Abstract
A major limitation to the administration of oral appliance therapy for obstructive sleep apnoea (OSA) is that therapeutic responses remain unpredictable. In the present study, we tested the hypotheses that oral appliance therapy (i) reduces pharyngeal collapsibility preferentially in patients with posteriorly-located tongue and (ii) is most efficacious (reduction in apnoea-hypopnea index; AHI) in patients with a posteriorly-located tongue and less-severe baseline pharyngeal collapsibility. Twenty-five OSA patients underwent upper airway endoscopy during natural sleep to assess tongue position (type I: vallecula entirely visible; type II: vallecula obscured; type III: vallecula and glottis obscured), as well as obstruction as a result of other pharyngeal structures (e.g. epiglottis). Additional sleep studies with and without oral appliance were performed to measure collapsibility (critical closing pressure; Pcrit) and assess treatment efficacy. Overall, oral appliance therapy reduced Pcrit by 3.9 ± 2.4 cmH2O (mean ± SD) and AHI by 69 ± 19%. Therapy lowered Pcrit by an additional 2.7 ± 0.9 cmH2O in patients with posteriorly-located tongue (types II and III) compared to those without (type I) (P < 0.008). Posteriorly-located tongue (p = 0.03) and lower collapsibility (p = 0.04) at baseline were significant determinants of (greater-than-average) treatment efficacy. Predicted responders (type II and III and Pcrit < 1 cmH2O) exhibited a greater reduction in the AHI (83 ± 9 vs. 48 ± 8% baseline, P < 0.001) and a lower treatment AHI (9 ± 6 vs. 32 ± 15 events h−1, P < 0.001) than predicted non-responders. The site and severity of pharyngeal collapse combine to determine oral appliance efficacy. Specifically, patients with a posteriorly-located tongue plus less-severe collapsibility are the strongest candidates for oral appliance therapy.
Introduction
Oral appliances are increasingly being prescribed for obstructive sleep apnoea (OSA) treatment in patients who do not tolerate first-line therapy with continuous positive airway pressure (CPAP) or are unwilling to try CPAP (Ramar et al. 2015). In unselected patients, oral appliances are less efficacious than CPAP (i.e. less reduction in apnoea-hypopnea index; AHI) but can potentially achieve similar effectiveness at improving health outcomes, probably as a result of superior adherence to oral appliance therapy (Phillips et al. 2013; Sutherland et al. 2015a). A major limitation to administration of oral appliances as a first-line therapy is that responses to therapy remain largely unpredictable (Bamagoos et al. 2016).
The available evidence suggests that at least two key pharyngeal factors determine oral appliance efficacy. First, patients with less-severe pharyngeal collapsibility have greater responses to therapy. Patients with a lower CPAP requirement, supine-dependent OSA or a lower body mass index (BMI), which are features of less-severe collapsibility, exhibit slightly better average responses (Hoekema et al. 2007; Tsuiki et al. 2010). Direct measurement of collapsibility (using CPAP drops; see Methods) has confirmed this relationship (Edwards et al. 2016), although the results have been inconsistent, indicating that collapsibility alone is not a sufficiently strong predictor (Randerath et al. 2002; Lam et al. 2011). Second, the available data suggest that the pharyngeal site or structure causing obstruction should be relevant. For example, in a small study (n = 12), oropharyngeal collapse (based on a multi-tip pharyngeal pressure catheter) was a predictor of oral appliance responses (Ng et al. 2006). A study with drug-induced sleep endoscopy suggested that oral appliances might not be effective as a first-line treatment for OSA patients with lateral wall obstruction (Park et al. 2016). Very recently, in a study run in parallel to our current investigation, tongue base obstruction (rather than palate, pharyngeal lateral walls, or epiglottis) seen on drug-induced sleep endoscopy was found to increase the probability of a strong oral appliance response (Op De Beeck et al. 2018) Intuitively, patients with tongue-related obstruction are expected to exhibit a preferential improvement in collapsibility with mandibular advancement. To date, no study has combined measures of structure and severity of collapsibility to explain the heterogeneity of oral appliance responses.
Accordingly, in the present study, we tested two specific hypotheses: (i) patients with a posteriorly-located tongue exhibit a greater improvement in collapsibility with therapy and (ii) the presence of a more posteriorly-located tongue and less-severe collapsibility are associated with a greater probability of efficacious oral appliance treatment (reduction in AHI). To test these hypotheses, patients underwent overnight sleep studies to assess the pharyngeal structure causing obstruction via natural sleep endoscopy. In addition, the acute effect of oral appliances on both collapsibility (pharyngeal critical closing pressure; Pcrit) and OSA severity, AHI, were also assessed. Aspects of this study have been presented previously in abstract form (Marques et al. 2017).
Methods
Ethical approval
The present study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by Partners Human Research Committee, Brigham and Women’s Hospital Institutional Review Board (protocol number: 2012P000957), except for registration in a database. Written informed consent was obtained from all subjects before participation in the study.
Subjects
Patients with a previous diagnosis of OSA of any severity were invited to participate in the study. The age range was 21–70 years. Exclusion criteria included heart failure, central sleep apnoea, periodontal disease, insufficient teeth (less than eight teeth in each maxillary and mandibular arch) and temporomandibular joint dysfunction.
The present study was registered at clinicaltrials.gov (NCT02489591). We emphasize that our study was not a trial aimed at assessing the effectiveness of oral appliances in general; rather, we aimed to explain the heterogeneity of oral appliance efficacy using gold standard measures of structure and severity of pharyngeal collapsibility. A detailed physiological study of effectiveness was considered highly challenging.
In total, we enrolled 43 subjects. Four patients had dental conditions inappropriate for oral appliance use. Four patients could not sleep during the endoscopy night and two patients could not sleep during the baseline night. Six patients did not exhibit OSA on the baseline night without the oral appliance (AHI < 10 events h−1) and were therefore ineligible. Two additional patients did not return for the sleep study with oral appliance (lost to follow-up). Twenty-five patients with OSA (AHI > 10 events h−1) completed the protocol. Twenty-two of 25 patients had an AHI > 20 events h−1. Initially, we aimed to study patients with AHI > 20 events h−1 but, to enhance recruitment for hypothesis 1 (effect of site on Pcrit improvement), we broadened criteria to include patients with 10 < AHI ≤ 20. Thus, the population for primary analysis differs between the two hypotheses (see below).
Protocol
The subjects underwent three overnight sleep studies at least 1 week apart: night 1, sleep endoscopy; night 2, baseline Pcrit determination and AHI; and night 3, oral appliance Pcrit determination and AHI.
Oral appliance
We intended for the results of our study to be as broadly applicable as possible. Thus, all subjects used a duobloc titratable oral appliance device holding the mandible fixed at their maximum comfortable protrusion. Patients who had their own custom-fit devices (n = 8/25: six SomnoDent Herbst and one SomnoDent Classic; SomnoMed, Crows, Nest, NSW, Australia; and one Narval CC; Resmed, San Diego, CA, USA) used their own devices for the oral appliance treatment periods. For those who did not have their own appliance, a customizable titratable thermoplastic device (BluePro, BlueSom) was provided (n = 17/25) patients during the overnight studies. This inclusive design strategy enabled us to assess a broader range of participants (beyond those who might be clinically offered a device). Devices were administered only for the single night of the oral appliance study.
Instrumentation
On all study nights, subjects were instrumented with electrodes for sleep staging: electroencephalography (C4-A1, O2-A1), left and right electrooculography, and submental electromyography (125 Hz). Subjects breathed via a sealed nasal mask connected to a pneumotachometer (Hans-Rudolph, Kansas City, MO, USA; Validyne, Northridge, CA, USA) to measure airflow (500 Hz).
Night 1: Sleep endoscopy
We performed natural sleep endoscopy at atmospheric pressure (off CPAP) to define the primary structure(s) contributing to pharyngeal obstruction for each participant. A 2.8 mm diameter pediatric bronchoscope (model BFXP-160F; Olympus, Tokyo, Japan) was inserted through the right nostril following topical application of a decongestant (oxymetazoline 0.05%) and anesthetic (lidocaine 4%). Endoscopic images (30 frames s−1) were synchronized with the sleep and respiratory signals and saved using Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
The subjects were asked to sleep in the supine position with the neck in a neutral position. Initially, the tip of the bronchoscope was placed in the nasopharynx above the palate to observe the pattern of velopharyngeal narrowing/obstruction. The tip was then advanced to the oropharynx to observe the oropharyngeal and hypo-pharyngeal structures.
In all subjects, tongue position was categorized into one of three types based on the relationship between the tongue base and the epiglottis during end-expiration (Li et al. 2014): In type I, the tongue base was clearly separated from the epiglottis, and the entire vallecula and glottis were visible. In type II, the tongue base was touching the epiglottis (obliterating the vallecula) but not pushing the epiglottis posteriorly, thus allowing the glottis to be clearly seen. Finally, in type III, the tongue base was pressing the epiglottis posteriorly such that the glottis could not be seen. The classification was performed by two investigators, and discrepancies were resolved by a third investigator. The tongue was assumed to be a contributing factor in obstruction in patients with a type II or type III tongue position. In addition to classifying the tongue position, other structures (palate, lateral walls and epiglottis) involved in collapse were also classified (Genta et al. 2017). Of note, we considered palatal collapse to be ‘isolated’ only if the tongue position was type I or II.
Nights 2 and 3: Collapsibility and OSA severity
On each night, the participants slept for the entire night either without or with their oral appliance device (baseline, oral appliance, respectively). For the first ~2 h of sleep, we assessed Pcrit and, for the remainder of the night (~4 h), we assessed AHI and other sleep characteristics.
Patients fell asleep in the supine position on 4 cmH2O of CPAP delivered via a modified device (Pcrit 3000; Philips Respironics, Murrysville, PN, USA) capable of delivering pressures between +20 and −20 cmH2O. Once asleep, CPAP was increased to the minimum level that eliminated snoring, hypopneas and inspiratory flow limitation (‘holding pressure’). Once stable stage N2 or N3 sleep was observed at the holding pressure, CPAP was abruptly dropped to various subtherapeutic levels for five breaths, with at least 1 min at the holding pressure in between drops. CPAP was dropped to progressively lower levels until complete obstructive apnoea occurred. If there was an arousal during any pressure drop, the CPAP was returned to the holding pressure until stable sleep resumed. To quantify collapsibility, we assessed the passive pharyngeal critical closing pressure (Pcrit): data were manually-selected using custom-designed software (Matlab; MathWorks, Natick, MA, USA) to determine the peak inspiratory flow for breaths 3 and 4 during each pressure drop. Pcrit was determined as the zero-flow intercept from the linear regression of peak flow vs. nasal pressure, as described previously (Patil et al. 2004).
After ~2 h of sleep, CPAP was removed and the participants were left to breathe freely without CPAP (but still with the oral appliance if night 3) in the supine position to assess AHI. A registered polysomnography technician blind to the study condition scored the sleep studies according to standard criteria (Berry et al. 2012). Hypopneas were based on a ≥30% reduction in airflow with either a ≥3% desaturation or arousal.
Statistical analysis
Descriptive characteristics are presented as mean ± SD unless otherwise specified. Data analysis was performed using SPSS, version 20 (IBM Corp., Armonk, NY, USA) and MATLAB (Statistics and Machine Learning Toolbox; MathWorks). P < 0.05 was considered statistically significant.
Primary analysis.
First, to test the specific hypothesis that patients with a posteriorly-located tongue exhibit a greater improvement in collapsibility with oral appliances, data from all 25 subjects were assessed. Multivariable linear regression was used to test for an association between the change in collapsibility with intervention (Pcrit on oral appliance minus Pcrit at baseline) and tongue-related obstruction (tongue types I, II and III as a continuous variable, denoted by 0, 0.5 and 1, respectively), adjusting for additional contributors as needed (epiglottic, palatal, or lateral walls collapse). Second, to test the specific hypothesis that the presence of a posteriorly-located tongue and less-severe collapsibility promotes greater oral appliance treatment efficacy, our primary analysis was limited to patients with an AHI > 20 events h−1, to minimize the influence of random night-to-night variability (i.e. SD of AHI is ~9 events h−1) (Bittencourt et al. 2001; Levendowski et al. 2009). Logistic regression analysis was used to explain greater-than-average responses (‘responders’) vs. poorer-than-average responses (‘non-responders’) to oral appliance therapy; a 70% historical average reduction in AHI was used as the cut-off to define responders/non-responders and thereby maximize statistical power (Hosmer & Lemeshow, 2000). Baseline Pcrit and tongue-position category (continuous variable, as above) were used to explain responder/non-responder status, at the same time as adjusting for baseline AHI (potential confounder). Interaction terms were not analysed because of statistical power concerns.
Post hoc analysis.
To the above models, we sequentially included-then-removed the three additional endoscopic variables (presence vs. absence of isolated palate, lateral walls and epiglottic obstruction) to test whether these factors also contributed to change in collapsibility and AHI with intervention.
Secondary analysis.
We selected parameters using a forward stepwise procedure to predict responder/non-responder status. Receiver operating characteristic analysis was used to select the optimal cut-off (maximizing sensitivity plus specificity). The predictive value of this model was then tested using leave-one-out cross validation
Results
Twenty-five OSA patients (age 49 ± 11 years; eight female) were studied. Subjects’ characteristics are presented in Table 1. Two patients were using CPAP at the time of the study, and one of them reported irregular use (3 or 4 nights week−1, not for the entire night). Six patients had tried CPAP therapy before but were no longer using it. Ten patients had never initiated any treatment for their diagnosed OSA. Two patients who had their own custom-fit oral appliances were not currently using their devices. Among the other six patients, the average time of therapy was 5.0 ± 3.8 years. Patients’ maximal protrusion range was 7.3 ± 2.0 mm, and the maximum comfortable advancement with the oral appliance was 75 ± 13% of the total range. There was no difference in the maximal comfortable protrusion between the custom-fit devices and the thermoplastic devices (71 ± 15% vs. 78 ± 12% of total protrusion range; p = 0.2). On average, 140 ± 46 min of endoscopy video per subject in the supine position were obtained. The tongue position (at end-expiration) and the other structures contributing to airway collapse appeared consistent within each individual. Patients were categorized into three mutually-exclusive categories: tongue type I (n = 5); tongue type II (n = 8); and tongue type III (n = 12).
Table 1.
Participant characteristics
| Parameters | Patients with AHI > 10 (n = 25) | Patients with AHI > 20 (n = 22) |
|---|---|---|
| Age (years) | 49 ± 11 | 50 ± 12 |
| Sex, M:F (n) | 17:8 | 16:6 |
| Body mass index (kg m−2) | 32.0 ± 7 | 32.5 ± 6 |
| Neck circumference (cm) | 40.8 ± 4.8 | 41.6 ± 4.3 |
| Comorbidities, n (%) | ||
| Hypertension | 7 (28) | 6 (27) |
| Diabetes | 3 (12) | 2 (9) |
| Hypercholesterolaemia | 3 (12) | 3 (13) |
Data presented as the mean ± SD.
Role of pharyngeal structure on the change in upper airway collapsibility
Overall, oral appliance therapy reduced Pcrit by 3.9 ± 2.4 cmH2O, from −0.6 ± 1.9 cmH2O at baseline to −4.5 ± 2.7 cmH2O on therapy (n = 25; P < 0.001). A reduction in Pcrit was evident within all three tongue type categories: in those with tongue types I, II and III, oral appliance therapy lowered Pcrit by 2.6 ± 1.3 cmH2O (n = 5; p = 0.019), 4.5 ± 2.7 cmH2O (n = 8; P < 0.001) and 4.1 ± 2.5 cmH2O (n = 12; P < 0.001), respectively.
Primary analysis.
With tongue-related obstruction as a continuous variable (types I, II and III, denoted 0, 0.5 and 1), the ~2 cmH2O greater reduction in Pcrit with tongue-related obstruction was not significant in a bivariate analysis (Model 1) (Table 2). However, in a multivariable analysis that included epiglottic obstruction, the relationship became significant, indicating that tongue position, when controlled for the presence of epiglottic collapse, was an important predictor of the Pcrit response to oral appliance therapy (Model 2) (Table 2).
Table 2.
Effect of oral appliances on collapsibility by pharyngeal structure
| Parameters | Patients with AHI > 10* | Patients with AHI > 20 | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 25) | (n = 22) | |||||||
| n | β ± SEM | P | F test, P | n | β ± SEM | P | F test, P | |
| Model 1: | ||||||||
| Intercept | −3.0 ± 0.7 | 0.0003 | 0.14 | −3.1 ± 0.7 | 0.0004 | 0.10 | ||
| Tongue-related obstruction | 5:8:12 | −1.7 ± 1.1 | 0.14 | 5:7:10 | −2.0 ± 1.1 | 0.10 | ||
| Model 2: | ||||||||
| Intercept | −2.2 ± 0.8 | 0.018 | 0.022 | −2.4 ± 1.0 | 0.014 | 0.033 | ||
| Tongue-related obstruction | 5:8:12 | −2.7 ± 1.1 | 0.025 | 5:7:10 | −2.9 ± 1.2 | 0.026 | ||
| Epiglottic obstruction | 21:4 | −1.8 ± 1.1 | 0.09 | 18:4 | −1.4 ± 1.0 | 0.18 | ||
| Model 3: | ||||||||
| Intercept | −3.7 ± 0.8 | <0.0001 | 0.072 | −4.0 ± 0.8 | 0.0001 | 0.14 | ||
| Epiglottic obstruction | 21:4 | −2.1 ± 1.1 | 0.072 | 18:4 | −1.8 ± 1.2 | 0.14 | ||
| Model 4: | ||||||||
| Intercept | −4.4 ± 0.6 | <0.0001 | 0.17 | −5.1 ± 1.0 | <0.0001 | 0.5 | ||
| Lateral wall obstruction | 18:7 | 1.2 ± 0.9 | 0.17 | 17:5 | 1.3 ± 1.8 | 0.5 | ||
| Model 5: | ||||||||
| Intercept | −4.0 ± 0.7 | <0.0001 | 0.9 | −2.4 ± 1.0 | 0.014 | 0.8 | ||
| Isolated palate obstruction | 18:7 | −0.1 ± 1.0 | 0.9 | 15:7 | 0.3 ± 1.0 | 0.8 | ||
| Model 6: | ||||||||
| Intercept | −2.6 ± 0.6 | 0.0002 | 0.035 | −2.6 ± 0.6 | 0.0002 | 0.016 | ||
| Tongue type II to III vs. type I | 20:5 | −1.8 ± 0.8 | 0.035 | 17:5 | −2.2 ± 0.8 | 0.016 | ||
| Model 7: | ||||||||
| Intercept | −1.9 ± 0.8 | 0.023 | 0.009 | −2.0 ±0.8 | 0.022 | 0.01 | ||
| Tongue type II to III vs. type I | 20:5 | −2.7 ± 0.9 | 0.008 | 17:5 | −2.9 ± 0.9 | 0.006 | ||
| Epiglottic obstruction | 21:4 | −1.5 ± 0.9 | 0.11 | 18:4 | −1.3 ± 1.0 | 0.20 | ||
Shading emphasizes columns describing the primary analysis with data from all 25 subjects with AHI > 10 at baseline. Multivariable linear regression model results are shown. The dependent variable is the change in collapsibility (critical collapsing pressure, Pcrit) on oral appliance therapy minus baseline (ΔPcrit). Model 1 describes the effect of tongue-related obstruction on ΔPcrit, with tongue-related obstruction modelled as a continuous variable (tongue types I, II and III were denoted by 0, 0.5 and 1, respectively). Model 2 is Model 1 plus the additional effect of epiglottic obstruction (selected using forward-stepwise inclusion); further inclusion of isolated palate and lateral wall status did not improve the model. Models 3–5 describe remaining bivariate relationships. Model 6 describes the effect of tongue-related obstruction with tongue types II and III pooled (i.e. type I is denoted by 0 and types II and III are denoted by 1). Model 7 is Model 6 with the additional effect of epiglottic obstruction. F test P values indicate significance of the overall model (vs. a constant). Model weights were used to balance influence of unevenly-distributed subgroups. Significant P values were marked in bold.
For patients with epiglottic collapse, there was a trend towards a better improvement in Pcrit with oral appliance therapy (~2 cmH2O, after adjusting for tongue base obstruction; Models 2 and 3) (Table 2). Lateral wall obstruction and isolated palate obstruction were not significant determinants of the change in Pcrit (+1.0 cmH2O for lateral wall obstruction; negligible effect of isolated palate). Pooling data from tongue types II and III led to stronger Models (compare Models 6 and 7 with Models 1 and 2) (Table 2).
Role of pharyngeal structure and collapsibility on the AHI response to therapy
Overall, oral appliance therapy reduced the AHI by 69 ± 19%, from 56.7 ± 21.5 events h−1 at baseline to 18.6 ± 15.8 events h−1 on therapy (n = 22, P < 0.001). The effects of oral appliances on polysomnographic parameters are shown in Table 3.
Table 3.
Sleep architecture and sleep disordered breathing parameters
| Patients with AHI > 10 (n = 25) | Patients with AHI > 20 (n = 22)* | |||||
|---|---|---|---|---|---|---|
| Baseline | Oral appliance | P value | Baseline | Oral appliance | P value | |
| Total sleep time (min) | 180 ± 73 | 218 ± 62 | 0.001 | 166 ± 64 | 212 ± 63 | <0.001 |
| Sleep efficiency (%) | 66 ± 18 | 77 ± 16 | <0.001 | 63 ± 17 | 76 ± 16 | <0.001 |
| NREM 1 (%)# | 38.8 ± 26.4 | 17.6 ± 13.3 | <0.001 | 40.5 ± 27.1 | 18.5 ± 13.7 | <0.001 |
| NREM 2 (%) | 44.1 ± 20.6 | 53.4 ± 12.9 | 0.05 | 43.4 ± 21.7 | 52.5 ± 12.4 | 0.02 |
| NREM 3 (%) | 4.6 ± 7.8 | 10.1 ± 12.0 | 0.01 | 4.0 ± 6.4 | 10.8 ± 12.6 | 0.004 |
| REM (%) | 13.1 ± 10.7 | 18.0 ± 9.3 | 0.02 | 12.8 ± 10.6 | 17.1 ± 9.2 | 0.07 |
| Arousal index (events h−1)# | 57.0 ± 30.2 | 29.5 ± 20.2 | <0.001 | 60.9 ± 29.8 | 31.6 ± 20.7 | <0.001 |
| Total AHI (events h−1)† | 51.6 ± 24.4 | 17.4 ± 15.3 | <0.001 | 56.7 ± 21.5 | 18.6 ± 15.8 | <0.001 |
| Apnoea index (events h−1) | 23.4 ± 17.9 | 4.4 ± 7.6 | <0.001 | 26.3 ± 17.1 | 4.3 ± 7.9 | <0.001 |
| Hypopnea index (events h−1) | 23.4 ± 16.4 | 10.3 ± 13.3 | 0.005 | 25.2 ± 16.7 | 11.4 ± 13.8 | 0.011 |
| AHINREM (events h−1) | 52.0 ± 25.1 | 14.6 ± 15.8 | <0.001 | 57.3 ± 21.7 | 16.1 ± 16.2 | <0.001 |
| AHIREM (events h−1) | 36.7 ± 25.4 | 24.8 ± 19.5 | 0.05 | 38.2 ± 26.2 | 26.0 ± 19.6 | 0.08 |
| Mean (%) | 97.5 ± 1.5 | 97.4 ± 1.9 | 0.88 | 97.3 ± 1.5 | 97.2 ± 4.0 | 0.70 |
| Minimum (%) | 85.7 ± 8.7 | 87.8 ± 7.6 | 0.19 | 86.2 ± 7.5 | 87.1 ± 7.8 | 0.38 |
| Pcrit (cmH2O) | −0.6 ± 1.9 | −4.5 ± 2.7 | <0.001 | −0.3 ± 1.9 | −4.6 ± 2.8 | <0.001 |
Shading emphasizes the columns illustrating the primary analysis with data from subjects with AHI > 20 at baseline.
Primary outcome variable.
Secondary outcome variable. Data are presented as the mean ± SD. Significant P values were marked in bold. AHI, apnoea-hypopnea index; NREM, non-rapid eye movement sleep; REM, rapid eyes sleep; , arterial blood oxygen saturation.
A reduction in AHI with oral appliance therapy was evident within all three tongue type categories: in those with tongue types I, II, and III, oral appliance therapy lowered AHI by 49 ± 10% (n = 5; p = 0.002), 85 ± 9% (n = 7; P < 0.001) and 68 ± 19% (n = 10, P < 0.001), respectively. There was a significant difference in the AHI reduction between tongue types (p = 0.002), with a greater reduction in those with tongue type II compared to tongue type I (p = 0.002) (Figs 1 and 2).
Figure 1. Examples of endoscopic views of two representative patients without and with oral appliance.
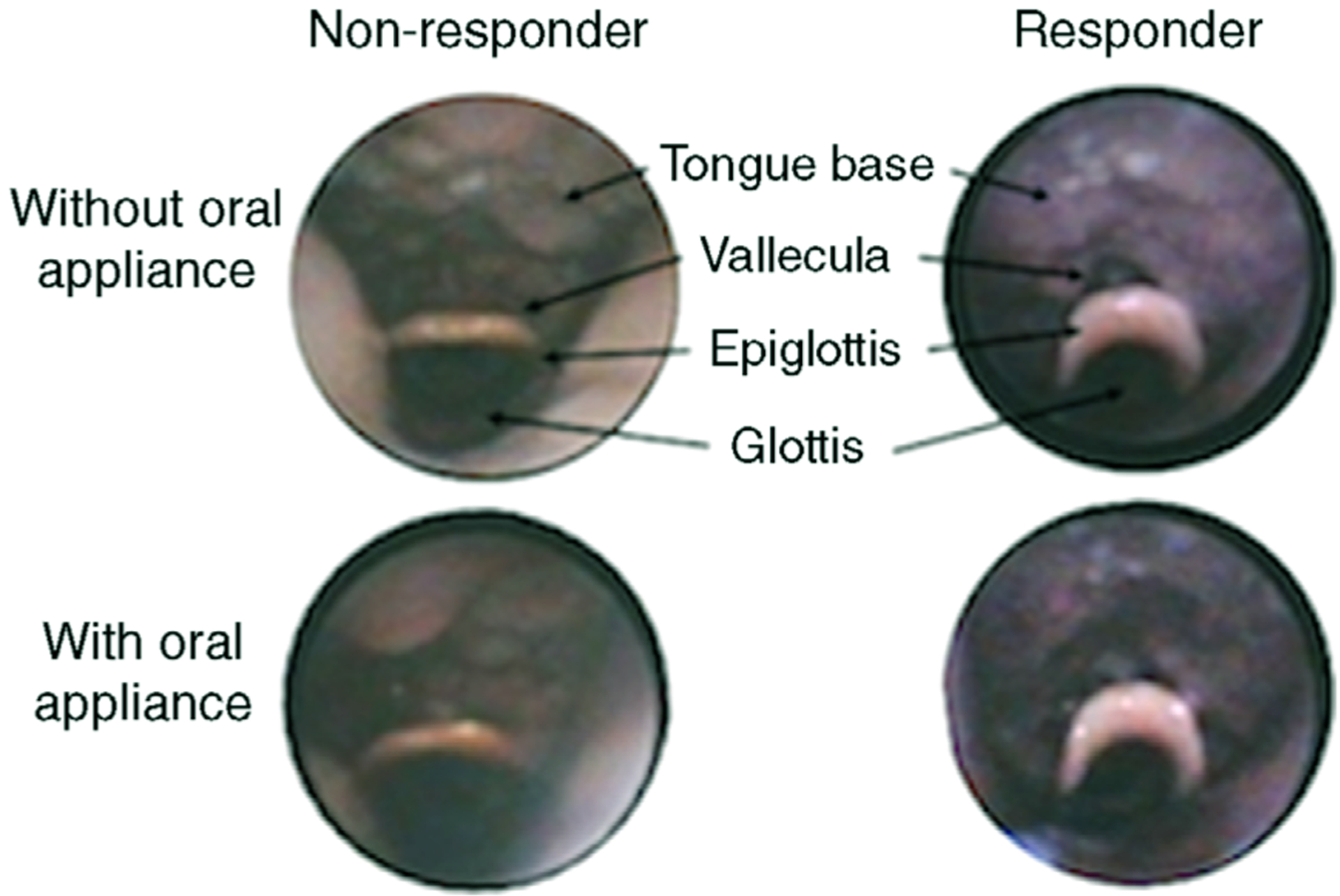
The patient who did not respond to therapy was classified (off oral appliance) with tongue type I; Pcrit decreased from −1.7 to −5.2 cmH2O, and the AHI reduced from 54 to 30 events h−1 with oral appliance. The patient who responded to therapy was classified with tongue type II, Pcrit decreased from −1.0 to −9.0 cmH2O, and the AHI reduced from 64 to 6 events h−1 with oral appliance.
Figure 2. AHI reduction.
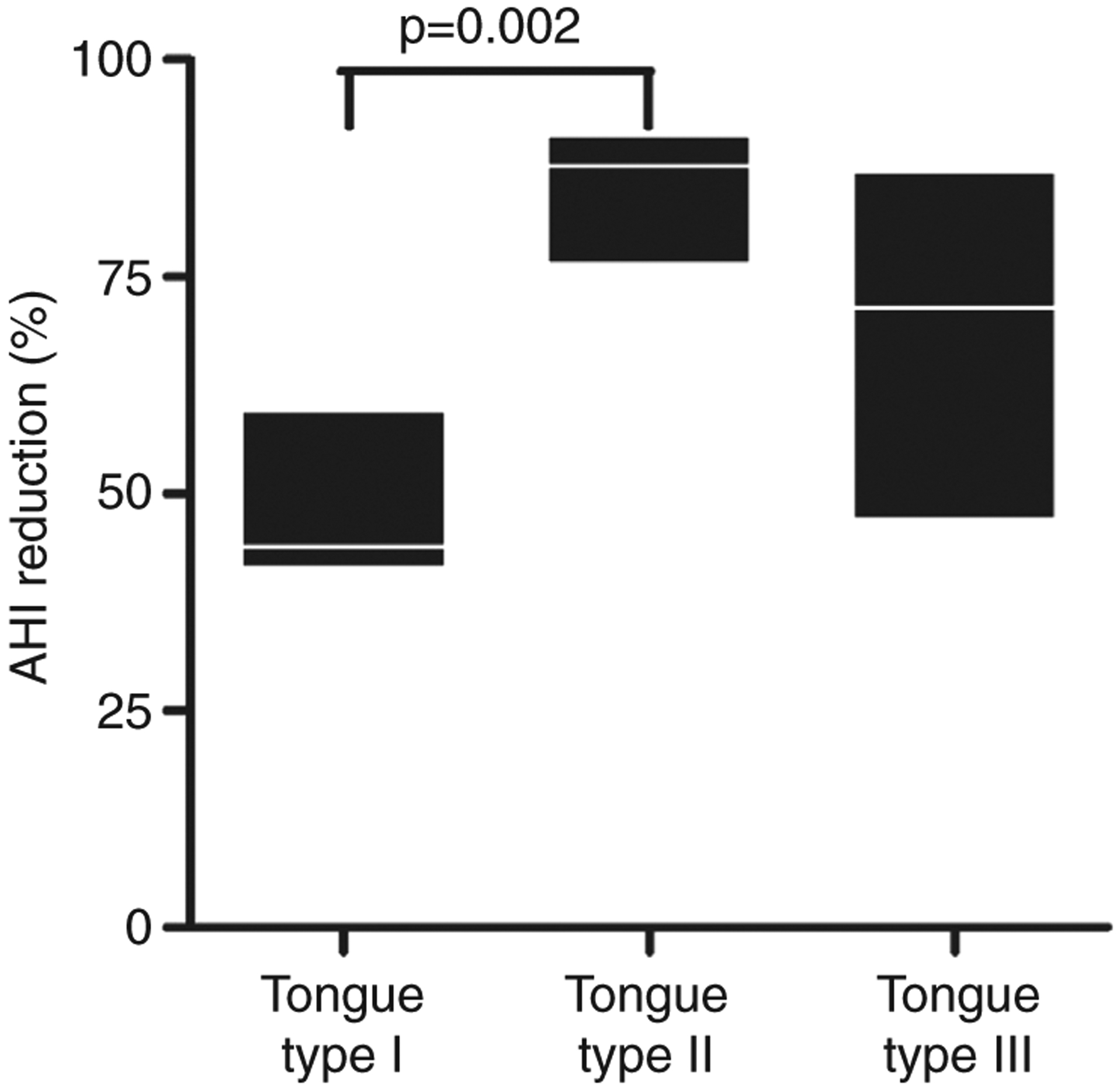
Differences in AHI reduction between tongue groups.
Primary analysis.
Posteriorly-located tongue (continuous variable; see above) and lower baseline collapsibility were both significantly associated with greater odds of being a responder vs. non-responder to oral appliance therapy (Model 1; multivariable logistic regression) (Table 4), after adjusting for baseline AHI.
Table 4.
Structure and severity of obstruction explain oral appliance responses
| Parameters | Patients with AHI >10 (n = 25) | Patients with AHI > 20* (n = 22) | ||||||
|---|---|---|---|---|---|---|---|---|
| β | Odds ratio (95% CI) | P | F test, P | β | Odds ratio (95% CI) | P | F test, P | |
| Model 1: | ||||||||
| Intercept | −3.2 | 0.10 | 0.039 | −6.9 | 0.10 | 0.0005 | ||
| Posteriorly-located tongue | 3.6 | 37 (1.3–1064) | 0.036 | 13.5 | 7.5 × 105 (2.7–2.0 × 1011) | 0.034 | ||
| Collapsibility, Pcrit (cmH2O) | −0.7 | ÷2.1 (0.92–4.8) | 0.075 | −3.6 | ÷37 (1.14–1.1 × 103) | 0.042 | ||
| Baseline AHI (events/hr) | 0.0 | 0.6 | 0.0 | 0.4 | ||||
Shaded columns shows the primary analysis with data from subjects with AHI>20 at baseline. AHI, apnoea-hypopnea index; β represents the non-standardized regression coefficients. Odds ratios for Pcrit are shown as the increased odds for being a responder (greater reduction in AHI than a 70% historical average reduction) per unit reduction in Pcrit (denoted by division symbol ‘÷’). CI, confidence interval. Additional inclusion of epiglottic obstruction (p = 0.36), isolated palate obstruction (p = 0.21) or lateral wall obstruction (p = 0.9) to Model 1 was not significant. Significant P values were marked in bold.
Epiglottic obstruction, lateral wall obstruction and isolated palatal collapse were not independently associated with responses.
Defining the structure and collapsibility phenotype of responders to therapy
We developed a phenotypic predictive model to define a subgroup of ‘predicted responders’ and ‘predicted non-responders’ based on pharyngeal structure and severity of obstruction. Forward stepwise logistic regression selected just two variables for inclusion: posteriorly-located tongue (defined as type II or III rather than type I) and Pcrit. The final prediction model was equivalent to: predicted responders were defined by a posteriorly-located tongue (tongue-related type II or III) and less-severe collapsibility (baseline Pcrit < 1 cmH2O) (Fig. 3). Before cross-validation, this approach detected responders accurately in 19/22 patients (86%), with a positive predictive value of 11/13 (85%) and a negative predictive value of 8/9 (89%). When this approach was repeated using left-out data (cross-validation), the results were similar: Accuracy was 18/22 (82%), positive predictive value was 10/12 (83%) and negative predictive value was 8/10 (80%). Notably, ‘predicted responders’ exhibited a median reduction in AHI that was substantially greater than in ‘predicted non-responders’ (83% vs. 48%; P < 0.001). This finding was upheld when including subjects with 10 < AHI ≤ 20 events h−1 (76% vs. 48%; p = 0.003).
Figure 3. Individual data illustrating the effect of oral appliances on collapsibility (Pcrit) by tongue type.
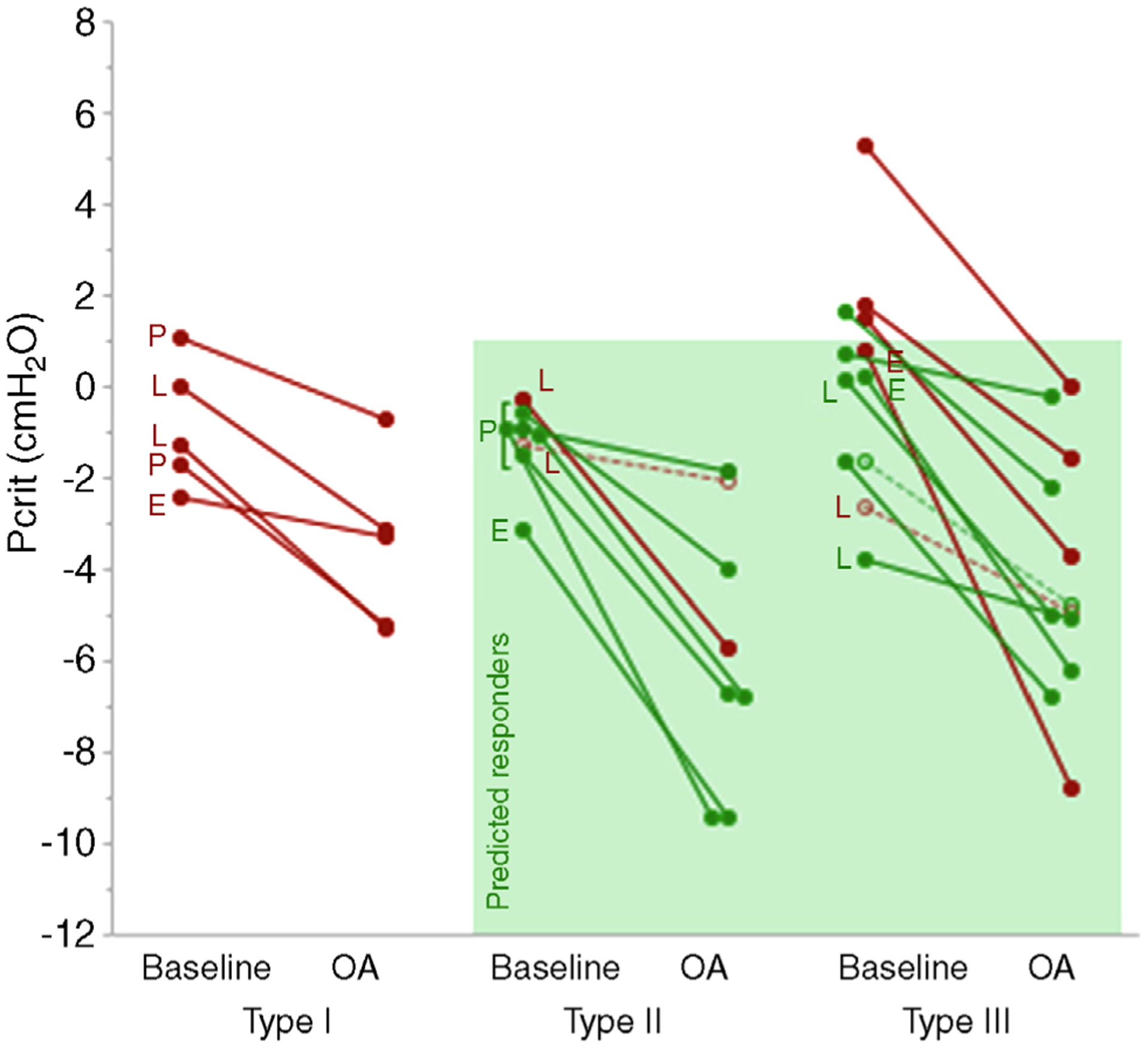
Presence of additional sites of collapse (P, palate; L, lateral walls; E, epiglottis) is marked along the corresponding patient circle. Open circles correspond to the patients with baseline AHI < 20 events h−1. Responders to oral appliance therapy are represented in green and non-responders are represented in red. The shaded box indicates the ‘predicted responders’ to oral appliance therapy (tongue type II or III and baseline Pcrit < 1 cmH2O).
Additional analysis and potential confounders
There were no significant differences in key potential confounders (BMI, age, use of provisional thermoplastic device vs. custom devices) between patients with posteriorly-located tongue (tongue types II and III) vs. anteriorly-located tongue (type I).
In multivariable analysis (Model 7 + potential confounder) (Table 2), we observed no association between the change in Pcrit with treatment and either increased BMI (β ± SEM = −1.1 ± 0.8 cmH2O per 10 kg m−2; p = 0.17; a negative value indicates a greater beneficial effect), older age (−0.3 ± 0.9 cmH2O per 20 years; p = 0.8) or use of provisional device (0.0 ± 1.3 cmH2O; P > 0.9). After adjustment for these potential confounders (BMI, age, use of provisional), posteriorly-located tongue (type II or III vs. type I) remained clearly associated with a greater treatment effect on Pcrit (β for posteriorly-located tongue: −2.5, −2.7 and −2.7 cmH2O, respectively; compare with −2.6 cmH2O in Table 2).
In bivariate analysis, we found no significant association between oral appliance treatment efficacy (>70% reduction in AHI) and lower BMI (p = 0.17), younger age (p = 0.07) or use of the provisional device (p = 0.23) (presented to illustrate that these ‘confounders’ were probably not the source of the associations seen between efficacy and tongue type or Pcrit; larger samples would be needed to assess these associations per se). In multivariable analysis, adding potential confounders to the primary logistic regression model (n = 22) (Table 4), in the context of perfect/near-perfect group separation, yielded large SEM estimates for all parameters. Adding potential confounders to the secondary logistic regression models for all subjects (n = 25) (Table 4) confirmed that posteriorly-located tongue remained significantly associated with treatment efficacy after adjusting for BMI, age and provisional device use (β for posteriorly located tongue remained at 3.8 in each model); the trend for Pcrit was unaffected (β = −0.7, −0.6 and −0.8, respectively; compare with −0.7 in Table 4); none of the potential confounders exhibited a significant association with efficacy.
Discussion
The major findings of the present study are:
Patients with a posteriorly-located tongue (type II or III) exhibited a greater improvement in pharyngeal collapsibility with oral appliance therapy.
Patients with the combination of a posteriorly-located tongue and a less-severe collapsibility (baseline Pcrit < 1 cmH2O) experienced greater oral appliance efficacy.
The efficacy of oral appliances in OSA treatment is highly variable. Reported mean reductions in AHI range between 24% and 72% (Bamagoos et al. 2016). Therefore, investigators have sought predictors of oral appliance therapy success. In a large retrospective study, Sutherland et al. (2015b) investigated predictors using a classification and regression tree model that included anthropometric and polysomnographic variables (e.g. AHI and body-mass index). The final model resulted in a correct classification between responders and non-responders of only 64% (area under the curve = 66%). Upper airway endoscopy has also been used to predict oral appliance therapeutic success based on the improvement of pharyngeal narrowing with mandibular advancement. A recent study using upper airway endoscopy during wakefulness failed to predict response to oral appliances (Sutherland et al. 2018). Another study performed during drug-induced sleep had a reasonable sensitivity to predict therapeutic success (0.71%) but low specificity (0.33%) (Vroegop et al. 2013). In the present study, a phenotypic predictive model that included the pharyngeal structure causing collapse identified during natural sleep endoscopy in combination with baseline pharyngeal collapsibility demonstrated good accuracy (82%) to detect responders to oral appliance therapy.
Posteriorly-located tongue and oral appliance therapy
Magnetic resonance imaging studies have shown that oral appliances induce anterior movement of the tongue base (Brown et al. 2013; Ogawa et al. 2015). A greater rate of success with oral appliances have been reported among patients with primarily oropharyngeal collapse rather than velopharyngeal collapse (Ng et al. 2006; Bosshard et al. 2011; Shen et al. 2012). Ng et al. (2006) investigated 12 patients using multisensor catheters with and without mandibular advancement to determine upper airway closing pressures and the sites of collapse. They found that all patients with oropharyngeal collapse achieved an AHI < 5 events h−1 with the oral appliance therapy compared to one with velopharyngeal collapse. Using bilateral anterior magnetic stimulation of the phrenic nerve during wakefulness to identify the site of upper airway collapse, Bosshard et al. (2011) also found that patients with oropharyngeal collapse had better responses compared to those with velopharyngeal collapse. However, neither study evaluated the specific pharyngeal structures causing obstruction (e.g. tongue vs. epiglottis vs. lateral walls). Shen et al. (2012) evaluated lateral cephalometric radiographs in 52 patients (upright and awake) and found a strong relationship between greater oral appliance efficacy and a reduced distance between the tongue and posterior pharyngeal wall (7.1 ± 2.9 mm in responders and 9.8 ± 3.4 mm in non-responders; mean ± SD). The present study adds to these previous observations by showing that patients with a posteriorly-located tongue during sleep had greater improvements in collapsibility and OSA severity with oral appliance therapy.
Pharyngeal collapsibility and oral appliance therapy
Oral appliances are known to improve pharyngeal collapsibility in general (Kato et al. 2000; Ng et al. 2003; Bamagoos et al. 2017). Kato et al. (2000) evaluated six patients under general anesthesia with and without an oral appliance. They produced progressively increasing amounts of mandibular protrusion and found a dose-dependent improvement in pharyngeal closing pressure. Similarly, Ng et al. (2003) found a significant decrease in upper airway closing pressure (−1.6 ± 0.4 to −3.9 ± 0.6 cmH2O) in 10 patients with oral appliance therapy. In the present study, we found that oral appliances reduced Pcrit overall by 3.9 ± 2.4 cmH2O. This finding is congruent with a recent study measuring Pcrit in seven patients at two different mandibular positions: (i) neutral bite and (ii) 100% mandibular advancement. The change in Pcrit was 0.7 ± 2.7 cmH2O at neutral bite compared to −5.7 ± 4.1 cmH2O at 100% mandibular advancement (Bamagoos et al. 2017). Therefore, oral appliances are capable of significantly improving pharyngeal collapsibility in most patients. However, these were small studies that did not stratify patients. The present study extends these previous observations by showing that oral appliances preferentially improve the airway in patients with a posteriorly-located tongue. Furthermore, from our phenotypic model, we were also able to select a Pcrit cut-off of +1cmH2O as a determinant of a poor AHI response to oral appliance therapy.
We also sought to further examine the strength of the evidence that a posteriorly-located tongue acts, mechanistically, via a greater reduction Pcrit to yield a lower Pcrit value and ultimately greater oral appliance efficacy. If this conceptual model were true, then we would expect responders to exhibit a lower on-treatment Pcrit than non-responders. We note that there was a physiologically-significant (but not statistically-significant) lower on-treatment Pcrit in responders vs. non-responders (−5.6; −3.1 to 6.8 cmH2O vs. −3.5; −5.3 to −1.6 cmH2O; expressed as median; interquartile range; difference −2.1 cmH2O, p = 0.18), with the average value being normal (< −5 cmH2O) in responders but not non-responders. Interestingly, the on-treatment Pcrit did not allow full clarification. We observed a trend for an independent effect of tongue-type on treatment efficacy after adjusting for on-treatment Pcrit and baseline AHI (p = 0.066, F statistic vs. model without tongue-type; model right-side repeated using on-treatment Pcrit instead of baseline Pcrit) (Table 4). This finding, and the weaker associations between efficacy and on-treatment Pcrit, suggests that there may be some additional advantage of tongue-base collapse for oral appliance therapy that may not be being captured by effects on (hypotonic) Pcrit alone. Such effects may be a result of measurement noise in the Pcrit, or physiological (e.g. greater mechanical efficiency, such as improved airflow, of genioglossus recruitment with tongue-related obstruction).
Oral appliances in severe OSA patients
Although previous studies have shown that some patients with severe OSA can be treated successfully with oral appliances, they have generally failed to predict the ideal candidate (Lam et al. 2011;Gjerde et al. 2016). In the present study, a reduction of 80% in AHI from 53 to 9 events h−1 with oral appliance therapy was observed in the subgroup of predicted responders. Notably, 92% of these patients had severe OSA. These findings suggest that a prediction model including the presence of a posteriorly-located tongue and a low pharyngeal collapsibility could be used to enable oral appliances to be recommended for treatment of OSA patients of any severity.
Limitations
The present study has several limitations. First, because of the invasiveness of the studies, the sample size was necessarily small. However, the different structures causing pharyngeal collapse were well represented, and clear changes in the outcome variables were observed. Second, the sleep parameters were obtained during a shorter period than usual. However, we included these data to compare sleep characteristics during both study nights, showing that, overall, the conditions were similar, other than the use of oral appliance. Third, the type of oral appliance was not standardized. Despite this limitation, we achieved sizable reductions in AHI with comparable mandibular advancement in all groups, suggesting that the treatment was generally effective. Moreover, the findings were observed even in the presence of differences in device type, treatment duration and advancement magnitude. Fourth, we studied patients on oral appliances for a single night of therapy. It is often considered that mandibular advancement should be titrated gradually over weeks to yield maximum benefit; however, we emphasize that our average single-night treatment effect (69% reduction in AHI) is similar to the ~70% reduction observed in longer trials (Pitsis et al. 2002; Petri et al. 2008). A gradual titration approach is probably helpful for subjective benefits. Fifth, upper airway endoscopy during natural sleep and Pcrit measurement using CPAP drops are physiological methods not suitable for clinical practice. However, methods to assess the pharyngeal structure causing collapse using non-invasive airflow shape analysis have been developed (Azarbarzin et al. 2017; Genta et al. 2017; Azarbarzin et al. 2018). Similalrly, pharyngeal collapsibility can now be estimated from routine diagnostic polysomnography (Azarbarzin et al. 2016; Sands et al. 2018). By identifying the phenotypic characteristics of responders, the present study provides the foundation for the focused development of new methods to identify patients who have a posteriorly-located tongue less severely collapsible airway.
Conclusions
In conclusion, the pharyngeal structure causing obstruction and upper airway collapsibility are strong determinants of oral appliance efficacy. In particular, the presence of a posteriorly-located tongue and a less-collapsible upper airway predicts oral appliance therapeutic success.
Key points.
Some patients with obstructive sleep apnoea (OSA) respond well to oral appliance therapy, whereas others do not for reasons that are unclear.
In the present study, we used gold-standard measurements to demonstrate that patients with a posteriorly-located tongue (natural sleep endoscopy) exhibit a preferential improvement in collapsibility (lowered critical closing pressure) with oral appliances.
We also show that patients with both posteriorly-located tongue and less severe collapsibility (predicted responder phenotype) exhibit greater improvements in severity of obstructive sleep apnoea (i.e. reduction in event frequency by 83%, in contrast to 48% in predicted non-responders).
The present study suggests that the structure and severity of pharyngeal obstruction determine the phenotype of sleep apnoea patients who benefit maximally from oral appliance efficacy.
Funding
This work was performed at Brigham and Women’s Hospital and was supported by the American Thoracic Society Foundation (Sands, Marques), National Institutes of Health (Wellman: R01HL128658, R01HL102321; Harvard Catalyst: UL1TR001102). Drs. Marques and Genta were supported by the Sao Paulo Research Foundation (FAPESP). Dr S. A. Sands and L. Taranto-Montemurro were also supported by the American Heart Association (15SDG25890059, 17POST33410436). BlueSom provided oral appliance devices (BluePro) for our investigation at no cost.
Competing interests
Drs M. Marques, P. R. Genta and L. Messineo declare that they have no competing interests. Dr L. Taranto-Montemurro received personal fees as a consultant for Novion Pharmaceuticals and Cambridge Sound Management outside the submitted work. Dr S. A. Sands received personal fees as a consultant for Cambridge Sound Management, Nox Medical and Merck outside the submitted work, and receives grant funding from Apnimed Corp. Dr A. Azarbarzin received personal fees as a consultant for Somnifix outside the submitted work. Dr A. Wellman received personal fees as a consultant for Bayer, Somnifix, Cambridge Sound Management and Nox Medical outside the submitted work. Drs A. Wellman and L. Taranto-Montemurro have a financial interest in Apnimed Corp., a company developing pharmacologic therapies for sleep apnoea. Their interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr D. P. White is the Chief Scientific Officer for Philips Respironics and is a consultant to Night Balance.
Biography

Melania Marques is a Brazilian physician who received her MD degree at University of Minas Gerais, Brazil, in 2007. After obtaining her specialty training in Otolaryngology and Sleep Medicine, she completed a postdoctoral research fellowship in the Division of Sleep Medicine at Brigham and Women’s Hospital & Harvard Medical School, Boston, MA, USA. In 2018, she concluded a PhD at the University of Sao Paulo, Brazil. She is currently working in clinical care and research focused in obstructive sleep apnea pathophysiology and alternative treatments.
References
- Azarbarzin A, Marques M, Sands SA, De Beeck SO, Genta PR, Taranto-Montemurro L, De Melo CM, Messineo L, Vanderveken OM, White DP & Wellman A (2017). Predicting epiglottic collapse in patients with obstructive sleep apnoea. Eur Respir J 50, 1700345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Sands SA, Marques M, Genta PR, Taranto-Montemurro L, Messineo L, White DP & Wellman A (2018). Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J 51, 1701419. 10.1183/13993003.01419-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbarzin A, Sands SA, Taranto-Montemurro L, Oliveira Marques MD, Genta PR, Edwards BA, Butler J, White DP & Wellman A (2016). Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep 40, zsw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamagoos AA, Cistulli P, Sutherland K, Ngiam J, Burke P, Bilston L, Butler JE & Eckert DJ (2017). Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle activity in obstructive sleep apnea. Am J Respir Crit Care Med 195, A6972. [DOI] [PubMed] [Google Scholar]
- Bamagoos AA, Sutherland K & Cistulli PA (2016). Mandibular advancement splints. Sleep Med Clin 11, 343–352. [DOI] [PubMed] [Google Scholar]
- Op De Beeck S, Dieltjens M, Verbruggen AER, Vroegop AV & Hamans E (2018). Characteristics of upper airway collapse assessed during drug- induced sleep endoscopy predict response and deterioration under mandibular advancement device therapy. Am J Respir Crit Care Med 197, A5936. [Google Scholar]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Ward SLD & Tangredi MM (2012). Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 8, 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt L, Suchecki D, Peres A, Togeiro M & Tufik S (2001). The variability of the apnoea-hypopnoea index. J Sleep Res 10, 245–251. [DOI] [PubMed] [Google Scholar]
- Bosshard V, Masse JF & Sériés F (2011). Prediction of oral appliance efficiency in patients with apnoea using phrenic nerve stimulation while awake. Thorax 66, 220–225. [DOI] [PubMed] [Google Scholar]
- Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC & Bilston LE (2013). Tongue and lateral upper airway movement with mandibular advancement. Sleep 36, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS & Wellman A (2016). Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in obstructive sleep apnea patients. Am J Respir Crit Care Med 194, 143–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta PR, Sands SA, Butler JP, Loring SH, Katz ES, Demko BG, Kezirian EJ, White DP & Wellman A (2017). Airflow shape is associated with the pharyngeal structure causing OSA. Chest 152, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde K, Lehmann S, Berge ME, Johansson AK & Johansson A (2016). Oral appliance treatment in moderate and severe obstructive sleep apnoea patients non-adherent to CPAP. J Oral Rehabil 43, 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekema A, Doff MHJ, de Bont LGM, van der Hoeven JH, Wijkstra PJ, Pasma HR & Stegenga B (2007). Predictors of obstructive sleep apnea-hypopnea treatment. J Dent Res 86, 1181–1186. [DOI] [PubMed] [Google Scholar]
- Hosmer DW & Lemeshow S (2000). Applied Logistic Regression. Danvers, MA: Wiley Series in Probability and Statistics. [Google Scholar]
- Kato J, Isono S, Tanaka A, Watanabe T, Araki D, Tanzawa H & Nishino T (2000). Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest 117, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Lam B, Sam K, Lam JCM, Lai AYK, Lam C-L & Ip MSM (2011). The efficacy of oral appliances in the treatment of severe obstructive sleep apnea. Sleep Breath 15, 195–201. [DOI] [PubMed] [Google Scholar]
- Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, Zavora T & Westbrook PR (2009). Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath 13, 163–167. [DOI] [PubMed] [Google Scholar]
- Li S, Wu D, Jie Q, Bao J & Shi H (2014). Lingua-epiglottis position predicts glossopharyngeal obstruction in patients with obstructive sleep apnea hypopnea syndrome. Eur Arch Otorhinolaryngol 271, 2737–2743. [DOI] [PubMed] [Google Scholar]
- Marques M, Genta P, Sands S, Taranto-Montemurro L, Azarbarzin A, De Melo C, White D & Wellman A (2017). Characterizing site and severity of upper airway collapse to guide patient selection for oral appliance therapy for obstructive sleep apnea. Am J Respir Crit Care Med 195, A2584. [Google Scholar]
- Ng AT, Gotsopoulos H, Qian J & Cistulli PA (2003). Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med 168, 238–241. [DOI] [PubMed] [Google Scholar]
- Ng AT, Qian J & Cistulli PA (2006). Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep 29, 666–671. [PubMed] [Google Scholar]
- Ogawa T, Long J, Sutherland K, Chan ASL, Sasaki K & Cistulli PA (2015). Effect of mandibular advancement splint treatment on tongue shape in obstructive sleep apnea. Sleep Breath 19, 857–863. [DOI] [PubMed] [Google Scholar]
- Park P, Jeon HW, Han DH, Bin Won T, Kim DY, Rhee CS & Kim HJ (2016). Therapeutic outcomes of mandibular advancement devices as an initial treatment modality for obstructive sleep apnea. Medicine 95, e5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL & Schwartz AR (2004). A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170, 86–93. [DOI] [PubMed] [Google Scholar]
- Petri N, Svanholt P, Solow B, Wildschiødtz G & Winkel P (2008). Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res 17, 221–229. [DOI] [PubMed] [Google Scholar]
- Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB & Cistulli PA (2013). Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 187, 879–887. [DOI] [PubMed] [Google Scholar]
- Pitsis AJ, Ali Darendeliler M, Gotsopoulos H, Petocz P & Cistulli PA (2002). Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med 166, 860–864. [DOI] [PubMed] [Google Scholar]
- Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM & Chervin RD (2015). Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 11, 773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath WJ, Heise M, Hinz R & Ruehle K-H (2002). An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest 122, 569–575. [DOI] [PubMed] [Google Scholar]
- Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, Hess L, White DP & Wellman A (2018). Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med 197, 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-L, Wen Y-W, Chen N-H & Liao Y-F (2012). Craniofacial morphologic predictors of oral appliance outcomes in patients with obstructive sleep apnea. J Am Dent Assoc 143, 1209–1217. [DOI] [PubMed] [Google Scholar]
- Sutherland K, Chan ASL, Ngiam J, Darendeliler MA, Cistulli PA & Sutherland K (2018). Qualitative assessment of awake nasopharyngoscopy for prediction of oral appliance treatment response in obstructive sleep apnoea. Sleep Breath 22, 8–15. [DOI] [PubMed] [Google Scholar]
- Sutherland K, Phillips CL & Cistulli PA (2015a). Efficacy versus effectiveness in the treatment of sleep apnea: CPAP and oral appliances. JDSM 2, 175–181. [Google Scholar]
- Sutherland K, Takaya H, Qian J, Petocz P, Ng AT & Cistulli PA (2015b). Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med 11, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiki S, Kobayashi M, Namba K, Oka Y, Komada Y, Kagimura T & Inoue Y (2010). Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J 35, 1098–1105. [DOI] [PubMed] [Google Scholar]
- Vroegop AVMT, Vanderveken OM, Dieltjens M, Wouters K, Saldien V, Braem MJ & Van de heyning PH (2013). Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res 22, 348–355. [DOI] [PubMed] [Google Scholar]


