Abstract
Electrokinetic supercharging (EKS) is known as one of the most effective online electrophoretic preconcentration techniques, though pairing with it with mass spectrometry has presented challenges. Here, EKS is successfully paired with ESI-MS/MS to provide a sensitive and robust method for analysis of biogenic amines in biological samples. Injection parameters including electric field strength and the buffer compositions used for the separation and focusing were investigated to achieve suitable resolution, high sensitivity, and compatibility with ESI-MS. Using EKS, the sensitivity of the method was improved 5,000-fold compared to a conventional hydrodynamic injection (HDI) with capillary zone electrophoresis (CZE). The separation allowed for baseline resolution of several neurotransmitters within 16 min with limits of detection down to 10 pM. This method was applied to targeted analysis of seven biogenic amines from rat brain stem and whole Drosophila tissue. This is the first method to use EKS with CE-ESI-MS/MS to analyze biological samples.
Keywords: Biological Samples, CE, CE-MS, Electrokinetic Supercharging, Neurotransmitters
Introduction
CE has become a popular technique for analysis of complex biological mixtures due to its fast and efficient separations as well as its small sample volume requirements [1–3]. A limitation for CE, until recently, was the difficulty of hyphenation with MS. The advent of robust and commercially available electrospray ionization (ESI) interfaces for CE-MS has opened many new uses in fields such as proteomics [4], food science [5], drug analysis [6], and genomics [7]. Recently, CE-MS has also gained momentum in metabolomics and neuroscience because of its ability to efficiently analyze many compounds simultaneously from small volumes of complex samples [4, 8, 9].
Currently two types of CE-MS interfaces are commonly used: sheath-flow and sheathless [10]. Sheathflow interfaces utilize coaxial conduits to add solvent and gas flow to assist with ESI. In contrast, sheathless interfaces generate stable ESI or nESI directly from the separation capillary to minimize dilution. Though sheathless methods are more difficult to achieve and operate routinely, they can still be robust and provide reasonable detection limits. For example, in a quantitative metabolomic study using a sheathless CE-MS interface, limits of detection (LOD) down to 60 nM were reported for several amino acids and metabolite compounds [11]. These LODs are relatively high compared to other separation and detection methods because of the small volumes injected into the capillary. Preconcentration methods are needed to allow lower detection limits and higher sensitivity by CE-MS.
A variety of on-line preconcentration techniques have been developed to improve the detection sensitivity for CE, including field amplification sample stacking (FASS), field amplification sample injection (FASI), transient isotachophoresis (tITP), pH-mediated stacking, large volume sample stacking (LVSS), and sweeping [12, 13]. Many of these techniques have been reportedly used with CE-MS in order to achieve acceptable limits of detection, including FASI, dynamic pH junction, LVSS, and more [14–18]. Among the preconcentration techniques available for CE, electrokinetic supercharging (EKS) is one of the most powerful, as it can achieve more than 5 orders of magnitude improvement in detection sensitivity over conventional injections [19]. EKS combines FASI and tITP. This approach overcomes the limits on FASI, wherein a limited amount of sample can be injected before excessive band broadening reduces separation quality, by using tITP to re-concentrate such bands. Since its introduction in 2003 [20], EKS has been combined with UV absorbance detection to measure low abundance analytes such as rare earth metals [21], environmental contaminants [22], protein complexes [23], DNA [24], peptides [25], and drugs [26].
To perform EKS, a small plug of high ionic strength leading electrolyte (LE) is loaded into the separation capillary, followed by a small plug of water. A long electrokinetic injection (EKI) is then performed. During this step, FASI occurs, where charged analytes preferentially enter the capillary and then reduce migration rate as they contact the relatively low electric field in the LE zone. A final small plug of low ionic strength terminating electrolyte (TE) is loaded and the separation voltage is applied. Here tITP occurs, where the fast LE and slow TE cause a gradient of the electric field across the sample zone, allowing the analytes to further concentrate into distinct zones. As the zones progress, the LE and TE eventually dissipate and a CZE separation occurs in the remainder of the capillary. To enhance the amount of preconcentration and maintain resolution, it is desirable to limit the movement of the stacking boundary into the capillary during injection by using counter-flow or reduced electroosmotic flow [21–24].
Little work has been reported on interfacing EKS to CE-MS. This is due to several key challenges, including obtaining proper buffer compatibility, controlling flow, and maintaining a robust ground at the ESI interface. Pairing with MS requires volatile buffers that will be acceptable for ESI without ionization suppression. Choosing MS compatible buffers limits the number of buffer options available, which is an important selection in EKS for achieving a compatible LE and ionic strength requirements for stacking. Using counter-flow to suppress analyte migration down the capillary is not easily implemented with MS detection, where the capillary outlet is interfaced to an ionization source. Without the option of counter-flow, reducing migration during injection is largely dependent on buffer composition. For example, if a low pH buffer is used for EKS it will reduce the EOF, allowing for a longer injection without losing effective capillary length for subsequent tITP; but, reduced EOF comes at the cost of reduced separation speed and/or efficiency. Finally, maintaining a robust ground for stable separation current during EKS with CE-MS is challenging. The grounded sheath liquid is responsible for grounding the separation and producing stable electrospray ionization; but, concentrated zones of ions generated from EKS can interfere with grounding and ESI stability. The interface requires careful selection of sheath liquid composition and its flow rates throughout a separation, as well as a reliable assembly of the interface.
One use of EKS with CE-MS has been reported [27]. This pioneering method achieved baseline resolution analysis of five hypolipidemic drugs with 1000-fold improvements in detection sensitivity over conventional injections. Though drastically lowering detection limits for these drugs, this method required regular disassembly of the CE-MS interface for capillary treatments with EOF reversal agents as well as an applied pressure during separation to produce and maintain a reversed EOF system necessary to analyze the desired anionic compounds. Such steps compromise robustness, throughput, maximum injection duration, and efficiency of the method.
In this work, we describe a new approach to EKS that is compatible with CE-MS. The method provides an online system by using a buffer that permits normal (cathodic) but suppressed EOF, allowing for sensitive and efficient analysis without regular capillary treatment or pretreatment of aqueous samples as previously reported. Suppressing EOF allows for longer FASI injections and subsequently lower LODs in this method. The preconcentration allows for a 5000-fold improvement in detection limits compared to CZE with HDI. This EKS-MS achieves LODs down to 10 pM in a 16-min separation for seven biogenic amine neurotransmitters.
As a demonstration, the method is applied to determination of several neurotransmitters (Table S1) in tissue sample extracts. Previous work has reported EKS-CE-UV analysis of low abundance neurotransmitters [28, 29]; however, the steps required to achieve sufficient LODs, namely counterflow to achieve longer injections, made the methods incompatible with MS detection, hence limiting the number of possible analytes and selectivity of detection. Measuring neurochemical concentrations can provide important insights into pathological states, pharmacological treatments, and brain functions [30]. Biogenic amines are implicated in many different diseases/dysfunctions and drug therapies, including Parkinson’s Disease [31], Alzheimer’s Disease [32], and depression [33]. Studying changes in the composition of the brain and the amine-based neurochemicals can lead to better understanding of these systems and diseases. Analyzing neurochemicals from tissue samples demonstrates the utility of the method to quantify low concentration compounds from limited sample volume.
Materials and Methods
Reagents and Materials
All chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Formic acid (99%) was from Acros Organics (Geel, Belgium). Stable-isotope labeled internal standards were purchased from CDN Isotopes (Quebec, Canada). Neurotransmitter standards and internal standards were prepared in HPLC-grade water as 100 μM and 100 nM stocks (respectively), aliquoted, and stored at -80 °C. The standard and internal standard aliquots were thawed daily (single use) and diluted for use. Standard mixes consisted of the seven neurotransmitters of interest and internal standard mixes of deuterated versions of the compounds of interest, excluding tyramine and octopamine. BGE contained 50 mM ammonium formate (pH 2.5) and 40% MeOH (v/v) and LE contained 250 mM ammonium formate (pH 2.5). Buffers were prepared fresh twice weekly, adjusting the pH by formic acid, sonicating for 10 min, and filtering through 0.45 μm membrane filter. Sheath-flow buffer contained 5 mM ammonium formate and 50% MeOH (v/v).
Instrumentation
Electrophoretic separations were performed on an Agilent 7100 Capillary Electrophoresis System using Agilent Masshunter software for CE-MS and Agilent ChemStation software for CE-UV. Electrophoresis experiments were performed in 80 cm of 50 μm inner diameter (id) and 360 μm outer diameter (od) fused silica capillaries coated with polyimide from Polymicro Technologies (Phoenix, AZ, USA). New capillaries were treated by flushing the capillary with 1 M NaOH for 20 min, HPLC-grade water for 5 min, and BGE for 10 min. ESI was carried out using an Agilent CE ESI-MS Sprayer and an Agilent 1260 Infinity Isocratic Pump to control the sheath-flow. MS detection was performed on an Agilent 6410 Triple Quadrupole, using Agilent Masshunter software.
HDI
The capillary was filled with BGE. A short plug of HPLC-grade water was introduced at the inlet at 50 mbar for 1 s. Sample was injected at 50 mbar for 65 s to fill 5% of the capillary volume. A separation of 30 kV was applied.
EKS
The capillary was filled with BGE, and a plug of LE was injected at 50 mbar for 30 s. A plug of HPLC-Grade water was hydrodynamically introduced at the inlet at 50 mbar for 1 s. Sample was then injected electrokinetically at 30 kV (375 V cm−1) for 150 s. A separation voltage of 30 kV was applied.
FASI
The capillary was filled with BGE. A short plug of HPLC-Grade water was hydrodynamically introduced at the inlet at 50 mbar for 1 s. Sample was then injected electrokinetically at 30 kV (375 V cm−1) for 30 s, (injections ranged from 5 – 50 s for comparisons). A separation voltage of 30 kV was applied.
FASS
The capillary was filled with BGE. A short plug of HPLC-Grade water was hydrodynamically introduced at the inlet at 50 mbar for 1 s. Sample was then injected hydrodynamically at 50 mbar for 195 s to fill 15% of the capillary volume (injections filling 5 – 25% capillary volume tested for comparisons). A separation voltage of 30 kV (375 V cm−1) was applied.
ESI-MS
For ESI, sheath liquid flow rate was set to 10 μL min−1 and nebulizer gas flow at 12 psi. Drying gas was used to assist with desolvation with a flow rate of 8 L min−1 at 300 C. The electrospray potential was set to 4 kV. For MS, multiple reaction monitoring (MRM) mode was used to enhance specificity. Precursor ions were selected in quadrupole one and product ions in quadrupole three for each compound (Table S1) and collision energies were optimized to produce the highest abundance of each product ion with dwell times of 200 ms. The source and drying gas temperature was 250 °C.
Sample Preparation
Rat Brain Stem
Homogenate was prepared from a whole rat brain stored at -80 °C. After thawing, the brain stem was sliced off and homogenized using a pestle homogenizer in a vial with cold ACN (10 μL mg−1). The homogenate was centrifuged 13 × 103 x g for 5 min, and the supernatant was collected. The supernatant was dried with nitrogen and resuspended in water (10x original volume). The sample was then aliquoted (100 μL aliquots) into vials which were subsequently frozen and stored at -80 °C until analysis. After thawing, 1% internal standard (v/v) was added prior to analysis. Whole Fly. Homogenate was prepared by homogenizing 10 whole Drosophila (male) in 150 μL of cold ACN, centrifuging, and removing the supernatant. Aliquots were stored at -80 °C until day of analysis. After thawing, 1% internal standard (v/v) was added and they were dried with nitrogen and resuspended in water (same volume) for immediate analysis.
LC-MS/MS Analysis
Chromatographic separations were conducted using a Waters nanoAcquity UPLC using a 1.0 × 100 mm column with HSS T3 1.8 μm particles interfaced to a mass spectrometer. Mobile phase A and B consisted of 5 mM ammonium formate with 0.15% formic acid and neat ACN, respectively. The gradient ran from 5% to 19% B in 0.01 min, 19% to 26% in 0.67 min, 26% to 75% B in 0.375 min, 75% to 100% B in 0.75 min, and stayed at 100% B for 0.1 min. The flow rate was set at 0.6 mL min−1.
For LC-MS analysis of the brain stem homogenate supernatant (as prepared above, excluding internal standard addition), a benzoylation reaction was implemented [34]. Derivatization involved mixing 2 volumes of sample (aqueous supernatant) with 1 volume of 100 mM sodium carbonate to raise the pH. Then 1 volume of 2% (v/v) benzoyl chloride in ACN was added followed by 1 volume of internal standard in 1% sulfuric acid (v/v) in 20/80 MeOH/water. The resulting mixture was analyzed by LC-MS. The internal standard mixture is comprised of analyte standards derivatized with the same procedure using C13 benzoyl chloride as the derivatizing agent.
Results and Discussion
We sought to develop an EKS method that was compatible with CE-MS. The approach presented here maintains resolution after preconcentration to provide high sensitivity and selectivity of MS/MS detection. The method is demonstrated to be suitable for analysis of tissue extracts for selected neurochemicals.
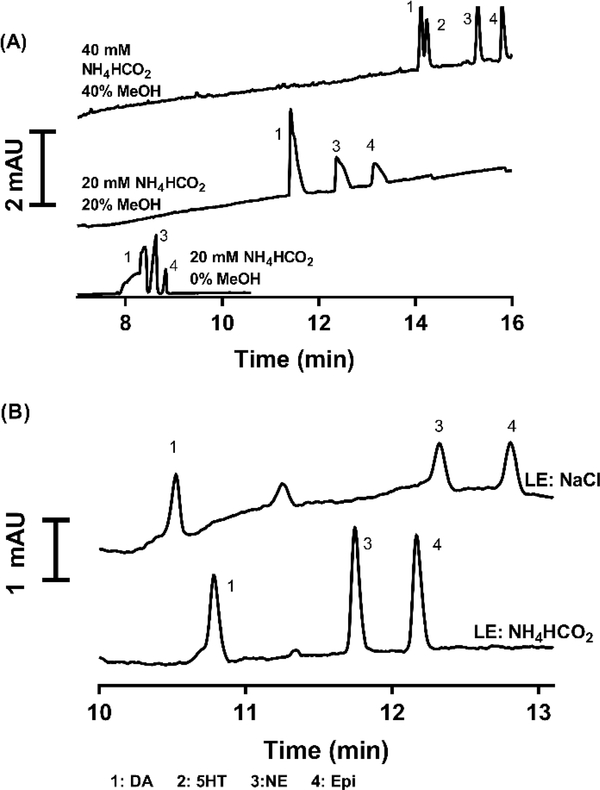
Initial experiments were performed with UV detection and a subset of our test compounds including dopamine (DA), epinephrine (EPI), norepinephrine (NE), and serotonin (5HT). These experiments focused on using low pH electrophoresis buffers to both facilitate analyte ionization during electrospray ionization and to suppress EOF [35]. Suppressing the EOF is required to allow for a long EKI without excessive migration down the capillary which would limit resolving power for positively charged analytes. 20 mM ammonium formate at pH 2.5 was initially selected due to its buffer capacity at low pH and compatibility with ESI. Aqueous ammonium formate did not allow for baseline resolution of all four compounds. To further lower EOF and improve selectivity, methanol was added to the buffer and the ammonium formate concentration of the water/methanol solution was increased to 50 mM to maintain comparable conductivity to buffer without methanol (Figure 1A). The final buffer chosen consisted of 50 mM ammonium formate in water/MeOH (60:40 v/v) with pH 2.5. This buffer offers a low EOF, which will allow for longer injections during preconcentration without diminishing resolution.
Figure 1.
(A) Effect of buffer on CE separation of 1 μM DA, 5HT, EPI, and NE at 20 kV in 60 cm long capillary. Increasing ionic strength and methanol allow for increased selectivity to resolve overlapping peaks in the original separation. (B) Comparison of 200 mM ammonium formate and 100 mM sodium chloride as LE during a tITP injection. Analytes 1 μM DA, E, and NE dissolved in water. Separation was performed at 20 kV in a 60 cm capillary using the previously chosen background electrolyte. Ammonium formate LE offers increased peak height and decreased broadness.
Using the above electrophoresis buffer and samples consisting of standards dissolved in water, it was possible to use FASI. Under these conditions FASI is limited to < 30 s injection before peak broadening and tailing becomes apparent and affects resolution (See Figure S1). Peak tailing is a limitation of FASI here, as it reduces achievable resolution. Utilizing a leading electrolyte and terminating electrolyte to achieve EKS can allow longer injections. LE buffer was chosen by testing the electrolytes sodium chloride and ammonium formate. 250 mM ammonium formate, pH 2.5, showed the best results as a LE, nearly double the peak signal than with 100 mM NaCl (See Figure 1B). Additionally, ammonium formate is more compatible than NaCl for MS due to its volatility. A 30 s injection at 50 mbar was used for LE loading, filling the capillary 2.3% to obtain a sufficient LE zone without drastically affecting the separation current.
Terminating Electrolyte
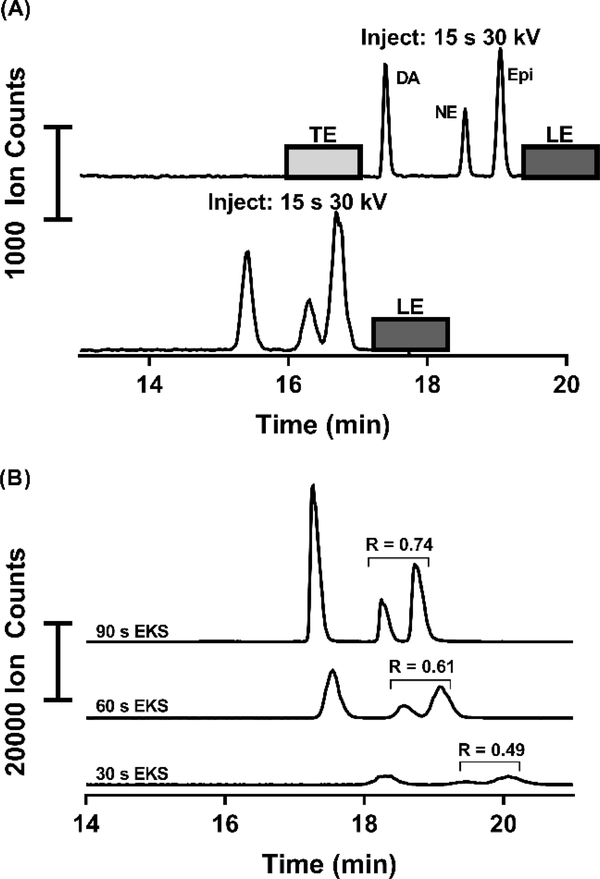
Use of 5 mM taurine (50 mbar, 10 s) loaded as a TE improved peak widths as expected for tITP. For example, a 15 s injection at 30 kV without a loaded TE gave DA peak width of 10.4 s, while the same injection and separation with a short plug of 5 mM taurine as a terminator loaded post-injection provided a peak width of 5.4 s (Figure 2A).
Figure 2:
(A) Shows the comparison of a 15 s injection with and without a loaded terminating electrolyte (5 mM taurine). Substantial peak broadening and loss of resolution can be seen without a TE loaded after the injection, indicating a loss of tITP. (B) This figure shows the EKS method with the only adjustment of different injection durations. As injection duration increases, more resolution is gained and the peaks become narrower, indication the formation of a system-induced TE and subsequent tITP at longer injection durations.
Interestingly however, we also observed that if TE was omitted, peaks became narrower and taller as injection time increased (Figure 2B). Intentional addition of a TE did not improve injections at these long times. We hypothesize that this result is due to a system-induced terminating electrolyte [21]. System-induced terminating electrolyte formation is a previously reported phenomenon which can result in tITP behavior such as that seen here [36, 37]. All further work used 30 kV, 150 s injections with no added TE.
We tested the method on 7 analytes. Applying the LE and TE parameters determined above, at a separation voltage of 30 kV using an 80 cm capillary with 150 s 30 kV injection, 5 of the 7 analytes were baseline resolved in a 16-min separation with LODs down to 10 pM using MS detection (Figure S2).
EKS vs. Other injection Methods
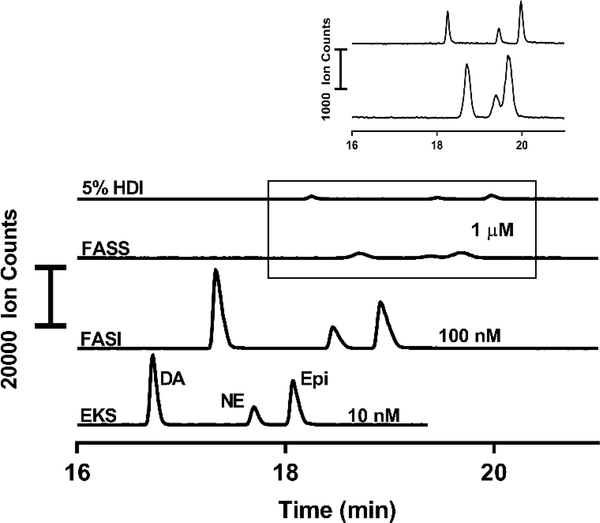
To determine the effectiveness of the EKS method, we compared it to a conventional HDI, FASI, and FASS (Table 1) (LODs reported in table 1 are improved in this comparison over LODs determined from calibration standards due to increased scan rate in the MS/MS detection, reducing the LOD to 10 pM in this comparison for DA). HDI is a commonly used mode of sample loading in CZE. It is useful for injecting all the analytes present without bias for the higher mobility compounds, though the maximum injection volume is limited due to peak broadening at larger capillary occupancy. Generally, injection volumes occupying over 5% of the capillary volume can lead to detrimental effects on the efficiency of an electrophoretic separation [38]. As shown in table 1, we observed a 5000-fold enhancement in the detection limits in EKS compared to HDI, and substantial enhancements over FASI and FASS. EKS offers the lowest limits of detection compared to the other forms of preconcentration while maintaining the narrowest peaks and highest resolution (Figure 3). While EKS maintains relatively Gaussian peak shapes, FASI and FASS have apparent broadening and display peak tailing, which can be detrimental to separation efficiency and resolution.
Table 1.
The developed EKS method compared to other forms of injection and preconcentration. LODs, resolution, and peak width are compared for each method. Enhancement factor is the quotient of a method’s LOD over the LOD from HDI
| Method | LOD (nM)a | Enhancement | FWHM (s) | Res |
|---|---|---|---|---|
| HDIb | 50 | - | 4.7 | 1.09 |
| FASSc | 20 | 2.5 | 9.6 | 0.37 |
| FASI (30 s) | 0.07 | 7.1 × 102 | 5.5 | 0.95 |
| EKS (150 s) | 0.01 | 5 × 103 | 4.2 | 1.02 |
LODs approximated using S/N = 3
HDI loads 5% of capillary volume
FASS fills 15% of capillary volume
Figure 3.
Comparison of EKS method with conventional HDI injection and other common forms of preconcentration in CE-MS. Concentrations of standards chosen to fit well within dynamic range for each method. For all, EKS background electrolyte was used with 30 kV separation voltage in an 80 cm capillary. Leading electrolyte only used in the EKS method. FASI performed at 30 kV for 30 s and EKS injection performed at 30 kV for 150 s. HDI and FASS injections performed using 50 mbar to fill 5% and 15% of the capillary volume, respectively.
Application to Biological Samples
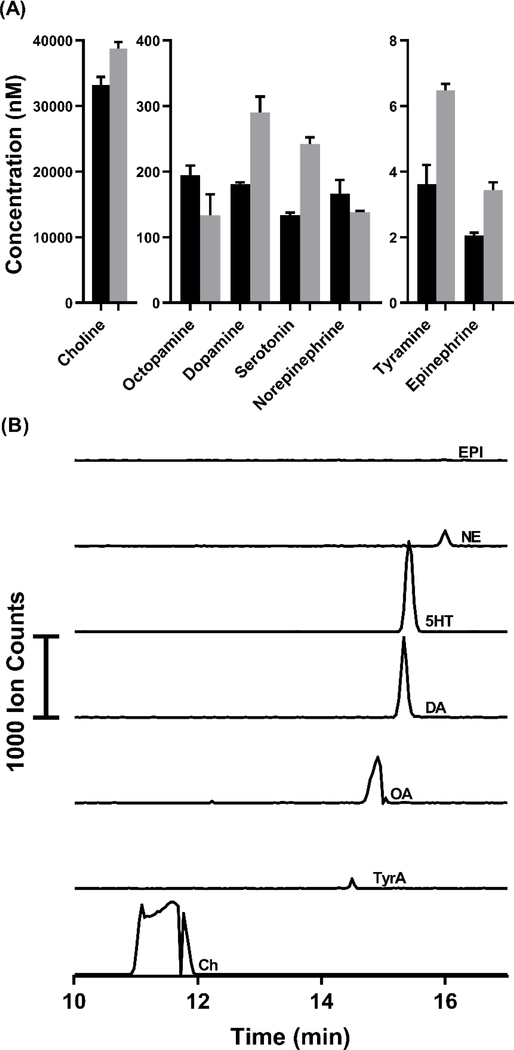
The limits of detection determined for the compounds makes this method potentially suitable for detecting trace compounds. Calibrations showed linearity (R2 = 0.99) from a 0.1 – 100 nM range of concentrations for most compounds and LODs from 30 – 140 pM (Table S2). To demonstrate the utility of the method to determine several neurochemicals in tissue samples, the method was applied to both rat brain tissue and whole Drosophila tissue. Supernatant from a rat brain stem homogenate was analyzed and all seven neurochemicals in the method were quantified from triplicate injections (Figure 4). To successfully determine these chemicals, a 10-fold dilution of the supernatant in water was necessary to avoid the negative effects of the sample matrix on the stacking. Figure 4A shows the concentrations after accounting for the 10-fold dilution, reflecting the original concentrations in the tissue extract. In Figure 4B, each trace shows the detection of one of the seven analytes from the brain tissue. Choline (Ch) overloads and interacts with capillary surface at high concentrations resulting in poor peak shape; however, this effect did not affect linearity of calibration (see Table S2).
Figure 4:
(A) Quantification of compounds in the rat brain stem. Concentrations determined by LC-MS/MS assay used for verification (grey bars). Each EKS injection used 100 μL of sample and LC injections used 5 μL of sample per injection. Measurements were made from three different aliquots of a single brain stem homogenate supernatant for each method. The error bars represent the standard deviation in each method determined for each compound from triplicate measurements. (B) Extracted electropherograms overlaid to show separation (Ch trace reduce by a factor of 250 for scaling).
Supernatant of whole Drosophila homogenate was also analyzed to measure the neurotransmitters in a sample that contains lower concentrations. Figure S3 shows the four neurochemicals and their concentrations in the supernatant. The concentrations correspond to an average of 0.17, 26, 0.010 and 0.046 pmol/fly of DA, Ch, octopamine (OA) and 5HT, respectively. All seven analytes could not be detected as EPI and NE are not present in flies, and tyramine (TyrA) was below the LOD in this analysis. In this tissue Ch, DA, 5HT and OA were able to be quantified with good repeatability, with RSD ranging from 4.8% – 17.3%. In this analysis, the method demonstrated its ability to simultaneously measure pM (OA) and μM (Ch) concentrations, as well as the range between.
LC-MS Method Validation
To verify the concentrations quantified by the EKS method, the same rat brain homogenate supernatant was analyzed using a published LC-MS/MS method [39]. Shown in Figure 4A, the concentrations determined by LC-MS/MS were comparable to the values determined by EKS, where concentrations determined differed by 14% – 46% between the two methods. Catecholamines DA, Epi, and NE differed between the two methods by 37%, 40% and -20% respectively. Catecholamine analogues TyrA and OA differed by 44 and -45% respectively and indolamine 5HT by 45%. Ch differed by 14%. Choline, the only compound without oxidizable moieties, had the most similar measured concentration between the methods.
A possible source of the difference in results between CE-MS and LC-MS methods is sample stability [40]. For CE-MS, samples were kept at room temperature for ~4 h before analysis. In contrast, for LC-MS samples were derivatized immediately before analysis. Derivatization has previously been shown to stabilize many of the oxidation prone neurotransmitters such as catecholamines and indoleamines [39]. All compounds but OA and NE were measured to be higher concentrations by LC-MS, and OA and NE are within error of concentrations determined by each method. To test if sample stability contributes to error between methods, rat brain homogenate supernatant was analyzed by LC-MS after sitting at room temperature for up to 4 h before derivatization (Figure S4). DA, NE, Epi, and 5HT saw loss of concentrations by 20%, 11%, 24%, and 3.5%, respectively between 0 hours (n = 5) and 4 hours (n = 4). This loss of concentration for several of the analytes accounts for a substantial portion of error seen in between the methods. Other potential sources of error include differences in the sample treatment and separation parameters for each method. These results suggest that more rapid analysis or more care taken in preventing oxidation may be important in increasing the accuracy of the CE-MS method. Though all sources of the differences between these two methods is not completely understood, this comparison proves the utility of the presented method, showing comparable accuracy to existing methods. Being a CE-based method, this can inject from and uses less sample volume than LC-based methods, making it advantageous for low sample volume situations. This EKS method can also provide complimentary information that cannot be obtained by LC-MS, due to its orthogonality as a separation method, i.e. resolution of isomers OA and DA (Figure 4B).
Concluding Remarks
A sensitive EKS method has been developed that can successfully interface to CE-MS. This method overcomes many challenges and previous limitations of pairing this method of preconcentration to MS through careful selection of buffer systems and positively charged analytes that can be analyzed by the method. LODs in this system are sufficient to analyze small molecules in many different samples, with detection limits down to 10 pM concentrations from standards, showing 5000-fold enhancements in detection sensitivity over conventional injections. This method has been applied to biogenic amine neurotransmitters from tissue samples. In principle the method could be applied to other tissues. Improvements in protection against oxidation would be required for better accuracy. Samples containing high ionic strength matrices such as microdialysate and plasma can also be measured in this method, however they would require a pretreatment to remove or exchange the sample matrix prior to EKS.
Supplementary Material
Acknowledgement
This work was supported by NIH 2R01EB003320-21 (RTK). The authors acknowledge Agilent Technologies for loans of electrophoresis instrumentation on which all experiments were performed.
Abbreviations
- 5HT
serotonin
- Ch
choline
- DA
dopamine
- EKI
electrokinetic injection
- EKS
electrokinetic supercharging
- EPI
epinephrine
- FASI
field-amplified sample injection
- FASS
field-amplified sample stacking
- HDI
hydrodynamic injection
- LE
leading electrolyte
- MeOH
methanol
- MRM
multiple reaction monitoring
- nESI
nano-electrospray ionization
- NE
norepinephrine
- OA
octopamine
- TE
terminating electrolyte
- tITP
transient isotachophoresis
- TyrA
tyramine
- UV
ultraviolet
References
- [1].Chen G, Ewing AG Crit Rev Neurobiol. 1997, 11, 59. [DOI] [PubMed] [Google Scholar]
- [2].Lapainis T, Sweedler JV, J Chromatogr A 2008, 1184, 144–158. [DOI] [PubMed] [Google Scholar]
- [3].Monton MRN, Soga T, Chromatogr J. A 2007, 1168, 237–246. [DOI] [PubMed] [Google Scholar]
- [4].Faserl K, Sarg B, Sola L, Lindner HH, Proteomics 2017, 17, 1–5. [DOI] [PubMed] [Google Scholar]
- [5].Mateos-Vivas M, Domínguez-Álvarez J, Rodríguez-Gonzalo E, Carabias-Martínez R, Food Chem. 2017, 233, 38–44. [DOI] [PubMed] [Google Scholar]
- [6].Maráková K, Piešťanský J, Zelinková Z, Mikuš P, Molecules 2017, 22, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Faserl K, Sarg B, Gruber P, Lindner HH, Electrophoresis 2018, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T, Proteome Res J. 2003, 2, 488–94. [DOI] [PubMed] [Google Scholar]
- [9].Ramautar R, Shyti R, Schoenmaker B, De Groote L, Derks R, Ferrari M, Maagdenberg A, Deelder A, Mayboroda O, Anal. Bioanal. Chem. 2012, 404, 2895–2900. [DOI] [PubMed] [Google Scholar]
- [10].Lindenburg PW, Haselberg R, Rozing G, Ramautar R, Chromatographia 2014, 78, 367–377. [Google Scholar]
- [11].Hirayama A, Abe H, Yamaguchi N, Tabata S, Tomita M, Soga T, Electrophoresis 2018, 1–8. [DOI] [PubMed] [Google Scholar]
- [12].Šlampová A, Malá Z, Gebauer P Electrophoresis 2019, 40, 40–54. [DOI] [PubMed] [Google Scholar]
- [13].Breadmore MC, Grochocki W, Kalsoom U, Phung SC, et al. , 2019, 17–39. [DOI] [PubMed] [Google Scholar]
- [14].Kim J, Choi K, Chung DS, Anal. Bioanal. Chem. 2019, 1067–1073. [DOI] [PubMed] [Google Scholar]
- [15].Wuethrich A, Haddad PR, Quirino JP, Electrophoresis 2016, 1139–1142. [DOI] [PubMed] [Google Scholar]
- [16].Zhu G, Sun L, Yan X, Dovichi NJ, Anal. Chem. 2014, 6331–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pu F, Zhang W, Bateman KP, Liu Y, Helmy R, Ouyang Z, 2017, 9, 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai T, Chromatography 2017, 1–8. [Google Scholar]
- [19].Dawod M, Chung DS, J. Sep. Sci. 2011, 34, 2790–2799. [DOI] [PubMed] [Google Scholar]
- [20].Hirokawa T, Okamoto H, Gaš B, Electrophoresis 2003, 24, 498–504. [DOI] [PubMed] [Google Scholar]
- [21].Xu Z, Kawahito K, Ye X, Timerbaev AR, Hirokawa T, Electrophoresis 2011, 32, 1195–1200. [DOI] [PubMed] [Google Scholar]
- [22].Chui MQ, Thang LY, See HH, Chromatogr J. A 2017, 1481, 145–151. [DOI] [PubMed] [Google Scholar]
- [23].Xu Z, Ando T, Nishine T, Arai A, Hirokawa T, Electrophoresis 2003, 24, 3821–3827. [DOI] [PubMed] [Google Scholar]
- [24].Xu Z, Nishine T, Arai A, Hirokawa T, Electrophoresis 2004, 25, 3875–3881. [DOI] [PubMed] [Google Scholar]
- [25].Busnel JM, Lion N, Girault HH, Electrophoresis 2008, 29, 1565–1572. [DOI] [PubMed] [Google Scholar]
- [26].Botello I, Borrull F, Calull M, Aguilar C, J. Sep. Sci. 2013, 36, 524–531. [DOI] [PubMed] [Google Scholar]
- [27].Dawod M, Breadmore MC, Guijt RM, Haddad PR, Electrophoresis 2010, 31, 1184–1193. [DOI] [PubMed] [Google Scholar]
- [28].Kwon JY, Chang SB, Jang YO, Dawod M, Chung DS, J. Sep. Sci. 2013, 36, 1973–1979. [DOI] [PubMed] [Google Scholar]
- [29].Wang W, Ju F, Ran Y, Zhang H, Chen X, Analyst 2016, 141, 956–962. [DOI] [PubMed] [Google Scholar]
- [30].Stuart JN, Hummon AB, Sweedler JV, Anal. Chem. 2008, 76, 120 A–128 A. [PubMed] [Google Scholar]
- [31].Song J, Kim J, Front. Aging Neurosci. 2016, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Querfurth HW, LaFerla FM, Engl N. J. Med. 2010, 362, 329–344. [DOI] [PubMed] [Google Scholar]
- [33].Dagyte G, Den Boer JA, Trentani A, Behav. Brain Res. 2011, 221, 574–582. [DOI] [PubMed] [Google Scholar]
- [34].Wong JMT, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy R, J Chromatogr. A 2016, 1446, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simpson SL, Quirino JP, Terabe S, Chromatogr J. A 2007, 1184, 504–541. [DOI] [PubMed] [Google Scholar]
- [36].Malá Z,Gebauer P Electrophoresis 2019, 40, 55–64. [DOI] [PubMed] [Google Scholar]
- [37].Křivánková L, Foret F, Gebauer P, Boček PJ Chromatogr. 1987, 390, 3–16. [Google Scholar]
- [38].Guan Q, Henry CS, Electrophoresis 2009, 30, 3339–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Song P, Mabrouk OS, Hershey ND, Kennedy RT, Anal. Chem. 2013, 84, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Miki K, Sudo A, Clin. Chem. 1998, 44, 1759–1762. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.