Abstract
Transcatheter aortic valve implantation (TAVI) has become a useful and effective treatment for surgical high-risk patients with severe aortic valve stenosis (AS). Stroke is one of the most frequent complications associated with TAVI. Shaggy and porcelain aortas are a risk factor for procedure-related strokes. Preventing brain embolism is one of the most important goals in patients with diseased aortas. We present a case where we performed TAVI in an 89-year-old man with severe AS, a shaggy aorta, a porcelain aorta, and congestive heart failure. TAVI via a transfemoral approach was performed using a modified isolation technique with cannulation from bilateral axillary arteries and cardiopulmonary bypass to prevent brain embolism. The catheter-delivered embolic protection device is necessary to pass the diseased aorta, but the modified isolation technique can be used without any contact with the shaggy aorta. Embolism did not occur, and his heart failure improved immediately.
<Learning objective: Transcatheter aortic valve implantation (TAVI) is a therapeutic option for patients with severe aortic stenosis considered to be at high risk for aortic valve replacement. Cerebral embolism is the most frequent complication of TAVI. A shaggy or porcelain aorta is associated with a high risk of stroke during TAVI. We report a case of TAVI in a shaggy and porcelain aorta and prevention of cerebral embolism by adopting a modified isolation technique without using conventional protection devices.>
Keywords: Transcatheter aortic valve implantation, Shaggy aorta, Porcelain aorta
Introduction
Transcatheter aortic valve implantation (TAVI) is less invasive compared to surgical aortic valve replacement and is indicated for patients with symptomatic aortic valve stenosis (AS) at high operative risk. The incidence of cerebral embolism following the procedure is reported to be over 70% as evaluated by diffusion-weighted magnetic response imaging, which includes silent stroke [1]. The large Transcatheter Valve Therapies registry showed that the composite outcome of mortality and stroke occurs in 26.0% of patients over 1 year of follow-up [2]. Various devices that are designed to prevent emboli into the cerebral arteries need to be inserted before navigating through the aortic arch. However, inserting such protective devices into a diseased aorta is regarded as a risk factor for stroke.
Here, we report a case of TAVI in a patient with an extremely shaggy and porcelain aorta and congestive heart failure using a modified isolation technique with cardiopulmonary bypass (CPB). The patient consented to the publication of this report.
Case report
An 89-year-old man was seen by a family physician for complaints of nocturnal dyspnea. He had a history of brain infarction and chronic kidney disease (CKD). He was diagnosed with severe AS and moderate mitral valve insufficiency with a left ventricle ejection fraction (EF) of 30% by transthoracic echocardiography (TTE) and was admitted to our hospital. The mean aortic pressure gradient measured 50 mmHg and the calculated aortic valve area was 0.8 cm2 (Fig. 1). Chest radiography showed congestive heart failure. Computed tomography (CT) showed a porcelain and shaggy aorta (Fig. 2). AS was determined to be the cause of his heart failure and our heart team scheduled a TAVI procedure. He was a high-risk candidate for surgical aortic valve replacement because of his high logistic EuroSCORE and STS score (92.5% and 25%, respectively), frailty, heart failure, and CKD. Although there was a discussion regarding the approach site, we chose the transfemoral (TF) approach because it is less invasive compared to the transapical (TA), which can damage the cardiac muscle. Because the risk of cerebral infarction is high in patients with a shaggy aorta, a modified isolation technique was adopted with cannulation of the bilateral axillary arteries and using CPB to prevent embolism to the brain.
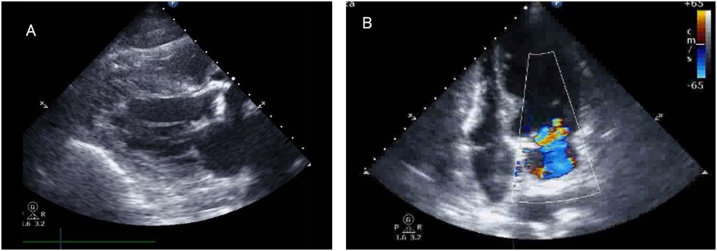
Fig. 1.
Preoperative echocardiography. (A) Echocardiographic examination before treatment showed aortic valve stenosis in the parasternal long-axis view. (B) A moderate amount of mitral regurgitation was observed in the four-chamber view.
Fig. 2.
Preoperative computed tomography (CT). (A) The CT axial and sagittal image showed thrombus in the ascending aorta and the aortic arch, and this was finding of shaggy aorta. (B) The aorta generally showed a high degree of calcification, a finding of porcelain aorta.
Pre-procedural anatomical measurement, including the virtual basal ring at the nadir of the aortic cusps, was obtained from multidetector CT. The device selected by the heart team after discussion was the SAPIEN 3 transcatheter heart valve (Edwards Lifesciences, Inc., Irvine, CA, USA), which has the flex system and an active 3-dimensional positional catheter. The TAVI procedure was performed under general anesthesia. The bilateral axillary arteries, the femoral arteries, and the right femoral vein were exposed. After general heparinization, the arterial cannulation was performed using a 7-mm knitted prosthesis that was anastomosed to the bilateral axillary arteries, while the venous cannulation was performed through the right femoral vein. The intraoperative placement of equipment was shown in Fig. 3. The perfusion flow of CPB was set at 1.5–2.0 L/min. Normally, the flow mixing zone is confirmed by angiography; however, due to the presence of CKD, it was confirmed by transesophageal echocardiography in this case. By increasing venous drainage using CPB, the cardiac output was decreased and the blood flow through the left common carotid artery (CCA) became retrograde. Adjustment of CPB was necessary as it may cause embolism into the coronary artery if the cardiac output is abolished. As a precautionary measure, the left CCA was manually pressed to prevent embolism as the catheter was passed into the diseased aorta. We punctured the left femoral artery to insert a 5-Fr pigtail catheter and placed it in the right coronary sinus. A 14-Fr expandable sheath was inserted into the right femoral artery to gain access. The valve was crossed with an Argon Spring Wire with the support of a 5-Fr multipurpose A2 catheter, which was subsequently exchanged for a Safari 2 pre-shaped guidewire with an extra small curve (Boston Scientific, Natick, MA, USA) after pressure measurement. The peak pressure gradient between the left ventricle and the aorta was 60 mmHg. Pre-dilatation was omitted to reduce the rapid pacing time for low EF. Prior to device deployment, the right ventricle was paced at 180 bpm for 15 s using a temporary pacing wire. The CPB was stopped during device deployment. During the rapid pacing period, the 26-mm Sapien 3 was implanted with careful attention to position. Only a trace of paravalvular leakage was observed, and the pressure gradient disappeared after deployment. The CPB was removed and the patient was moved to the cardiac care unit. His heart failure also improved after procedure and the patient was extubated on postoperative day 3. There were no new cerebral infarctions and neurological abnormalities. The postoperative TTE showed an aortic valve area of 1.84 cm2, an EF improved of 50%, and amelioration of the mitral valve insufficiency. He recovered uneventfully and was discharged home on day 12.
Fig. 3.
Photograph of the operative setup. The cardiopulmonary bypass machine was positioned behind the monitor, and to the left side of the patient. The anesthesia apparatus and transesophageal echocardiography machine were placed near the patient’s head.
Discussion
The available embolic protection devices (EPDs), which have been widely adopted in recent years, are designed to capture embolic debris and protect the supra-aortic vessels during TAVI [3]. Various types of EPDs can be deployed into the aortic arch or the ostium of the brachiocephalic trunk and left common carotid artery [3]. While they may not reduce the number of embolic lesions, systematic reviews and meta-analyses have shown that they may reduce their total volume [3], [4], [5]. The disadvantage of these EPDs is that they must be inserted into the aorta at the time of use. In our case, the aortic arch was very shaggy and porcelain. Shaggy aorta is indicated by severe arterial degeneration of the aorta, the surface of which is extremely friable and likely to cause atheroembolism [6]. Normally, TF-TAVI is contraindicated in shaggy aorta, and insertion of these EPDs into the aortic arch would also increase the risk of cerebral embolism. Comparing the two methods, the TF approach was needed because of the use of large catheters in the aortic arch and retrograde crossing of the native aortic valve. The TA approach is not associated with a reduction in new post-procedural cerebral lesions compared with the TF approach. Furthermore, the 30-day mortality was higher when using the TA approach than when using the TF approach [7]. Another report pointed out that the TA approach is associated with increased biomarkers of cardiac tissue injury and left ventricular damage compared with the TF approach [8]. Based on these facts, we chose TF-TAVI for this patient with a low EF. Regarding device selection, CoreValve (Medtronic, Minneapolis, MN, USA) has a more rigid catheter system than the Sapien. Therefore, we believed that CoreValve was likely to scrape the greater curvature of the aortic arch and cause embolism. The Sapien has a catheter flex system that prevents it from scraping the aortic arch and was thus deemed more suitable. The disadvantage of the Sapien device is that rapid pacing is necessary even in patients with a low EF.
The isolation technique has been usually performed for total aortic arch replacement with a mobile atheroma [9]. The original modified technique is setting of arterial cannulations from bilateral axillary arteries and left CCA. The brain perfusion flow is set at 1.5 L/min using two pumps [10]. We further simplified this method and established isolated brain blood flow through bilateral axillary artery perfusion. In theory, if the cardiac output is decreased by venous drainage using CPB, the blood flow through the left CCA would flow in a retrograde manner, thus protecting it. The advantage of this technique is the avoidance of contact with the shaggy aorta using CPB. Extracorporeal membranous oxygenator and CPB can provide temporary cardiorespiratory support in case of hemodynamic instability during the procedure.
Although there were no cerebral complications and the postoperative course was good, this approach should be further developed to enable complete left CCA protection. Although the blood flow through the left CCA was theoretically protected by CPB, it is difficult to evaluate it angiographically during the procedure. Although the effect of compressing the carotid artery is unknown, the left CCA was manually pressed during the procedure prophylactically; however, it may be unnecessary. Protection of the left CCA needs to be investigated in the future. Although invasive, clamping the CCA directly or inserting a direct occlusion balloon to prevent embolism are potential approaches.
Conclusion
TAVI was performed safely without complications for severe AS with extremely shaggy and porcelain aorta using a modified isolation technique. Although this modified technique does not completely protect the left CCA, it is effective for the prevention of cerebral embolism in patients with a diseased aorta and it provides hemodynamic support during the TAVI procedure.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
None.
References
- 1.Kahlert P., Knipp S.C., Schlamann M., Thielmann M., Al-Rashid F., Weber M. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic response imaging study. Circulation. 2010;121:870–878. doi: 10.1161/CIRCULATIONAHA.109.855866. [DOI] [PubMed] [Google Scholar]
- 2.Holmes D.R., Jr, Brennan J.M., Rumsfeld J.S., Dai D., O’Brien S.M., Vemulapalli S. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Jawad Altisent O., Puri R., Rodés-Cabau J. Embolic protection devices during TAVI: current evidence and uncertainties. Rev Esp Cardiol (Engl Ed) 2016;69:962–972. doi: 10.1016/j.rec.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 4.Bagur R., Solo K., Alghofaili S., Nombela-Franco L., Kwok C.S. Cerebral embolic protection devices during transcatheter aortic valve implantation: systematic review and meta-analysis. Stroke. 2017;48:1306–1315. doi: 10.1161/STROKEAHA.116.015915. [DOI] [PubMed] [Google Scholar]
- 5.Giustino G., Sorrentino S., Mehran R., Faggioni M., Dangas G. Cerebral embolic protection during TAVR: a clinical event meta-analysis. J Am Coll Cardiol. 2017;69:465–466. doi: 10.1016/j.jacc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Hollier L.H., Kazmier F.J., Ochsner J., Bowen J.C., Procter C.D. “Shaggy” aorta syndrome with atheromatous embolization to visceral vessels. Ann Vasc Surg. 1991;5:439–444. doi: 10.1007/BF02133048. [DOI] [PubMed] [Google Scholar]
- 7.Panchal H.B., Ladia V., Amin P., Patel P., Veeranki S.P., Albalbissi K. A meta-analysis of mortality and major adverse cardiovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using edwards valve for severe aortic stenosis. Am J Cardiol. 2014;114:1882–1890. doi: 10.1016/j.amjcard.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Barbash I.M., Dvir D., Ben-Dor I., Badr S., Okubagzi P. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:1337–1343. doi: 10.1016/j.amjcard.2012.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Shiiya N., Kunihara T., Kamikubo Y., Yasda K. Isolation technique for stroke prevention in patients with a mobile atheroma. Ann Thorac Surg. 2001;72:1401–1402. doi: 10.1016/s0003-4975(01)02922-8. [DOI] [PubMed] [Google Scholar]
- 10.Shiiya N. Aortic arch replacement for degenerative aneurysms: advances during the last decade. Gen Thorac Cardiovasc Surg. 2013;61:191–196. doi: 10.1007/s11748-012-0166-4. [DOI] [PubMed] [Google Scholar]