Abstract
Background and Aims
We investigated neurological events, graft patency, major adverse cardiovascular events (MACEs), and mortality at 1 year following coronary artery bypass grafting (CABG) surgery using automated proximal anastomotic devices (APADs) and compared the overall rates with the current literature.
Methods
A systematic review of all available reports of APADs use in the literature was conducted. Cumulative incidence and 95% confidence interval (CI) were the main statistical indexes. Nine observational studies encompassing a total of 718 patients were included at the end of the selection process.
Results
The cumulative event rate of neurological complications was 4.8% (lower‐upper limits: 2.8‐8.0, P < .001; I2 = 72.907%, P = .002; Egger's test: intercept = –2.47, P = 0.16; Begg and Mazumdar test: τ = −0.20, p = 0.57). Graft patency was 90.5% (80.4 to 95.7, P < .001; I2 = 76.823%, P = .005; Egger's test: intercept = –3.04, P = .10; Begg and Mazumdar test: τ = −0.67, P = .17). Furthermore, the overall incidence of MACEs was 3.7% (1.3‐10.4, P < .001; I2 = 51.556%, P = .103; Egger's test: intercept = –1.98, P = < .11; Begg and Mazumdar test: τ = −0.67, P = .17). Finally, mortality within 1 year was 5% (3.5‐7, P < .001; I2 = 29.675%, P = .202; Egger's test: intercept = –0.91, P = .62; Begg and Mazumdar test: τ = −0.04, P = .88).
Conclusions
APADs do not seem to be correlated with a reduction of either neurological events or mortality. By contrast, these tools showed satisfactory one‐year graft patency and a low incidence of MACEs. Further research on this topic is warranted.
Keywords: coronary artery bypass grafting, coronary artery disease, proximal anastomoses
1. INTRODUCTION
Neurological complications represent a fearsome consequence of coronary artery bypass grafting (CABG).1, 2 Aortic manipulation has been identified as the primary cause of neurological adverse events.1, 2 Indeed, handling of the aorta can lead to embolus formation and further damage of pre‐existing lesions.3 Compared with off‐pump CABG (OPCAB), on‐pump CABG has been shown to be a risk factor for embolization that contributes to an increased number of embolic events.4 Hence, the clamp on the aorta and the quality of the perfusion are determinant in the onset of neurological complications.3, 4, 5
Automated proximal anastomotic devices (APADs) represent a noninvasive and innovative option for the creation of proximal anastomoses.6 They can be employed both in traditional CABG and OPCAB. Automated devices in OPCAB do not require an aortic clamp, thus reducing aortic manipulation and, consequently, the potential risk of neurological events.7, 8, 9 This characteristic is critical in the case of the diseased or calcified aorta because the endothelium is not further damaged.7, 8, 10, 11 Moreover, APADs have been demonstrated to have optimal results in terms of the patency rate.12, 13
Nonetheless, little recent information is available on the follow‐up of APADs, with the majority of studies focused on immediate postoperative results.
Therefore, the aim of our systematic review and metanalysis is to investigate neurological events, graft patency, major adverse cardiovascular events (MACEs), and mortality at 1 year following surgery using APAD and to compare these findings to the current literature.
2. MATERIAL AND METHODS
2.1. Search strategy
The literature search was performed in conformity with the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA).14 The literature search was conducted by adopting the PubMed and Google Scholar Databases. The search strategy included the use of Boolean logic as well as MeSH terms in the PubMed Database. Further research was performed manually through consultation of the reference lists in the original relevant sources. The search strategy was determined by two authors (FM and OP) and consequently approved by a third reviewer (CT).
Search terms compliant with Boolean logic were “Automatic device” OR “Automatic device proximal anastomoses” AND “Cerebrovascular OR Stroke” AND “Off‐pump”; “Automatic device proximal anastomoses” AND “Cerebrovascular OR Stroke” AND “Off‐pump” AND “CABG”. MeSH terms adopted were “Anastomosis, surgical” [MeSH] AND “Coronary Artery Bypass, off‐pump/instrumentation” [Majr]; “Anastomosis, surgical” [MeSH] AND “Coronary Artery Bypass, off‐pump” [MeSH] AND “Equipment and supplies” [MeSH]; “Anastomosis, surgical/instrumentation” [MeSH] AND “Coronary Artery Bypass, off‐pump/instrumentation” [MeSH] OR “Coronary Artery Bypass, off‐pump/methods” [MeSH]; “Anastomosis, surgical/instrumentation” [MeSH] AND “Coronary Artery Bypass/methods” [MeSH].
2.2. Selection criteria and quality assessment
Studies were selected if the following conditions were met: (a) human studies, (b) articles about automated devices employed for proximal anastomoses, (c) English articles, and (d) articles published in the last 15 years. Exclusion criteria were as follows: (a) animal studies, (b) articles about automated devices employed for distal anastomoses, (c) articles about semiautomated anastomotic devices, (d) case reports, (e) studies including groups of patients with ≤10 individuals, (f) previous reviews and/or metanalyses, (g) articles in languages other than English, and (h) articles published more than 15 years ago.
Two authors (LM and FL) established the inclusion and exclusion criteria. These authors also performed the research on patients’ medical histories and results. Two reviewers (MdJ and AM) evaluated the eligibility and the risk of bias of the studies.
2.3. Methodological quality assessment
The quality of the studies included in the present metanalysis was evaluated by means of the Downs and Black's Checklist for Measuring Quality. The aforementioned checklist is composed of 27 items assessing the quality of randomized and nonrandomized studies in terms of reporting, external validity, internal validity, bias, and power. Each component of the checklist is rated on a binary basis (0/1), except two items that are respectively rated on a scale from 0 to 2 or from 0 to 5.15 We employed a revised version that includes 18 items, and we established a binary rating of the item related to power. Two researchers (MdJ and AM) were responsible for conducting the ratings, whereas a third party was involved in review (SG). The agreement was quantified by means of Cohen's kappa.
2.4. Endpoints
The primary endpoints of our study were the incidence of neurological events, graft patency, MACEs, and mortality of any cause within the first postoperative year.
Neurological events were defined as the onset of global or circumscribed neurological damage occurring immediately after the procedure or later on in patients who initially showed no neurological deficit after awakening.3 The neurological complications examined were cerebral anoxia subsequent to transient ischemic attack (TIA) or stroke, as well as delirium and cognitive impairment. We decided to include the latter phenomena because in all of them aortic manipulation can be indicated as an influencing factor.1, 16
Graft patency was described as the absence of graft failure due to atherosclerosis, technical complications related to the graft, and intimal hyperplasia resulting from the increased pressure.17 Long‐term graft patency influences the prognosis of the CABG procedure.18
We defined MACEs as comprehensive of cardiac death, target vessel revascularization, and myocardial infarction.19
Since cardiac death was included in the definition of MACEs, we decided to examine death from all causes. This decision was dictated by the assumption that analyzing all‐cause death gives less biased outcomes. In fact, bias could result from the classification of the causes of death.20
2.5. Statistical analysis
Metanalysis was conducted by means of Comprehensive Meta‐Analysis v.2.2 (Biostat, Englewood, New Jersey). Cumulative incidence and 95% confidence interval (CI) were the main statistical indexes. Heterogeneity of the sources was evaluated by considering the statistical inconsistency test I.2, 21 Publication bias was mathematically assessed using Egger regression and the Begg‐Mazumdar rank correlation test. Further graphic assessment was applied using a funnel plot and a high‐resolution plot for each of the variables examined. Statistical significance was established for P values < .05.
3. RESULTS
3.1. Characteristics of the studies
The study selection process is represented in the PRISMA flow diagram shown in Figure 1. The total number of studies found was 1072. Among these articles, 1029 were excluded either due to a lack of relevance to the topic or inability to access their full texts. At the end of this first selection, 43 were identified as potentially pertinent articles. However, four reviews and metanalyses were eliminated and thus not included in our metanalytic study. Since the focus of our study was to investigate proximal automated anastomotic devices, articles about hand‐sewn anastomotic devices and distal automated anastomotic devices were excluded. Two articles were added from the references of the original sources. As a result, this selection led to the inclusion of nine relevant articles. The selected studies were prospective observational studies encompassing a total of 718 patients.7, 8, 9, 10, 11, 12, 13, 22, 23 Table 1 shows the characteristics of the study populations included in every study. In addition, a description of the operative data is provided in Table 2.
Figure 1.
PRISMA flow diagram of the selection process
Table 1.
Patients’ demographics
| Female (%) | Age (y) mean ± SD | BMI (kg/m2) mean ± SD | Hyperlipidemia (%) | Hypertension (%) | Diabetes (%) | Smoker (%) | Family History of CAD (%) | LVEF (%, mean ± SD) | Prior MI (%) | Prior PCI (%) | Prior CVA (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bassano et al n = 1528 | 15.8 | 68.4 ± 9 | 27.3 ± 3.6 | 75 | 88.1 | 39.5 | 23 | 38.1 | 53.2 ± 8.2 | 27.6 | 13.8 | 13.8 |
| Demertzis et al n = 10013 | 18 | 68.9 ± 12 | ‐ | 66 | 94 | 45 | 31 | 57.8 ± 13 | 44 | 26 | ‐ | |
| Dohmen et al n = 1710 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Puskas et al n = 2207 | 22.3 | 68.1 ± 7.3 | 28.8 ± 4.5 | 72.7 | 85.9 | 36.8 | 40.9 | ‐ | 56.5 ± 12.2 | 34.5 | ‐ | 6.8 |
| Kai et al n = 6612 | 24.2 | 69.7 ± 10.3 | ‐ | 51.5 | 63.6 | 37.9 | ‐ | ‐ | ‐ | 34.8 | ‐ | 12.1 |
| Kempfert et al n = 519 | 19.6 | 74.5 ± 0.6 | 28 ± 0.5 | 84.3 | ‐ | ‐ | ‐ | ‐ | 59.4 ± 2 | ‐ | ‐ | ‐ |
| Bergmann et al n = 4222 | 37.5 | 70.9 ± 7.8 | ‐ | 33.3 | 75 | 20.83 | ‐ | ‐ | 63 ± 12.3 | 33.3 | ‐ | ‐ |
| Gummert et al n = 5411 | 15 | 69 ± 7 | ‐ | 81 | ‐ | 39 | 41 | ‐ | 61 ± 14 | ‐ | ‐ | ‐ |
| Skjelland et al n = 1623 | 87.5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 18.75 | ‐ | 6.25 |
Abbreviations: BMI, body mass index; CAD, cardiac artery disease; CVA, cerebrovascular accident; LVEF, left ventricle ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Table 2.
Operative data
| Device implanted | On‐pump coronary artery bypass (%) | Off‐pump coronary artery bypass (%) | Grafts (per patient) mean ± SD | Venous graft (%) | Arterial graft (n) | |
|---|---|---|---|---|---|---|
| Bassano et al n = 1528 | Cardica Pas‐Port ® | ‐ | ‐ | ‐ | ‐ | ‐ |
| Demertzis et al n = 10013 | Cardica Pas‐Port ® | 24 | 76 | ‐ | ‐ | ‐ |
| Dohmen et al n = 1710 | Cardica Pas‐Port ® | ‐ | ‐ | ‐ | ‐ | ‐ |
| Puskas et al n = 2207 | Cardica Pas‐Port ® | 36.4 | 63.6 | |||
| Kai et al n = 6612 | Cardica Pas‐Port ® | 4.5 | 95.5 | ‐ | ‐ | ‐ |
| Kempfert et al n = 519 | Cardica Pas‐Port ® | 7.8 | 92.2 | ‐ | ‐ | ‐ |
| Bergmann et al n = 4222 | Symmetry | 75 | 25 | ‐ | 100 | ‐ |
| Gummert et al n = 5411 | Cardica Pas‐Port ® | ‐ | ‐ | 3.2 ± 0.5 | ‐ | ‐ |
| Skjelland et al n = 1623 | Symmetry | ‐ | 100% | ‐ | ‐ | ‐ |
Abbreviations: SD, standard deviation.
3.2. Methodological quality
The average overall quality rating was 0.81 ± 0.47 with values ranging from 0.39 to 1.56. Appendix 1 presents the average scores on the items of the checklist. The table reveals lower scores for the item assessing whether the studies tested a representative group of the populations. The low values can be attributed to the fact that the characteristics of the samples were not exhaustively determined in each study examined. Furthermore, low scores emerged in relation to the item estimating the appropriateness of data collection. Acceptable interrater agreement was found (κ = 0.63).
3.3. Main endpoints
The incidence of neurological accidents was considered by six of the selected studies.7, 8, 9, 11, 12, 23 As shown in Figure 2A, the cumulative event rate of neurological complications was 4.8% (lower‐upper limits: 2.8‐8.0, P < .001; I2 = 72.907%, P = .002; Egger's test: intercept –2.47, P = .16; Begg and Mazumdar test: τ = −0.20, P = .57).
Figure 2.
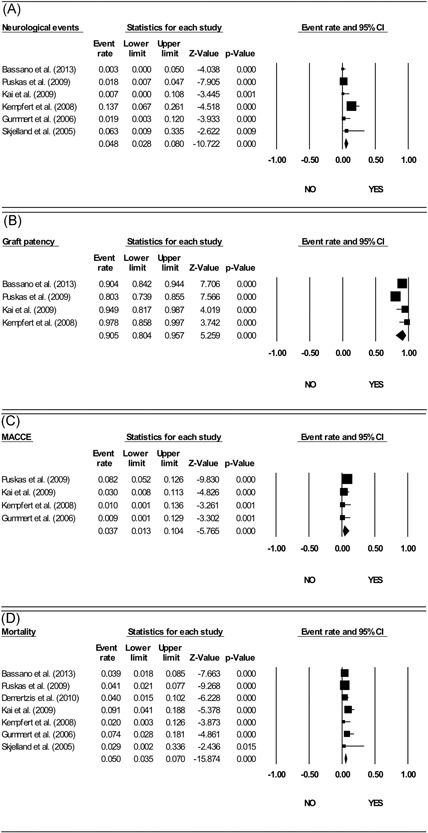
Forest plot event rates of the main endpoints. A, Neurological events. B, Graft patency. C, Major adverse cardiovascular events (MACEs). D, Mortality
Twelve‐month graft patency was assessed by only four of the selected studies.7, 8, 9, 12 The cumulative graft patency (Figure 2B) was 90.5% (80.4‐95.7, P < .001; I2 = 76.823%, P = .005; Egger's test: intercept –3.04, P = .10; Begg and Mazumdar test: τ = −0.67, P = .17).
Furthermore, four papers reported MACEs.7, 9, 11, 12 The overall incidence of MACE (Figure 2C) was 3.7% (1.3‐10.4, P < .001; I2 = 51.556%, P = .103; Egger's test: intercept –1.98, P = .11; Begg and Mazumdar test: τ = −0.67, P = .17).
Finally, mortality within 1 year was described by seven of the selected studies.7, 8, 9, 11, 12, 13, 23 The overall incidence of mortality (Figure 2C) was 5% (3.5‐7, P < .001; I2 = 29.675%, P = .202; Egger's test: intercept –0.91, P = .62; Begg and Mazumdar test: τ = −0.04, P = .88).
4. DISCUSSION
Because of the paucity of information related to automated proximal anastomotic devices (APADs), this review and metanalysis was aimed at examining the incidence of neurological events, graft patency, MACEs, and mortality within 1 year from surgery employing these devices, which is the longest common follow‐up time in studies on the topic.
APADs were introduced in clinical practice to reduce the incidence of neurological complications. The manipulation of the aorta represents a well‐known and consistent risk factor for the development of perioperative and postoperative stroke, both in the short‐ and long‐term.3, 5 Manipulating the aorta can cause the embolization of pre‐existing atheromatic lesions in the vessel and contributes to the formation of thrombi and emboli, whose nature can be gaseous or solid.3, 24 These phenomena are associated with the placement of the clamp on the aorta as well as its removal, because debris belonging to the atheroma can be dislocated.25 Indeed, Eldaif et al24 reported that 79% of the emboli detected in their study originated at the removal of the aortic clamp. The incidence of cerebrovascular accidents is correlated with the presence of extensive atherosclerotic disease that is not merely circumscribed to the coronaries and the aorta.3 For this reason, Nakamura et al26 underline the importance of accurate preoperative screening. Matsuura et al27 note the importance of not touching the aorta for reducing the risk of stroke. Furthermore, avoiding manipulation of the aorta is critical in patients with “porcelain” aorta, since the risk of causing the mobilization of atheromatous material is higher in these patients.10 Hence, creating anastomoses with the use of APADs should theoretically reduce the risk of stroke and neurological complications.8 However, the creation of proximal anastomoses itself can be responsible for embolus formation.2
The selected papers used two types of such devices: the St Jude Symmetry (St Jude Medical, Inc, Minneapolis, MN) and the Cardica PAS‐Port (Cardica, Inc., Redwood City, CA).6 Studies of the Symmetry device have shown poor results in terms of graft patency and number of emboli, which compromise patient safety.6, 23 As a consequence, the device has been withdrawn from the market.6, 7 Indeed, Skjelland et al23 observed a significant growth in the number of emboli, both solid and gaseous, in the group of patients treated with the Symmetry connector device. The phenomenon of solid embolus formation is related to the perforation of atheromatous material during insertion of the device. The creation of gaseous emboli might be caused by the inflation of air coming from the device itself during its attachment or by the shift in aortic diameter following the introduction of the device.23 By contrast, the employment of the PAS‐Port device has given acceptable results. In fact, some studies claim a reduction in the frequency of cerebrovascular accidents by adopting the PAS‐Port device.3, 8, 9 This might depend upon the speed of implantation and involvement of the minimal aortic area during the procedure. These characteristics are crucial in the presence of severe calcification or atherosclerosis of the aortic vessel.8, 28
The main finding of our study is the not‐negligible overall incidence of neurological events, which was 4.8% (2.8‐8.0) evaluated on 559 patients.7, 8, 9, 11, 12, 23 This is in contrast with the lower rates reported in the literature. Indeed, Misfeld et al29 showed an incidence of 0.5% for a cohort of 5619 patients in terms of neurological events using the off‐pump “anaortic” approach to coronary artery bypass grafting (OPCAB). For traditional on‐pump coronary artery bypass grafting (CABG) with a cross‐clamp, Calafiore et al3 described an incidence of cerebrovascular events of 1.2% for 2233 patients. The SYNTAX Trial identified an incidence of stroke of 2.3% in 897 individuals.30 However, our results were strongly influenced by the findings of Kempfert et al,9 who described six episodes of postoperative delirium in six different patients and one episode of fatal stroke within the first year, reaching a total event rate of 13.7% in 51 subjects.
Second, we showed that the overall event rate of graft patency was 90.5%.7, 8, 9, 12 This figure is inferior compared with the pure “anaortic” technique. Actually, Halbersma et al30 found that 99.2% of grafts were patent after OPCAB performed with the no‐touch technique. However, our figure compares favorably to the results from PREVENT IV, which reports a one‐year venous graft patency of 58.3% in a cohort of 951 patients31 after traditional CABG surgery. The graft patency reported in our study is strongly influenced by the poor performance of the Symmetry device. Bergmann et al22 reported stenoses in the proximity of device implantation, thus suggesting its involvement in the process. Histopathological evidence seems to confirm the endothelial damage caused by the device, as well as dissection of the tunica media in some cases.32 Puskas et al7 attributed these changes to endothelial damage because of the quantity of external material implanted.
Third, from our metanalysis emerged the superiority of anastomotic devices in reducing the occurrence of MACEs. Indeed, we found an incidence of 3.7% in a group of 391 patients,7, 9, 11, 12 which compares favorably to the current literature. Indeed, PREVENT IV reported an incidence of MACEs of 8.1% among 1506 patients undergoing traditional on‐pump CABG.31 Furthermore, SYNTAX published a rate of MACEs of 12.4% in 897 patients. The frequency reported in our paper is also inferior to the off‐pump “anaortic” approach, which was associated with a prevalence of MACEs of 5.3% in a group of 400 patients.30
Finally, the incidence of death at 1 year was 5% in a group of 659 patients.7, 8, 9, 11, 12, 13, 23 This compares unfavorably to both no‐touch OPCAB and traditional CABG. In fact, the SYNTAX trial evaluating traditional CABG established an event rate of 3.5% in 897 patients.30 In off‐pump no‐touch procedures, Halbersma et al30 showed that in a pool of 400 patients, the frequency of all‐cause mortality was 1.8%.
5. LIMITATIONS
This metanalysis has five main limitations that must be pointed out. First, the sample size is small, and high heterogeneity was detected. However, the heterogeneity was addressed by employing a random model. Second, the potential bias due to the small sample does not allow us to draw final conclusions. Third, we could not compare results with conventional surgery given the limited number of cases analyzed. Fourth, the four main endpoints were not always reported in the studies examined. Finally, delirium and cognitive impairment may be underestimated since only one paper referred to a systematic neurocognitive test.
6. CONCLUSIONS
In conclusion, APADs do not seem to be correlated to a reduction of either neurological events or mortality. By contrast, these tools showed satisfactory one‐year graft patency and a low incidence of MACEs. Further research on this topic is warranted.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
FUNDING
The authors received no financial support for the research and/or authorship of this article.
ACKNOWLEDGMENTS
We thank the Wiley Editing Services team for the English revision of the manuscript.
APPENDIX 1. QUALITY ASSESSMENT
| Item | M | SD | |
|---|---|---|---|
| 1 | Study hypothesis/aim/objective described? | 0.89 | 0.32 |
| 2 | Main outcomes described in the introduction or methods? | 0.61 | 0.50 |
| 3 | Participant characteristics described? | 0.89 | 0.32 |
| 4 | Contacted participants representative? | 0.56 | 0.51 |
| 5 | Prepared participants representative? | 0.44 | 0.51 |
| 6 | Participants recruited from the same population? | 0.67 | 0.49 |
| 7 | Participants recruited over the same time? | 0.78 | 0.43 |
| 8 | Measures and experimental tasks described? | 1.00 | 0.00 |
| 9 | Main outcome measures valid and reliable? | 0.94 | 0.24 |
| 10 | Task engagement assessed? | 0.78 | 0.43 |
| 11 | Confounders described and controlled for? | 1.56 | 0.51 |
| 12 | Statistical tests appropriate? | 0.94 | 0.24 |
| 13 | Main findings described? | 1.00 | 0.00 |
| 14 | Estimates of the random variability in data main outcomes? | 0.89 | 0.32 |
| 15 | Probability values reported? | 0.67 | 0.49 |
| 16 | Withdrawals and drop‐outs reported? | 0.89 | 0.32 |
| 17 | Data dredging made clear? | 0.39 | 0.50 |
| 18 | Sufficient power analysis provided? | 0.67 | 0.49 |
Notes: All items have a maximum score of 1.00 except for item 11, which has a maximum score of 2.00.
Micali LR, Matteucci F, Parise O, et al. Clinical outcomes of automated anastomotic devices: A metanalysis. J Card Surg. 2019;34:1297‐1304. 10.1111/jocs.14186
References
REFERENCES
- 1. McDonagh DL, Berger M, Mathew JP, Graffagnino C, Milano CA, Newman MF. Neurological complications of cardiac surgery. Lancet Neurol. 2014;13(5):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmerini T, Savini C, Di Eusanio M. Risks of stroke after coronary artery bypass graft ‐ recent insights and perspectives. Interv Cardiol (London, England). 2011;9(2):77‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calafiore AM, Di Mauro M, Teodori G, et al. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg. 2002;73(5):1387‐1393. [DOI] [PubMed] [Google Scholar]
- 4. Bassano C, Bovio E, Uva F, et al. Partially anaortic clampless off‐pump coronary artery bypass prevents neurologic injury compared to on‐pump coronary surgery: a propensity score‐matched study on 286 patients. Heart Vessels. 2016;31(9):1412‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kapetanakis EI, Stamou SC, Dullum MKC, et al. The impact of aortic manipulation on neurologic outcomes after coronary artery bypass surgery: a risk‐adjusted study. Ann Thorac Surg. 2004;78(5):1564‐1571. [DOI] [PubMed] [Google Scholar]
- 6. Puskas JD. The current status of anastomotic devices for coronary artery bypass surgery. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 2009;4(1):9‐12. [DOI] [PubMed] [Google Scholar]
- 7. Puskas JD, Halkos ME, Balkhy H, et al. Evaluation of the PAS‐Port proximal anastomosis system in coronary artery bypass surgery (the EPIC trial. J Thorac Cardiovasc Surg. 2009;138(1):125‐132. [DOI] [PubMed] [Google Scholar]
- 8. Bassano C, Bovio E, Sperandio M, et al. Five‐year clinical outcome and patency rate of device‐dependent venous grafts after clampless OPCAB with PAS‐Port automated proximal anastomosis: the PAPA study. J Card Surg. 2014;29(3):325‐332. [DOI] [PubMed] [Google Scholar]
- 9. Kempfert J, Opfermann UT, Richter M, Bossert T, Mohr FW, Gummert JF. Twelve‐month patency with the PAS‐Port proximal connector device: a single center prospective randomized trial. Ann Thorac Surg. 2008;85(5):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 10. Dohmen G, Hatam N, Goetzenich A, Mahnken A, Autschbach R, Spillner J. PAS‐Port® clampless proximal anastomotic device for coronary bypass surgery in porcelain aorta. Eur J Cardiothorac Surg. 2011;39(1):49‐52. [DOI] [PubMed] [Google Scholar]
- 11. Gummert JF, Opfermann U, Jacobs S, et al. Anastomotic devices for coronary artery bypass grafting: technological options and potential pitfalls. Comput Biol Med. 2007;37(10):1384‐1393. [DOI] [PubMed] [Google Scholar]
- 12. Kai M, Hanyu M, Soga Y, et al. Midterm patency rate after saphenous vein grafting with a PAS‐Port device. J Thorac Cardiovasc Surg. 2009;137(2):503‐504. [DOI] [PubMed] [Google Scholar]
- 13. Demertzis S, Trunfio R, Faletra F, Wyttenbach R, Siclari F. Sutureless proximal anastomosis using the PAS‐port system: six‐month patency and five‐year follow‐up in “all‐comers”. Ann Thorac Surg. 2010;90(5):1507‐1513 [DOI] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sabol F, Bilý B, Artemiou P, et al. Incidence and risk factors of delirium in patients after cardiac surgery: modifiable and non‐modifiable factors. Cor Vasa. 2015;57(3):e168‐e175. [Google Scholar]
- 17. Sabik Joseph F. Understanding saphenous vein graft patency. Circulation. 2011;124(3):273‐275. [DOI] [PubMed] [Google Scholar]
- 18. Tinica G, Chistol RO, Enache M, Leon Constantin MM, Ciocoiu M, Furnica C. Long‐term graft patency after coronary artery bypass grafting. Effects of morphological and pathophysiological factors. The Anatolian Journal of Cardiology. 2018;20(5):275‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovascular studies. J Am Coll Cardiol. 2008;51(7):701‐707. [DOI] [PubMed] [Google Scholar]
- 20. Chang M, Ahn J‐M, Lee CW, et al. Long‐term mortality after coronary revascularization in nondiabetic patients with multivessel disease. J Am Coll Cardiol. 2016;68(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergmann P, Meszaros K, Huber S, et al. Forty‐one‐month follow‐up of the symmetry aortic connector system for proximal venous anastomosis. J Thorac Cardiovasc Surg. 2007;134(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 23. Skjelland M, Bergsland J, Lundblad R, et al. Cerebral microembolization during off‐pump coronary artery bypass surgery with the Symmetry aortic connector device. J Thorac Cardiovasc Surg. 2005;130(6):1581‐1585. [DOI] [PubMed] [Google Scholar]
- 24. Eldaif SM, Thourani VH, Puskas JD. Cerebral emboli generation during off‐pump coronary artery bypass grafting with a clampless device versus partial clamping of the ascending aorta. Innovations. 2010;5(1):7‐11. [DOI] [PubMed] [Google Scholar]
- 25. Barbut D, Hinton RB, Szatrowski TP, et al. Cerebral emboli detected during bypass surgery are associated with clamp removal. Stroke. 1994;25(12):2398‐2402. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura M, Okamoto F, Nakanishi K, et al. Does intensive management of cerebral hemodynamics and atheromatous aorta reduce stroke after coronary artery surgery? Ann Thorac Surg. 2008;85(2):513‐519. [DOI] [PubMed] [Google Scholar]
- 27. Matsuura K, Mogi K, Sakurai M, Kawamura T, Takahara Y. Medium‐term neurological complications after off‐pump coronary artery bypass grafting with and without aortic manipulation. Coron Artery Dis. 2013;24(6):475‐480. [DOI] [PubMed] [Google Scholar]
- 28. Patel NC, Hemli JM. Anastomotic devices in coronary artery surgery: it is about the anastomosis? Multimedia Manual Cardio‐Thoracic Surgery. 2013;2013:mmt019. [DOI] [PubMed] [Google Scholar]
- 29. Misfeld M, Brereton RJL, Sweetman EA, Doig GS. Neurologic complications after off‐pump coronary artery bypass grafting with and without aortic manipulation: meta‐analysis of 11,398 cases from 8 studies. J Thorac Cardiovasc Surg. 2011;142(2):e11‐e17. [DOI] [PubMed] [Google Scholar]
- 30. Halbersma WB, Arrigoni SC, Mecozzi G, et al. Four‐year outcome of OPCAB no‐touch with total arterial Y‐Graft: making the best treatment a daily practice. Ann Thorac Surg. 2009;88(3):796‐801. [DOI] [PubMed] [Google Scholar]
- 31. Alexander JH, Hafley G, Harrington R, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446‐2454. [DOI] [PubMed] [Google Scholar]
- 32. Farhat F, Chalabreysse L, Diab C, Aubert Sp, Jegaden O. Histological aspects of the saphenous vein damage with the use of the symmetry® aortic connector system. Interact Cardiovasc Thorac Surg. 2004;3(2):373‐375. [DOI] [PubMed] [Google Scholar]


