Highlights
-
•
Age and gender moderate associations between ERN and anxiety in youth.
-
•
Smaller ERN associated with more anxious symptoms in preschool-aged girls.
-
•
A larger ERN associated with more anxious symptoms in school-aged girls.
Keywords: ERN, Error-related negativity, Anxiety, Moderator, Children, Development
Abstract
The error-related negativity (ERN) is a neurophysiologic response to errors that associates with anxiety. Despite the potential relevance of the ERN for understanding mechanisms of early anxiety problems in the developing brain, the relation between ERN and anxious symptoms in young children remains poorly understood. Emerging evidence suggests that ERN-anxiety associations could vary by developmental stage, but this work requires replication and consideration of gender effects, given earlier maturation of the ERN and higher rates of anxiety problems in girls relative to boys. To address this gap, the ERN was collected in 49 preschool- to school-aged children (ages 4–9; 26 girls) sampled across a wide range of anxiety severity. Regression analyses revealed that ERN - anxiety associations depended on age and gender. Specifically, larger (more negative) ERN associated with more anxiety in older girls, whereas smaller ERN associated with more anxiety symptoms in younger girls. No ERN-anxiety association was found in boys. These findings suggest that age and gender moderate the direction of the relation between ERN and anxiety in early childhood and could have important implications for the development of ERN-based risk identification and targeted treatment strategies tailored to individual children.
1. Introduction
Early anxiety, even at subclinical levels, increases risk for clinically significant internalizing problems (i.e., anxiety and depressive disorders) in mid-childhood and into adult life (Kroes et al., 2002; Mesman et al., 2001; Moffitt et al., 2007; Petty et al., 2008). As a result, early detection and prevention efforts have been widely endorsed (Novins et al., 2013) but are limited by clinical screening methods that more accurately identify children who will not develop illness than those who will Petty et al., 2008; Najman et al., 2008). Furthermore, current assessment methods do not link to neural mechanisms and thus have limited utility in guiding the development of more effective intervention or prevention strategies (Insel, 2014). To address these issues, the Research Domain Criteria (RDoC) has been championed as a framework for linking symptoms (e.g., anxiety), across the normal to abnormal range, to neural circuits underlying constructs of relevance to psychopathology (e.g., cognitive control). RDoC posits that pinpointing associations between symptoms and neurobehavioral markers of psychologically relevant constructs will guide the design of novel strategies to treat and prevent psychopathology (Insel, 2014).
To realize this promise, candidate biomarkers of RDoC constructs and related neural circuits must be understood in the context of age and gender differences known to characterize psychopathology (Casey et al., 2014; Woody and Gibb, 2015). For instance, the error-related negativity (ERN) is a neurophysiological marker of cognitive control associated with anxiety (Vaidyanathan et al., 2012) that may differentially associate with anxiety symptom severity in children compared to adolescents and adults (Meyer, 2017; Moser, 2017), and in women compared to men (Moser et al., 2016). Examining the effects of age and gender on ERN-anxiety associations in early childhood, when anxiety symptoms first present, represents an important step towards characterizing the ERN for developmentally informed application within the RDoC framework.
1.1. ERN as an RDoC biomarker associated with anxiety
The ERN is a negative deflection in the event-related brain potential (ERP) that occurs within 100 ms following an erroneous response (Gehring et al., 1993), is typically maximal at frontocentral scalp locations, and is believed to be generated by anterior cingulate cortex (ACC), among other regions (Mathalon et al., 2003). Prominent theories suggest that the ERN may index error signaling to recruit cognitive control (Yeung and Summerfield, 2012) and/or may reflect the rapid evaluation of the motivational significance of a mistake (Weinberg et al., 2016a). These processes are of phenomenological relevance for anxiety given that worry and rumination may reflect an inability to recover from real life mistakes (Fitzgerald and Taylor, 2015), and/or hypersensitivity to the importance of mistakes (Weinberg et al., 2016a). The ERN has gained attention as an RDoC biomarker for anxiety disorders given that clinically-affected adolescents and adults, particularly those who experience high levels of anxious apprehension (i.e., worry), exhibit an enlarged ERN compared to healthy individuals (Vaidyanathan et al., 2012; Cavanagh and Shackman, 2015; Moser et al., 2013). Indeed, consistent with the RDoC framework (Cuthbert, 2014), the ERN tracks anxiety symptoms across the normal to abnormal range in community samples, with larger ERN relating to more anxiety symptoms from early adolescence into adulthood (Bress et al., 2015; Moadab et al., 2010; Moran et al., 2015; Olvet and Hajcak, 2008). While increased ERN amplitude has been most consistently associated with anxiety (Carrasco et al., 2013; Hajcak et al., 2008; Ladouceur et al., 2006; Meyer et al., 2013; Santesso et al., 2006), some studies have also found increased ERN in relation to depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2010), whereas others show decreased ERN in depression (Weinberg et al., 2016a; Ladouceur et al., 2012; Weinberg et al., 2012a, b) or no relation between ERN and depressive symptoms (Bress et al., 2015).
1.2. ERN, anxiety and development
A gradual increase in ERN magnitude with age has been well-documented (Tamnes et al., 2013), but the influence of development on ERN-anxiety associations is less understood. When studied dimensionally in a community sample, greater anxiety was related to a larger ERN in older children (11–13 years), but to a marginally (i.e., trend-level-significant) smaller ERN in younger children (8–10 years) (Meyer et al., 2012), suggesting a reversal of the adult-like associations between anxiety symptoms and ERN in younger children. Consistent with this suggestion, higher levels of separation anxiety were related to a smaller ERN in a community sample of children ages 5–7 years (Lo et al., 2016). Moreover, Torpey and colleagues (Torpey et al., 2013) found that fearful temperament at aged 3 was associated with a smaller ERN among 6-year-old children, but with a larger ERN when children were re-assessed at aged 9 (Meyer et al., 2018). However, when anxiety was examined categorically, six-year-olds who met criteria for clinically significant anxiety demonstrated larger ERN compared to healthy children, consistent with the pattern of group differences observed when older patients are compared to healthy controls (Meyer et al., 2012). Collectively, these findings raise the possibility that ERN-anxiety associations may shift with age and symptom severity in childhood (Meyer, 2017; Moser, 2017(Meyer et al., 2018), and underscore the importance of examining the relationship between ERN and anxiety symptoms in young children across the full spectrum of severity.
1.3. Gender effects on the ERN and early expression of anxiety problems
Converging lines of evidence suggest that characterization of the ERN as a developmentally sensitive biomarker of anxiety symptoms should consider interactive effects of age and gender. Not only is there evidence to suggest earlier maturation of the ERN in girls than boys (Davies et al., 2004) but anxiety problems are more common in females than males across the lifespan. It is well-documented that fear and anxiety affect more girls than boys beginning in childhood (Craske, 2003; Ollendick et al., 2002), and foreshadow higher rates of anxiety and depression in females compared to males from adolescence (Costello et al., 2005; Pine et al., 1998) into adulthood (Beesdo et al., 2009). The higher frequency of internalizing problems in females than males may be influenced by gender differences in neural circuitry (Bangasser and Valentino, 2014). While it remains unknown whether the ERN indexes a process that is responsible for higher rates of anxiety disorders in females than males, recent meta-analytic evidence suggests that the association of larger ERN with anxiety in adulthood is characteristic of women, not men (Moser et al., 2016; Moran et al., 2012). Gender effects on the relation between ERN and anxiety symptoms have yet to be examined in children as a function of age.
1.4. The current study
The ERN has been previously posited as an RDoC-relevant biomarker of anxiety symptoms (Vaidyanathan et al., 2012; Weinberg et al., 2015); but, despite the common emergence of anxiety in early to middle childhood (Beesdo et al., 2009) and developmental change in ERN magnitude during this period (Tamnes et al., 2013), the association between ERN and anxiety symptoms in young children is not well-characterized. Better understanding how ERN-anxiety associations shift with age, gender and symptom severity will be important for refining the ERN as an early biomarker of anxiety risk and potential treatment target. Thus, we sought to examine age and gender effects on the relationship between ERN and anxiety symptoms in children, ages four to nine, sampled across the non-clinical to clinical range of severity. Prior work has suggested a reversal of the adult pattern of ERN-anxiety associations at approximately age 10 years (Meyer et al., 2012), however, neural networks for cognitive control (Tamnes et al., 2013) and behavioral capacity for this function (Diamond, 2013) undergo dramatic development between early childhood and preadolescence. Thus, we tested for an age-related reversal in the association between ERN and anxiety between ages 4–9 years, remaining agnostic to precisely when this reversal might occur. In addition, based on findings from an adult meta-analysis (Moser et al., 2016), we hypothesized that the relationship of ERN with anxiety might be further moderated by gender such that ERN-anxiety associations would be stronger in girls than boys.
2. Method
2.1. Participants
Participants included 56 children (30 girls) sampled from the community and the University of Michigan Child and Adolescent Psychiatry Clinic to capture the full spectrum of anxiety symptoms severity. Participants were 6.87 years old on average (SD = 1.39, range = 4.1–9.7); 77% Caucasians, 5% African American, 16% bi-racial and 2% “Other”. To be eligible for participation, children had to be between 4–9 years old and to have no history of head injury, serious medical illness, neurodevelopmental delay (autism spectrum disorder or mental retardation) and not taking medications that affect central nervous system functioning.
After data cleaning, the final sample consisted of 49 participants (26 girls; mean = 6.99 +/- 1.32 years, range 4.1–9.7). Six children (mean = 5.44 +/- .76 years, 4 girls) made fewer than six errors on the ERN-eliciting task and were excluded from further analysis based on standard convention (Olvet and Hajcak, 2009). One additional child, whose ERN amplitude was more than three standard deviations from the mean, was excluded (age = 9.42; male). No differences with respect to age, gender and anxiety symptoms were found between children who were in the final sample compared to those excluded (all ps > .05). The age distribution was comparable across gender (girls: mean age in years 6.63 +/- 1.11.; boys: mean age in years 7.31 +/- 1.43, t(47) = 1.82, p = n.s.).
Based on the effect size (partial r = .28) observed previously in a community sample of 55 children, 8- to 13-years old (Meyer et al., 2012), power analysis conducted in G*Power suggested a sample size of 75 is needed for detecting interactive effects of age x ERN on anxiety in that age group (with a power of .80). However, no prior developmental studies have investigated the relation between ERN and age on anxiety in younger, preschool- to school-aged children or the moderating role of gender, thus precluding direct comparison with the present study. Moreover, inclusion of anxious children recruited from a Child and Adolescent Psychiatry Clinic enabled sampling into the clinical range of anxiety severity (i.e., increased variance), distinguishing the sample presented here from prior work. Thus, analyses were conducted to test a priori hypotheses involving both age and gender effects on ERN-anxiety associations to generate preliminary results in the unique sample collected here.
2.2. Task
Participants performed the child-friendly Go/No-go “Zoo” task (Grammer et al., 2014). In the Zoo task, children were asked to help a zookeeper return loose animals to their cages, except three friendly orangutans who are the zookeeper’s “helpers” and should remain free. Children were asked to put the loose animals back in their cages by pressing a button as quickly as they could every time an animal picture was presented (Go Trials), but to withhold their response each time they saw an orangutan (No-Go trials). No-Go trials on the Zoo Task have been previously shown to produce error rates that are sufficient to elicit the ERN (Grammer et al., 2014).
Children completed 8 blocks of the task, each including 30 Go trials and 10 No-Go trials for a total of 320 trials. For each trial, a fixation cross was presented for 200–300 milliseconds (ms), followed by an animal image presented for 750 ms, and a blank screen for 500 ms. Responses could be made during the animal image and blank screen presentation. Each block consisted of novel sets of animal images, balanced on color, animal type and size. The task was presented using Eprime software (Psychology Software Tools, Inc.: Pittsburg, PA). Before the experimental trials of the Zoo task, children practiced on a set of 12 trials, 3 with orangutans and 9 with other animals and could practice multiple times until they understood the task.
2.3. Procedure
The study was approved by the University of Michigan Medical School Institutional Review Board. Initially, phone screening was conducted to determine that the child met study inclusion criteria. After written informed consent and oral assent were obtained from parents and children, respectively, children were brought to a child-friendly EEG booth by experimenters while parents filled out questionnaires. EEG experiments were conducted using the BioSemi ActiveTwo recording system (see below). Children were seated on a comfortable chair in front of a computer screen while watching cartoons during experimental set-up. To reduce fidgeting and increase compliance during ERP recording, children were given brief breaks between blocks and animal stickers as tokens for every block they completed. Verbal and visual feedback (in the form of a zoo map) were provided between blocks to remind children to stay still during blocks and monitor their progress through the zoo. Families received monetary incentives and children received toys for their participation.
2.4. Electrophysiological recording, data reduction and analysis
The EEG was recorded from 34 Ag/AgCI scalp electrodes and two mastoid electrodes, using BioSemi ActiveTwo recording system. Electro-oculogram (EOG) data were recorded from electrodes placed above and below the right eye and at the outer canthi of both eyes to capture vertical EOG and horizontal EOG, respectively. Data were referenced to a ground formed from a common mode sense active electrode and driven right leg passive electrode (see http://www.biosemi.com/faq/cms&drl.), and sampled at 1024 Hz. For analysis, EEG data were referenced to averaged mastoid electrodes, and band-pass filtered 0.05–30 Hz using zero-phase shift butterworth filters. EEG data were screened using automated algorithms that rejected epochs in which the absolute voltage range exceeded 500 u V for midline channels (Fz, FCz, Cz and Pz), consistent with prior work (Grammer et al. (2014)). Ocular movement artifacts were then corrected using a regression-based algorithm (Gratton et al., 1983). After ocular correction, individual trials were rejected if any amplitudes were greater than 100 u V, differed by more than 50 u V from the previous time point, or were less than 0.5 μV in magnitude in any midline electrode.
2.4.1. Behavioral measures
Correct trials included correct response to Go trials (button press when viewing any animal that was not an orangutan) and correct inhibition of response to No-Go trials (withholding button press to orangutan stimuli). Only the number of correct Go trials were evaluated. Errors were evaluated only for No-go trials, defined as errors of commission when children incorrectly responded to an orangutan (Grammer et al., 2014). Response times were evaluated for correct Go trials.
2.4.2. ERP measures
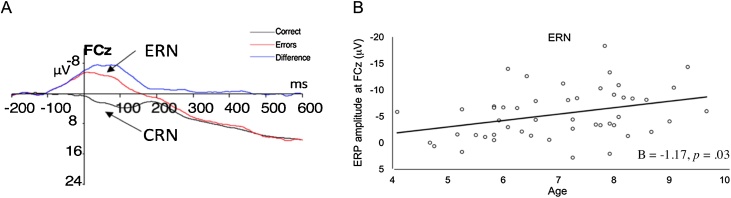
Response-locked ERP components were quantified using mean amplitude measurements relative to a pre-response baseline -200 to −100 ms, consistent with prior work in young children (Grammer et al., 2014). The mean amplitude of the ERN was computed for commission errors in a window 0–50 ms after the incorrect button response on No-Go trials (Grammer et al., 2014). ERN was measured at Fz (mean amplitude: -5.0 ± 5.1), FCz (mean amplitude: -5.4 ± 4.7) and Cz (mean amplitude: -3.6 ± 5.1). Overall amplitude at each of these locations was more negative on error relative to correct trials measured in the same time window (i.e., ERN effect, p’s < 0.001). As with prior work in this age group (Grammer et al., 2014), ERN at FCz (Fig. 1) had the highest mean amplitude and increased with age (Tamnes et al., 2013); thus, ERN measured at FCz was used in all subsequent analyses. When participants with higher numbers of errors were considered (e.g., > 16), the split-half reliability of the ERN increased and was comparable with prior studies (e.g., Schroder et al., 2017). Importantly, main findings remained significant in secondary analysis of subjects who made more errors (e.g., > 16) and therefore we report results with the full sample (> 6 errors; N = 49) and provide sub-sample analyses (> 16 errors; N = 33) in Supplementary Table 2.
Fig. 1.
Panel A: ERN and CRN waveforms at FCz electrode. Panel B: ERN amplitude increased (more negative) with age.
2.4.3. Child anxiety symptoms
Parent report, using the Child Behavior Checklist (CBCL/1-5 years and CBCL/6-18 years) (Achenbach and Rescorla, 2001), is commonly used to measure psychopathology in young children who struggle to provide accurate self-report (Tandon et al., 2009). Thus, given the inclusion of the children as young as 4 years and the lack of validated self-report anxiety measures for children at this age (Birmaher et al., 1997; March et al., 1997), child anxiety was measured by parent report on the CBCL DSM-oriented Anxiety Problems subscale (CBCL-AP ; α = .79) (Achenbach et al., 2003) combined with the CBCL Somatic Problems (α = .64) subscale (Kendall et al., 2007) by averaging the T-scores from the two subscales. The composite of these scales was used because young children often express anxiety as somatic complaints, and combination of the Anxiety and Somatic Problems subscales of the CBCL has been found to better capture anxiety severity than the CBCL-AP subscale alone (Kendall et al., 2007). Moreover, the correlation between the two subscales were moderately high (r = .48, p = .001) and reliability showed that the combined scale (with all the anxiety and somatic subscale items together) showed adequate reliability (α = .78). Of the 49 children who provided ERN data, CBCL data was missing for one (7.3-year-old boy), but was imputed using the Expectation-Maximization (EM) algorithm (Dong and Peng, 2013); therefore, the final sample remained 49 children.
2.4.4. Child depressive symptoms and attention problems
Parent report on the DSM-oriented Depression Problems (α = .64) and Attention Problems (α = .76) subscales from the CBCL were also examined given prior work suggesting the ERN may be differentially modulated by these phenomena (Weinberg et al., 2016a; Ladouceur et al., 2012; Weinberg et al., 2012a; Albrecht et al., 2008; Weinberg et al., 2012b).
2.5. Data analysis plan
All predictor variables were mean centered prior to the analyses. Pearson correlation was first conducted to examine inter-correlation among all study variables (Table 1). Hayes’ (2013) PROCESS (Model 3) was used to test for conditional effects of age and gender as moderators of the relation between ERN and child anxiety symptom severity. Specifically, the PROCESS model considered all two-way (Age X ERN, Gender X ERN, Age X Gender) and the three-way (Age X Gender X ERN) interactions as predictors of child anxiety symptoms. The main effect of age was treated as a continuous variable in the model.
Table 1.
Descriptive statistic and Pearson correlation among all study variables.
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 6.99 | 1.32 | — | |||||||||||
| 2. #Correct Go Trials | 231.59 | 8.94 | .45 | *** | — | |||||||||
| 3. Correct Go RT | 544 | 65.9 | −.55 | *** | −.45 | *** | — | |||||||
| 4. #NoGo Errors | 23.98 | 11.4 | −.17 | −.32 | * | −.29 | * | — | ||||||
| 5. ERN | −5.40 | 4.70 | −.34 | * | −.12 | .21 | .19 | — | ||||||
| 6. Anxiety | 53.63 | 5.21 | −.18 | .08 | −.09 | .00 | −.02 | — | ||||||
| 7. Depression | 53.37 | 5.19 | −.18 | −.03 | −.03 | −.03 | .11 | .67 | *** | — | ||||
| 8. Attention Prob. | 54.47 | 5.79 | −.09 | −.28 | .15 | −.05 | .09 | .38 | * | .51 | *** |
Note. RT is reaction time in millisecond. #No Go Errors is the number commission errors on No Go trials. *p < .05, **p < .01, ***p < .001.
Two additional PROCESS models (Model 3) were also conducted to test for conditional effects of age and gender on associations of ERN with child depressive and attention problems. To aid interpretation of results, linear regression was used to test for effects of age, gender and the interaction on behavioral measures; if not significant, the interaction term was dropped from the model. For comparison with prior work, effects of age, gender and performance on ERN were also assessed. Unstandardized beta coefficients were reported in all PROCESS and regression models.
3. Results
3.1. Behavioral
Participants committed an average of 23.98 (SD = 11.40; range = 6–63) commission errors on 80 total No-Go trials, and responded correctly to 231.59 (SD = 8.94; range = 194–240) of 240 total Go trials. On No-go trials, the number of commission errors were fewer in girls than boys (M = 18.6; SD = 6.1 for girls, M = 30; SD = 13 for boys; B = -11.19, p = .000), but did not vary by age (B = -.36, p = .75). On Go trials, a greater number of correct trials (B = 2.88, p = .003) and faster response times (B = -29.51, p = .000) occurred with older age, but there was no effect of gender on either measure (number of correct Go trials: B = 1.47, p = .55; response times: B = 21.25, p = .20). There were no age X gender interactions on any measure of behavioral performance.
3.2. Age, gender and performance effects on ERN
Consistent with prior work (Tamnes et al., 2013), older age associated with larger (i.e. more negative) ERN amplitude (B = -1.17, p = .03, total R2 = .14; Fig. 1), controlling for the effects of gender and performance. There were no significant gender or age X gender interaction effects on ERN amplitude (ps > 0.05). Performance (i.e., number of No Go commission errors, number of correct Go trials and Go response time) did not associate with ERN amplitude (Table 1) and there were no age X performance interaction effects on ERN amplitude (ps > 0.05).
3.3. Differential relation of ERN with anxiety by age and gender
As shown in Table 2, there were no significant main effects of age, gender, ERN or two-way interaction terms as predictors of child anxiety symptoms (all p > .05). However, there was a significant 3-way interaction effect of Age X Gender X ERN on child anxiety symptoms (B = -.91, p = .002) that explained 13% of the variance (F (1, 41) = 11.19, p = .002; effect size: partial r = .35; observed power = .92) in the model (total R2 = .24; F (7, 41) = 4.85, p = .0005).
Table 2.
Regression predicting child’s anxiety symptoms.
| Variables | B | SE | |
|---|---|---|---|
| Age | −1.04 | .68 | |
| Gender | −2.97 | 1.68 | |
| ERN | .08 | .17 | |
| Age X Gender | .86 | 1.44 | |
| Gender X ERN | .59 | .36 | |
| Age X ERN | .05 | .13 | |
| Age X Gender X ERN | −.91 | ** | .27 |
| R2 | .24 | ||
| F | 4.85 | *** |
Note. * p < .05, ** p < .01, *** p < .001. Male gender is the reference group.
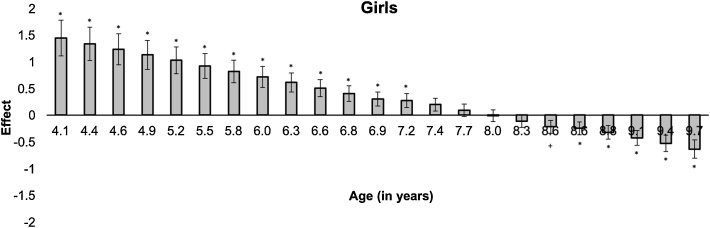
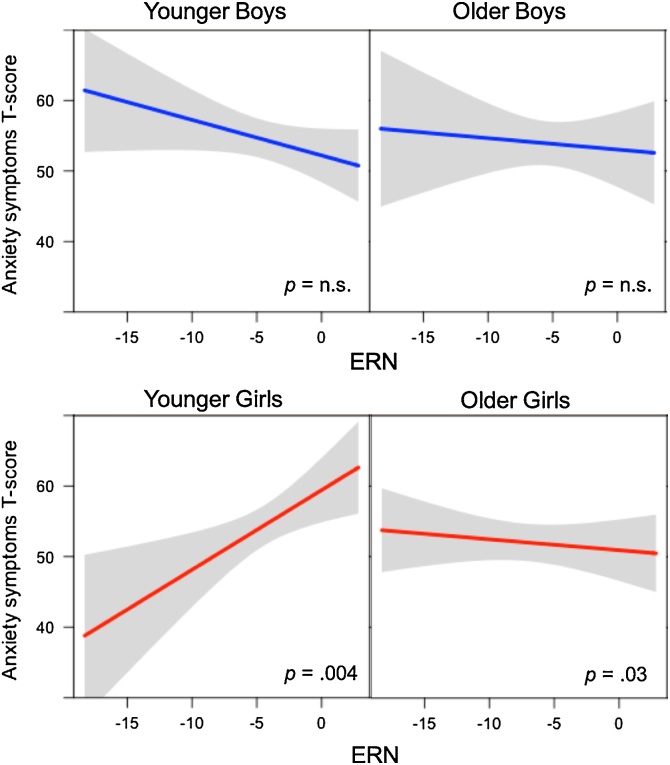
To explore the 3-way interaction on child anxiety, Johnson-Neyman (J-N) analyses were first conducted using PROCESS (Model 1) to characterize the conditional effects of age (in years) on the relationship of ERN with anxiety, separately for each gender. The J-N analysis for girls revealed a significant shift in the directionality of the ERN effect on anxiety between 7.2 and 8.6 years (Fig. 2). There was no J-N significance region (or significance transition points) across the study age range (all ps > .05) in boys. Next, based on the J-N defined age split, post-hoc simple slope analyses within PROCESS were conducted. Partial residuals from this analysis were plotted using the Visreg package in R to visualize how JN-defined age groups and gender moderate the relation of ERN and anxiety symptoms (Fig. 3). As shown in Fig. 3, smaller (i.e., less negative) ERN associated with more anxiety symptoms in younger girls (effect= .90, SE = .30, p =.004) whereas, in older girls, larger (i.e., more negative) ERN associated with more anxiety symptoms (effect = - .16, SE = .07, p = .03). In boys, ERN- anxiety associations were not significantly moderated by age (younger: effect = -.53, se = .42, p = .21; older: effect = -.13, se = .42, p = .74). Including the number of No go errors in the PROCESS models did not change primary results, and did not reveal any main effect of error rates. For comparison, Supplementary Fig. 1 plots raw data showing the ERN plotted against anxiety for each gender and age group.
Fig. 2.
Johnson-Neyman analysis of the conditional effect of age on the relationship between ERN and anxiety severity in girls. *p < .05, +p < .08.
Fig. 3.
Plot of partial residuals (derived from post-hoc simple slope analyses within PROCESS) depicts how age and gender moderate the relation of ERN and anxiety symptoms. Expected values are presented as lines; confidence intervals are presented in gray band. n.s. = not significant.
Finally, there were no significant associations between any behavioral measures (number of correct trials or reaction time) and anxiety symptoms, and no two- or three-way interaction effects of any behavioral measure with age and/or gender on anxiety.
3.4. Relation of ERN with depression and attention problems by age and gender
No significant model was found for predictors of child depressive symptoms (F (1, 41) = 1.11, p = .37) and child attention problems (F (1, 41) = 1.29, p = .28).
4. Discussion
As operationalized in the RDoC framework (Cuthbert, 2014), characterizing the neural circuits that relate to psychopathology should yield synergistic advances in the understanding and treatment of psychiatric illness, including anxiety (Pine, 2007). However, to realize this promise, converging lines of evidence suggest that effects of age and gender on neural circuits must be considered (Casey et al., 2014; Woody and Gibb, 2015). Towards this end, the current study examined whether age and/or gender moderated the association between the ERN and anxiety in girls and boys, ages 4–9 years, when anxiety often first presents (Beesdo et al., 2009). Results support prior work implicating ERN as a neural correlate of anxiety in children (Meyer et al., 2013, 2012; Lo et al., 2016) and substantiate the possibility of an age-related reversal in the association of ERN and anxiety (Meyer, 2017; Moser, 2017). Specifically, the adult-like pattern of larger ERN with greater anxiety severity (Moser et al., 2013; Olvet and Hajcak, 2008; Weinberg et al., 2015; Endrass and Ullsperger, 2014; Weinberg et al., 2010; Xiao et al., 2011) was observed in older girls, but reversed in younger girls in our sample. In contrast, there were no significant associations between the ERN and anxiety in younger or older boys, consistent with recent work showing ERN-anxiety association in women, but not men (Moser et al., 2016). These results suggest that the ERN-anxiety link is not only developmentally sensitive, but may also be gender specific. Thus, our findings highlight the importance of considering both age and gender differences known to characterize psychopathology when examining neural markers of RDoC constructs (Casey et al., 2014; Woody and Gibb, 2015).
4.1. Cognitive control theory of ERN and anxiety
Cognitive control functions begin to develop in early childhood (Diamond, 2013). Moreover, age-related change in cortical networks has been found to contribute to increased neural capacity for cognitive control (Bunge and Wright, 2007; Casey et al., 2005; Menon, 2013; Posner et al., 2014). Accumulating evidence shows that, while neural networks that support cognitive control continue to mature from childhood into adolescence and early adulthood (Luna et al., 2015), key components of these networks (e.g., ACC), are already involved in the implementation of control functions in preschool-aged children (Petrican et al., 2017). A widely accepted index of cognitive control, the ERN, occurs in response to errors, localizes to the ACC, and can be elicited as early as 3 years of age (Tamnes et al., 2013; Grammer et al., 2014; Ferdinand and Kray, 2014). Consistent with maturational trajectories for cognitive control, prior studies have found developmental increase in ERN amplitude from childhood (Torpey et al., 2012) and throughout adolescence (Tamnes et al., 2013; Davies et al., 2004; Lo, 2018). Although we did not find a relationship between larger ERN and better performance (nor age moderation of this relationship) in the current study, likely due to insufficient power, prior work has found that age-related increases of ERN magnitude associates with faster RTs and higher accuracy (Torpey et al., 2012). Taken together, this prior work suggests that the age-related increase in ERN amplitude may index the maturation of neural substrate for cognitive control, especially in performance monitoring of errors (Tamnes et al., 2013; Ferdinand and Kray, 2014 (Torpey et al., 2012).
Cognitive control may play a key role in facilitating the inhibition and/or regulation of negative/threatening thoughts (i.e., rumination and worry) (Derryberry and Rothbart, 1997; Eisenberg et al., 2009), and behavioral adaptation to reduce anxiety problems (Ip et al., 2019; Lemery-Chalfant et al., 2008; Lengua, 2003; Riggs et al., 2004). The directional shift of the ERN-anxiety relationship from younger to older girls may therefore mark the developmental transition of increasing neural capacity of using ERN to signal the need for cognitive control (Moser, 2017), such that an enlarged ERN allows greater recruitment of cognitive control (Gehring et al., 1993; Debener et al., 2005; West and Travers, 2007). In theory, low levels of ERN-indexed cognitive control in younger children may leave early anxiety symptoms unchecked, whereas the reversal of this relationship at older ages may reflect a compensatory process by which increasing neural capacity for cognitive control is leveraged to maintain adequate performance on task (Moser, 2017) and/or reduce anxiety symptom severity (Fitzgerald and Taylor, 2015).
Notably, we found a significant relationship between ERN-anxiety problems in girls but not in boys. Our gender specific finding is consistent with a meta-analysis in adults showing the presence of ERN- anxiety problems in women, but not men (Moser et al., 2016). In epidemiologic work, anxiety disorders have been demonstrated to be more common in girls than boys from childhood, throughout adolescence and into adulthood (Ollendick et al., 2002; Costello et al., 2005; Beesdo et al., 2009). Indeed, it has been suggested that biological mechanisms for processing threat and anxiety-inducing stimuli may be more sensitive in girls than boys from the earliest stages of development (Lebron-Milad et al., 2012; Ruigrok et al., 2014). With a greater biological sensitivity to threatening stimuli, girls may have to recruit greater cognitive control (as indexed through ERN) to maintain task goals and/or reduce anxiety than boys. Therefore, the association of ERN with greater anxiety severity in girls (but not boys) may suggest that girls are more dependent on ERN-indexed ACC network to suppress sensitivity to and/or interference from anxiety.
On experimental tasks requiring cognitive control, girls exhibit better performance than boys (e.g., higher accuracy, fewer commission errors), consistent with the greater cognitive control capabilities that have been previously demonstrated in preschool-aged girls compared to boys (Else-Quest et al., 2006). It is possible that ERN-indexed systems for cognitive control are more available to manage behavior, including behaviors related to fear and anxiety, in females than males over the course of development, which may be related to gender differences in the functional maturation of ACC (Christakou et al., 2009; Liu et al., 2012). Further research, across a broader age range, is needed to understand whether the maturation of ACC influences the relationship between the ERN and anxiety symptoms and whether this relationship differs by gender.
4.2. Developmental transition of anxiety phenomenon and ERN
We hypothesize that the developmental shift in ERN-anxiety relationship in girls may reflect a developmental change in neural capacity for cognitive control, however, other interpretations are possible. A larger ERN has been found to have a stronger association with worry/anxious apprehension than other forms (e.g., fear) of anxiety-related symptoms in meta-analysis (Moser et al., 2013). Some have argued that with the natural transition of anxiety phenomena from more fear-based disorders at younger ages (e.g. phobias) to worry-related disorders at older ages (e.g. generalized anxiety), a smaller ERN in younger, more anxious children may reflect sensitivity to acute, external threat (i.e., fear), whereas a larger ERN in older, anxious children may relate to the greater relevance of internal threat (i.e., worry (Meyer, 2017; Weinberg et al., 2016a)). Thus, the shift of the ERN-anxiety relationship across ages may reflect the changing nature of anxiety symptom phenomenology with age (Meyer, 2017; Meyer et al., 2018).
Finally, it is important to note that although we do not find a significant ERN-anxiety link in boys, a non-significant pattern of larger ERN with greater anxiety severity was observed in younger boys (b = -.53, p = .21, see Fig. 3). This pattern is consistent with previous work showing a larger ERN in clinically anxious compared to healthy 6-year-old children in a sample comprised of more boys (˜2/3) than girls (˜1/3) (Meyer et al., 2013). It is possible that these previously reported findings were male-driven. Alternatively, ERN-anxiety association may also be influenced by anxiety severity (i.e., clinical vs subclinical) and further study in larger samples will be needed to understand whether age and gender effects on ERN-anxiety association shift with anxiety severity.
4.3. Limitations
Our findings should be viewed with caution given our small sample size. Power analysis based on the effect size (partial r = .28) observed in prior work37 suggests that a sample size of 75 (power = .80) is needed to detect the interactive effect of age and ERN on anxiety in 8 - 13-year-old children. However, no prior developmental studies have investigated moderating effects of age and gender on ERN-anxiety relations among younger children, precluding direct comparison. In fact, in the current study, we observed a larger effect size (partial r = .35; observed power = .92) than previously reported (Meyer et al., 2012), raising the possibility that a smaller sample may be appropriate when considering the interactive effects of age and gender on ERN-anxiety associations in preschool- to school-aged children (ages 4–9 years). On the other hand, small samples can produce statistically significant results that do not reflect a true effect due to outliers (Button et al., 2013). Thus, even though we did not observe any outlier(s) driving the findings presented here, it is important to view our results as preliminary, requiring confirmation with a larger sample. Nonetheless, our results should guide future research to consider both age and gender when examining the ERN as a neurophysiological marker of anxiety in children.
Several other limitations deserve consideration. First, our study used a cross-sectional design and therefore is unable to infer longitudinal relationship or causality of ERN and anxiety symptoms. Second, our study relied on parent-report of anxiety symptoms as there is no validated self-report measure that reliably assesses anxiety symptoms in preschool-aged children. Third, we did not include a cognitive control measure outside of performance on the Go No Go task used to elicit the ERN. Further research that includes other behavioral measures of cognitive control is needed to more fully understand the relations among ERN, cognitive control and anxiety in children and to test the prediction that a smaller ERN may reflect less capacity for cognitive control in more anxious, younger girls (i.e., a 4-way interaction).
4.4. Conclusion
Our findings represent an important first step towards clarifying the relation of the ERN and anxiety symptoms in children. If, as suggested by RDoC, indices of error-processing (e.g., ERN) in children with sub-clinical anxiety symptoms fall on a continuum between clinically affected children at one end and healthy children at the other, this information would justify future testing of strategies to shift ERN along the continuum to reduce subclinical symptoms and prevent the progression to illness. Specifically, cognitive control training or other interventions designed to increase ERN might help to reduce anxiety symptoms among preschool-aged girls. By contrast, given the opposite pattern of the ERN- anxiety relationship in school-aged girls, strategies to decrease ERN may hold more promise for anxiety symptom reduction (Schroder et al., 2018). On the other hand, if greater ERN is an adaptive response to reduce anxiety, then interventions should be designed to increase ERN across early to late childhood and across genders. Future longitudinal work is needed to understand how changes in ERN associate with changes in anxiety symptoms over time and should consider age and gender to identify whether the ERN should be targeted differently in different children to reduce and prevent anxiety problems.
Acknowledgements
This work was supported by the National Institutes of Mental Health (R03MH102648; MPI: Katherine Rosenblum, Kate Fitzgerald), the Brain Behavior Research Foundation (Kate Fitzgerald), the One Mind- AIM Sullivan Family Rising Star Award (Kate Fitzgerald), and the Michigan Institute for Clinical Health Research (UL1TR000433-06; MPI: Kate Fitzgerald, Jason Moser).
References
- Kroes M. A longitudinal community study: do psychosocial risk factors and child behavior checklist scores at 5 years of age predict psychiatric diagnoses at a later age? J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:955–963. doi: 10.1097/00004583-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Mesman J., Bongers I.L., Koot H.M. Preschool developmental pathways to preadolescent internalizing and externalizing problems. J. Child Psychol. Psychiatry. 2001;42:679–689. [PubMed] [Google Scholar]
- Moffitt T.E. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch. Gen. Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Petty C.R. The child behavior checklist broad-band scales predict subsequent psychopathology: a 5-year follow-up. J. Anxiety Disord. 2008;22:532–539. doi: 10.1016/j.janxdis.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novins D.K., Green A.E., Legha R.K., Aarons G.A. Dissemination and implementation of evidence-based practices for child and adolescent mental health: a systematic review. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:1009–1025. doi: 10.1016/j.jaac.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najman J.M. Screening in early childhood for risk of later mental health problems: a longitudinal study. J. Psychiatr. Res. 2008;42:694–700. doi: 10.1016/j.jpsychires.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am. J. Psychiatry. 2014;171:395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Oliveri M.E., Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol. Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Woody M.L., Gibb B.E. Integrating NIMH research domain criteria (RDoC) into depression research. Curr. Opin. Psychol. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U., Nelson L.D., Patrick C.J. Clarifying domains of internalizing psychopathology using neurophysiology. Psychol. Med. 2012;42:447–459. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. A biomarker of anxiety in children and adolescents: a review focusing on the error-related negativity (ERN) and anxiety across development. Dev. Cogn. Neurosci. 2017;27:58–68. doi: 10.1016/j.dcn.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S. The nature of the relationship between anxiety and the error-related negativity across development. Curr. Behav. Neurosci. Rep. 2017;4:309–321. doi: 10.1007/s40473-017-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Kneip C., Schroder H.S., Larson M.J. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: a meta-analytic review. Psychophysiology. 2016;53:21–29. doi: 10.1111/psyp.12509. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Mathalon D.H., Whitfield S.L., Ford J.M. Anatomy of an error: ERP and fMRI. Biol. Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Yeung N., Summerfield C. Metacognition in human decision-making: confidence and error monitoring. Philos. Trans. R. Soc. B. 2012;367:1310–1321. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53:372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K.D., Taylor S.F. Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS Spectr. 2015;20:346–354. doi: 10.1017/S1092852915000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Shackman A.J. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J. Physiol. Paris. 2015;109:3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. 2013. On the Relationship Between Anxiety and Error Monitoring: a Meta-analysis and Conceptual Framework. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J.N., Meyer A., Hajcak G. Differentiating Anxiety and Depression in Children and Adolescents: Evidence From Event-Related Brain Potentials. J. Clin. Child Adolesc. Psychol. 2015;44:238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Moadab I., Gilbert T., Dishion T.J., Tucker D.M. Frontolimbic activity in a frustrating task: covariation between patterns of coping and individual differences in externalizing and internalizing symptoms. Dev. Psychopathol. 2010;22:391–404. doi: 10.1017/S0954579410000131. [DOI] [PubMed] [Google Scholar]
- Moran T.P., Bernat E.M., Aviyente S., Schroder H.S., Moser J.S. Sending mixed signals: worry is associated with enhanced initial error processing but reduced call for subsequent cognitive control. Soc. Cogn. Affect. Neurosci. 2015;10:1548–1556. doi: 10.1093/scan/nsv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin. Psychol. Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depress. Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Franklin M.E., Foa E.B., Simons R.F. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am. J. Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J. Child Psychol. Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Meyer A. Increased error-related brain activity in six-year-Old children with clinical anxiety. J. Abnorm. Child Psychol. 2013;41:1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses are enhanced in children with obsessive–Compulsive behaviors. Dev. Neuropsychol. 2006;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Chiu P.H., Deldin P.J. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Holmes A.J., Pizzagalli D.A. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cogn. Affect. Behav. Neurosci. 2010;10:119–128. doi: 10.3758/CABN.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D. Altered error-related brain activity in youth with major depression. Dev. Cogn. Neurosci. 2012;2:351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Klein D.N., Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J. Abnorm. Psychol. 2012;121:885. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Liu H., Shankman S.A. Blunted neural response to errors as a trait marker of melancholic depression. Biol. Psychol. 2016;113:100–107. doi: 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Torstveit M., Sells V.T., Fjell A.M. Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev. Cogn. Neurosci. 2013;6:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev. Cogn. Neurosci. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.L. Associations between disorder-specific symptoms of anxiety and error-monitoring brain activity in young children. J. Abnorm. Child Psychol. 2016:1–10. doi: 10.1007/s10802-016-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. J. Child Psychol. Psychiatry. 2013;54:854–862. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Dev. Psychobiol. 2018 doi: 10.1002/dev.21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of Response-Monitoring ERPs in 7- to 25-Year-Olds. Dev. Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Craske M.G. Elsevier; 2003. Origins of Phobias and Anxiety Disorders: Why More Women Than Men? [Google Scholar]
- Ollendick T.H., King N.J., Muris P. Fears and Phobias in Children: Phenomenology, Epidemiology, and Aetiology. Child Adolesc. Ment. Health. 2002;7:98–106. [Google Scholar]
- Costello E.J., Egger H.L., Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc. Psychiatr. Clin. 2005;14:631–648. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Knappe S., Pine D.S. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr. Clin. North Am. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T.P., Taylor D., Moser J.S. Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. Int. J. Psychophysiol. 2012;85:188–194. doi: 10.1016/j.ijpsycho.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Dieterich R., Riesel A. Error-related brain activity in the age of RDoC: a review of the literature. Int. J. Psychophysiol. 2015;98:276–299. doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Grammer J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Achenbach T.M., Rescorla L. 2001. ASEBA School-age Forms & Profiles. (Aseba Burlington. [Google Scholar]
- Tandon M., Cardeli E., Luby J. Internalizing disorders in early childhood: a review of depressive and anxiety disorders. Child Adolesc. Psychiatr. Clin. 2009;18:593–610. doi: 10.1016/j.chc.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- March J.S., Parker J.D., Sullivan K., Stallings P., Conners C.K. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Achenbach T.M., Dumenci L., Rescorla L.A. DSM-oriented and empirically based approaches to constructing scales from the same item pools. J. Clin. Child Adolesc. Psychol. 2003;32:328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- Kendall P.C. Assessing anxiety with the child behavior checklist and the teacher report form. J. Anxiety Disord. 2007;21:1004–1015. doi: 10.1016/j.janxdis.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dong Y., Peng C.-Y.J. Principled missing data methods for researchers. SpringerPlus. 2013;2:222. doi: 10.1186/2193-1801-2-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biol. Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Riesel A., Hajcak G. Integrating multiple perspectives on error-related brain activity: the ERN as a neural indicator of trait defensive reactivity. Motiv. Emot. 2012;36:84–100. [Google Scholar]
- Pine D.S. Research Review: a neuroscience framework for pediatric anxiety disorders. J. Child Psychol. Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Endrass T., Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2014;46(Part 1):124–138. doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Olvet D.M., Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychol. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Xiao Z. Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Galvan A., Hare T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17:627–640. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. Developing attention: behavioral and brain mechanisms. Adv. Neurosci. 2014;2014 doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrican R., Taylor M.J., Grady C.L. Trajectories of brain system maturation from childhood to older adulthood: implications for lifespan cognitive functioning. Neuroimage. 2017;163:125–149. doi: 10.1016/j.neuroimage.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Ferdinand N.K., Kray J. Developmental changes in performance monitoring: how electrophysiological data can enhance our understanding of error and feedback processing in childhood and adolescence. Behav. Brain Res. 2014;263:122–132. doi: 10.1016/j.bbr.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A., Klein D.N. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Dev. Psychobiol. 2012;54:139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.L. A meta-analytic review of the event-related potentials (ERN and N2) in childhood and adolescence: providing a developmental perspective on the conflict monitoring theory. Dev. Rev. 2018 [Google Scholar]
- Derryberry D., Rothbart M.K. Reactive and effortful processes in the organization of temperament. Dev. Psychopathol. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Dev. Psychol. 2009;45:988. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip K.I., Jester J.M., Sameroff A., Olson S.L. Linking Research Domain Criteria (RDoC) constructs to developmental psychopathology: the role of self-regulation and emotion knowledge in the development of internalizing and externalizing growth trajectories from ages 3 to 10. Dev. Psychopathol. 2019:1–18. doi: 10.1017/S0954579418001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K., Doelger L., Goldsmith H.H. Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant Child Dev. 2008;17:365–385. doi: 10.1002/icd.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua L.J. Associations among emotionality, self-regulation, adjustment problems, and positive adjustment in middle childhood. J. Appl. Dev. Psychol. 2003;24:595–618. [Google Scholar]
- Riggs N.R., Blair C.B., Greenberg M.T. Concurrent and 2-Year longitudinal relations between executive function and the behavior of 1st and 2nd grade children. Child Neuropsychol. 2004;9:267–276. doi: 10.1076/chin.9.4.267.23513. [DOI] [PubMed] [Google Scholar]
- Debener S. Trial-by-Trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R., Travers S. Tracking the temporal dynamics of updating cognitive control: an examination of error processing. Cereb. Cortex. 2007;18:1112–1124. doi: 10.1093/cercor/bhm142. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol. Mood Anxiety Disord. 2012;2:7. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A.N.V. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest N.M., Hyde J.S., Hill H., Van Hulle C.A. Gender differences in temperament: a meta-analysis. Psychol. Bull. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Christakou A. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Liu J., Zubieta J.-K., Heitzeg M. Sex differences in anterior cingulate cortex activation during impulse inhibition and behavioral correlates. Psychiatry Res. Neuroimaging. 2012;201:54–62. doi: 10.1016/j.pscychresns.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Schroder H.S., Moran T.P., Moser J.S. The effect of expressive writing on the error-related negativity among individuals with chronic worry. Psychophysiology. 2018;55 doi: 10.1111/psyp.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]