Abstract
Purpose of review
One of the most vexing problems for gastroenterologists is what actions to take after receiving a histological diagnosis of gastric intestinal metaplasia. We approach the problem by starting with suggesting a biopsy protocol that ensures obtaining the biopsies required for diagnosis, assessing the status of the gastric mucosa, and effective communication with the pathologist and patient.
Recent findings
The rediscovery and integration of the long history of gastric damage and repair resulting in pseudopyloric metaplasia (called SPEM) into the thinking of investigators working with animal models of gastric cancer has resulted in improved ability to separate changes associated with benign repair from those associated with inflammation-associated gastric carcinogenesis.
Summary
Gastric intestinal metaplasia is a potential reversible product of injury and repair and not directly connected with carcinogenesis. Intestinal metaplasia is a biomarker for prior gastric injury and repair. The risk of gastric cancer is best assessed in relation to the severity, extent, and, most importantly, the cause of the atrophic changes.
Keywords: atrophy, autoimmune gastritis, cancer, gastric, gastric biopsy, Helicobacter pylori, injury and repair, intestinal metaplasia, pseudopyloric metaplasia, risk assessment, spasmolytic polypeptide-expressing metaplasia
INTRODUCTION
Only 50 years ago, gastric adenocarcinoma was the most common cause of cancer death in many western countries [1,2]. Although it has declined considerably, its incidence remains high in many parts of the world. The discovery that gastric cancer was caused by infection with Helicobacter pylori led to the new paradigm ‘no H. pylori, no gastric cancer’ and has made eradication of H. pylori a basic strategy for primary cancer prevention [3]. With few exceptions, it is now accepted that the discovery of H. pylori infection should prompt treatment and that a search and treat approach is reasonable in potential high-risk groups [4,5]. H. pylori can cause progressive damage and genetic instability such that H. pylori eradication per se does not automatically equate with elimination of gastric cancer risk. Here we discuss how to identify and manage patients in relation to the risk for gastric cancer after H. pylori eradication. We focus on practical advice for those patients where there is the most confusion (i.e. those in whom intestinal metaplasia was identified in at least one gastric biopsy). There are a large number of recently published articles, symposia, and guidelines [6■■,7■■,8,9■,10,11,12■■,13■,14■]. The American Gastrointestinal Institute has also announced it will soon publish a new technical review on gastric metaplasia.
INTESTINAL METAPLASIA IN THE PATHOGENESIS OF GASTRIC CANCER
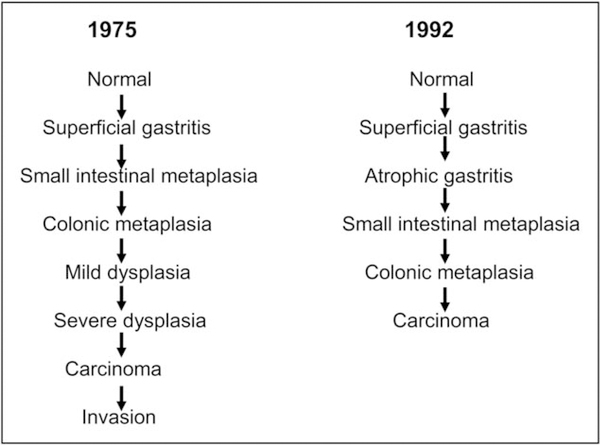
After more than 70 years of research, the progression of normal gastric mucosa to gastric cancer was described as a multistep, multifactorial progression. The sequence is frequently termed the Correa cascade and includes intestinal metaplasia as a precursor for gastric cancer (Fig. 1) [15,16]. The original versions of the cascade focused on nitrites but provided a useful frame for subsequent studies and hypothesis testing. However, several of its elements, such as atrophy being multifocal and intestinal metaplasia directly leading to cancer, are no longer accepted [14■]. For example, Correa-stained gastric gross specimens for sucrase activity (associated with intestinal metaplasia) to determine the extent of gastric atrophy. The multifocal distribution of the stain reflected the extent of intestinalized mucosal islands within a lawn of pseudopyloric metaplasia described as an atrophic front [1]. The placement of intestinal metaplasia immediately before gastric cancer in the cascade model resulted in the widespread misconception that intestinal metaplasia was preneoplastic and directly progressed to gastric cancer. This notion was then often uncritically incorporated in the body of knowledge of both gastroenterologists and pathologists, making intestinal metaplasia a feared entity that requires management. This produced one of the most vexing problems encountered by gastroenterologists: what to tell patients and what should be done when a pathology report notes the presence of intestinal metaplasia in a gastric biopsy.
FIGURE 1.
Correa cascades from 1975 and updated version from 1992. The term ‘small intestinal metaplasia’ is used as a synonym for ‘complete type’ or ‘type II’ intestinal metaplasia; ‘colonic metaplasia’ indicates ‘incomplete type’ or ‘type III’ intestinal metaplasia.
The main goal of this review is to provide evidence-based yet practical advice regarding the management of intestinal metaplasia, including recommendations for specimen collection and suggestions for improving the communication with pathologists and patients. The first step is to place intestinal metaplasia in its in-vivo context of gastric atrophy and learn to decode pathology reports that mention intestinal metaplasia.
TOPOGRAPHY AND HISTOLOGY OF THE NORMAL STOMACH
The stomach can be divided in two main and one minor mucosal regions, distinguished by their gross appearance, histology, and functional profile. The mucosa of the gastric body (oxyntic mucosa, grossly structured as gastric folds) superficially consists of pits (‘foveolae’) in which mucus-secreting columnar epithelia transition into pariet al cells that are responsible for the secretion of acid, enterochromaffin-like cells that secrete histamine, and chief cells that secrete pepsin and intrinsic factor. The oxyntic mucosa gradually transitions into the funnel-shaped antral area of the distal (antral and pyloric) stomach. In antral mucosa, pariet al and chief cells are rare and the mucosa primarily consists of coiled tubular mucus-secreting glands along with an endocrine cell population (the gastrin-secreting G cells and the somatostatin-secreting D cells), which play pivotal roles in the regulation of acid secretion. The most proximal region is the cardia, a short (typically <2 cm) tubular segment bridging the esophageal squamous with the oxyntic mucosa.
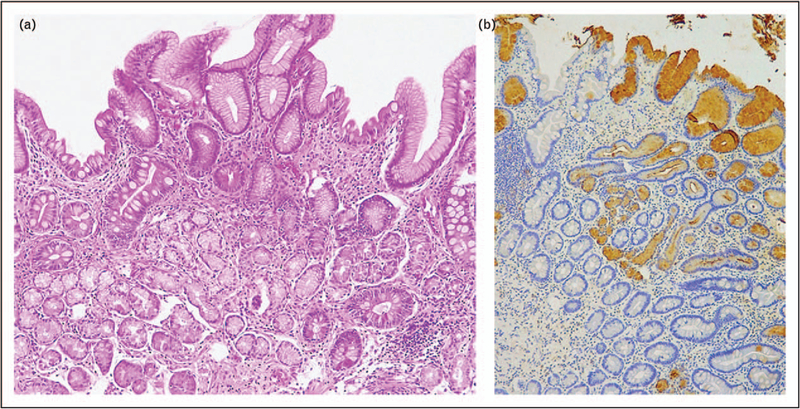
The gastric epithelium is resistant to acid and digestive juices and rapidly repairs any injury, whether chemical (e.g. NSAIDs), physical (biopsy), or inflammatory. The reparative process is similar to that seen in the intestine, except for the location of its proliferative compartment, which in the body of the stomach lies at the glandular necks [9■]. The regenerated mucosa typically assumes the phenotype of the pyloric (mucosecreting) epithelia and expresses trefoil factor II (TFF2 or spasmolytic peptide) (Fig. 2). The new metaplastic mucosa, which in humans is called pseudopyloric mucosa, mucus metaplasia, or pyloric metaplasia, recapitulates the antral mucosa and is considered as reliable marker of oxyntic mucosa repair [14■,17]. In animal studies, the reparative mucosa is known as spasmolytic peptide expressing mucosa (SPEM) [7■■]. Depending on the etiological agent, its presence can be either temporary or permanent. The depth, chronicity, and extent of the damage/inflammation associated with pseudopyloric metaplasia determine whether it progresses to further metaplastic transformation by expressing goblet cells similar to those seen in the intestine and become intestinal metaplasia. Although the repair process of the oxyntic mucosa may result in the recovery of its native commitment, residual islands of metaplastic mucosa may persist possibly indefinitely [14■]. These patches, which do not contain native gastric mucosa, are the morphologic and functional expression of gastric atrophy [18].
FIGURE 2.
Biopsy sample obtained from antral-appearing oxyntic mucosa. Intestinalized glands coexist with extensive pseudopyloric metaplasia (SPEM). The latter is stained brown by the TFF2 immunohistochemical stain, which stain both SPEM and superficial epithelia. Intestinalized glands do not show a TFF2-positive immunoreaction (a: H&E, original magnification 10×; (b) TFF2 immunohistochemical staining; original magnification 5×).
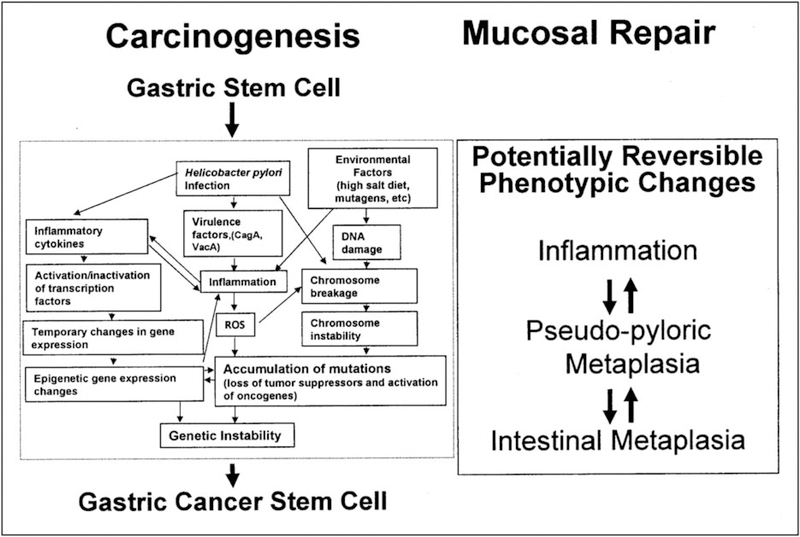
Both observational and experimental evidence has now made clear that intestinal metaplasia is reversible and, crucially, does not progress directly to gastric cancer (Fig. 3) [3,14■]. Rather, its presence is a biomarker for prior gastric injury and repair. Gastric cancer risk is associated with the severity, extent, and, most importantly, the cause of the atrophic changes. In specific circumstances, it may signal an increased risk for the development of epithelial (adenocarcinoma) or neuroendocrine (carcinoid) neoplasms [14■].
FIGURE 3.
Helicobacter pylori-associated gastric cancer is an inflammation associated cancer in which genetic instability is exaggerated by H. pylori and environmental factors. Metaplastic gastric epithelium (pseudopyloric and intestinal) reflects mucosal damage independent of whether is or is not associated with a gastric cancer risk. In H. pylori infection, it is best considered as a biomarker of potential cancer risk. Adapted with permission from [14■].
The discovery of intestinal metaplasia in a gastric biopsy specimen should prompt the clinical question of whether it is a small remnant of a prior repair process and, therefore, clinically insignificant, or the harbinger of possible harm. In the latter case, an assessment of future risk for gastric cancer (risk stratification) is needed.
INTESTINAL METAPLASIA IS BEST EVALUATED BY THE COMPANY IT KEEPS
Gastric atrophy is defined as any type of loss of the native glands and has two main causes: H. pylori infection and autoimmunity [18]. Each of them is expressed by a distinct topographic distribution of atrophy and is associated with different levels and types of cancer [19,20].
H. pylori causes long-standing, nonself-limiting inflammation and progressive mucosal damage initially largely restricted to the antrum, an area where the organisms can interact with both surface and foveolar cells. In the corpus, the highly concentrated acid produced by the oxyntic mucosa effectively restricts H. pylori to the surface epithelium [21]. However, any impairment of acid secretion, whether caused by fever, malnutrition, or antisecretory drugs, may upset this balance [22,23]. Under normal conditions, H. pylori induced inflammatory mucosal damage that spreads from the antrum into the corpus as an advancing front of inflammation. As it moves proximally, it leaves behind an atrophic corpus mucosa, in which pariet al and chief cells become rare or absent (i.e. becomes pseudopyloric metaplasia) [17]. The distance between the gastric angle and the esophagogastric junction is shorter in the lesser than in the greater curvature, and this may explain in part why the atrophic process advances more rapidly in the former. The incisura angularis is, thus generally the first site where atrophy appears and is a targeted biopsy site in the Sydney biopsy system [24■].
In autoimmune gastritis, the essential lesion is a destructive lymphocytic infiltration of the oxyntic mucosa [25■]. Over a period of decades, the immune-mediated damage results in the disappearance of the native oxyntic cells, which are replaced by mucus-producing cells; as the disease progresses, the gastric corpus is overtaken by large areas of pseudopyloric and intestinal metaplasia. The resulting hypochlorhydria induces hyperplasia and in some cases, dysplasia of the enterochromaffin-like cells. In the absence of concurrent H. pylori infection, autoimmune gastritis is strictly limited to the corpus. The antral mucosa usually takes a reactive appearance with only mildly increased chronic inflammation.
RECOGNIZING GASTRIC ATROPHY
Although gastric atrophy was described as early as 1854 [26], reliable histology had to await the more modern era in which formalin was added to the stomach immediately after death [27]. Currently, the histological spectrum of atrophy includes any histologically proved loss of native glands with disappearance of glandular units or with metaplastic transformation (intestinal and pseudopyloric metaplasia) of native glands. Most frequently, the different variants of atrophy coexist.
Atrophy of the oxyntic mucosa, particularly in its most advanced stages and when atrophic-meta-plastic lesions are restricted to the corpus, is easily recognizable, even without ancillary histological stain. In antral biopsy samples, fibrosis of the lamina propria, particularly when it separates the deeper part of the glandular coils from the muscularis mucosae, is a reliable indicator of atrophy [28]. In such biopsy specimens, however, dense inflammatory infiltrates may push apart the glandular units, mimicking glandular loss. Because of the uncertainty this creates, codified in the term ‘indefinite for atrophy’, most pathologists rely on the detection of intestinal metaplasia for a diagnosis of atrophic gastritis in the antrum.
PUTTING INTESTINAL METAPLASIA INTO CLINICAL CONTEXT
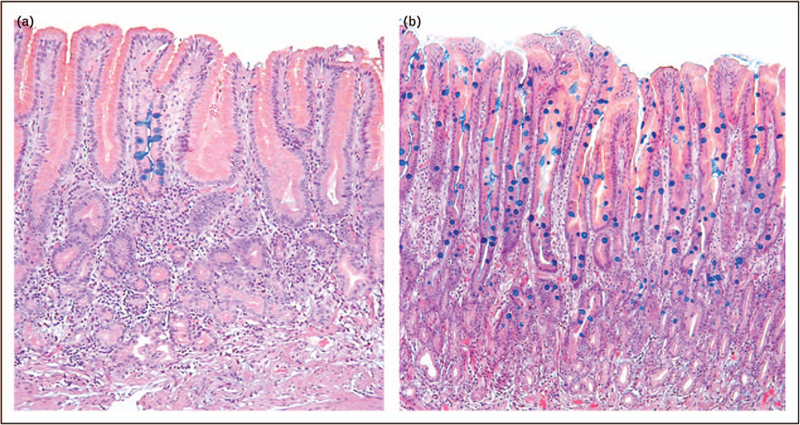
The level of clinical alarm associated with intestinal metaplasia, and its ultimate prognosis, are based on the answers to these two questions: what is the cause of the damage that triggered a metaplastic response?, and is it still ongoing? Resolving the nature of intestinal metaplasia and predicting its clinical importance includes evaluating three aspects: extent, location, and the background in which it arises (Table 1). For example, focal damage, such as an NSAID-induced gastric ulcer, may be repaired by a scar within otherwise normally appearing mucosa. However, a biopsy may show a small focus of intestinal metaplasia (Fig. 4a). Although this can be noted in the pathology report, it has neither functional nor clinical significance. In contrast, in a patient with H. pylori gastritis, multiple foci or large patches of intestinal metaplasia in biopsy specimens from antrum and corpus indicate an ongoing destructive process that may carry an increased risk for gastric cancer (Fig. 4b). Intestinal metaplasia limited to the corpus with normal antral mucosa should prompt an investigation for autoimmune gastritis, which has a low-to-absent risk of adenocarcinoma, but a high likelihood of neuroendocrine proliferations [25■].
Table 1.
Checklist for atrophy/intestinal metaplasia
| Location (antrum, corpus, both) |
| Extent (locally and of stomach) |
| Type(s) (nonmetaplastic atrophy, pseuduopyloric metaplasia, intestinal metaplasia) |
| Cause (H. pylori, autoimmune, toxic injury, etc.) |
| Risk stratification score (endoscopic, histologic) |
H. pylori, Helicobacter pylori.
FIGURE 4.
These two images illustrate the concept of focal (panel a where only one foveola is affected) versus diffuse (panel b where the entire mucosa is intestinalized) metaplasia. However, one must interpret these designations with skepticism, as the true extent of intestinal metaplasia in a stomach can never be assessed accurately by biopsy sampling. Careful gastric biopsy mapping, however, allows pathologists to make reasonable estimates.
OBTAINING INTERPRETABLE AND USEFUL GASTRIC BIOPSIES
Endoscopy is an invasive and expensive procedure not without risk. Modern endoscopes with magnifying and narrow band imaging allow much better identification of mucosal abnormalities, and thus for targeted biopsies to be taken [29,30,31■,32■]. Among the many indications for gastric biopsy, the most common are the presence of an abnormal endoscopic finding thought to need histologic study and the search for H. pylori in patients with dyspepsia. A disturbingly common description accompanying gastric samples is ‘random biopsies taken’. The thought behind this approach seems to be the widespread misconception that a good pathologist can sort out where the biopsies were obtained and what they mean [33]. Although it may be inconsequential when the specimens are normal, it can make the diagnosis of important conditions, particularly atrophy, difficult or impossible, thus defeating the main goal of the biopsy, which is to obtain sufficient data to prevent the need to perform a second procedure. Pseudopyloric metaplasia, so named because corpus biopsies histologically appear as antral, ideally requires the pathologist to use special stains, such as pepsinogen I or for TFF2 or spasmolytic peptide [9■,34■].
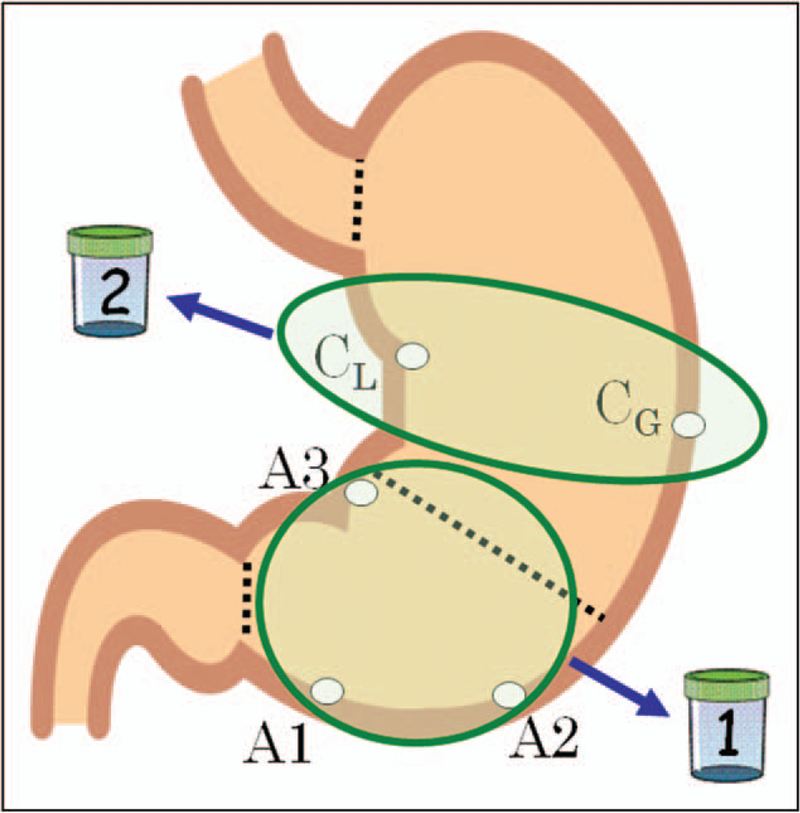
Almost irrespective of the condition seen or suspected, when one takes biopsies, one should provide sufficient information so that the pathologist can make a statement about the status of the gastric mucosa. For example, a biopsy from an abnormal-appearing site should be accompanied by samples from the surrounding mucosa, which may hold the key to the cause of a lesion. The status of the entire stomach mucosa is best assessed using the Sydney System biopsy protocol, which consists of three biopsies from the antrum (lesser and greater curve of the mid-antrum and incisura angularis) and two from the corpus (mid-lesser and greater curve) (Fig. 5). Antral and corpus biopsies should be placed in two separate containers because an atrophic corpus, particularly one with pseudopyloric metaplasia, may sometimes be histologically similar to the antrum, and a less experienced pathologist might misinterpret this finding (Figs. 2 and 6). The biopsy set for histologic assessment of the stomach should not be combined with specimens from targeted lesions.
FIGURE 5.
Illustration showing the Sydney system biopsy sites and the use of separate bottles for the antral and corpus specimens.
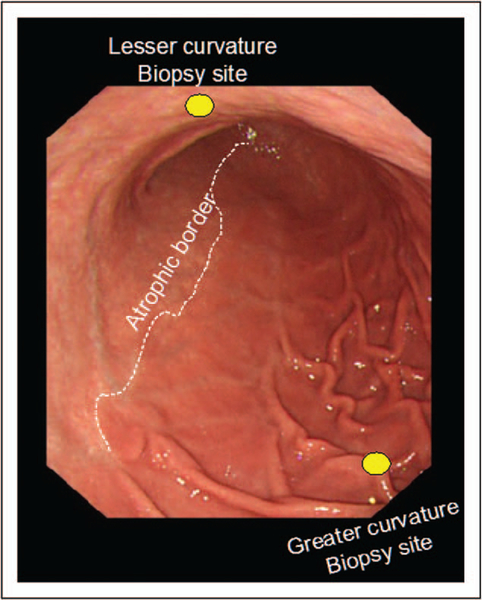
FIGURE 6.
Endoscopic picture looking from the proximal to the distal stomach in a patient with extensive atrophy extending along the lesser cure. Biopsy of the atrophic lesser curve corpus likely shows pseudopyloric metaplasia whereas the greater curve biopsy would be nonatrophic. Placing all five biopsies in one bottle would likely confuse the pathologist. If in separate bottles, the pathologist could determine that the patient had extensive antral and lesser curve corpus atrophy.
Topographic and contextual clinical information (where were biopsies taken, why were they taken, what was seen endoscopically, and what result is expected) should always be included in the requisition accompanying the specimens. Information that is especially useful in assisting the pathologist to provide a clinically helpful report are listed in Table 2 and Fig. 7. Appropriately labeled good-quality photographs will also assist both the pathologist as well as the next endoscopist who examines that stomach. All this will allow a pathologist who detects intestinal metaplasia to make educated guesses on its location, extension, origin, and possible clinical significance.
Table 2.
Etiquette of gastric biopsying
| Use jumbo forceps (the largest that will fit in the channel) |
| Do not mix biopsies from normal appearing mucosa with those where there is a question |
| Do not mix antral and corpus biopsies |
| Take an adequate number of biopsies |
| Put no more than four or five biopsies in one bottle |
| Identify and separate biopsies from mucosal abnormalities (erosions, ulcer, lumps and bumps) |
| If the status of the mucosa is or may become a question: take the five Sydney targeted samples |
| Take good-quality photographs of the abnormalities biopsied |
| Provide your opinion and why question(s) you have (i.e. possible pancreatic rest, gastric ulcer, etc.) |
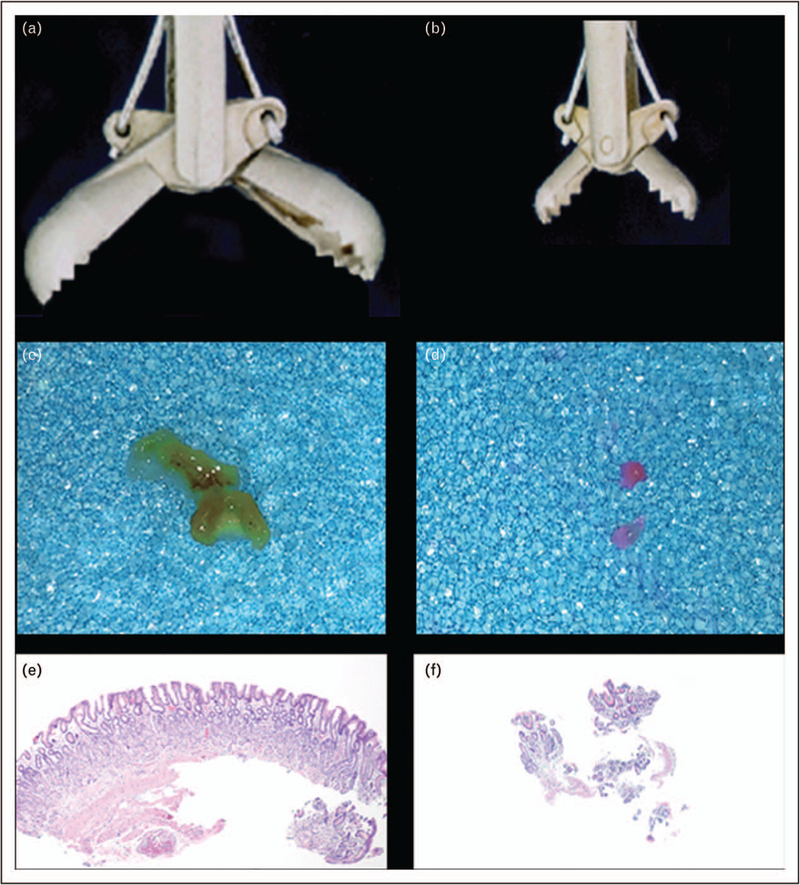
FIGURE 7.
Illustration of the importance of using large forceps. The sequence (a), (b), and (c) illustrates the relative biopsy size, tissue specimen, and tissue section with a large forceps contrasted with sequence (d), (e), and (f) in which a small forceps is used. A larger specimen (as in c) also allows a better orientation and, consequently, a better interpretation than can be made by examining the disorderly fragments of mucosa depicted in panel f.
CLINICAL INTERPRETATION OF INTESTINAL METAPLASIA AND EXPECTATIONS FROM THE PATHOLOGY REPORT
The presence of intestinal metaplasia only signifies that gastric mucosal injury has occurred and has been repaired. The clinical question is, therefore, whether the injury was a onetime event or was part of an ongoing process. Clinicians should agree with their pathologists what an optimal report should look like. A useful report is usually the result of a productive relationship between clinician and pathologist. With respect to intestinal metaplasia, the histopathology report should indicate its location and extension, extrapolated by the percentage of specimens in which it is present. Typing intestinal metaplasia is not yet within the reach of most pathologists and most clinicians would not be sure on how to act on that information. In general, the extent of intestinal metaplasia predicts the odds of finding advanced metaplasia, and thus typing itself adds little [35]. We suggest that phenotyping intestinal metaplasia should be limited to research settings.
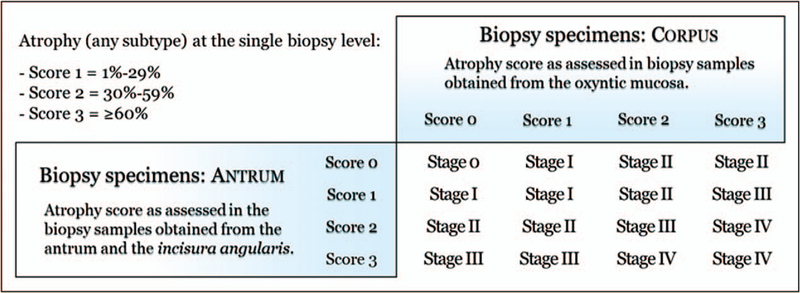
Excluding syndromic cancers (less than 5% of gastric adenocarcinomas), mucosal atrophy - almost invariably metaplastic - is the cancerization field where more than 90% of gastric epithelial malignancies develop. This finding provides the rationale for gastritis staging, which has been shown to be a reliable predictor of the risk for gastric cancer. In East Asian countries, the extent of atrophy and its associated cancer risk is often assessed endoscopically using the Takemoto-Kimura classification [36■,37]. In the west, biopsy assessment is preferred, and when an adequate number of biopsy specimens is available, the Operative Link for Gastritis Assessment of Atrophic Gastritis (OLGA) or the Operative Link for Gastritis Assessment of Intestinal Metaplasia (OLGIM) risk assessment systems can be used [38–40]. OLGA staging is based on a combined score of atrophy, as obtained from the biopsy samples taken from oxyntic and antral mucosa (Sydney System protocol). The combined score values, expressed as the stage, represent a semiquantitative message on the severity of the disease, which parallels the risk of malignancy (OLGA stages o—I-II: from very low to low cancer risk; OLGA-stages III-IV: high risk of gastric cancer) (Fig. 8). Both cross sectional and prospective studies have supported the prognostic value of gastritis staging and its suitability in clinical practice [20,41]. An alternative staging system, OLGIM, is only based on the intestinal metaplasia. Because of its intestinal metaplasia-restricted score, the OLGIM sensitivity in the assessment of atrophy is likely lower than the more ‘global’ OLGA approach [42].
FIGURE 8.
The Operative Link for Gastritis Assessment of Atrophic Gastritis system of staging risk for developing gastric cancer. Atrophy scores from antral and corpus specimens are plotted on the table to reach a stage. For example, a set of biopsies with a score of 2 in the antrum and 3 in the corpus will have an OLGA Stage IV. Adapted with permission from [40]. OLGA, the Operative Link for Gastritis Assessment of Atrophic Gastritis.
CONCLUSION
Gastric intestinal metaplasia is a potential reversible product of injury and repair and not directly connected with carcinogenesis. Think ahead, your biopsy protocol should provide samples sufficient to answer whether there is damage/atrophy, what the cause that triggered the metaplastic response, whether it is ongoing. If atrophy is present, what is the topographic distribution. The pathologist must have appropriate samples to be able to make a statement about the status of the gastric mucosa.
KEY POINTS.
Gastric intestinal metaplasia is a potential reversible product of injury and repair and not directly connected with carcinogenesis.
Random gastric biopsies rarely provide definitive results and often prompt another procedure to collect appropriate specimens. The biopsy protocol should be able to answer whether there is damage/atrophy, what the cause that triggered the metaplastic response, whether it is ongoing and if atrophy is present, what is the topographic distribution such that the pathologist can make a statement about the status of the gastric mucosa. Biopsies of an abnormal appearing site should be accompanied by biopsies of the surrounding mucosa.
The requisition accompanying the specimens should contain sufficient topographic and contextual clinical information (where were biopsies taken, why were they taken, what was seen endoscopically, and what result is expected) to assist the pathologist in understanding what information is needed.
Acknowledgements
The authors thank Dr Akira Horiuchi for supplying the photograph used in Figure 6.
Financial support and sponsorship
D.Y.G. is supported in part by the Research Service Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
D.Y.G. is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel Helicobacter pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. R.M.G. is a consultant for RedHill Biopharma.
Footnotes
Conflicts of interest
M.R. has no conflicts to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Tan MC, Balakrishnan M, Graham DY. Gastric cancer worldwide except Japan In: Shiotani A, editor. Gastric cancer with special focus on studies from Japan. Singapore: Springer; 2018. pp. 17–28. [Google Scholar]
- 2.Rugge M, Fassan M, Graham DY. Epidemiology of gastric cancer In: Strong VE, editor. Gastric cancer: principles and practice. Cham: Springer International Publishing; 2015. pp. 23–34. [Google Scholar]
- 3.Graham DY. Helicobacterpylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719.e3–731.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019; 51:365–388.■■ 2019 update of the European guideline regarding management of precancerous and potentially precancerous lesions of the stomach. An up-to-date overview with general guidelines, such as frequency of follow-up for those followed up is indicated.
- 7.Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol 2018; 245: 132–137.■■ Excellent review of gastric mucosal repair and the histologic types involved in both humans and animals.
- 8.Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol 2017; 4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol 2018; 596:3861–3867.■ Review of gastric mucosal injury and repair.
- 10.Trieu JA, Bilal M, Saraireh H, et al. Update on the diagnosis and management of gastric intestinal metaplasia in the USA. Dig Dis Sci 2019; 64:1079–1088. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the stomach-precursor of gastric cancer? Int J Mol Sci 2017; 18:; pii: E2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018; 21:579–587.■■ Systemic review and meta-analysis confirming the value of the OLGA and OLGIM gastric cancer risk stratification systems.
- 13.Waddingham W, Graham D, Banks M, et al. The evolving role of endoscopy in the diagnosis of premalignant gastric lesions. F1000Res 2018; 7:; pii: F1000 Faculty Rev–715.■ Update of endoscopic methods of assessing premalignant and potentially premalignant lesions in the stomach
- 14.Graham DY, Zou WY. Guilt by association: intestinal metaplasia does not progress to gastric cancer. Curr Opin Gastroenterol 2018; 34:458–464.■ Review of intestinal metaplasia as a premalignant condition with data about reversibility and the importance of cause in prognosis.
- 15.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet 1975; 2:58–60. [DOI] [PubMed] [Google Scholar]
- 16.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52:6735–6740. [PubMed] [Google Scholar]
- 17.Ricuarte O, Gutierrez O, Cardona H, et al. Atrophic gastritis in young children and adolescents. J Clin Pathol 2005; 58:1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther 2002; 16:1249–1259. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Genta RM, Fassan M, et al. OLGA gastritis staging for the prediction of gastric cancer risk: A long-term follow-up study of 7436 patients. Am J Gastroenterol 2018; 113:1621–1628. [DOI] [PubMed] [Google Scholar]
- 20.Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut 2019; 68:11–17. [DOI] [PubMed] [Google Scholar]
- 21.Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology 1989; 96:615–625. [DOI] [PubMed] [Google Scholar]
- 22.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology 1997;113:1983–1991. [DOI] [PubMed] [Google Scholar]
- 23.Graham DY, Opekun AR, Yamaoka Y, et al. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther 2003; 17:193–200. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20:1161–1181.■ Classic Sydney system classification paper.
- 25.Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: pathologist’s view-point. World J Gastroenterol 2015; 21:12179–12189.■ Recent update on autoimmune gastritis.
- 26.Jones CH, Sieveking EH. A manual of pathological anatomy. London: Churchill; 1854. [Google Scholar]
- 27.Faber K. Gastritis and its consequences. Paris: Oxford University Press; 1935 [Google Scholar]
- 28.Ruiz B, Garay J, Johnson W, et al. Morphometric assessment of gastric antral atrophy: comparison with visual evaluation. Histopathology 2001; 39:235–242. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Liu Y, Lu Y, et al. Ability of blue laser imaging with magnifying endoscopy for the diagnosis of gastric intestinal metaplasia. Lasers Med Sci 2018; 33:1757–1762. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Wu X, Liu Y, et al. Blue laser imaging with acetic acid enhancement improved the detection rate of gastric intestinal metaplasia. Lasers Med Sci 2019; 34:555–559. [DOI] [PubMed] [Google Scholar]
- 31.Ono S, Kato M, Tsuda M, et al. Lavender color in linked color imaging enables noninvasive detection of gastric intestinal metaplasia. Digestion 2018; 98:222–230.■ Update on recent advances of endoscopic imaging for detection of intestinal metaplasia.
- 32.White JR, Sami SS, Reddiar D, et al. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand J Gastroenterol 2018; 53:1611–1618.■ Update on recent advances of endoscopic imaging for detection of intestinal metaplasia.
- 33.Huang RJ, Hwang JH. Routine gastric biopsies: should we be doing more? Gastrointest Endosc 2019; 89:1150–1151. [DOI] [PubMed] [Google Scholar]
- 34.El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 2002; 94:1428–1436.■ Classic paper showing that atrophic gastritis is not a multifocal condition.
- 35.Cassaro M, Rugge M, Gutierrez O, et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol 2000; 95:1431–1438. [DOI] [PubMed] [Google Scholar]
- 36.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 1:87–97.■ Classic paper on the endoscopic system of gastric cancer risk stratification.
- 37.Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology 1972; 63:584–592. [PubMed] [Google Scholar]
- 38.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007; 56:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010; 71:1150–1158. [DOI] [PubMed] [Google Scholar]
- 40.Rugge M, Correa P, DiMario F, et al. OLGA staging for gastritis: atutorial. Dig Liver Dis 2008; 40:650–658. [DOI] [PubMed] [Google Scholar]
- 41.Rugge M, Sugano K, Scarpignato C, et al. Gastric cancer prevention targeted on risk assessment: gastritis OLGA staging. Helicobacter 2019; 24:e12571. [DOI] [PubMed] [Google Scholar]
- 42.Rugge M, Fassan M, Pizzi M, et al. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol 2011; 17:4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]