Abstract
Cystic echinococcosis (CE), as a zoonotic helminthic infection, imposes a large socioeconomic burden to societies throughout the world. This study aimed to analyze the demographic, clinical, diagnostic and therapeutic data of CE patients across all provinces in Iran. In this cross-sectional study, the mentioned data were routinely collected by provincial medical universities during the time from March 2016 to March 2017. The provincial population census was used to calculate the prevalence of CE per 1,000,000 populations for all provinces. T test and Chi squared test were used to compare variables between genders. Statistical analysis was done at 95% significant level using STATA 14 software. The overall prevalence of CE was 6.8 cases per 1,000,000 populations in Iran. The highest and lowest prevalence was reported for Northeast (15.2) and southeast (0.7) of Iran, respectively. There was a significant difference in the prevalence between male and female (5.8 vs. 7.9, p < 0.001). An increasing trend of the prevalence was found by age in both males and females. Abdominal pain was reported as the highest proportion of symptom (39.0%) and the liver was a commonly infected organ (62.7%). CT scan (39.0%) and MRI (1.9%) had the highest and lowest proportion among types of CE diagnosis, respectively. About 67% of the patients washed the vegetable by water without any disinfectant. Due to global efforts on the control and prevention of CE, it still remains endemic in many countries throughout the world. Incidence trend of CE in Iran showed being endemic for CE and more investigations are needed on all aspects of the disease.
Keywords: Echinococcus, Cystic echinococcosis, Epidemiology, Prevalence, Iran
Introduction
Echinococcus granulosus, a tapeworm belonged to Taeniidae family, causes cystic echinococcosis (CE) or hydatidosis throughout the world (Thompson 2017). CE, as a zoonotic helminthic infection, imposes a large socioeconomic burden to societies, especially in developing countries, including Iran (Sadjjadi 2006; Rokni 2009; Deplazes et al. 2017). Larval stage (metacestode) of this parasite could be dwell in the visceral organs of intermediate hosts while the adult worm is in the intestine of canine-definitive hosts. Domestic or wild ungulate (accidentally human), as intermediate hosts form cysts in their organs after eating eggs of the parasite (Thompson 2017).
CE patients face different clinical syndromes that are dependent on cyst size and involved organ. Infection with CE can cause morbidity and mortality if left untreated (Eckert and Deplazes 2004; Mansour-Ghanaei et al. 2012; Budke et al. 2013). Molecular and phylogenetic studies on E. granulosus showed 10 genotypes of this parasite, G1–G3 (common sheep strains), G4 (pig strain), G5 (cattle strain), G6–G10 (Nakao et al. 2006). The latest group previously, named E. canadensis but the taxonomy of this is controversial (Thompson 2008). In Iran, G1–G3 and G6 have been reported from human (Sadjjadi 2006; Nikmanesh et al. 2014; Rostami et al. 2015)and genotypes, G1, G3, G4, G5, G6, and G7 have been reported from different herbivore intermediate hosts (Rokni 2009; Sharbatkhori et al. 2010, 2011; Sharafi et al. 2014; Fadakar et al. 2015; Ebrahimipour et al. 2017b; Matini et al. 2018).
In recent years, the diagnosis of CE is based on the integration of imaging and serological techniques (McManus et al. 2012; Hadipour et al. 2016). The decision for the effective management of CE patients is strongly dependent on location, severity and life-threatening of the cyst. Watch and wait, chemotherapy, and surgery could be used for treatment (McManus et al. 2012; Larrieu et al. 2018).
As an endemic area for CE, studies on epidemiological aspects are very important for combating this disease in Iran. These finding could be used in evidence-based control and management programs (Craig et al. 2017). Epidemiology of CE have stressed out in many studies in Iran but the data are not updated.
This study aimed to analyze the demographic and diagnostic data of CE patients in Iran. Descriptive and analytical statistics were done on the mentioned data collected from all provinces of Iran during the time from March 2016 to March 2017.
Methods
Data collection
In this cross-sectional study, we used CE data from all provinces of Iran during the time from March 2016 to March 2017.
Geographical, occupational data, and diagnostic techniques and demographic data (age and gender of CE cases) are routinely collected by provincial medical universities.
Population and geographical regions
The data on gender and age distribution of the population from all provinces were obtained from the 2016 Population and Housing Censuses of Iran. Age was classified into three categories of 1–19, 20–44, and ≥ 45 years.
All provinces of Iran were divided into eight geographical regions including the northwest (i.e., Ardebil, East Azarbaijan, Gilan, West Azarbaijan, Zanjan), west (i.e., Hamadan, Ilam, Kermanshah, Kordestan, Lorestan), central (i.e., Alborz, Qazvin, Qom, Tehran), southeast (i.e., Hormozgan, Kerman, Sistan-va-Baluchestan), southwest (i.e., Chaharmahal-va-Bakhtiari, Esfahan, Khuzestan, Markazi), north (i.e., Golestan, Mazandaran, Semnan), northeast (North Khorasan, Razavi khorasan, South khorasan), and south (i.e., Bushehr, Fars, Kohgiluyeh-va-Buyer Ahmad, Yazd).
Statistical analysis
We used the provincial population census (as the denominator) to calculate the prevalence of CE per 1,000,000 populations for all provinces and eight geographical regions. The overall prevalence was also reported by gender and age groups. The Cochran-Armitage test was used for trend analysis according to age groups and monthly trend. Descriptive statistics were done as number (%) and mean (standard deviation) for qualitative and quantitative variables, respectively. T test and Chi squared test were used to compare variables between genders. Statistical analysis was done at 95% significant level using STATA 14 software.
Results
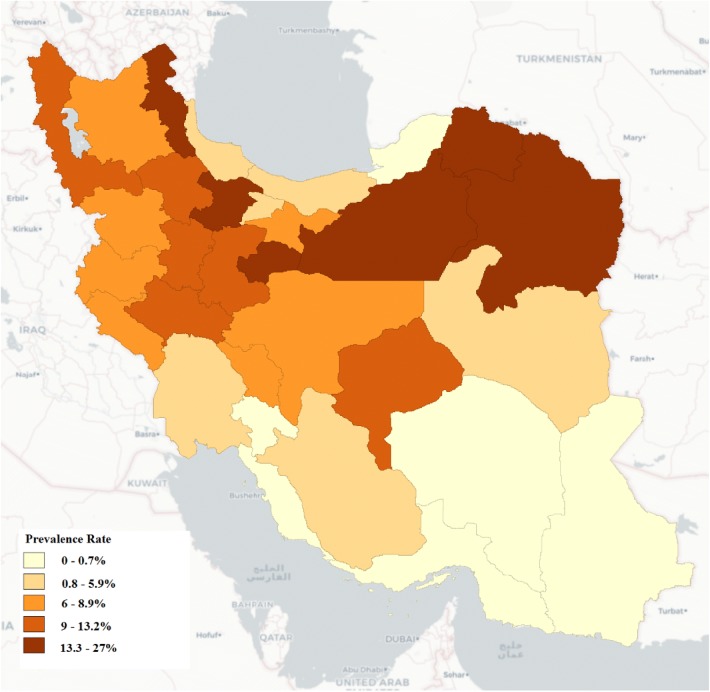
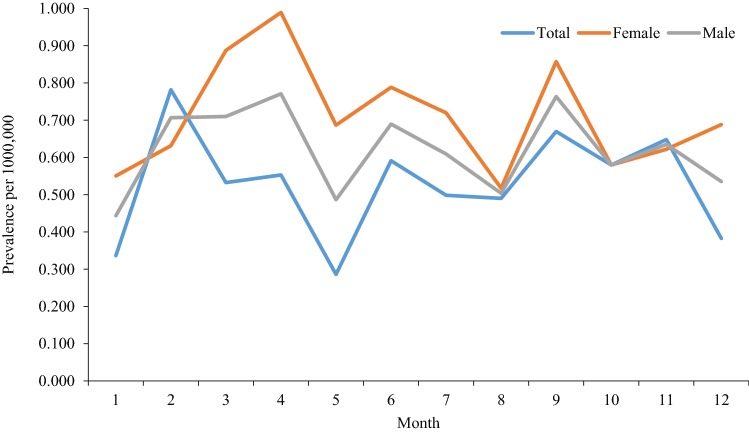
The prevalence rates of Human Cystic Echinococcosis per 1,000,000 populations across the Provinces and by eight geographical regions are shown in Table 1. The overall prevalence of CE was 6.8 cases per 1,000,000 populations in Iran in 2016. The highest prevalence was reported for Northeast (15.2) and Northwest (9.9) of Iran, respectively (Fig. 1). The lowest prevalence of 0.7 per 1,000,000 was for Southeast of the country. There was a significant difference in the prevalence between males and females (5.8 vs. 7.9, p < 0.001). An increasing trend of the prevalence was found by age in both males and females (Table 2). Figure 2 shows a stable trend of monthly prevalence of CE by sex in 2016, but there is a slight difference between genders.
Table 1.
The prevalence rate of human cystic echinococcosis per 1,000,000 populations across the Provinces in Iran
| Provinces | No. | Population | Prevalence | |
|---|---|---|---|---|
| Northwest | Ardebil | 35 | 1,302,701 | 26.9 |
| East Azarbaijan | 33 | 3,892,407 | 8.5 | |
| Gilan | 5 | 2,589,706 | 1.9 | |
| West Azarbaijan | 32 | 3,217,514 | 10.0 | |
| Zanjan | 14 | 1,059,425 | 13.2 | |
| Total | 119 | 12,061,752 | 9.9 | |
| West | Hamadan | 17 | 1,836,337 | 9.3 |
| Ilam | 4 | 580,722 | 6.9 | |
| Kermanshah | 14 | 2,032,527 | 6.9 | |
| Kurdistan | 13 | 1,561,671 | 8.3 | |
| Lorestan | 17 | 1,828,489 | 9.3 | |
| Total | 65 | 7,839,747 | 8.3 | |
| Central | Tehran | 77 | 12,720,950 | 6.0 |
| Alborz | 6 | 2,519,078 | 2.4 | |
| Qazvin | 20 | 1,255,615 | 15.9 | |
| Qom | 17 | 1,200,682 | 14.2 | |
| Total | 120 | 17,696,325 | 6.8 | |
| Southeast | Hormozgan | No data | ||
| Kerman | 2 | 3,068,409 | 0.7 | |
| Sistan-va-Baluchestan | No data | |||
| Total | 2 | 3,068,409 | 0.7 | |
| Southwest | Chaharmahal-va-Bakhtiari | 8 | 933,864 | 8.6 |
| Isfahan | 32 | 5,093,089 | 6.3 | |
| Khuzestan | 4 | 4,732,099 | 0.9 | |
| Markazi | 13 | 1,475,348 | 8.8 | |
| Total | 57 | 12,234,399 | 4.7 | |
| North | Golestan | 1 | 1,852,032 | 0.5 |
| Mazandaran | 8 | 3,209,666 | 2.5 | |
| Semnan | 13 | 659,198 | 19.7 | |
| Total | 22 | 5,720,896 | 3.9 | |
| Northeast | North Khorasan | 12 | 902,473 | 13.3 |
| Razavi Khorasan | 103 | 6,262,380 | 16.5 | |
| South Khorasan | 4 | 690,588 | 5.8 | |
| Total | 119 | 7,855,442 | 15.2 | |
| South | Bushehr | No data | ||
| Fars | 12 | 4,802,728 | 2.5 | |
| Kohgiluyeh-va-Buyer Ahmad | No data | |||
| Yazd | 11 | 1,122,206 | 9.8 | |
| Total | 23 | 5,924,934 | 3.9 | |
| Total | 533 | 78,460,246 | 6.8 | |
Fig. 1.
Map depicting prevalence rate of human cystic echinococcosis per 1,000,000 populations across the provinces in Iran
Table 2.
The prevalence rate of human cystic echinococcosis by sex and age group in Iran in 2016
| Age | Cases | Population (× 1000) | Prevalence (per 1,000,000) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | Female | Male | P value | ||
| 1–19 | 34 | 41 | 75 | 12,390 | 12,810 | 25,200 | 2.7 | 3.2 | 0.506 | 3.0 |
| 20–44 | 127 | 98 | 225 | 18,007 | 18,165 | 36,172 | 7.1 | 5.4 | 0.045 | 6.2 |
| + 45 | 144 | 89 | 144 | 8400 | 8505 | 16,905 | 17.1 | 10.5 | < 0.001 | 8.5 |
| P-trend | – | – | – | – | – | – | < 0.001 | < 0.001 | – | < 0.001 |
| Total | 305 | 228 | 533 | 38,797 | 39,480 | 78,277 | 7.9 | 5.8 | < 0.001 | 6.8 |
Fig. 2.
Trend of monthly prevalence of human cystic echinococcosis by sex in Iran in 2016
Abdominal pain was reported as the highest proportion of symptom (39.0%) among the population and the liver was a common organ (62.7%) which infected by CE (Table 3). The frequency of CE by the type of diagnosis and treatment are shown in Table 4. CT scan (39.0%) and MRI (1.9%) had the highest and lowest proportion among types of CE diagnosis, respectively. More than half of the patients were treated by both surgery and drug therapy. The proportion of death was 1.3% in the patients which all of them were female. About 67% of the patients washed the vegetable by water without any disinfectant liquid (Table 5).
Table 3.
Frequency of human cystic echinococcosis by symptom and infected organ in Iran
| No. | % | ||
|---|---|---|---|
| Symptom | Hepatomegaly | 41 | 7.7 |
| Abdominal pain (AP) | 208 | 39.0 | |
| Hepatomegaly and AP | 77 | 14.4 | |
| Cough | 43 | 8.1 | |
| Chest pain | 33 | 6.2 | |
| Cough and Chest pain | 49 | 9.2 | |
| AP and Chest pain | 59 | 11.1 | |
| AP and Cough | 23 | 4.3 | |
| Organs | Lungs | 99 | 18.6 |
| Abdominal | 19 | 3.5 | |
| Liver | 334 | 62.7 | |
| Spleen | 10 | 1.9 | |
| Kidney | 8 | 1.5 | |
| More than two organs | 57 | 10.7 | |
| Another organ | 6 | 1.1 |
Table 4.
Frequency of human cystic echinococcosis by type of diagnosis and treatment in Iran
| No. | % | ||
|---|---|---|---|
| Type of diagnosis | MRI | 10 | 1.9 |
| During surgery | 27 | 5.1 | |
| Radiotherapy | 123 | 23.0 | |
| CT | 192 | 36.0 | |
| Sonography | 41 | 7.7 | |
| Combine methods | 140 | 26.3 | |
| Type of treatment | Surgery | 197 | 37.0 |
| Drug therapy | 68 | 12.8 | |
| Both | 268 | 50.2 | |
| Treatment outcome | Improved | 526 | 98.7 |
| Death | 7 | 1.3 |
Table 5.
Frequency of human cystic echinococcosis by behavior factors
| No. | % | ||
|---|---|---|---|
| Washing vegetables | Water | 357 | 67.0 |
| Water and disinfectant | 25 | 4.7 | |
| Water and scourer | 130 | 24.4 | |
| Three methods | 21 | 3.9 | |
| Contact animal | No | 275 | 51.6 |
| Yes | 258 | 48.4 |
The result of gender differences is shown in Table 6. A significant difference was found between males and females according to mean age, contact animal, and treatment outcome.
Table 6.
Result of gender differences by the variables
| Female | Male | P value | ||
|---|---|---|---|---|
| Age | Mean ± SD | 42.7 ± 19.3 | 39.1 ± 20.7 | 0.041 |
| Contact animal | No | 174 (57.1) | 101 (44.3) | 0.004 |
| Yes | 131 (42.9) | 127 (55.7) | ||
| Treatment outcome | Improved | 298 (97.7) | 228 (100.0) | 0.022 |
| Death | 7 (2.3) | 0 (0.0) | ||
| Place | Urban | 183 (60.2) | 131 (58.0) | 0.605 |
| Rural | 121 (39.8) | 95 (42.0) |
Discussion
In spite global efforts on control and management of CE, it remains endemic and even hyper endemic in many countries throughout the world like Iran (Craig et al. 2017; Deplazes et al. 2017). Given the demographic and geographical differences between and among countries, different aspects of CE including epidemiological and clinical status should be more investigated in each region (Ebrahimipour et al. 2019). Remaining of CE could be a detriment not only as medical but also as the veterinary industry in the endemic regions (Budke et al. 2017; Motazedian et al. 2018).
As an endemic area for CE, the incidence rate of this infection remains high in Iran from 1995 to 2017. In addition, the trend of CE incidence showed the CE as important health threatening infection in Iran (Khademvatan et al. 2018; Khalkhali et al. 2018). Accordingly, the main source of infection for human and animal hosts should be more emphasized in this country.
In this study, the rate of infection varied from 0.5 in Golestan Province to 26.9 per 1,000,000 populations in Ardabil Province (Table 1). The high prevalence of CE in northeast and northwest of Iran is in line with the previous studies conducted in Iran (Rokni 2009; Andalib Aliabadi et al. 2015; Khalkhali et al. 2018). A similar study conducted in Khorasan Razavi Province, Northeast of Iran, from 2011 to 2014 showed a high prevalence of CE which conclude that it should be considered as an alarm for health policy-makers (Khazaei et al. 2016).
As with previous studies in Iran, human CE could more occur in females. Housewives are more infected with CE. It could be due to consuming raw and unwashed vegetable infected with parasite eggs (Rokni 2009; Moshfe et al. 2018). Contacting with mouth during washing of vegetables is considered as an important risk factor for this groups (Thompson 2008).
In the recent study, an increasing trend of the prevalence was found by age in both males and females. The highest rate of CE infection was found in over 45-year group that is in concordant with previous studies that showed the highest in 20–40 year age group (Rokni 2009; Sarkari et al. 2010).
As with previous studies, the liver was the most infected organ in human CE in Iran (Sadjjadi 2006; Rokni 2009; Sarkari et al. 2010; Chalechale et al. 2016). The highest complaint of patients was abdominal pain. CT scan was the most used diagnostic method for human diagnosis in the current study. Previous studies in Iran showed sonography, CT scan, and X-ray as more frequent methods for diagnosis of human CE. (Ebrahimipour et al. 2017a; Khalkhali et al. 2018) It was showed this method in concomitant with sonography were most used diagnostics methods (Rokni 2009).
Surgery and using Albendazole were used for treatment. It is emphasized that only in life-threatening cyst surgical should be done. Watch and wait and no necessity for treatment is needed in above 60% of CE cases. This is needed especially in liver cysts (McManus et al. 2012; Larrieu et al. 2018).
Nowadays, the diagnosis of CE is based on the integration of imagining and serological techniques. Serological techniques used as complementary, especially in cases with cyst lesions. Due to no responses of serology in the initial (CE1) and calcifying (CE4 and CE5) cysts, relying just on these techniques, couldn’t be satisfactory. (Kern et al. 2017; Siles-Lucas et al. 2017). In Iran, most surgeons decided based on just imaging without considering serological techniques.
The finding of this study showed a total of 67% of cases washed vegetables only with water. Washing and disinfecting were used just in 4.7% of infected persons. Many important risk factors were considered for getting the disease in the endemic areas (Rokni 2009; Khazaei et al. 2016; Thompson 2017). The finding of the recent study indicated most CE cases did not use disinfectants for washing the vegetables. Health education about properly washing of vegetable could have an effect on decreasing of CE prevalence in endemics. Hence, focusing on improvement in health educations in high-risk groups such as housewives, farmers, and ranchers could be more effective (Pozio 2003; Daryani et al. 2008; Abougrain et al. 2010; Romig et al. 2017).
High prevalence of CE in other occupations like students, farmers, ranchers, and other groups in endemic areas including Iran, showed that this infection should be considered as important health-threatening for all peoples in both rural and urban dwellers. Although living in lower hygiene areas where contacting with infected dogs and infected foods in more, the probability of getting the infection is more, but all individuals in endemic areas are in danger. This is due to the transmission patterns of this infection (Sadjjadi 2006; Rokni 2009; Romig et al. 2017). Although this study provide critical information to authorities for management of CE, it does have its limitation. The most noticeable limitation is short review period of CE cases.
Conclusion
Due to global efforts on control and management of CE, it is endemic and even hyper endemic in many countries throughout the world. Different aspects of CE including epidemiological and clinical should be more investigated in each region. Trend of CE incidence in Iran showed being endemic for CE and more investigations are needed on all aspects of the disease.
Acknowledgements
This study was funded by AJA University of Medical Sciences, Tehran, Iran. The authors acknowledge the personnel of Zoonosis Control Department, Iran Ministry of Health for their useful collaboration.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abougrain AK, Nahaisi MH, Madi NS, Saied MM, Ghenghesh KS. Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 2010;21:760–762. doi: 10.1016/j.foodcont.2009.11.005. [DOI] [Google Scholar]
- Andalib Aliabadi Z, Berenji F, Fata A, Jarahi L. Human hydatidosis/echinococosis in north eastern Iran from 2003 to 2012. Iran J Parasitol. 2015;10:658–662. [PMC free article] [PubMed] [Google Scholar]
- Budke CM, et al. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am J Trop Med Hyg. 2013;88:1011–1027. doi: 10.4269/ajtmh.12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke CM, Casulli A, Kern P, Vuitton DA. Cystic and alveolar echinococcosis: successes and continuing challenges. PLoS Negl Trop Dis. 2017;11:e0005477. doi: 10.1371/journal.pntd.0005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalechale A, Hashemnia M, Rezaei F, Sayadpour M. Echinococcus granulosus in humans associated with disease incidence in domestic animals in Kermanshah, west of Iran. J Parasit Dis. 2016;40:1322–1329. doi: 10.1007/s12639-015-0681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig PS, Hegglin D, Lightowlers MW, Torgerson PR, Wang Q. Echinococcosis: control and prevention. Adv Parasitol. 2017;96:55–158. doi: 10.1016/bs.apar.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Daryani A, Ettehad G, Sharif M, Ghorbani L, Ziaei H. Prevalence of intestinal parasites in vegetables consumed in Ardabil. Iran Food control. 2008;19:790–794. doi: 10.1016/j.foodcont.2007.08.004. [DOI] [Google Scholar]
- Deplazes P, et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Ebrahimipour M, Budke CM, Najjari M, Yaghoobi K. Surgically managed human cystic echinococcosis in north-eastern Iran: a single center’s experience from 2001 to. J Parasit Dis. 2017;41:883–887. doi: 10.1007/s12639-017-0911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimipour M, Sadjjadi SM, Yousofi Darani H, Najjari M. Molecular studies on cystic echinococcosis of camel (Camelus dromedarius) and report of Echinococcus ortleppi in Iran. Iran J Parasitol. 2017;12:323–331. [PMC free article] [PubMed] [Google Scholar]
- Ebrahimipour M, Afgar A, Barati M, Mohammadi MA, Harandi MF. Evaluation of the antigenic epitopes of EgAgB/1 and EgAgB/4 subunit antigens in G1 and G6 genotypes of Echinococcus granulosus using bioinformatics. Gene Rep. 2019 [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadakar B, Tabatabaei N, Borji H, Naghibi A. Genotyping of Echinococcus granulosus from goats and sheep indicating G7 genotype in goats in the Northeast of Iran. Vet Parasitol. 2015;214:204–207. doi: 10.1016/j.vetpar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Hadipour M, Nazari M, Sanei B, Ghayour Z, Sharafi SM, Yazdani H, Darani HY. Immunological diagnosis of human hydatid cyst using Western immunoblotting technique. J Res Med Sci. 2016;21:130. doi: 10.4103/1735-1995.196612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, Menezes da Silva A, Akhan O, Mullhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. 2017;96:259–369. doi: 10.1016/bs.apar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Khademvatan S, Majidiani H, Foroutan M, Hazrati Tappeh K, Aryamand S, Khalkhali HR. Echinococcus granulosus genotypes in Iran: a systematic review. J Helminthol. 2018 doi: 10.1017/S0022149X18000275. [DOI] [PubMed] [Google Scholar]
- Khalkhali HR, Foroutan M, Khademvatan S, Majidiani H, Aryamand S, Khezri P, Aminpour A. Prevalence of cystic echinococcosis in Iran: a systematic review and meta-analysis. J Helminthol. 2018;92:260–268. doi: 10.1017/S0022149X17000463. [DOI] [PubMed] [Google Scholar]
- Khazaei S, et al. Epidemiological and clinical characteristics of patients with hydatid cysts in Khorasan Razavi Province, from 2011 to 2014. Iran J Parasitol. 2016;11:364–370. [PMC free article] [PubMed] [Google Scholar]
- Larrieu E, Uchiumi L, Salvitti JC, Sobrino M, Panomarenko O, Tissot H, Herrero E. Epidemiology, diagnosis, treatment and follow-up of cystic echinococcosis in asymptomatic carriers. Trans R Soc Trop Med Hyg. 2018;113(2):74–80. doi: 10.1093/trstmh/try112. [DOI] [PubMed] [Google Scholar]
- Mansour-Ghanaei F, Joukar F, Soati F, Javadi M. Clinical features of hydatid disease in Guilan (the North Province of Iran): a ten-year study. Arch Clin Infect Dis. 2012;7:119–123. doi: 10.5812/archcid.15089. [DOI] [Google Scholar]
- Matini M, Roostaei M, Fallah M, Maghsood AH, Saidijam M, Fasihi Harandi M. Genetic identification of Echinococcus granulosus isolates in Hamadan, Western Iran. Iran J Parasitol. 2018;13:423–429. [PMC free article] [PubMed] [Google Scholar]
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ. 2012;344:e3866. doi: 10.1136/bmj.e3866. [DOI] [PubMed] [Google Scholar]
- Moshfe A, et al. Seroepidemiological study of cystic echinococcosis in nomadic communities in the southwest of Iran: a population-based study. J Immunoassay Immunochem. 2018 doi: 10.1080/15321819.2018.1547974. [DOI] [PubMed] [Google Scholar]
- Motazedian M, Najjari M, Zarean M, Karimi G, Karimazar M, Ebrahimipour M. An abattoir survey of hydatid and liver fluke disease in slaughtered cattle in Alborz Province, Iran. Comp Clin Pathol. 2018 [Google Scholar]
- Nakao M, McManus D, Schantz P, Craig P, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2006;134:713–722. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- Nikmanesh B, et al. Genotyping of Echinococcus granulosus isolates from human clinical samples based on sequencing of mitochondrial genes in Iran, Tehran. Iran J Parasitol. 2014;9:20–27. [PMC free article] [PubMed] [Google Scholar]
- Pozio E. Foodborne and waterborne parasites. Acta Microbiol Pol. 2003;52:83–96. [PubMed] [Google Scholar]
- Rokni MB. Echinococcosis/hydatidosis in Iran. Iran J Parasitol. 2009;4:1–16. [Google Scholar]
- Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, de la Rue M (2017) Ecology and life cycle patterns of Echinococcus species. In: Thompson RCA, Deplazes P, Lymbery AJ (eds) Advances in parasitology, vol 95. Academic Press, pp 213–314 [DOI] [PubMed]
- Rostami S, Shariat Torbaghan S, Dabiri S, Babaei Z, Ali Mohammadi M, Sharbatkhori M, Fasihi Harandi M. Genetic characterization of Echinococcus granulosus from a large number of formalin-fixed, paraffin-embedded tissue samples of human isolates in Iran. Am J Trop Med Hyg. 2015;92:588–594. doi: 10.4269/ajtmh.14-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006;55(Suppl):S197–S202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Sarkari B, Sadjjadi SM, Beheshtian MM, Aghaee M, Sedaghat F. Human cystic echinococcosis in Yasuj District in Southwest of Iran: an epidemiological study of seroprevalence and surgical cases over a ten-year period. Zoonoses Public Health. 2010;57:146–150. doi: 10.1111/j.1863-2378.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- Sharafi SM, Rostami-Nejad M, Moazeni M, Yousefi M, Saneie B, Hosseini-Safa A, Yousofi-Darani H. Echinococcus granulosus genotypes in Iran. Gastroenterol Hepatol Bed Bench. 2014;7:82–88. [PMC free article] [PubMed] [Google Scholar]
- Sharbatkhori M, et al. Echinococcus granulosus genotypes in livestock of Iran indicating high frequency of G1 genotype in camels. Exp Parasitol. 2010;124:373–379. doi: 10.1016/j.exppara.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Sharbatkhori M, Fasihi Harandi M, Mirhendi H, Hajialilo E, Kia EB. Sequence analysis of cox1 and nad1 genes in Echinococcus granulosus G3 genotype in camels (Camelus dromedarius) from central Iran. Parasitol Res. 2011;108:521–527. doi: 10.1007/s00436-010-2092-7. [DOI] [PubMed] [Google Scholar]
- Siles-Lucas M, Casulli A, Conraths FJ, Müller N (2017) Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. In: Thompson RCA, Deplazes P, Lymbery AJ (eds) Advances in parasitology, vol 96. Academic Press, pp 159–257 [DOI] [PubMed]
- Thompson RC. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Thompson RCA. Biology and systematics of Echinococcus. Adv Parasitol. 2017;95:65–109. doi: 10.1016/bs.apar.2016.07.001. [DOI] [PubMed] [Google Scholar]