Abstract
Disease states and cellular compartments can display a remarkable amount of heterogeneity, and truly appreciating this heterogeneity requires the ability to detect and probe each subpopulation present. A myriad of recent single-cell assays has allowed for in-depth analysis of these diverse cellular populations; however, fully understanding the interplay between each cell type requires knowledge not only of their mere presence but also of their spatial organization and their relation one to the other. Immunohistochemistry allows for the visualization of cells and tissue; however, standard techniques only allow for the use of very few probes on a single specimen, not allowing for in-depth analysis of complex cellular heterogeneity. A number of multiplex imaging techniques, such as immunofluorescence and multiplex immunohistochemistry, have been proposed to allow probing more cellular markers at once; however, many of these techniques still have their limitations. The use of fluorescent markers has an inherent limitation to the number of probes that can be simultaneously used due to spectral overlap. Moreover, other proposed multiplex IHC methods are time-consuming and require expensive reagents. Still, many of the methods rely on frozen tissue, which deviates from standards in human pathological evaluation. Here, we describe a multiplex IHC technique, staining for consecutive markers on a single slide, which utilizes similar steps and similar reagents as standard IHC, thus making it possible for any lab with standard IHC capabilities to perform this useful procedure. This method has been validated and confirmed that consecutive markers can be stained without the risk of cross-reactivity between staining cycles. Furthermore, we have validated that this technique does not lead to decreased antigenicity of subsequent epitopes probed, nor does it lead to steric hindrance.
Keywords: Multiplexed immunohistochemistry, Chromogenic immunohistochemistry, Immunostaining, Whole slide imaging, Consecutive staining, Serial staining, Single slide, Image analysis, Positive cell detection, Histology, Morphology, Cell segmentation, Machine learning, Random forest, Cancer immunotherapy, Immuno-oncology, Biomarkers, In situ markers, PD-L1, PD-1, CD3, CD8, FOXP3, CD20, CD66b, CD68
1. Introduction
Characterization and spatial organization of molecular targets and cellular subsets in and around tissue lesions is of great significance, as shown by examples in tumor immunology such as the immunoscore [1], that is, the quantification of CD8 memory T cells in and around colorectal tumors as a prognostic marker [2], or as PD-L1 as a predictive marker of various solid tumors responding to immune checkpoint blockade of the PD-1 pathway [3, 4]. This characterization requires high-dimensional analysis of immune cells and cells from the tissue microenvironment, including diseased cells (tumor), immune cells, stroma, and vasculature, to elucidate their lineage by detecting specific markers of these cells in situ. Single-cell methods such as time-of-flight mass cytometry (CyTOF) can analyze cells of a given tissue with high precision and accuracy but require fresh tissue and do not provide information about tissue architecture [5]. Geographic location of cells and their relationship with other cells is also of interest. Many methods allowing for high-dimensional tissue analyses often rely on expensive equipment and proprietary reagents [6–10]. There is a need for an assay capable of showing multiple marker expressions on a single cell level including spatial features but concurrently affordable and simple, that can be performed in most labs with minimal requirements. Chromogen-based immunohistochemistry from formalin-fixed, paraffin embedded tissues is a perfect fit for this definition and it is used routinely in every pathology laboratory worldwide in daily diagnostic routines. However, it is most commonly used as a singleplex staining in which single antibody is performed on a single section and this approach is limited by tissue availability and insufficient for complex exploration such as immune cell subpopulations or colocalization of multiple markers in the tissue microenvironment. We have developed a multiplex immunostaining system named “multiplexed immunohistochemical consecutive staining on the same slide (MICSSS)” as simple and cost-effective as singleplex immunohistochemistry but robust and powerful at the same time for characterizing up to ten markers on the same tissue section [11]. MICSSS can be done on any formalin-fixed, paraffin-embedded (FFPE) section and it relies on iterative cycles of immunostaining with various antibodies that can be selected in a mix-and-match fashion, scanning, and removal of chromogenic substrate (Fig. 1). High-dimensional coexpression analysis can be done on processed whole slide images since all antibodies are applied on the same section. Moreover, this method does not negatively affect marker intensity and can be performed for up to ten markers even on delicate tissue structures. Whole slide images belonging to the same section can be analyzed by AI-powered image analysis software such as QuPath to reveal markers within the microenvironment of a tissue lesion with spatial features visually and to further analyze in the form of data files rather than images [12]. MICSSS provides a tremendous opportunity and potential for tissue-based research worldwide as it makes multiplexed analysis of tissue markers affordable and simple.
Fig. 1.
MICSSS pipeline
1.1. Immunostaining
1.1.1. Iterative Cycles of Immunostaining
MICSSS is a sample-sparing multiplex immunohistochemical method developed for formalin-fixed, paraffin-embedded (FFPE) tissue sections based on iterative cycles of immunostaining with primary and secondary antibodies using standard chromogen amplification, scanning, and destaining of tissue slides (Fig. 1). Each staining cycle starts with optimized pH and heat-induced antigen retrieval conditions to reveal epitopes tailored to the primary antibody, followed by imaging of slides. Organic solvent soluble chromogens are used for MICSSS protocol since chromogenic substrate must be easily removable by destaining between cycles. Amino ethyl carbazole (AEC) is a red-colored chromogen ideal for this purpose; however, any soluble chromogen can be used for MICSSS immunostaining. Harris hematoxylin is used for counterstaining in each cycle and it includes ethanol in its preparation formula; one should be careful for the duration of hematoxylin counterstaining since it can decrease the intensity of chromogenic color due to the effect of ethanol. While this bleaching step removes the chromogenic substrate, it does not guarantee removal of all antibody bonds to antigen on a given cellular compartment along with the chromogenic substrate. Heat-induced antigen retrieval likely contributes to additional partial removal of primary and secondary antibody pairs. Nevertheless, proper blocking must be included in the protocol after the first cycle and thereafter to block all residual antibodies belonging to the previous cycle. Peroxidase block and serum-free protein block are used in all cycles including the first one to eliminate possible nonspecific background staining. MICSSS system is capable of performing up to ten antibodies by this method without the interference of residual antibodies from previous cycles of staining, and before tissue damage from repeated cycles may start precluding analyses. Each cycle lasts for an average of 6–7 h, and approximately 30 slides can be processed simultaneously. Automated immunostaining platforms allow for batch process, and multiple slides can be stained with different antibodies simultaneously, in contrast to manual immunostaining in which just one antibody can be performed on multiple slides simultaneously.
1.1.2. Does Not Affect Antigenicity/No Steric Hindrance
Antigen quality loss can be a major concern since destaining and heat exposure are used after every cycle of MICSSS immunostaining. MICSSS method has been validated with multiple markers constitutively expressed on immune cells by testing them in permutation of order of staining on consecutive slides from the same tissue block. Number of cells per mm2 for each marker tested was comparable for each marker regardless whether immunostaining was performed first, second, or even after 10 cycles, thereby establishing that MICSSS can be used as a reliable test for markers used to detect immune cells. MICSSS also provides similar signal intensity level for a selected marker after several cycles of immunostaining and it is validated by testing signal intensity for various antibodies in permutation of order by performing color deconvolution and comparing signal intensity histograms for different orders. This validation has revealed MICSSS provides robust evidence for antibody signal preservation even after tenth cycle and there was no significant difference between different orders of a selected immunostain. However, quantitative markers relying not only on density but on signal intensity, like PD-L1 and HER-2, should be prioritized, preferentially first in the iterative cycles before performing destaining, since even minimal signal intensity loss could affect interpretation based on guidelines for these markers. Another concern of iterative cycles can be damage on tissue sections after prolonged handling, in particular due to repeated need for removal of coverslips, which contributes to the recommended limit of a maximum of ten markers per slide.
MICSSS panels can include immune markers that stain different compartments of a cell like nuclear, cytoplasmic, and nuclear. While other multiplexing methods with depot effect amplification could experience steric hindrance, MICSSS does not appear to suffer from this problem due to effective destaining and blocking between cycles. MICSSS allows for routine testing of membranous expression of CD2, CD3, CD8, PD-1 quadruple positive T cells or cytoplasmic expression of HLA-DR, CD206, CD68, CD163 quadruple positive histiocytes without obvious steric hindrance.
1.1.3. Can Be Performed Along with a Fixed Marker
Pathology archives host a tremendous amount of IHC slides worldwide which are acquired during diagnostic workflows. They are mostly singleplex immunostained slides in which brown-colored DAB is used as a chromogen, as it is the most commonly used chromogen in the world. However, DAB is a permanent chromogen and is not removable by organic solvents the way AEC can be. MICSSS may be performed along with DAB staining using sets of antibodies from non-cross-reactive species in the first cycle, and double-colored (brown-red) multiplexed images can be acquired. This may be useful for example when performing cytokeratin immunostaining for tumor cells with DAB brown staining which remains preserved throughout MICSSS (insensitive to solvent-based destaining), while in parallel querying iteratively several markers for the immune microenvironment using standard MICSSS with AEC red staining.
1.2. Image Acquisition
Image acquisition is done after every cycle of immunostaining since bleaching takes place between cycles and removes the immunostain (Fig. 2). It is performed by a whole slide scanner in which slides are scanned at 20× or 40× magnification and saved as full images in formats depending on the scanner type/brand. Certain points should be taken into consideration during the whole slide scanning process. Proper naming of image files is important to prevent any confusion, and it should ideally include at least antibody name, cycle number, and date, and including important immunostaining conditions like antibody incubation duration and dilution levels can be useful. Every project should have a specific folder/subfolder system, and they should be kept in a cloud with backup system. Slide labels should also be included in the scanning since they can provide an image info backup. The size of image files can become an issue in time by accumulating data from several projects. Instead of scanning the entire slide area, selecting actual tissue area first and limiting the scan to this region can save some disk space without affecting the analysis. Tissue selection can be made by manual annotation or automatically. Most scanning systems provide automatic tissue detection feature. If automatic tissue recognition is used for detecting the scanned area and tissue is thin or faintly stained, thresholding should be used. Thresholding can also be used for the opposite scenario of having dark-colored dirt or pen lines on the slides. Artifacts on the slide like dust, excessive residual stain, and/or curled tissue areas should be discarded as much as possible since they can create false-positive detection during image analysis on the next step. Focusing is important since blurry areas negatively affect the image analysis quality. It can be done automatically or manually. Putting multiple focus points on the tissue area can minimize blurring in newer scanners. When there is more than one tissue on the slide, each tissue piece can be selected and focused separately to increase quality, otherwise the scanner recognizes all tissue pieces as a single tissue and sets one common focal plane. There are many whole slide image formats depending on the selection of digital slide scanner. Scanner specific formats usually have file size limitation advantages when compared with uncompressed raw images. Selected objective magnification level is the main factor for resolution of images and digital slide scanners most commonly have 20× and 40× options. 20× is the most commonly used magnification level of scanning since it usually provides enough detail for image analysis in addition to providing images with optimal file sizes.
Fig. 2.
Comparison of several markers on the same ROI of a triple negative breast cancer case
1.3. Image Analysis
MICSSS produces individual images of full tissue immunostaining per marker, up to ten markers per slide (Fig. 2). A challenge is then to be able to analyze these images, either individually to establish density (per mm2) or percentage (out of all nucleated cells) of stained cells expressing the marker, or for coexpression of markers on the same cell. Manual counting is feasible for individual markers but can quickly become overwhelming, pointing to the growing use of digital pathology solutions when multiple markers are involved. MICSSS-generated image analysis can be performed with a variety of software tools and suites, most of which include steps of image segmentation based on nucleus detection (hematoxylin or optical density), detection of chromogen, and, in some cases, image coregistration. Because one of the goals of MICSSS is to democratize access to multiplex immunohistochemistry, the following pipeline is based on open-source software developed by Dr. Bankhead named QuPath [12].
1.3.1. Color Vectors
QuPath performs color deconvolution for channel separation for RGB images. Default stain vectors for MICSSS brightfield images are hematoxylin, AEC chromogen, and residual channel (Fig. 3). Default values for these stain vectors should always be checked since accuracy of cell segmentation and positive cell detection depends on these vector values. QuPath has a useful feature named “estimate stain vectors” in which color vector values can be automatically estimated depending on a sampling ROI on the whole slide image. However, this sampling ROI must be small and include a balanced level of positive and negative cells as well as a blank white area.
Fig. 3.
The ROI selected above is used for estimating stain vectors (hematoxylin, AEC, residual). Hematoxylin and AEC channels are produced by using these stain vectors with color deconvolution
1.3.2. Annotation
Scanned tissue area can include many different tissue compartments such as tumor, normal organ parenchyma, normal epithelium, and tumor/normal stroma. Annotation of compartments of interest correctly holds significance since annotated areas are used in cell segmentation and further image analysis. QuPath has many tissue annotation features including rectangle, circular selection tools in addition to special annotation tools like wand tool which can detect the sharp tissue compartment borders like tumor or normal parenchymal border automatically and makes the annotation around these tissue compartment types easier (Fig. 4). Annotation is also performed or double checked by a pathologist to prevent errors. Any error made in this step can lead to cascade of errors image analysis since everything is dependent on annotated areas. If annotation of specific area of interest is not considered and just whole tissue annotation is needed, QuPath has a “simple tissue detection” feature that can automatically annotate the whole tissue and this feature can be used in batch mode to make whole tissue annotation for all project images in one run. However, double check must be done to prevent errors that might happen during batch run. Peritumoral lymphocytic accumulation can be significant in some tumors and special annotation methods can be used to analyze peritumoral region. QuPath allows to expand the tumor border outward and/or inward for a selected diameter, and this feature allows for quantifying the tumor border TILs and comparing them with intratumoral ones in detail. This feature can provide important data in terms of geographic configuration of TILs.
Fig. 4.
Various annotation tools provided by QuPath are demonstrated here including line, circle, rectangle, wand, polygon, and brush tools
1.3.3. Cell Segmentation
Cell segmentation refers to identification of cells in an image, and it is one of the most important steps of image analysis. It is performed as nuclear segmentation, and cytoplasmic and membranous portions are included in the segmentation by expanding nuclear segmentation border for a selected diameter. Hematoxylin and chromogen color intensity values for nuclear, cytoplasmic, and total cellular compartment in addition to several shape features are recorded in each cellular detection of segmentation. QuPath provides two types of segmentation based on optical density and hematoxylin color intensity (Fig. 5). Cell segmentation quality depends on multiple factors including accuracy of stain vector values, image scanning quality, and number of artifacts. Some dense tissue types like lymph node require diligent setting testing to reach optimal segmentation. If cell segmentation is done on each IHC image belonging to the same slide separately, this can cause slight change in number of total cells per image. Moreover, chromogen color can obscure the nuclear details for some antibodies like CD68 and cause false positive segmented cells. Pure hematoxylin staining without immunostaining can be included in MICSSS panel as a separate cycle and this channel can be used as a common segmentation layer. Once cell segmentation is done on a hematoxylin-stained image, segmentation layer can be moved onto other images of the project. Uniform total cell number can be calculated by using this methodology; however, it requires processing on all images to fix minimal elastic changes occur during cycles of MICSSS. Fiji software’s Trakem2 plug-in can be used for fixing the elastic changes and aligning all images and ultimately all cells on the same coordinates [13, 14].
Fig. 5.
Colorectal carcinoma stained with Ki-67. Cell segmentation is performed based on optical density, and positive cell detection is made by finding a cutoff value with using the mean nuclear chromogen color intensity feature
1.3.4. Phenotyping
Phenotyping refers to detection of certain type of cell(s) depending on their features including color intensity and shape features; however, it is most commonly used for detection of positive cells in an image (Fig. 5). Correct cell segmentation and recorded shape and intensity values in segmented cells are extremely important for the accuracy of phenotyping. Thresholding is the most commonly used phenotyping method; however, machine learning-based classifier methods can be performed for phenotyping as well. Thresholding is simpler and faster method than the latter; however, it bears some caveats as well. Thresholding basically uses feature values recorded in each cell during cell segmentation including shape features like nuclear size and nuclear/cellular size rate and intensity features like nuclear chromogen intensity or total cellular intensity. Operator finds a cutoff value for a desired feature to differentiate them from other cells by using QuPath’s measurement maps function. Once the cutoff value is achieved, that value can be used for phenotyping. For example, CD3 positive cells can easily be differentiated from other cells by using thresholding on cellular chromogen color mean value. Requiring minimal tweaks for different slides stained with the same antibody in a project is the major drawback of this method and it leads to bias. Machine learning classifier method can be performed to prevent bias for a project including a lot of case (Fig. 6). This method depends on training a classifier by selecting features like intensity and/or shape values and using them in training by processing with a selected machine learning or deep learning algorithm. For example, detection of positive immunostained cells can be made by using chromogen color intensity features. A classifier can be created after training it with a proper number of positive and negative cells, and this classifier can be used throughout the project in an unbiased way. Thresholding method allows only dual classification like positive and negative, whereas a trained classifier is capable of classifying infinite number of categories depending on the training. This can help us phenotyping not just positive and negative but also subcategories like positive immune cells or morphological categories like tumor, normal epithelium, and normal parenchyma. It can even allow us to train for artifacts on tissue section and categorize them. Moreover, shape and intensity features like nuclear-to-cellular ratio and intensity values can be combined, and this helps in yielding more precise results for immunostains like cytokeratin to detect tumor cells.
Fig. 6.
ROI from a normal tonsil tissue including a germinal center which is diffusely positive stained with Ki67 as expected. Yellow circle shaped dots are used to train the machine learning based classifier for positive and negative cells. After marking 11 cells as positive and negative, this algorithm classified the rest of the cells correctly
1.3.5. Multiplexing MICSSS Images Belonging to the Same Slide
Multiplexing methods bear the purpose of demonstrating coexpression data of cells in a tissue, and MICSSS is capable of providing it with spatial information in a unique way. However, a visual demonstration of multiplexed immunostaining requires image processing steps since MICSSS is based on cycles of immunostaining and removal of antibodies between these cycles. This causes elastic changes on tissue at the microscopic level. The placement of the slide in the scanner after every immunostaining cycle can also be slightly different which can cause the tissue section to be located in a slightly different area in the image. All these conditions should be fixed to align IHC images belonging to the same tissue section for visualizing multiple immune markers on the same image. The purpose of alignment is to fix coordinates of the same cells on different IHC images belonging to the same section for visualizing coexpressions. Fiji Trakem2 plug-in is a good option for alignment of images automatically and/or manual by using landmarks [14]. After alignment, images must be registered on top of each other; however, since every image uses the same red-colored AEC chromogen, registration of these raw images do not give the desired multiplexed image which has a different color code for each antibody. Color deconvolution is performed for this purpose to extract hematoxylin and AEC chromogen channels as 8-bit images. Hematoxylin channel can be yielded best from pure hematoxylin stained image and chromogen channels are extracted from immunostained sections. After assigning different color codes for each IHC image, color inversion takes place to achieve pseudofluorescent channels ready for merging. Merging these channels after these steps will result in a perfect image visualizing all antibody expression in one focal plane. Cell segmentation is made on hematoxylin channel to achieve the most accurate results. Cell segmentation performed on hematoxylin channel will record all intensity values for other IHC channels, and this allows to obtain detailed coexpression data for each cell. This method’s drawback is being able to perform only in a selected ROI. However, whole slide image coexpression data without visual image can be still provided by using same cell segmentation layer for all images. This whole slide image data can be analyzed more in detail by mass cytometry approaches like viSNE analysis.
2. Materials
2.1. Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue Sections
MICSSS assay is performed on 4-μm-thick FFPE sections.
2.2. Antigen Retrieval
Antigen Retrieval Solution (pH 9): Dako Target Retrieval Solution, pH 9.
Antigen Retrieval Solution (pH 6): Dako Target Retrieval Solution, pH 6.
2.3. Blocking Reagents
Peroxidase Suppressor (Thermo Scientific).
Protein Block Serum-Free Ready-to-Use (Agilent).
Biotin Blocking System (Agilent).
AffiniPure Fab Fragment Donkey anti-mouse IgG (H+L) (Jackson Immuno Research).
AffiniPure Fab Fragment Donkey anti-rabbit IgG (H+L) (Jackson Immuno Research).
AffiniPure Fab Fragment Donkey anti-rat IgG (H+L) (Jackson Immuno Research).
2.4. Secondary Antibody and Horseradish Peroxidase (HRP)
HRP-labeled anti-mouse secondary antibody: EnVision™+System-HRP Labelled Polymer Anti-mouse (Agilent).
HRP-labelled anti-rabbit secondary antibody: EnVision™+System-HRP Labeled Polymer Anti-Rabbit (Agilent).
HRP-labelled anti-rat secondary antibody: ImmPRESS HRP Anti-Rat IgG, Mouse adsorbed (Peroxidase) Polymer Detection Kit (Vector Laboratories).
Streptavidin/HRP (Agilent).
Biotin-SP-AffiniPure Donkey anti-Rabbit IgG (H+L) (Jackson Immuno Research).
2.5. Chromogen
AEC Peroxidase Substrate Kit (Vector Laboratories).
2.6. Counterstain
Hematoxylin Solution, Harris Modified (Sigma-Aldrich).
2.7. Mounting Media
Glycergel Mounting Media (Agilent).
2.8. Other Reagents
Ethanol, Absolute (200 Proof), Molecular Biology Grade, (Fisher BioReagents™, Fisher Scientific).
Dako Antibody Diluent (Agilent).
Tween™ 20 Surfact-Amps™ Detergent Solution (Fisher BioReagents™, Fisher Scientific).
Tris hydrochloride (Fisher Scientific).
Sodium chloride, Fisher BioReagents™ (Fisher Scientific).
Sodium hydroxide (NF/EP/BP/FCC) 10N, Fisher Chemical (Fisher Scientific).
Hydrochloric acid solution 6N (Fisher Scientific).
Alfa Aesar 3P Xylenes Mixed 97+%2.5L (Fisher Scientific).
2.9. Other Materials and Equipment
Beakers and Measuring Cylinders.
Coverslip 40 × 24 mm (Fisher Scientific).
H2O (Milli-Q Water Purification System).
Vortex machine.
Refrigerator (2°–8°).
Slide stain tray.
Water bath (Fisherbrand Isotemp).
Staining racks.
Slide boxes.
Slide scanner NanoZoomer S60 (Hamamatsu).
Scott C-fold paper towels (Fisher Scientific).
Nuova II stir plate (Thermolyne).
3. Methods
3.1. Immunostaining
3.1.1. Slide Preparation
Prior to staining your sample, the tissue must be prepared by removing the paraffin and progressively rehydrating the tissue which had been previously undergone dehydration for paraffin embedding. Due to the cross-linking of proteins that occurs with formalin-fixation, the desired epitopes on the sample are not readily available for binding to a primary antibody, and they must first undergo an unmasking procedure, known as heat-induced epitope retrieval (HIER).
Deparaffinization and Rehydration
Incubate the slides at 37 °C overnight to allow the tissue to adhere better to the slides during the staining/destaining/restaining process.
Immerse the slides in 100% xylene for 5 min. Repeat this step two more times, gently shaking off excess liquid in between steps.
- The slides will be immersed in progressively more dilute ethanol solutions and ultimately immersed in water to rehydrate the tissue.
- Immerse the slides in 100% ethanol for 5 min.
- Immerse the slides in 90% ethanol for 5 min.
- Immerse the slides in 70% ethanol for 5 min.
- Immerse the slides in 50% ethanol for 5 min.
- Immerse the slides in dH2O for 5 min. Repeat.
Heat-Induced Epitope Retrieval (HIER)
Depending on the antigen, the Retrieval Solution (RS) with appropriate pH should be chosen. In our experience, most antigens will require pH 6 citrate buffer or pH 9 EDTA buffer, but some will require pH 8.
Dilute 10× RS to 1× in dH2O. Prepare enough solution so that the slides can be completely submerged in the solution.
Preheat the solution to 95 °C using a water bath. Be careful not to allow the water in the water bath to mix with and further dilute the retrieval solution.
Incubate the slides in the RS at 95 °C in the water bath for 30 min.
Remove the slides from the water bath, leaving them in the RS, and allow the solution and the slides to cool to RT for 30 min. If the slides are in a container with a cover, remove the cover at this point to facilitate cooling.
Rinse the slides with 1× Tris Buffered Saline (TBS).
Remove excess liquid from the slides. Be careful not to disturb the tissue, and do not allow the tissue to become dry (see Notes 1–3).
Blocking
In order to decrease nonspecific binding of primary and secondary antibodies, the tissue must be blocked with a protein solution to bind to nonspecific sites. Because signal amplification and subsequent detection relies on the chemical reaction involving horseradish peroxidase (HRP), the tissue’s endogenous peroxidase activity should also be blocked to avoid false signals and/or increased background signals.
Cover the tissue sample with 3% hydrogen peroxide (H2O2) and incubate for 15 min at room temperature.
Rinse the slides in TBS and remove excess liquid from the slides.
Cover the tissue sample with Serum-Free Protein Block and incubate for 30 min at room temperature.
Rinse the slides in TBS and remove excess liquid from the slides.
3.1.2. Primary Antibody Staining
Dilute primary antibody in REAL Antibody Diluent to working concentration.
Cover tissue sample with diluted primary antibody solution and incubate for 1 h at room temperature. Some antigens may require overnight incubation with primary antibody, in which case, the incubation should be performed at 4 °C.
Immerse the slides in TBS + 0.04% Tween 20 (TBS20) for 5 min. Remove excess liquid from the slides.
3.1.3. Secondary Antibody Staining
Dilute secondary antibody in TBS to working concentration and cover tissue sample with secondary antibody solution (biotinylated anti-rabbit or polymer anti-mouse 1:200, polymer anti-rat 1:500 dilution) and incubate for 30 min. Secondary antibody should be against species of origin of primary antibody (i.e., anti-mouse secondary antibody if primary antibody is from mouse species), but the secondary antibody species of origin should not be from same species of origin as the primary antibody.
Immerse the slides in TBS20 for 5 min at room temperature. Remove excess liquid from the slides.
If a biotinylated antibody is used, dilute streptavidin-HRP to 1:300 concentration in TBS.
Cover tissue sample with HRP solution and incubate at room temperature for 30 min.
Immerse the slides in TBS20 for 5 min. Repeat this washing step and remove excess liquid from the slides.
3.1.4. Antigen Detection
Prepare fresh AEC solution for each staining: In 5 mL of dH2O, add 2 drops of Buffer Stock Solution, 3 drops of AEC Stock solution, and 2 drops of 3% H2O2. Cover tissue sample with prepared AEC solution and incubate for at least 5 min, but no more than 30 min. Check the slide under light microscope periodically to ensure there is no excessive background staining, which will appear as diffuse pink/red staining. If background tissue remains transparent with minimum background staining, incubation with AEC solution can continue up to 30 min in order to maximize signal.
Rinse the slide with dH2O to stop the chromogen reaction.
Perform counterstaining by immersing the slide in 100% hematoxylin for 5 s. Thoroughly rinse the slide by immersing in H2O. This may require several liters of water depending on the number of slides in order to adequately remove excess hematoxylin.
Mount the slides using a coverslip and Aqueous Mounting Medium. Be careful not to create bubbles over the tissue sample. Mounting medium needs to be warmed before use so that it is of correct consistency (a viscous solution).
Incubate the slides at room temperature until mounting medium solidifies.
3.1.5. Dehydration/Destaining/Rehydration
After image acquisition of the first staining, the slides are ready to be processed for destaining and subsequently restained for the next antigen of interest.
Incubate the slides in hot water (56 °C) for 5–10 min and gently remove the cover slip without damaging the underlying tissue sample.
Immerse the slides in cold water to clean off aqueous mounting medium.
Immerse the slides in 50% ethanol for 2 min.
Immerse the slides in 70% with 1% HCl (12 N) for 2 min. It is imperative to add the HCl to the ethanol in this step, as this will remove the hematoxylin from previous counterstaining.
Immerse the slides in 100% ethanol for 5 min.
Immerse the slides in 70% ethanol (without HCl) for 2 min.
Immerse the slides in dH2O for 5 min.
Using correct antigen retrieval solution with the appropriate pH for the new antigen, repeat HIER steps. Only incubate the slides in the RS for 10 min as opposed to 30 min as in original HIER step.
3.1.6. Blocking (Subsequent Cycles)
In this step, it is necessary to block any residual HRP that was not inactivated during the HIER. Exposed Fc segments of the prior primary antibody will also need to be blocked in addition to repeating the general blocking steps that were performed during the initial staining.
Cover the tissue with 3% H2O2 and incubate for 15 min at room temperature. Rinse the slide with 1× TBS and remove excess liquid from the slide.
Cover the tissue with Serum-Free Protein Block and incubate for 30 min. Rinse the slide with 1× TBS and remove excess liquid from the slide.
Cover the tissue with monovalent Fab fragment directed against the species of origin of the previous primary antibody and incubate for 30 min at room temperature. (For Life Technology FAB-Fragment Donkey, anti-mouse, a 1:50 dilution in TBS is recommended.)
Wash the slide in TBS20 for 5 min. Repeat wash step two times.
3.1.7. Antibody Staining and Antigen Detection (Subsequent Cycles)
Repeat steps of “Primary Staining,” “Secondary Antibody Staining,” and “Antigen Detection.”
For every subsequent staining, the procedure should be repeated beginning with “Dehydration/Destaining/Rehydration” step.
3.2. Scanning
After every cycle of immunostaining, scanning of immunostained slide is carried out in a whole slide scanner (Hamamatsu NanoZoomer S60). NDPScan is used as the scanning software in our workflow.
The glass slide is inserted in the tray and loaded into the scanner.
Destination location for output image, and slide name which includes antibody name, order of cycle, and protocol details like primary antibody incubation duration and concentration optionally are set.
Magnification level of scanning is selected as 20× or 40×. 20× is preferred since it results in optimal file sizes.
Scan area is set by automatic tissue recognition or manual selection.
Focusing is done by focus points. It can be done automatically by selecting the number of focus points or manually. These focus points can be reviewed to increase the quality and prevent any sort of blurriness.
NDPI extension is used by Hamamatsu scanner in default mode; however, it can be changed to raw image extensions like TIFF images. NDPI is the preferred extension type since it provides small file sizes and acceptable image quality together.
Output images are reviewed for quality control.
3.3. Image Analysis
Image analysis is performed by using QuPath (0.1.2) which is an open-source software.
3.3.1. Project Management
Regardless of organized image folders for each project, QuPath has a feature of project management feature to group images belonging to a project together and save every single image analysis data in that project to work on them anytime needed. It creates “qpdata” file for every image in a project to save image analysis data like annotations, cell segmentation, and phenotyping data. QuPath stores project folder in a different location than the image files.
Project is created with a specific name.
Related images are imported into the project. QuPath provides several methods to import images like selecting files one by one or importing a selected folder of images altogether.
Metadata like antibody name, date, and order of cycle are encoded in each image to sort them in different desired orders.
3.3.2. Stain Vectors
Chromogenic immunohistochemistry images are basically RGB images per their background pixel values, and three important stain vectors (hematoxylin, chromogen, and residual) are used for immunostaining separation. Since QuPath uses color deconvolution for stain separation, stain vector values are extremely important for further steps of image analysis. QuPath has a very useful feature for estimating these values named “Estimate Stain Vectors” for the purpose of improving stain separation.
Default stain vector type should be selected as Brightfield H-DAB.
Representative tissue ROI is selected on tissue. This ROI must include a balanced amount of positive and negative cells as well as a blank (white) area. Moreover, it should not be a large region since it results in downsampling of pixel values and it should be avoided for best results.
“Estimate Stain Vectors” feature is used for automatic estimation of stain vectors. Results are shown in 2D scatterplots belonging to three vectors.
Scatterplot shows stain vector values by dots and two stain vectors per scatterplot. Most of the dots should fall ideally between two stain vectors in each scatterplot.
Quality control is made by visualizing each vector in Brightness/Contrast tool.
NOTE: If there are many images in a project that are acquired with a similar staining, one representative ROI can be used to estimate stain vectors in batch mode by using a script.
3.3.3. Annotation
Annotation can be made in different ways according to the purpose of a project. It is needed for further steps of image analysis since analysis is performed on created annotations in the image.
Simple tissue detection: This tool can automatically recognize and annotate whole tissue on the slide (Fig. 7).
Annotation tools: QuPath provides many basic annotation tools like rectangle, circle, and line tool as well as more advanced ones like wand tool which detects sharp changes in tissue and annotate it by moving the pointer around the interested area (Fig. 4).
TMA annotation: TMA cores can automatically be annotated by the TMA support of QuPath (Fig. 8).
Annotation of specific tissue structures like tumor should be done by using the annotation tools mentioned above by a pathologist to prevent possible errors.
Fig. 7.
Normal tonsil tissue is annotated by “Simple Tissue Detection” feature of QuPath
Fig. 8.
Automatic TMA annotation
NOTE: Tumor annotation can be expanded inward and outward for a desired diameter to analyze the tumor border and peritumoral regions (Fig. 9).
Fig. 9.
Expansion of a malignant melanoma annotation inward and outward by 100 μm. This feature can be useful to study peritumoral immune cell population located at the tumor border
3.3.4. Segmentation
Cell segmentation refers to detection of cells in an image. QuPath basically makes nuclear segmentation and expands the nuclear border of detection by a selected diameter to create a cytoplasmic zone.
Detection image type is set to either “Hematoxylin OD” or “Optical density sum.”
- Nuclear parameters are tweaked according to the tissue type.
- Background radius value estimates the background color and improves the cell segmentation. It makes this estimation pixel by pixel and propagates the information throughout the tile by using the background radius value. This works in images with a lot of texture in the background or images with background staining; however, it is not helpful on all occasions, so its use is optional.
- Median filter and Gaussian filter values are used to smooth the image to ease the minimal details inside the cellular compartments to segment cells properly and accurately. High values are especially helpful in segmenting large cells like CD68 positive histiocytes.
- Minimum and maximum nuclear area values are tweaked for discarding small and large artifacts and set the segmentation algorithm just to segment real nuclei.
- There are two different intensity parameters;
- Threshold for selected detection image is one of the most important settings in cell segmentation and it basically sets an intensity cutoff for detection of positive immune cells. Multiple cutoff values can be set for multiple intensity levels. Threshold can also be used with morphological features to differentiate cells with various shapes like large anaplastic tumor cells and lymphocytes from each other.
- Maximum background intensity value can be useful to get rid of artifacts by decreasing its value.
Cell expansion value sets the area of cytoplasmic region of segmented cells; however, if cells are squeezed together in a dense tissue, adjacent cells automatically decrease the size of this expansion area and limit it in their neighborhood.
3.3.5. Phenotyping
Phenotyping can be performed by two different methods.
Thresholding
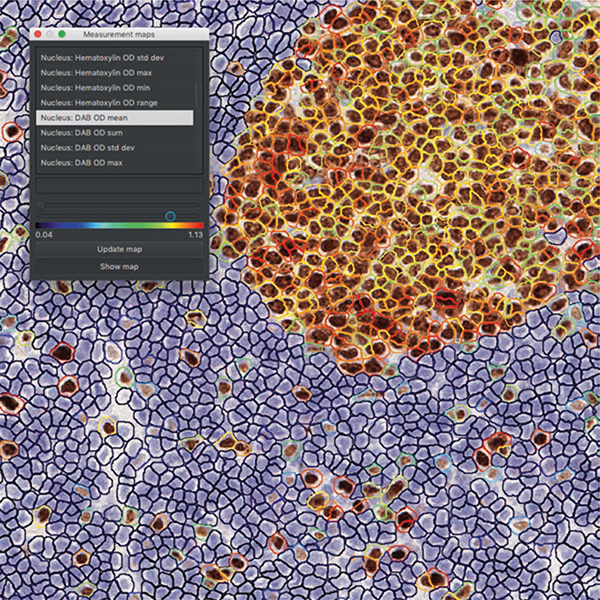
Measurement maps are used to set a cutoff value for a desired feature (Fig. 10).
Once a cutoff value is set, it is applied to the image to differentiate the cells for a selected feature like shape or intensity.
Data can be extracted from annotation measurements or detection measurements, and it can be exported to an Excel file.
Fig. 10.
ROI from a normal tonsil tissue including a germinal center which is diffusely positive stained with Ki67 as expected. Measurement maps tool helps with finding a cutoff value for a selected feature to classify the cells. “Nucleus: DAB OD mean” is selected here to find a cutoff value and make a positive/negative classification
Machine Learning-Based Classifier
Use of this method varies according to the purpose of analysis. If morphological classification is the interest, “add smoothed features” option can be used to improve the classification. However, if the aim is just obtaining results for positive cell detection, smoothing process is usually not needed (except markers like cytokeratin which stains group of cells). Smoothing reduces the noise of measurement of shape features like nucleus–cell ratio. It takes weighted average of detection measurements of adjacent cells.
Smoothed features can be added according to the aim of the project.
Detection classifier is created by selecting a classifier type. Random forest method is the default classifier algorithm.
Desired features are selected from advanced options according to the aim of the project.
Cell/tissue type classes are created in annotations tab.
Different cell classes are annotated using annotation tools. Using dots for different classes is useful for detection of positive immune cells.
The classifier system will autoupdate classification in annotations which include segmented cells during the annotation of different classes. Once the desired classification is achieved, classifier and training objects are saved separately.
Saved classifier can be applied to other images in the project file. Batch run of a saved classifier can be performed to save time; however, review is always needed for quality control.
Training objects can be loaded any time on other images to continue training the classifier, if there is any inaccurate classification in other images in the project.
3.3.6. Data Analysis
Final data can be extracted as annotation or detection measurements and exported as a data file.
4. Notes
It is important to ensure that the tissue on the slide at no point becomes dry. The slide or the tissue should remain covered in the solution/reagent from the previous step until it is ready to proceed with the next step. Avoid processing too many slides at once in between steps without applying the next appropriate reagent/solution in order to prevent tissue desiccation.
If a certain antibody produces a faint signal at baseline, staining with this antibody should be performed as one of the initial rounds of staining with this consecutive staining method.
AEC is toxic and carcinogenic. Make sure to use appropriate personal protective equipment when handling the solution or slides that have been treated with this solution.
References
- 1.Galon J et al. (2012) Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remark R et al. (2013) Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 19:4079–4091 [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD (2014) Immune modulation in cancer with antibodies. Annu Rev Med 65:185–202 [DOI] [PubMed] [Google Scholar]
- 5.Spitzer MH, Nolan GP (2016) Mass cytometry: single cells, many features. Cell 165:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson D, Savage K, Reis-Filho JS, Isacke CM (2008) Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol 9:13–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giesen C et al. (2014) Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 11:417–422 [DOI] [PubMed] [Google Scholar]
- 8.Gerdes MJ et al. (2013) Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A 110:11982–11987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stack EC, Wang C, Roman KA, Hoyt CC (2014) Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70:46–58 [DOI] [PubMed] [Google Scholar]
- 10.Angelo M et al. (2014) Multiplexed ion beam imaging of human breast tumors. Nat Med 20:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remark R et al. (2016) In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci Immunol 1:aaf6925–aaf6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bankhead P et al. (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rueden CT; Schindelin J & Hiner MC et al. (2017), “ImageJ2: ImageJ for the next generation of scientific image data”, BMC Bioinformatics 18:529, doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardona Albert, Saalfeld Stephan, Schindelin Johannes, Arganda-Carreras Ignacio, Preibisch Stephan, Longair Mark, Tomancak Pavel, Hartenstein Volker and Douglas Rodney J.. 2012. TrakEM2 Software for Neural Circuit Reconstruction. PLoS ONE 7(6): e38011. [DOI] [PMC free article] [PubMed] [Google Scholar]