Abstract
Vaccination continues to be the single most important and successful public health intervention, due to its prevention of morbidity and mortality from prevalent infectious diseases. Severe immunologically mediated reactions are rare and less common with the vaccine than the true infection. However, these events can cause public fearfulness and loss of confidence in the safety of vaccination. In this paper, we perform a systematic literature search and narrative review of immune‐mediated vaccine adverse events and their known and proposed mechanisms, and outline directions for future research. Improving our knowledge base of severe immunologically mediated vaccine reactions and their management drives better vaccine safety and efficacy outcomes.
Keywords: adverse drug reactions, allergy, drug allergy, hypersensitivity, immunology, vaccines
1. INTRODUCTION/BACKGROUND
Vaccination is 1 of the most important and successful public health interventions. Globally, vaccines effect immunity in hundreds of millions of individuals each year.1 The sentinel successes of vaccination include eradication of smallpox, the confinement of polio to a tiny geographic footprint with hopes of eradication, and the aversion of millions of deaths due to measles.2, 3 An estimated 23.3 million global deaths will be averted by vaccination between 2011 and 2020.2
Despite, or perhaps because of these successes, increasing attention by the public has been focused on the infrequent risks of vaccination.4, 5 Patients sometimes perceive the risks of vaccination to be of greater concern than the benefits, and some groups currently promote avoidance of specific or all vaccines.4, 5, 6 This has been described previously as a crisis of confidence in vaccination.7, 8, 9 Currently, a global resurgence of measles is due in part to parents avoiding routine childhood immunizations for their children.10
Parents who report a child having a previous adverse event following immunization have increased hesitancy about future vaccination, although the vast majority of these events are usually the expected sequelae required for immunity, and of low severity.11 Worldwide, pharmacovigilance for these events is strengthened on a country‐by‐country basis by the World Health Organization through the Global Vaccine Safety Initiative.12 In the USA, adverse events are reported either by patients, family members or health care providers to the Vaccine Adverse Event Reporting System (VAERS) and can be evaluated by the multidisciplinary Clinical Immunization Safety Assessment network or the Vaccine Safety Datalink.13, 14 Loughlin et al. reported a sample of 100 patients from VAERS who were evaluated using the World Health Organization's adverse events from immunization causality assessment criteria, and only 3% of reported vaccine reactions could be definitely causally linked to the vaccine received.15 Of the remaining adverse events, 20% were classified as probably linked, 20% were classified as possibly linked, and the majority (53%) were classified as either unlikely to be linked or unrelated to a vaccine received.15 However, in light of unnecessary gaps in vaccination coverage it is increasingly important that any major side effects from vaccination should be separated from online myths of adverse effects and made predictable by science. This would ideally include both heightened education and elimination of the adverse effects.
In this paper we perform a systematic literature search and narrative review of immune‐mediated vaccine adverse events and their known and proposed mechanisms, and outline directions for future research. The scope of this review focuses on mechanisms of immediate and delayed hypersensitivity to vaccination, adverse outcomes in the immune suppressed, and discusses ways in which the field of vaccinology can move toward a goal of minimizing hypersensitivity reactions and other adverse events that lead to gaps in vaccination on a population level.
2. METHODS
We searched PubMed for relevant articles published between January 1945 and August 2018. Articles were selected as relevant if they provided a description of an immune‐mediated vaccine adverse event or shed light on a mechanism for an immune‐mediated vaccine reaction. We used various combinations of the following terms: ‘vaccine’, ‘vaccination’, ‘immunization’, ‘allergy’, ‘hypersensitivity,’ ‘Guillain‐Barré,’ ‘adverse’ and ‘anaphylaxis.’ In addition, we added supplemental search terms related to vaccine excipients; specifically: ‘gelatin,’ ‘egg,’ ‘alpha‐gal,’ ‘latex,’ ‘milk,’ ‘chicken,’ ‘yeast,’ ‘aluminum,’ ‘thimerosal,’ ‘phenoxyethanol,’ ‘neomycin,’ ‘polymyxin B,’ ‘kanamycin,’ ‘gentamicin,’ ‘streptomycin,’ ‘chlorotetracycline,’ ‘amphotericin B,’ ‘dextran’ and ‘polysorbate 80.’ In combination with the term ‘vaccine’ we also searched for reports of ‘acute generalized exanthematous pustulosis,’ ‘erythema nodosum,’ ‘granuloma annulare,’ ‘bullous pemphigoid,’ ‘Sweet's syndrome,’ ‘Gianotti‐Crosti,’ ‘lichenoid’ ‘cutaneous lupus,’ ‘lupus vulgaris’ and ‘serum sickness.’ Additional publications were sourced from the references in individual articles. Relevant articles were selected after reading through all titles and abstracts, and full texts were obtained if the information contained in the title or abstract was insufficient to exclude the study as relevant. Priority on relevance was given to: (i) publications containing cases of post‐vaccination immune‐mediated adverse events that provided evidence for a mechanism; followed by (ii) publications containing well described cases of postvaccination immune‐mediated adverse events and at least probable causality where mechanisms are currently unknown or poorly defined; and (iii) original research articles that examine the epidemiology of postvaccination immune‐mediated adverse events. Out of a total of 260 articles initially selected on abstract review as potentially relevant, 169 were included as references in this final narrative review manuscript.
2.1. Mechanisms of vaccine hypersensitivity
2.1.1. Antibody mediated hypersensitivity
Immediate hypersensitivity to vaccines is caused by the presence of immunoglobulin (Ig)E in a patient which can precipitate degranulation of mast cells and release of histamine (type I hypersensitivity) in response to an antigen within the vaccine.16 Although it is now known that there are mechanisms by which mast cell degranulation can occur without the presence of IgE,17 IgE‐mediated anaphylaxis is the most important and severe immediate reaction occurring after vaccination.18, 19 Symptoms have a rapid onset (typically <15 min) and include itching, urticaria, angioedema, nausea, vomiting, diarrhoea, wheezing, shortness of breath, hypotension, loss of consciousness and, in severe instances, death.16 Treatment of acute symptoms should always include early administration of intramuscular epinephrine.18, 19 Allergy testing using skin prick or intradermal testing read at 15–20 minutes with appropriate histamine and saline controls can be informative to evaluate this type of hypersensitivity.19 (Figure 1).
Figure 1.
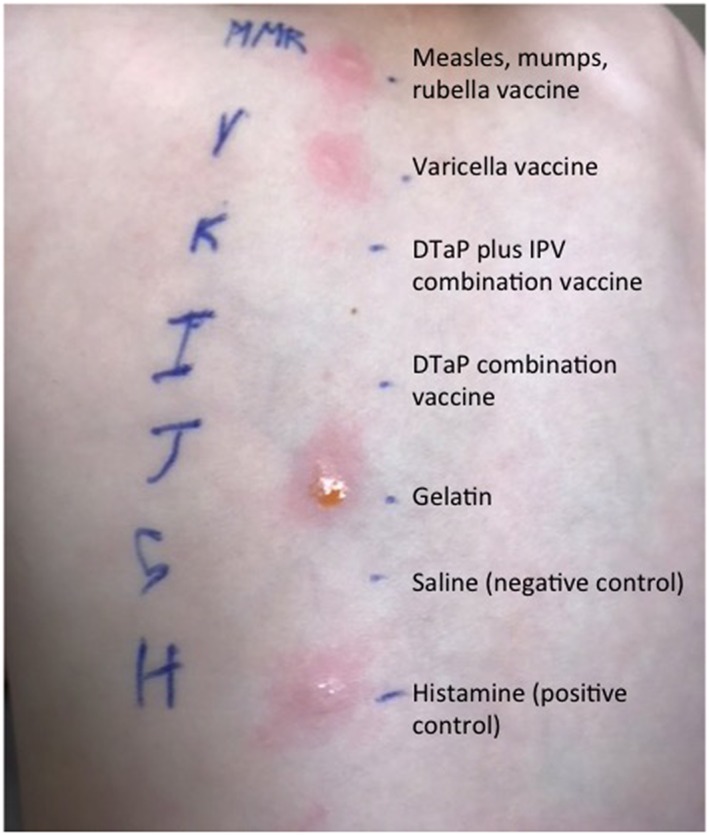
Immediate hypersensitivity skin testing in an alpha‐gal allergic patient with anaphylaxis after vaccination demonstrates a skin test positive response to gelatine containing vaccines, which were subsequently demonstrated to also contain alpha‐gal allergen. Image modified from Stone et al.28, 29 DTaP = diphtheria, tetanus, and acellular pertussis; IPV = inactivated polio vaccine
Because of the short time between immunization and onset of symptoms, causality assessment of true immediate hypersensitivity is often more straightforward, but there are reactions which may present with similar symptoms and signs which are not true IgE‐mediated reactions. Mimics of immediate hypersensitivity do not utilize an IgE mediated mechanism, and include vasovagal syncope (especially amongst teenagers) and hypotonic hyporesponsive episodes.20, 21 Delayed onset urticaria, irritability, drowsiness, hypotonia, and febrile seizures are other examples of more adverse events that may be falsely labelled as allergic.20, 21 Within a dedicated Italian vaccine adverse event clinic, Donà et al. report that IgE‐mediated anaphylaxis accounted for 10% of paediatric referrals, with the remainder comprised of these other causes.20 Within a random VAERS sample, there was 1 case of reported anaphylaxis out of 100 event reports.15
In addition, there are delayed antibody mediated hypersensitivity reactions that have been mechanistically suggested to be related to complement activation, immune complex deposition (type 3 hypersensitivity or an Arthus reaction) or other less‐well defined mechanisms, including T‐cell mediated processes or, less likely, late activation of the IgE system.22
2.2. Role of vaccine excipients in immediate hypersensitivity reactions
Unlike drugs, excipients represent a major contributor to specific IgE and immediate reactions associated with vaccines. However, immediate hypersensitivity reactions to vaccines which meet definitions for IgE‐mediated reactions or anaphylaxis are exceedingly rare, occurring in <1 case per million doses administered.23 The major common predictor and mechanism of immediate hypersensitivity during vaccination is the presence of pre‐existing allergy to a vaccine excipient, such as egg,24 gelatine25, 26, 27 and, most recently, galactose‐α, 1,3, galactose, commonly referred to as alpha‐gal.28, 29 This differs significantly from anaphylaxis related to drugs where anaphylaxis is usually related to the active drug component and not the excipient.
Ideally, identification and reporting of cases of vaccine excipient anaphylaxis should progress through recognizable stages (Figure 2). Cases should be evaluated using causality assessment and hypersensitivity should be confirmed using published immediate hypersensitivity skin testing strategies19 to prove immediate reactivity to a vaccine and/or a specific excipient.
Figure 2.
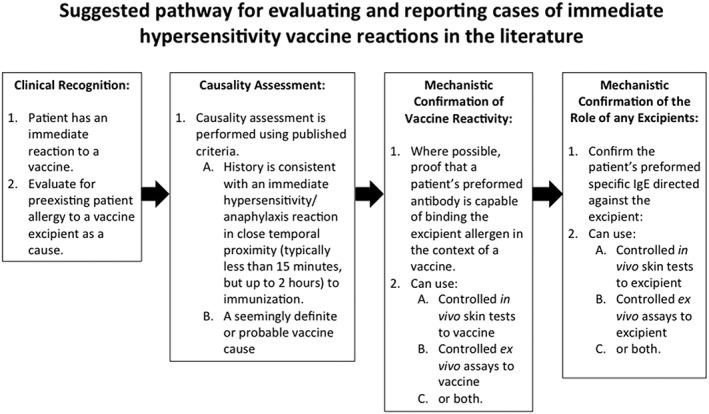
Suggested pathway for evaluating and reporting cases of immediate hypersensitivity vaccine reactions in the literature26, 27, 28, 29
We suggest that these criteria also be considered when interpreting the literature of vaccine anaphylaxis, especially when the number of reactions to an excipient is limited to isolated case reports. These criteria were utilized in the creation of Table 1.
Table 1.
Immediate and delayed excipient‐mediated reactions to vaccines
| Pre‐existing allergen | Excipient causes immediate vaccine reaction | Excipient causes delayed vaccine reaction | Relevant vaccines |
|---|---|---|---|
| Foods: | • MMR | ||
| Gelatine | Yes25, 26, 27, 28, 29, 32, 114, 115, 116, 117 | Not reported | • MMRV |
| • varicella | |||
| • yellow fever | |||
| • zoster | |||
| Alpha‐gal | Yes, in some allergic recipients28, 29, 118 alpha‐gal may confound the diagnosis for some gelatine allergies, or require co‐presence of gelatine allergy to cause reaction. | Not reported | • MMR |
| • MMRV | |||
| • varicella | |||
| • zoster | |||
| Potential concern: | |||
| • intranasal live attenuated influenza vaccine | |||
| • yellow fever | |||
| Egg | Yes24, 119 | Not reported | Potential concern: |
| • rabies | |||
| • yellow fever | |||
| Previous concern, no longer clinically relevant: | |||
| • influenza31, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130 | |||
| • MMR30, 131, 132 | |||
| Cow's milk (severe) | Possible133, 134, 135 | Not reported | • DTaP, Tdap |
| • OPV | |||
| Chicken | Possible136 | Not reported | • yellow fever |
| Yeast | Possible137 | Not reported | • hepatitis B |
| Nonfoods: | |||
|
Preservatives/adjuvants (aluminium, thimerosal, phenoxyethanol) |
Yes, thimerosal in 1 patient with preceding contact allergy34
Aluminium and phenoxyethanol |
Reports typically describe large local reaction with positive patch testing.140, 141, 142, 143, 144, 145, 146 Disseminated rash147, 148 and sterile granulomas/abscesses149, 150, 151 also reported. | Various19, 152, 153 |
| • aluminium used as adjuvant | |||
| • Thimerosal used as preservative; overall use is declining | |||
| • Phenoxyethanol used as preservative | |||
|
Antimicrobials (neomycin, polymyxin B, kanamycin, gentamicin, streptomycin, chlorotetracycline and amphotericin B) |
Possible154, 155 | Reports typically describe large local reaction and positive patch testing. | Various19, 152, 153 |
| Latex | Yes156, 157 | Contact allergy to latex very common but does not appear to increase vaccine reactions. |
Vaccines with rubber latex in syringes, vials, diluents, caps or packaging19 are becoming less common.
Most experts recommend vaccination followed by observation.19 |
| Dextran | Yes, possibly non‐IgE mediated.158, 159 | Not reported | No vaccines containing dextran currently available on the market. |
| Polysorbates/polyethylene glycols |
One case reported.160
Immediate IgE mediated hypersensitivity has recently been reported and sensitization has been demonstrated with positive vaccine skin test in a sensitized individual.161, 162, 163 |
Not reported |
• HPV vaccine reported160 |
MMR = measles, mumps, rubella; MMRV = measles, mumps, rubella, varicella; DTaP= diphtheria, tetanus and acellular pertussis; Tdap = tetanus, diphtheria and acellular pertussis; OPV = oral polio vaccine; HPV = human papilloma virus
2.2.1. Interventions to reduce excipient mediated immediate hypersensitivity
Intentional efforts to minimize egg protein and careful study have subsequently made the ongoing risk of egg allergy mediated reactions during vaccination with measles–mumps–rubella30 and influenza vaccines31 minimal. Efforts to reduce gelatine content in vaccines given in Japan and Germany subsequently reduced allergic vaccine adverse events.32, 33 These success stories provide a framework to consider when there is a need to reduce immediate hypersensitivity to a vaccine excipient.
2.2.2. T‐cell mediated hypersensitivity
Most delayed‐type hypersensitivity reactions (type IV hypersensitivity) are T‐cell mediated reactions that can be both CD4+ and/or CD8+ dependent, with a target allergen presented via major histocompatibility molecules to T‐cell receptors.16 Activation of CD4+ T cells results in cytokine mediated inflammation which is typically confined to a local area, but can sometimes be widespread.16 In reactions where CD8+ T cells are involved, the release of perforin and granzyme can lead to bystander cell injury and death by apoptosis.16 Symptoms of delayed hypersensitivity generally have onset within 6 hours to weeks and can range widely from localized skin symptoms to disseminated rashes with systemic symptoms and/or blistering of the skin and mucosal surfaces.34
Locally confined reactions to vaccines, with prolonged warmth, redness, swelling, rash or malaise is the most common type of immune‐mediated reaction after vaccination, and may represent the extreme spectrum of normal immune responses leading to immunity. Low‐acuity delayed‐type immune‐mediated reactions have a typical onset of hours to days of reactions, but can be delayed up to 2–3 weeks, making it difficult to determine definitive causality.15 The most common type of such reactions reported are delayed onset papular rashes,35 which can be confusing since these are a very common occurrence in childhood and generally associated with viruses, rather than the vaccine itself. Local delayed hypersensitivity reactions confined to the site of the vaccine are not a contraindication to future vaccination.36 A rare disseminated papular rash with delayed hypersensitivity features has been described in adult military recruits 10–18 days after receipt of the current smallpox vaccine (ACAM2000; Figure 3).37
Figure 3.
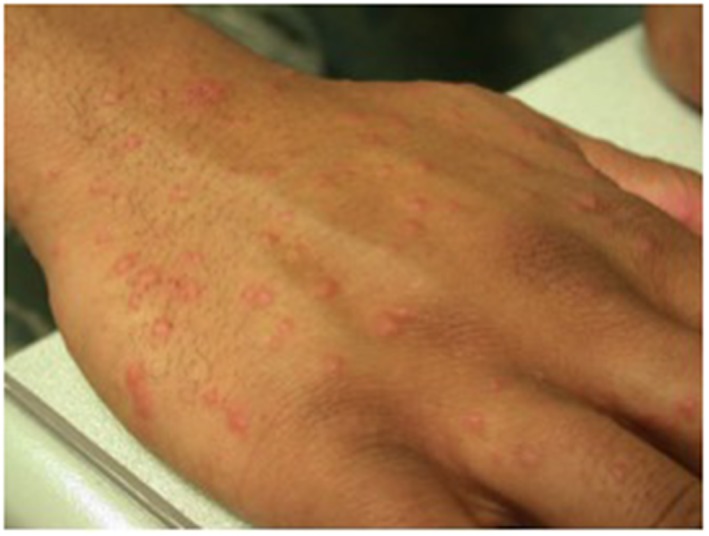
Disseminated papular rash after receipt of smallpox vaccine (ACAM2000), without detectable intralesional virus37
By contrast, more severe versions of delayed‐type hypersensitivity such as Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) have been reported in exceedingly rare cases only.38, 39, 40 It is thought in most cases that these are single events related to a response to virotopes present within the vaccine and unlikely to occur in the future. SJS/TEN needs to be differentiated from erythema multiforme major (EMM) which has been associated with a viruses such as herpes simplex virus 1, Mycoplasma pneumoniae as well as rarely with vaccines.41, 42 EMM, unlike SJS/TEN, is recurrent in the absence of re‐exposure to the initial inciting event.43
Other rare delayed cutaneous reactions potentially associated with vaccines have been reported (Table 2) and include acute generalized exanthematous pustulosis,44, 45 erythema nodosum,46, 47, 48, 49 granuloma annulare,50 bullous pemphigoid,51, 52, 53 Sweet's syndrome,54, 55, 56, 57, 58, 59 Gianotti–Crosti syndrome,60 lichenoid eruptions,46, 50, 61, 62, 63, 64, 65, 66 cutaneous lupus,46, 67 lupus vulgaris68, 69, 70 and serum sickness‐like reactions.71, 72, 73, 74, 75, 76 Similar to vaccine associated EMM, the presence of an ongoing infection prior to both vaccination and the development of these cutaneous syndromes is frequently reported in these cases. Most reports provide follow‐up data that there was no recurrence of symptoms upon subsequent booster doses of the associated vaccines. Causality assessment has only rarely been performed in these reports and underlying host risk factors including genetic predisposition are currently unknown.77, 78, 79, 80
Table 2.
Immunological reactions to vaccines, by associated vaccine
| Reaction | Associated vaccines |
|---|---|
| Immediate hypersensitivity164 | Hepatitis B |
| Influenza | |
| Measles, mumps and rubella (MMR) | |
| Tetanus toxoid containing vaccines | |
| Varicella | |
| Yellow fever | |
| Zoster | |
| Serum sickness or serum sickness‐like71, 72, 73, 74, 75, 76 | Hepatitis B |
| Influenza | |
| Influenza (H1N1) | |
| Pneumococcal | |
| Rabies | |
| Tetanus | |
| Severe delayed cutaneous hypersensitivity (SJS or TEN)38, 39, 40 | Hantavirus |
| Influenza | |
| MMR | |
| Rabies | |
| Smallpox | |
| Erythema multiforme major38, 39, 40 | Diphtheria and tetanus (DT) |
| Diphtheria, pertussis and tetanus (DPT) | |
| Haemophilus influenza B | |
| Hepatitis B | |
| Human papillomavirus (HPV) | |
| Influenza (H1N1) | |
| MMR | |
| Meningococcal | |
| Inactivated polio vaccine (IPV) | |
| Oral polio vaccine (OPV) | |
| Smallpox | |
| Varicella | |
| Guillain‐Barré syndrome86, 89, 90, 91, 92, 93, 164 | Influenza |
| OPV | |
| Rabies | |
| Tetanus toxoid containing vaccines | |
| Risk for disseminated/prolonged infection in severe cellular immunodeficiency107, 108, 109 | Bacille Calmette–Guérin (BCG) |
| Live attenuated influenza | |
| MMR | |
| OPV | |
| Oral typhoid vaccine | |
| Rotavirus | |
| Smallpox | |
| Varicella | |
| Yellow fever | |
| Risk for disseminated/prolonged infection in severe humoral immunodeficiency107, 108, 109 | Live attenuated influenza |
| Oral typhoid vaccine | |
| OPV | |
| Smallpox | |
| Yellow fever | |
| Acute generalized exanthematous pustulosis44, 45 | DPT |
| Influenza | |
| MMR | |
| Pneumococcal vaccine | |
| Erythema nodosum47, 48, 49, 165, 166 | BCG |
| Hepatitis B | |
| HPV | |
| Rabies | |
| Tetanus, diphtheria, and acellular pertussis (TDaP) | |
| Typhoid | |
| Granuloma annulare50, 167, 168, 169 | BCG |
| DT | |
| Hepatitis B | |
| Bullous pemphigoid51, 52, 53 | Hepatitis B |
| DT | |
| DPT | |
| Influenza | |
| MMR | |
| Meningococcal | |
| Pneumococcal | |
| Smallpox | |
| Sweet's syndrome54, 55, 56, 57, 58, 59 | BCG |
| Influenza | |
| Influenza (H1N1) | |
| Pneumococcal | |
| Gianotti‐Crosti syndrome60 | DPT |
| Hepatitis A | |
| Hepatitis B | |
| Influenza | |
| Japanese encephalitis | |
| MMR | |
| Lichenoid eruptions46, 50, 61, 62, 63, 64, 65, 66 | BCG |
| Hepatitis B | |
| HPV | |
| Influenza | |
| Pneumococcal | |
| Yellow fever | |
| Cutaneous lupus46, 67 | Hepatitis B |
| Influenza | |
| Lupus vulgaris68, 69, 70 | BCG |
SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis
2.3. Excipient allergies and delayed vaccine hypersensitivity
Delayed type hypersensitivity reactions against vaccine excipients have also been described (Table 1) and may present as a generalized reaction or a contact reaction over the site of the vaccine. In general, these reactions are of lesser severity and localized, but some reported excipient‐mediated vaccine reactions are consistent with disseminated cutaneous rashes or other symptomatology. Currently there have not been any major attempts to determine how often or the mechanism by which delayed hypersensitivity responses against a vaccine excipient occurs, due to the typically lower severity. There are currently no validated testing strategies for ascertaining cases of delayed hypersensitivity to a vaccine. Subsequent causality assessment should attempt to prove the likeliness of the role of the vaccine or excipient.81 If a vaccine excipient is suspected in particular there is a potential role for delayed intradermal skin testing and/or patch testing with the vaccine and the excipient separately (Figure 4).34
Figure 4.
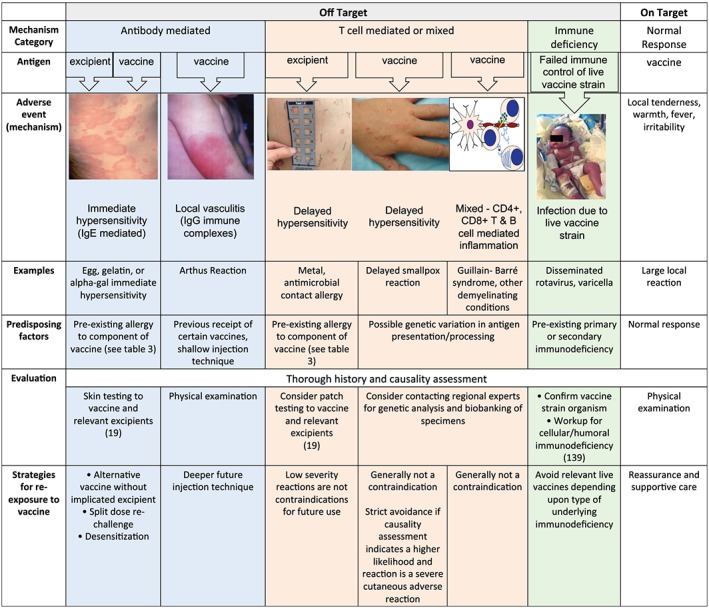
Mechanism category, adverse events, examples, predisposing factors, evaluation and strategies for management of immune‐mediated adverse events to vaccines. The blue column represents immediate‐type reactions, the pink columns represent delayed‐type reactions, and the green column represents failure to control live vaccine strain leading to a prolonged or disseminated vaccine strain infection19, 108
2.3.1. Guillain–Barré syndrome
Immunologically mediated neurological complications such as Guillain–Barré syndrome and other demyelinating neuropathies (Bell's palsy, acute disseminated encephalomyelitis, etc.) are a known and reported adverse event related to immunization,13 but such events are exceedingly rare, occurring after <1 per million doses of vaccines administered.23, 82, 83, 84, 85 Guillain–Barré syndrome suspected as a vaccine‐associated event through case reports.36, 86, 87 Symptoms include progressive neuromuscular weakness, usually beginning in the extremities and then centrally, with potential progression toward respiratory failure or cranial nerve weakness.88 Onset of symptoms is considered as possibly linked to vaccination if it occurs within 6 weeks after a dose of tetanus,89, 90 oral polio,86 rabies91 or influenza92, 93 containing vaccines. Postvaccination Guillain–Barré syndrome, similar to that occurring after an acute infection, is thought to be a mixed, delayed immune‐mediated reaction, which probably represents a T cell response where CD4+ and CD8+ T cells cross‐recognize specific virotopes and similar self‐antigens in the nervous system, leading to either an axonal or demyelinating clinical subtype.91 This mixed delayed inflammatory reaction is not easily characterized but has a high level of severity; 20–30% of cases develop respiratory failure.91 Due to the delayed onset of symptoms, a more thorough understanding of Guillain–Barré events is crucial for their diagnosis and to assess whether vaccination was in the causal pathway.13, 15 Other patient specific factors need to be taken into account, such as preceding infections with Campylobacter jejuni, cytomegalovirus, Epstein–Barr virus, influenza A virus, Mycoplasma pneumoniae or Haemophilus influenzae that could be the actual trigger.94, 95, 96 Interestingly, it is unknown whether the influenza vaccine may be protective against the subsequent development of Guillain–Barré during natural influenza A infection.91 It is known that influenza vaccination after a previous episode of Guillain–Barré syndrome does not precipitate recurrence of symptoms.97
2.3.2. Disseminated infections in immunocompromised populations
Disseminated or prolonged vaccine‐strain infections are an exceedingly rare complication after receiving a live vaccine. Symptoms are typically consistent with a primary infection from the organism, but with progression to a more severe outcome into an immunocompromised host. Such infections have been reported with smallpox,37 varicella,98 rotavirus,99, 100, 101 yellow fever,102 measles–mumps–rubella,103 oral polio104 and Bacille Calmette–Guérin (BCG) vaccines.105 While these cases are exceedingly rare amongst the general population, they are more common amongst those with either primary or acquired immunodeficiencies.98, 104, 106 Severe T‐cell immunodeficiency or a household member with a similar immunodeficiency is therefore a strict contraindication to immunization with any form of live vaccines (Figure 4).107, 108 Similarly, vaccination with mucosally‐administered vaccines (oral typhoid, oral polio, live attenuated influenza) and yellow fever vaccines is contraindicated in severe humoral immunodeficiency.109 Adverse outcomes of this type highlight the importance of newborn screening programmes for severe combined immunodeficiencies.99, 100, 101 Ideal case ascertainment should confirm detection of a vaccine strain organism in a patient with a confirmed immune deficiency and a confirmed vaccine receipt. Conversely, identification of a vaccine strain infection after receipt of a live vaccine should prompt evaluation for immune deficiency.
3. DISCUSSION
Adverse reactions to vaccines that are the result of either an immune‐mediated reaction to the vaccine excipient, the active components of the vaccine or related to host immunodeficiency are rare and occur in <1 per million vaccines administered. At the same time, increasing attention by the public is focused on these infrequent risks of vaccination.4, 5 The previously described crisis of confidence in vaccination largely centres around an overemphasis upon these rare events or upon a fear of other events such as autism for which an evidence base exists to show them as unrelated to vaccination.7, 8, 9, 110, 111, 112 The current unpredictability of vaccine‐related adverse events contributes to their occurrence and provides the opportunity for mistrust amongst segments of the public. With risk factor and mechanism identification comes the opportunity to avoid adverse events by alteration of vaccines or use of risk stratifying vaccination strategies, as has been demonstrated with egg and gelatine allergy.30, 31, 32, 33 There are excellent methods in place for assessing the causality of vaccine associated events in most countries. We believe that the future direction of this field will require strengthening these networks to include biobanking of specimens along with additional epidemiological, mechanistic and genetic study of true adverse events.43, 113 Such infrastructure will serve as the vehicle by which to drive the field of immunization ever closer to the patient centred goals of predictability, personalized medicine and minimized adverse vaccine reactions.
Given the importance of vaccination to public health, a nonspecific diagnosis of immediate vaccine allergy is too imprecise to guide efforts at quality and safety improvement, and may do more harm than good. Hence, as future cases of hypersensitivity emerge, we believe they should be evaluated with increasingly stringent criteria that require initial causality assessment followed by in vivo or in vitro assessment of mechanisms. Validated and widely accepted approaches for mechanistic assessment need to be defined. From the patient perspective, understanding and knowledge of the cause of a reaction provides reassurance and can guide selection of future vaccination approaches.
Postvaccination delayed‐type hypersensitivity reactions and immunologically mediated neurological conditions are currently under‐studied, and there are no a priori predictors or diagnostic tools available for these conditions. Future research directions involve understanding the immunopathogenesis of these reactions and appropriate biobanking of DNA and cellular materials for future genetic and mechanistic studies.
Similarly, while there are already criteria in place for administration of vaccines in immune deficient patients,19 additional mechanistic research into immune deficiency related postvaccination adverse events should be targeted toward identifying host immune risk factors that predict adverse events and lead to improved screening programmes.
The ability of the healthcare system and vaccine safety researchers to evolve in these future directions will help bolster trust in 1 of the most important public health interventions ever created.
4. CONCLUSIONS
In summary, adverse reactions to vaccines that are either the direct result of an immune‐mediated reaction to the vaccine excipient, the active components of the vaccine or related to host immunodeficiency are rare (Table 2) and fortunately defined diagnosis and management strategies exist (Figure 4). Although many immune‐mediated vaccine reactions lack risk factors and mechanisms, excipient allergy and immune deficiency are 2 known mechanisms by which the immune system precipitates adverse events after vaccination. The specific mechanisms and immunological risk factors by which T‐cell mediated illnesses such as SJS/TEN or inflammatory neurological conditions such as Guillain–Barré can occur after vaccination are not known but may be less commonly related to a vaccine than the natural viral illness. Where predictability has been obtained and the implementation of mitigation strategies was performed (egg, gelatine allergy), there have been associated increases in safety and confidence. Improved understanding of mechanistic risk factors for severe immunologically mediated vaccine reactions and a shift toward mechanistic causality assessment are crucial future directions for maintaining this vital public health intervention.
ACKNOWLEDGEMENTS
Dr Stone received funding support related to this project from NIH/NIGMS T32 GM007569.
Dr Phillips receives funding from: National Institutes of Health (1P50GM115305–01, R21AI139021 and R34AI136815–1), National Health and Medical Research Foundation of Australia.
COMPETING INTERESTS
There are no competing interests to declare.
There is no principal investigator for this study.
Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694–2706. 10.1111/bcp.14112
REFERENCES
- 1. World Health Organization . Immunization Coverage; 2018. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
- 2. CDC . Infographic: The Global Impact of Vaccines in Reducing Vaccine‐Preventable Disease Morbidity and Mortality; 2017. https://www.cdc.gov/globalhealth/infographics/immunization/global_impact_of_vaccines.htm: CDC.
- 3. Minor P. Live attenuated vaccines: historical successes and current challenges. Virology. 2015;479–480:379‐392. [DOI] [PubMed] [Google Scholar]
- 4. Karafillakis E, Larson H. Consortium a. the benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35(37):4840‐4850. [DOI] [PubMed] [Google Scholar]
- 5. Siddiqui M, Salmon D, Omer S. Epidemiology of vaccine hesitancy in the United States. Hum Vaccin Immunother. 2013;9(12):2643‐2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith P, Chu S, Barker L. Children who have received no vaccines: who are they and where do they live? Pediatrics. 2004;114(1):187‐195. [DOI] [PubMed] [Google Scholar]
- 7. Black S, Rappuoli R. A crisis of public confidence in vaccines. Sci Transl Med. 2010;2(61):61mr1. [DOI] [PubMed] [Google Scholar]
- 8. Larson H, Cooper L, Eskola J, Katz S, Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526‐535. [DOI] [PubMed] [Google Scholar]
- 9. Cooper L, Larson H, Katz S. Protecting public trust in immunization. Pediatrics. 2008;122(1):149‐153. [DOI] [PubMed] [Google Scholar]
- 10. Nelson R. US measles outbreak concentrated among unvaccinated children. Lancet Infect Dis. 2019;19(3):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parrella A, Gold M, Marshall H, Braunack‐Mayer A, Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine. 2013;31(16):2067‐2074. [DOI] [PubMed] [Google Scholar]
- 12. Maure C, Dodoo A, Bonhoeffer J, Zuber P. The global vaccine safety initiative: enhancing vaccine pharmacovigilance capacity at country level. Bull World Health Organ. 2014;92(9):695‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams S, Klein N, Halsey N, et al. Overview of the clinical consult case review of adverse events following immunization: clinical immunization safety assessment (CISA) network 2004‐2009. Vaccine. 2011;29(40):6920‐6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baggs J, Gee J, Lewis E, et al. The vaccine safety datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(Suppl 1):S45‐S53. [DOI] [PubMed] [Google Scholar]
- 15. Loughlin A, Marchant C, Adams W, et al. Causality assessment of adverse events reported to the vaccine adverse event reporting system (VAERS). Vaccine. 2012;30(50):7253‐7259. [DOI] [PubMed] [Google Scholar]
- 16. Abbas AK. Cellular and molecular immunology. In: Lichtman AH, Pillai S, Abbas AK, eds. . Ninth ed. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 17. Stone C Jr, Brown N. Angiotensin‐converting enzyme inhibitor and other drug‐associated angioedema. Immunol Allergy Clin North Am. 2017;37(3):483‐495. [DOI] [PubMed] [Google Scholar]
- 18. Joint Task Force on Practice Parameters . Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259‐273. [DOI] [PubMed] [Google Scholar]
- 19. Kelso J, Greenhawt M, Li J, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25‐43. [DOI] [PubMed] [Google Scholar]
- 20. Dona D, Masiero S, Brisotto S, et al. Special immunization service: a 14‐year experience in Italy. PLoS ONE. 2018;13(4):e0195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCallum A, Duncan C, MacDonald R, Jones M. A decade of vaccinating allergic travellers: a clinical audit. Travel Med Infect Dis. 2011;9(5):231‐237. [DOI] [PubMed] [Google Scholar]
- 22. Dreskin S, Halsey N, Kelso J, et al. International consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohlke K, Davis R, Marcy S, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112(4):815‐820. [DOI] [PubMed] [Google Scholar]
- 24. Kelso J. Raw egg allergy‐a potential issue in vaccine allergy. J Allergy Clin Immunol. 2000;106(5):990. [DOI] [PubMed] [Google Scholar]
- 25. Sakaguchi M, Yamanaka T, Ikeda K, et al. IgE‐mediated systemic reactions to gelatin included in the varicella vaccine. J Allergy Clin Immunol. 1997;99(2):263‐264. [DOI] [PubMed] [Google Scholar]
- 26. Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate‐type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol. 1996;98(6 Pt 1):1058‐1061. [DOI] [PubMed] [Google Scholar]
- 27. Kelso J, Jones R, Yunginger J. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993;91(4):867‐872. [DOI] [PubMed] [Google Scholar]
- 28. Stone C Jr, Commins S, Choudhary S, et al. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha‐gal allergy. J Allergy Clin Immunol Pract. 2018;7:322‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone C Jr, Hemler J, Commins S, et al. Anaphylaxis after zoster vaccine: implicating alpha‐gal allergy as a possible mechanism. J Allergy Clin Immunol. 2017;139(5):1710‐1713. e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen D, Jorgensen I. MMR vaccination of children with egg allergy is safe. Dan Med J. 2013;60(2):A4573. [PubMed] [Google Scholar]
- 31. Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn‐Lajeunesse M, Investigators S‐S. Safety of live attenuated influenza vaccine in young people with egg allergy: multicentre prospective cohort study. BMJ. 2015;351:h6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakayama T, Aizawa C. Change in gelatin content of vaccines associated with reduction in reports of allergic reactions. J Allergy Clin Immunol. 2000;106(3):591‐592. [DOI] [PubMed] [Google Scholar]
- 33. Zent O, Hennig R. Post‐marketing surveillance of immediate allergic reactions: polygeline‐based versus polygeline‐free pediatric TBE vaccine. Vaccine. 2004;23(5):579‐584. [DOI] [PubMed] [Google Scholar]
- 34. Phillips E, Bigliardi P, Bircher A, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. 2019;143(1):66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caubet J, Ponvert C. Vaccine allergy. Immunol Allergy Clin North Am. 2014;34(3):597‐613. ix [DOI] [PubMed] [Google Scholar]
- 36. National Center for I, Respiratory D . General recommendations on immunization ‐‐‐ recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2011;60(2):1‐64. [PubMed] [Google Scholar]
- 37. Beachkofsky T, Carrizales S, Bidinger J, Hrncir D, Whittemore D, Hivnor C. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146(6):656‐661. [DOI] [PubMed] [Google Scholar]
- 38. Ma L, Du X, Dong Y, et al. First case of Stevens‐Johnson syndrome after rabies vaccination. Br J Clin Pharmacol. 2018;84(4):803‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chahal D, Aleshin M, Turegano M, Chiu M, Worswick S. Vaccine‐induced toxic epidermal necrolysis: a case and systematic review. Dermatol Online J. 2018;3(1):1–10. pii: 13030/qt7qn5268. [PubMed] [Google Scholar]
- 40. Christou E, Wargon O. Stevens‐Johnson syndrome after varicella vaccination. Med J Aust. 2012;196(4):240‐241. [DOI] [PubMed] [Google Scholar]
- 41. Canavan T, Mathes E, Frieden I, Shinkai K. Mycoplasma pneumoniae‐induced rash and mucositis as a syndrome distinct from Stevens‐Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 42. Keller N, Gilad O, Marom D, Marcus N, Garty B. Nonbullous erythema Multiforme in hospitalized children: a 10‐year survey. Pediatr Dermatol. 2015;32(5):701‐703. [DOI] [PubMed] [Google Scholar]
- 43. White K, Abe R, Ardern‐Jones M, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6(1):38‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuo S, Nishizawa A, Oshio‐Yoshii A, Satoh T. Influenza vaccine‐induced acute generalized exanthematous pustulosis during pregnancy. J Dermatol. 2017;44(5):598‐599. [DOI] [PubMed] [Google Scholar]
- 45. Ersoy S, Paller A, Mancini A. Acute generalized exanthematous pustulosis in children. Arch Dermatol. 2004;140(9):1172‐1173. [DOI] [PubMed] [Google Scholar]
- 46. Stavrianeas N, Katoulis A, Kanelleas A, Hatziolou E, Georgala S. Papulonodular lichenoid and pseudolymphomatous reaction at the injection site of hepatitis B virus vaccination. Dermatology. 2002;205(2):166‐168. [DOI] [PubMed] [Google Scholar]
- 47. Sandre M, Poenaru S, Boggild A. Erythema Nodosum Leprosum triggered by antecedent influenza vaccine and respiratory tract infection: a case report. J Cutan Med Surg. 2018;1:114‐116. [DOI] [PubMed] [Google Scholar]
- 48. Cohen P. Combined reduced‐antigen content tetanus, diphtheria, and acellular pertussis (tdap) vaccine‐related erythema nodosum: case report and review of vaccine‐associated erythema nodosum. Dermatol Ther (Heidelb). 2013;3(2):191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Longueville C, Doffoel‐Hantz V, Hantz S, et al. Gardasil(R)‐induced erythema nodosum. Rev Med Interne. 2012;33(3):e17‐e18. [DOI] [PubMed] [Google Scholar]
- 50. Criado P, de Oliveira Ramos R, Vasconcellos C, Jardim Criado R, Valente N. Two case reports of cutaneous adverse reactions following hepatitis B vaccine: lichen planus and granuloma annulare. J Eur Acad Dermatol Venereol. 2004;18(5):603‐606. [DOI] [PubMed] [Google Scholar]
- 51. Sezin T, Egozi E, Hillou W, Avitan‐Hersh E, Bergman R. Anti‐laminin‐332 mucous membrane pemphigoid developing after a diphtheria tetanus vaccination. JAMA Dermatol. 2013;149(7):858‐862. [DOI] [PubMed] [Google Scholar]
- 52. Bisherwal K, Pandhi D, Singal A, Sharma S. Infantile bullous pemphigoid following vaccination. Indian Pediatr. 2016;53(5):425‐426. [DOI] [PubMed] [Google Scholar]
- 53. de la Fuente S, Hernandez‐Martin A, de Lucas R, et al. Postvaccination bullous pemphigoid in infancy: report of three new cases and literature review. Pediatr Dermatol. 2013;30(6):741‐744. [DOI] [PubMed] [Google Scholar]
- 54. Pedrosa A, Morais P, Nogueira A, Pardal J, Azevedo F. Sweet's syndrome triggered by pneumococcal vaccination. Cutan Ocul Toxicol. 2013;32(3):260‐261. [DOI] [PubMed] [Google Scholar]
- 55. Hali F, Sbai M, Benchikhi H, Ouakadi A, Zamiati S. Sweet's syndrome after H1N1 influenza vaccination. Ann Dermatol Venereol. 2010;137(11):740‐741. [DOI] [PubMed] [Google Scholar]
- 56. Jovanovic M, Poljacki M, Vujanovic L, Duran V. Acute febrile neutrophilic dermatosis (Sweet's syndrome) after influenza vaccination. J Am Acad Dermatol. 2005;52(2):367‐369. [DOI] [PubMed] [Google Scholar]
- 57. Carpentier O, Piette F, Delaporte E. Sweet's syndrome after BCG vaccination. Acta Derm Venereol. 2002;82(3):221. [DOI] [PubMed] [Google Scholar]
- 58. Maddox P, Motley R. Sweet's syndrome: a severe complication of pneumococcal vaccination following emergency splenectomy. Br J Surg. 1990;77(7):809‐810. [DOI] [PubMed] [Google Scholar]
- 59. Radeff B, Harms M. Acute febrile neutrophilic dermatosis (Sweet's syndrome) following BCG vaccination. Acta Derm Venereol. 1986;66(4):357‐358. [PubMed] [Google Scholar]
- 60. Retrouvey M, Koch L, Williams J. Gianotti‐Crosti syndrome after childhood vaccination. Pediatr Dermatol. 2012;29(5):666‐668. [DOI] [PubMed] [Google Scholar]
- 61. Karouni M, Kurban M, Abbas O. Lichen striatus following yellow fever vaccination in an adult woman. Clin Exp Dermatol. 2017;42(7):823‐824. [DOI] [PubMed] [Google Scholar]
- 62. Cohen P. Injection site lichenoid dermatitis following pneumococcal vaccination: report and review of cutaneous conditions occurring at vaccination sites. Dermatol Ther (Heidelb). 2016;6(2):287‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Laschinger M, Schleichert R, Green B. Lichenoid drug eruption after human papillomavirus vaccination. Pediatr Dermatol. 2015;32(2):e48‐e49. [DOI] [PubMed] [Google Scholar]
- 64. de Golian E, Brennan C, Davis L. Lichenoid drug reaction following influenza vaccination in an HIV‐positive patient: a case report and literature review. J Drugs Dermatol. 2014;13(7):873‐875. [PubMed] [Google Scholar]
- 65. Zaki S, Sanjeev S. Lichen striatus following BCG vaccination in an infant. Indian Pediatr. 2011;48(2):163‐164. [PubMed] [Google Scholar]
- 66. Hwang S, Ahn S, Lee S, Choi E. Lichen striatus following BCG vaccination. Clin Exp Dermatol. 1996;21(5):393‐394. [DOI] [PubMed] [Google Scholar]
- 67. Rosenblatt A, Stein S. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33(3):327‐332. [DOI] [PubMed] [Google Scholar]
- 68. Najem N, Zadeh V, Al‐Abdulrazzaq A, Al‐Otaibi S, Kadyan S, Joneja M. Bacillus Calmette‐Guerin vaccine‐ induced lupus vulgaris in a child. Acta Dermatovenerol Alp Panonica Adriat. 2009;18(4):195‐197. [PubMed] [Google Scholar]
- 69. Samuel A, Browning J, Campbell J, Metry D. Bacillus Calmette‐Guerin vaccine‐induced lupus vulgaris in a child adopted from China. Pediatr Dermatol. 2007;24(5):E44‐E46. [DOI] [PubMed] [Google Scholar]
- 70. Tan H, Seow C. A review of cutaneous granulomas and lupus vulgaris following BCG vaccination in a skin hospital in Singapore. Ann Acad Med Singapore. 2002;31(5):663‐665. [PubMed] [Google Scholar]
- 71. Chiong F, Loewenthal M, Boyle M, Attia J. Serum sickness‐like reaction after influenza vaccination. BMJ Case Rep. 2015;2015:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bonds R, Kelly B. Severe serum sickness after H1N1 influenza vaccination. Am J Med Sci. 2013;345(5):412‐413. [DOI] [PubMed] [Google Scholar]
- 73. Hengge U, Scharf R, Kroon F, Pfeffer K. Severe serum sickness following pneumococcal vaccination in an AIDS patient. Int J STD AIDS. 2006;17(3):210‐211. [DOI] [PubMed] [Google Scholar]
- 74. Arkachaisri T. Serum sickness and hepatitis B vaccine including review of the literature. J Med Assoc Thai. 2002;85(Suppl 2):S607‐S612. [PubMed] [Google Scholar]
- 75. Warrington R, Martens C, Rubin M, Rutherford W, Aoki F. Immunologic studies in subjects with a serum sickness‐like illness after immunization with human diploid cell rabies vaccine. J Allergy Clin Immunol. 1987;79(4):605‐610. [DOI] [PubMed] [Google Scholar]
- 76. Daschbach R. Serum sickness and tetanus immunization. JAMA. 1972;220(12):1619. [DOI] [PubMed] [Google Scholar]
- 77. Mallal S, Phillips E, Carosi G, et al. HLA‐B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568‐579. [DOI] [PubMed] [Google Scholar]
- 78. Karnes J, Miller M, White K, et al. Applications of Immunopharmacogenomics: predicting, preventing, and understanding immune‐mediated adverse drug reactions. Annu Rev Pharmacol Toxicol. 2019;59(1):463‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Konvinse K, Trubiano J, Pavlos R, et al. HLA‐A*32:01 is strongly associated with vancomycin‐induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. 2019;144(1):183‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Garon S, Pavlos R, White K, Brown N, Stone C Jr, Phillips E. Pharmacogenomics of off‐target adverse drug reactions. Br J Clin Pharmacol. 2017;83(9):1896‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Halsey N, Edwards K, Dekker C, et al. Algorithm to assess causality after individual adverse events following immunizations. Vaccine. 2012;30(39):5791‐5798. [DOI] [PubMed] [Google Scholar]
- 82. Vanlander A, Hoppenbrouwers K. Anaphylaxis after vaccination of children: review of literature and recommendations for vaccination in child and school health services in Belgium. Vaccine. 2014;32(26):3147‐3154. [DOI] [PubMed] [Google Scholar]
- 83. Stratton K, Howe C, Johnston R., Jr (Eds). Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 1994. [PubMed] [Google Scholar]
- 84. McNeil M, Weintraub E, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oberle D, Pavel J, Rieck T, et al. Anaphylaxis after immunization of children and adolescents in Germany. Pediatr Infect Dis J. 2016;35(5):535‐541. [DOI] [PubMed] [Google Scholar]
- 86. Stratton K, Howe C, Johnston R Jr. Adverse events associated with childhood vaccines other than pertussis and rubella. Summary of a report from the Institute of Medicine. JAMA. 1994;271(20):1602‐1605. [PubMed] [Google Scholar]
- 87. Pollard J, Selby G. Relapsing neuropathy due to tetanus toxoid. Report of a case. J Neurol Sci. 1978;37(1–2):113‐125. [DOI] [PubMed] [Google Scholar]
- 88. Stroke NNIoNDa . Guillain‐Barré Syndrome Fact Sheet; 2018. [4‐11‐2019]. Available from: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Guillain-Barr%C3%A9-Syndrome-Fact-Sheet.
- 89. Use of diphtheria toxoid‐tetanus toxoid‐acellular pertussis vaccine as a five‐dose series. Supplemental recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2000;49(RR‐13):1‐8. [PubMed] [Google Scholar]
- 90. Broder K, Cortese M, Iskander J, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2006;55(RR‐3):1‐34. [PubMed] [Google Scholar]
- 91. Willison H, Jacobs B, van Doorn P. Guillain‐Barre syndrome. Lancet. 2016;388(10045):717‐727. [DOI] [PubMed] [Google Scholar]
- 92. Grohskopf L, Sokolow L, Broder K, Walter E, Fry A, Jernigan D. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices‐United States, 2018‐19 influenza season. MMWR Recomm Rep. 2018;67(3):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Geier M, Geier D, Zahalsky A. Influenza vaccination and Guillain Barre syndrome. Clin Immunol. 2003;107(2):116‐121. [DOI] [PubMed] [Google Scholar]
- 94. Hughes R, Cornblath D. Guillain‐Barre syndrome. Lancet. 2005;366(9497):1653‐1666. [DOI] [PubMed] [Google Scholar]
- 95. Lehmann H, Hartung H, Kieseier B, Hughes R. Guillain‐Barre syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10(9):643‐651. [DOI] [PubMed] [Google Scholar]
- 96. Jacobs B, Rothbarth P, van der Meche F, et al. The spectrum of antecedent infections in Guillain‐Barre syndrome: a case‐control study. Neurology. 1998;51(4):1110‐1115. [DOI] [PubMed] [Google Scholar]
- 97. Kuitwaard K, Bos‐Eyssen M, Blomkwist‐Markens P, van Doorn P. Recurrences, vaccinations and long‐term symptoms in GBS and CIDP. J Peripher Nerv Syst. 2009;14(4):310‐315. [DOI] [PubMed] [Google Scholar]
- 98. Willis E, Woodward M, Brown E, et al. Herpes zoster vaccine live: a 10year review of post‐marketing safety experience. Vaccine. 2017;35(52):7231‐7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stone C Jr, Markert M, Abraham R, Norton A. A case of atypical, complete DiGeorge syndrome without 22q11 mutation. Ann Allergy Asthma Immunol. 2017;118(5):640‐642. e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Krantz M, Stone C Jr, Connelly J, Norton A, Khan Y. The effect of delayed and early diagnosis in siblings, and importance of newborn screening for SCID. Ann Allergy Asthma Immunol. 2018;122:(2)211‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: reports to the vaccine adverse events reporting system (VAERS). Vaccine. 2010;28(40):6609‐6612. [DOI] [PubMed] [Google Scholar]
- 102. Thomas R, Lorenzetti D, Spragins W, Jackson D, Williamson T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hyg. 2012;86(2):359‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bitnun A, Shannon P, Durward A, et al. Measles inclusion‐body encephalitis caused by the vaccine strain of measles virus. Clin Infect Dis. 1999;29(4):855‐861. [DOI] [PubMed] [Google Scholar]
- 104. Jorba J, Diop O, Iber J, et al. Update on vaccine‐derived polioviruses ‐ worldwide, January 2017‐June 2018. MMWR Morb Mortal Wkly Rep. 2018;67(42):1189‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marciano B, Huang C, Joshi G, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133(4):1134‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Su J, Ng C, Lewis P, Cano M. Adverse events after vaccination among HIV‐positive persons, 1990‐2016. PLoS ONE. 2018;13(6):e0199229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Advisory Committee on Immunization Practices. Contraindications and Precautions. General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP) [Internet]; 2018. 12‐3‐2018. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.pdf.
- 108. Bonilla F, Khan D, Ballas Z, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186‐1205. e1–78 [DOI] [PubMed] [Google Scholar]
- 109. Medical Advisory Committee of the Immune Deficiency F , Shearer W, Fleisher T, et al. Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol. 2014;133(4):961‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jain A, Marshall J, Buikema A, Bancroft T, Kelly J, Newschaffer C. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA. 2015;313(15):1534‐1540. [DOI] [PubMed] [Google Scholar]
- 111. Taylor L, Swerdfeger A, Eslick G. Vaccines are not associated with autism: an evidence‐based meta‐analysis of case‐control and cohort studies. Vaccine. 2014;32(29):3623‐3629. [DOI] [PubMed] [Google Scholar]
- 112. Wessel L. Vaccine myths. Science. 2017;356(6336):368‐372. [DOI] [PubMed] [Google Scholar]
- 113. Manolio T, Hutter C, Avigan M, et al. Research directions in genetic predispositions to Stevens‐Johnson syndrome/toxic epidermal necrolysis. Clin Pharmacol Ther. 2018;103(3):390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pool V, Braun M, Kelso J, et al. Prevalence of anti‐gelatin IgE antibodies in people with anaphylaxis after measles‐mumps rubella vaccine in the United States. Pediatrics. 2002;110(6):e71. [DOI] [PubMed] [Google Scholar]
- 115. Retterer M, Workman L, Bacon J, Platts‐Mills T. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol. 2018;141:1956‐1957. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sakaguchi M, Inouye S. IgE sensitization to gelatin: the probable role of gelatin‐containing diphtheria‐tetanus‐acellular pertussis (DTaP) vaccines. Vaccine. 2000;18(19):2055‐2058. [DOI] [PubMed] [Google Scholar]
- 117. Sakaguchi M, Miyazawa H, Inouye S. Specific IgE and IgG to gelatin in children with systemic cutaneous reactions to Japanese encephalitis vaccines. Allergy. 2001;56(6):536‐539. [DOI] [PubMed] [Google Scholar]
- 118. Pinson M, Waibel K. Safe administration of a gelatin‐containing vaccine in an adult with galactose‐α‐1,3‐galactose allergy. Vaccine. 2015;33(10):1231‐1232. [DOI] [PubMed] [Google Scholar]
- 119. Fasano M, Wood R, Cooke S, Sampson H. Egg hypersensitivity and adverse reactions to measles, mumps, and rubella vaccine. J Pediatr. 1992;120(6):878‐881. [DOI] [PubMed] [Google Scholar]
- 120. Chung E, Huang L, Schneider L. Safety of influenza vaccine administration in egg‐allergic patients. Pediatrics. 2010;125(5):e1024‐e1030. [DOI] [PubMed] [Google Scholar]
- 121. Clark A, Skypala I, Leech S, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40(8):1116‐1129. [DOI] [PubMed] [Google Scholar]
- 122. Cronin J, Scorr A, Russell S, McCoy S, Walsh S, O'Sullivan R. A review of a paediatric emergency department vaccination programme for patients at risk of allergy/anaphylaxis. Acta Paediatr. 2012;101(9):941‐945. [DOI] [PubMed] [Google Scholar]
- 123. Diseases. AAoPCoI . Recommendations for prevention and control of influenza in children, 2011–2012. Pediatrics. 2011;128(4):813‐825. [DOI] [PubMed] [Google Scholar]
- 124. Echeverria‐Zudaire L, Ortigosa‐del Castillo L, Alonso‐Lebrero E, et al. Consensus document on the approach to children with allergic reactions after vaccination or allergy to vaccine components. Allergol Immunopathol. 2015;43(3):304‐325. [DOI] [PubMed] [Google Scholar]
- 125. Gagnon R, Primeau M, Des Roches A, et al. Safe vaccination of patients with egg allergy with an adjuvanted pandemic H1N1 vaccine. J Allergy Clin Immunol. 2010;126(2):317‐323. [DOI] [PubMed] [Google Scholar]
- 126. Greenhawt M, Chernin A, Howe L, Li J, Sanders G. The safety of the H1N1 influenza a vaccine in egg allergic individuals. Ann Allergy Asthma Immunol. 2010;105(5):387‐393. [DOI] [PubMed] [Google Scholar]
- 127. Greenhawt M, Li J, Bernstein D, et al. Administering influenza vaccine to egg allergic recipients: a focused practice parameter update. Ann Allergy Asthma Immunol. 2011;106(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 128. Howe L, Conlon A, Greenhawt M, Sanders G. Safe administration of seasonal influenza vaccine to children with egg allergy of all severities. Ann Allergy Asthma Immunol. 2011;106(5):446‐447. [DOI] [PubMed] [Google Scholar]
- 129. Turner P, Southern J, Andrews N, Miller E, Erlewyn‐Lajeunesse M. Investigators. SS. Safety of live attenuated influenza vaccine in atopic children with egg allergy. J Allergy Clin Immunol. 2015;136(2):376‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Webb L, Petersen M, Boden S, et al. Single‐dose influenza vaccination of patients with egg allergy in a multicenter study. J Allergy Clin Immunol. 2011;128(1):218‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Baxter D. Measles immunization in children with a history of egg allergy. Vaccine. 1996;14(2):131‐134. [DOI] [PubMed] [Google Scholar]
- 132. James J, Burks A, Roberson P, Sampson H. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med. 1995;332(19):1262‐1266. [DOI] [PubMed] [Google Scholar]
- 133. Kattan J, Konstantinou G, Cox A, et al. Anaphylaxis to diphtheria, tetanus, and pertussis vaccines among children with cow's milk allergy. J Allergy Clin Immunol. 2011;128(1):215‐218. [DOI] [PubMed] [Google Scholar]
- 134. Slater J, Rabin R, Martin D. Comments on cow's milk allergy and diphtheria, tetanus, and pertussis vaccines. J Allergy Clin Immunol. 2011;128(2):434. author reply 5 [DOI] [PubMed] [Google Scholar]
- 135. Parisi C, Smaldini P, Gervasoni M, Maspero J, Docena G. Hypersensitivity reactions to the Sabin vaccine in children with cow's milk allergy. Clin Exp Allergy. 2013;43(2):249‐254. [DOI] [PubMed] [Google Scholar]
- 136. Kelso J, Cockrell G, Helm R, Burks A. Common allergens in avian meats. J Allergy Clin Immunol. 1999;104(1):202‐204. [DOI] [PubMed] [Google Scholar]
- 137. DiMiceli L, Pool V, Kelso J, Shadomy S, Iskander J. Team. VAERS. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS). Vaccine. 2006;24(6):703‐707. [DOI] [PubMed] [Google Scholar]
- 138. Zheng W, Dreskin S. Thimerosal in influenza vaccine: an immediate hypersensitivity reaction. Ann Allergy Asthma Immunol. 2007;99(6):574‐575. [DOI] [PubMed] [Google Scholar]
- 139. Nagao M, Fujisawa T, Ihara T, Kino Y. Highly increased levels of IgE antibodies to vaccine components in children with influenza vaccine‐associated anaphylaxis. J Allergy Clin Immunol. 2016;137(3):861‐867. [DOI] [PubMed] [Google Scholar]
- 140. Aberer W. Vaccination despite thimerosal sensitivity. Contact Dermatitis. 1991;24(1):6‐10. [DOI] [PubMed] [Google Scholar]
- 141. Audicana M, Munoz D, del Pozo M, Fernandez E, Gastaminza G. Fernandez de Corres L. allergic contact dermatitis from mercury antiseptics and derivatives: study protocol of tolerance to intramuscular injections of thimerosal. Am J Contact Dermat. 2002;13(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 142. Cox N, Forsyth A. Thiomersal allergy and vaccination reactions. Contact Dermatitis. 1988;18(4):229‐233. [DOI] [PubMed] [Google Scholar]
- 143. Noel I, Galloway A, Ive F. Hypersensitivity to thiomersal in hepatitis B vaccine. Lancet. 1991;338(8768):705. [DOI] [PubMed] [Google Scholar]
- 144. Patrizi A, Rizzoli L, Vincenzi C, Trevisi P, Tosti A. Sensitization to thimerosal in atopic children. Contact Dermatitis. 1999;40(2):94‐97. [DOI] [PubMed] [Google Scholar]
- 145. Rietschel R, Adams R. Reactions to thimerosal in hepatitis B vaccines. Dermatol Clin. 1990;8(1):161‐164. [PubMed] [Google Scholar]
- 146. Leventhal J, Berger E, Brauer J, Cohen D. Hypersensitivity reactions to vaccine constituents: a case series and review of the literature. Dermatitis. 2012;23(3):102‐109. [DOI] [PubMed] [Google Scholar]
- 147. Lee‐Wong M, Resnick D, Chong K. A generalized reaction to thimerosal from an influenza vaccine. Ann Allergy Asthma Immunol. 2005;94(1):90‐94. [DOI] [PubMed] [Google Scholar]
- 148. Vogt T, Landthaler M, Stolz W. Generalized eczema in an 18‐month‐old boy due to phenoxyethanol in DPT vaccine. Contact Dermatitis. 1998;38(1):50‐51. [DOI] [PubMed] [Google Scholar]
- 149. Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22(1):64‐69. [DOI] [PubMed] [Google Scholar]
- 150. Kaaber K, Nielsen A, Veien N. Vaccination granulomas and aluminium allergy: course and prognostic factors. Contact Dermatitis. 1992;26(5):304‐306. [DOI] [PubMed] [Google Scholar]
- 151. Lehman H, Faden H, Fang Y, Ballow M. A case of recurrent sterile abscesses following vaccination: delayed hypersensitivity to aluminum. J Pediatr. 2008;152(1):133‐135. [DOI] [PubMed] [Google Scholar]
- 152. Prevention CfDCa . Vaccine Excipient & Media Summary: Centers for Disease Control and Prevention; 2015. [Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf.
- 153. Safety IfV . Vaccine Excipients per 0.5ml dose; 2018. http://www.vaccinesafety.edu/components-Excipients.htm: Johns Hopkins Bloomberg School of Public Health
- 154. Kwittken P, Rosen S, Sweinberg S. MMR vaccine and neomycin allergy. Am J Dis Child. 1993;147(2):128‐129. [DOI] [PubMed] [Google Scholar]
- 155. Elliman D, Dhanraj B. Safe MMR vaccination despite neomycin allergy. Lancet. 1991;337(8737):365. [DOI] [PubMed] [Google Scholar]
- 156. Lear J, English J. Anaphylaxis after hepatitis B vaccination. Lancet. 1995;345(8959):1249. [DOI] [PubMed] [Google Scholar]
- 157. Russell M, Pool V, Kelso J, Tomazic‐Jezic V. Vaccination of persons allergic to latex: a review of safety data in the vaccine adverse event reporting system (VAERS). Vaccine. 2004;23(5):664‐667. [DOI] [PubMed] [Google Scholar]
- 158. Ponvert C, Scheinmann P. Vaccine allergy and pseudo‐allergy. Eur J Dermatol. 2003;13(1):10‐15. [PubMed] [Google Scholar]
- 159. Zanoni G, Puccetti A, Dolcino M, et al. Dextran‐specific IgG response in hypersensitivity reactions to measles‐mumps‐rubella vaccine. J Allergy Clin Immunol. 2008;122(6):1233‐1235. [DOI] [PubMed] [Google Scholar]
- 160. Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Stone C Jr, Liu Y, Relling M, et al. Immediate hypersensitivity to polyethylene glycols and Polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2018;n/a:n/a‐n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wenande E, Garvey L. Immediate‐type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907‐922. [DOI] [PubMed] [Google Scholar]
- 163. Wenande E, Kroigaard M, Mosbech H, Garvey L. Polyethylene glycols (PEG) and related structures: overlooked allergens in the perioperative setting. A A Case Reports. 2015;4(5):61‐64. [DOI] [PubMed] [Google Scholar]
- 164. Update: vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 1996;45(RR‐12):1‐35. [PubMed] [Google Scholar]
- 165. Di Giusto C, Bernhard J. Erythema nodosum provoked by hepatitis B vaccine. Lancet. 1986;2(8514):1042. [DOI] [PubMed] [Google Scholar]
- 166. Thomson B, Nuki G. Erythema nodosum following typhoid vaccination. Scott Med J. 1985;30(3):173. [DOI] [PubMed] [Google Scholar]
- 167. Yoon N, Lee N, Choi E. Generalized granuloma annulare after bacillus Calmette‐Guerin vaccination, clinically resembling papular tuberculid. J Dermatol. 2014;41(1):109‐111. [DOI] [PubMed] [Google Scholar]
- 168. Nomiyama T, Takenaka H, Kishimoto S, Katoh N. Granuloma annulare‐like reaction to the bacillus Calmette‐Guerin vaccination. Australas J Dermatol. 2013;54(1):e4‐e7. [DOI] [PubMed] [Google Scholar]
- 169. Baskan E, Tunali S, Kacar S, Adim S, Saricaoglu H. A case of granuloma annulare in a child following tetanus and diphtheria toxoid vaccination. J Eur Acad Dermatol Venereol. 2005;19(5):639‐640. [DOI] [PubMed] [Google Scholar]


