2015;466:443–454. doi: 10.1042/BJ20141171. [DOI] [PMC free article] [PubMed] [Google Scholar]