Abstract
DPP-4 inhibitors, used for treatment of type 2 diabetes, act by increasing the concentrations of intact glucagon-like peptide-1 (GLP-1), but at the same time, they inhibit secretion of GLP-1, perhaps by a negative feedback mechanism. We hypothesized that GLP-1 secretion is feedback regulated by somatostatin (SS) from neighboring D-cells, and blocking this feedback circuit results in increased GLP-1 secretion. We used a wide range of experimental techniques, including gene expression analysis, immunohistochemical approaches, and the perfused mouse intestine to characterize the paracrine circuit controlling GLP-1 and SS. We show that 1) antagonizing the SS receptor (SSTr) 2 and SSTr5 led to increased GLP-1 and SS secretion in the mouse, 2) SS exhibits strong tonic inhibition of GLP-1 secretion preferentially through SSTr5, and 3) the secretion of S was GLP-1 receptor dependent. We conclude that SS is a tonic inhibitor of GLP-1 secretion, and interventions in the somatostain-GLP-1 paracrine loop lead to increased GLP-1 secretion.
Keywords: GLP-1, somatostatin, paracrine loop, somatostatin receptors, SSTr antagonist
INTRODUCTION
Somatostatin (SS) is a peptide hormone, originally isolated from hypothalamic extracts during the search for regulators of growth hormone secretion (4). SS is also known to be produced in intestinal D-cells, which are scattered throughout the gastrointestinal tract and, to some extent, from D-cells in the pancreatic islets and from enteric neurons (4, 19, 46), in which it generally has inhibitory effects. The intestinal processing of proSS, the precursor protein, results in 2 biologically active isoforms, SS-14 and SS-28 (3, 16, 35, 43, 50), which bind to five SS receptor (SSTr) subtypes (denoted SSTr1–5) with different potencies (6, 39–41, 49, 56, 57). Among the potential targets for SS are the enteroendocrine L-cells secreting glucagon-like peptide-1 (GLP-1), an insulinotropic incretin hormone that plays an important role in regulating glucose tolerance. GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) and inhibitors of DPP-4, which increase concentrations of plasma GLP-1 and are used to improve glycemic control in patients with type 2 diabetes (8). However, in spite of increasing plasma concentrations of intact GLP-1, secretion of the hormone is actually reduced, suggesting that an inhibitory feedback mechanism might exist; the mechanism has been speculated to involve a paracrine circuit involving SS (5, 9, 12, 19, 20). Thus, interference with SS-SSTr regulation of L-cell function could lead to increases in the secretion of endogenous GLP-1 and might be of clinical relevance (58). We therefore decided to investigate and characterize this putative paracrine loop between SS-producing D-cells and specific SSTr subtypes located on GLP-1 producing L-cells in the intestine of the mouse. We analyzed the anatomical relationship between the two cell types in the proximal small intestine in mice and visualized the presence of GLP-1 receptors (GLP-1r) on surfaces of D-cells, as well as SS binding to L- and D-cells. Expression levels of Sstr1–5 were characterized in GLP-1-positive cells and validated by in situ hybridization. Functional studies were performed using isolated perfused mouse small intestine, the only experimental model that allows elucidation of paracrine relationships and their dynamic effects on secretion. Our results document the existence of a powerful paracrine relationship between the L- and D-cell and show that specific disruption of SSTr signaling with selective antagonists for the SSTr2 and SSTr5 receptors has important stimulatory implications for GLP-1 secretion.
MATERIALS AND METHODS
Animals.
All animal studies were carried out with permission from the Danish Animal Experiments Inspectorate (2013–15–2934–00833) and in accordance with the European Union Directive 2010/63/EU and guidelines of Danish legislation governing animal experimentation (1987) and the National Institutes of Health (publication no. 85–23). Wild-type male/female C57BL/6JRj mice (9–12 wk, 24–29/20–24 g) were purchased from Janvier, Le Genest-Saint-Isle, France. The GLU-Venus mice used for isolating proglucagon-positive and negative cells were from an in-house mouse breed, originally generated at University of Cambridge (45). All animals were kept under a 12-h light/dark cycle with free access to water and standard rodent chow. They were housed 2–8 mice per cage.
Test compounds.
The following test compounds were obtained from Bachem, Bubendorf, Germany: the macrocyclic SSTr2 antagonist (hereafter SSTr2a) PRL-2915 [cat. no. H-6056; H-p-Chloro-Phe-d-Cys-β-(3-pyridyl)-Ala-d-Trp-Lys-tBu-Gly-Cys-2-Nal-NH2 trifluoroacetate salt (disulfide bond) depicted in Supplemental Fig. S1A (https://doi.org/10.6084/m9.figshare.8307335)] (25), GLP-1 7–36 amide (cat. no. H-6995), SS-14 (cat. no. H-1490), SS-28 (cat. no. H-4455), exendin-(9–39) (cat. no. H-8740) (48), and bombesin (cat. no. H-2155). The SSTr5 antagonist (hereafter SSTr5a), Compound B, was a kind gift from F. Hoffmann-La Roche Ltd., Basel, Switzerland (51) (depicted in Supplemental Fig. S1B).
Immunohistochemistry.
Staining of D- and L-cells was performed on sections from the proximal small intestine (C57BL/6JRj female mice, n = 17). Intestinal segments were embedded in paraffin and sections of 4 μm were cut with a microtome. Sections were dewaxed and pretreated in a microwave oven for 15 min in an EGTA buffer, pH 9. Hereafter, the sections were washed in PBS, blocked with blocking buffer (2% bovine serum albumin, PBS) and incubated overnight with GLP-1 or SS primary antibody in blocking buffer at 4°C (dilution for SS; 1:10,000, GLP-1; 1:1:500, see Supplemental Table S1 [https://doi.org/10.6084/m9.figshare.8307326] for details of antibodies). The following day, the sections were washed and incubated for 1 h with a mix of AF568 donkey anti-rabbit IgG (1:500 Abcam, 175693, red) and AF488 donkey anti-mouse IgG (1:500 Abcam, 150109, green) in blocking buffer, then washed and coverslipped. The GLP-1r was first stained as described by Jensen et al. (27), after which SS-positive cells were stained (female mice, n = 8). The sections were washed in PBS and boiled for 15 min in a citrate buffer pH 6, blocked and incubated overnight with SS primary antibody (1758, in house, dilution 1:5,000) in blocking buffer. On the second day, sections were incubated with AF568 donkey anti-rabbit IgG (1:500, Abcam, 175693, red) and treated as described above. The slides were analyzed using Zeiss Axioskop 2 microscope, and images were taken using a CoolSNAP camera.
Autoradiography.
The procedures for the autoradiography studies and development of the autoradiography sections have been described previously by Jensen et al. (27). In brief, C57BL/6JRj male mice (n = 3) were anesthetized with intraperitoneal injection of ketamine-xylazine (0.1 mL/20 g) [ketamine 90 mg/kg (Ketaminol Vet; MSD Animal Health, Madison, NJ) and xylazine 10 mg/kg (Rompun Vet; Bayer Animal Health, Leverkusen, Germany)], the abdomen was opened, and 2,000,000 cycles/min 125I-labeled SS-28 (NEX084, Bachem) dissolved in 0.04 M phosphate buffer with 1% human serum albumin (pH 7.5) was administered intravenously into the inferior vena cava. Half of the animals received a 1,000-fold excess of unlabeled peptide (SS-28 cat. no. 4031270, Bachem) in combination with 125I-labeled SS-28 in the same injection to test for specificity. After 5 (n = 3) or 10 (n = 3) min, blood was flushed out by infusing 0.9% saline at a constant flow rate through a catheter placed into the left cardiac ventricle, with the blood leaving via an opening of the right atrium. Hereafter, mice were perfusion fixed by flushing the vascular system with ice-cold 4% paraformaldehyde (PFA). The proximal small intestine was excised and stored in 4% PFA before further processing. Tissue specimens were embedded in paraffin and histological sections were cut and prepared with film emulsion as described by Jensen et al. (27). The sections were developed for 6–8 wk at –20°C and stained with hematoxylin, followed by examination using a light microscope. Sections were then scanned in a Zeiss Axio Scan.Z1 slide scanner before further analysis by immunohistochemistry following the methods described by Jensen et al. (27). Areas with grains, representing SS binding, were randomly selected, and the number of cells that showed both SS binding as well as either GLP-1 or SS immune staining was counted.
Cell isolation and FACS.
Intestinal segments of ~2–3 cm, taken from duodenum, jejunum, ileum, and colon of GLU-Venus mice (n = 30), were used to investigate the expression of SSTrs on L-cells (45). The mice were euthanized by cervical dislocation, and the intestinal segments were flushed with Dulbecco’s modified Eagle’s medium (DMEM) 1885 to remove luminal contents. Thereafter, the intestinal pieces were turned inside out and inflated with DMEM 1885 and placed in DMEM 1885 containing 0.13 Wünsch units of Liberase (125 µL 200 × stock solution Liberase + 25 mL DMEM or, for colon, 250 µL 200 × stock solution Liberase + 25 mL DMEM; Roche, Indianapolis, IN). The tissues were then incubated for 25 min in a water bath (37°C) with slow shaking interspersed with vigorous shaking for 5 s every 5 min to allow digestion. The tissue was then placed in a fresh DMEM 1885-Liberase solution, and the incubation process was repeated 3 times in total. The cells were then filtered through a 70-μm pore diameter cell strainer and centrifuged at 1,500 revolutions/min for 5 min. Next, the cells were trypsinized for 2 min at 37°C, and the pellet was resuspended in DMEM 1885 with 5% fetal bovine serum. Finally, the suspension was passed through a 30-μm pore diameter filter at the top of the FACS sorting tube to form a single-cell suspension. The cells were FACS sorted using a BD FACSAria II (BD Biosciences, Palo Alto, CA), yielding GLU-Venus-positive and GLU-Venus-negative cells. The positive cells constituted, on average, 0.3% of the cells (except for duodenum, which was 0.14%) released from the mucosa by enzymatic digestion. After FACS sorting, the cells were more than 90% pure, as determined by microscopy (10, 47).
RNA extraction and quantitative PCR.
Total RNA was extracted using NucleoSpin RNA XS kit (REF. 740902 Macherey-Nagel, Düren, Germany). RNA concentration was measured, and the volume (µL) needed to yield a concentration of 250 ng was calculated and used for generation of cDNA using QuantiTect Whole Transcriptome kit (cat. no. 207043, QIAGEN, Hilden, Germany). For colon, 100 ng was used because of the low concentration of isolated RNA in nanograms per microliter. The expression of SSTr1–5 was investigated using custom-designed 384-well quantitative PCR plates from Lonza, Denmark. Data are shown as expression relative to a mean of three housekeeping genes, 18S ribosomal RNA, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (YWHAZ), and hypoxanthine phosphoribosyltransferase 1 (HPRT1) (15, 55), and calculated using the Livak method (34). The sequences used for the five receptors and the housekeeping genes are listed in Supplemental Table S2 (https://doi.org/10.6084/m9.figshare.8307386) and published in Ref. 13.
In situ hybridization.
Male C57BL/6JRj mice (n = 4) were euthanized by cervical dislocation, and the tissue of interest was rinsed with cold PBS and transferred to fresh 4% PFA/PBS for 24 h at room temperature. Hereafter, the tissue samples were transferred to 70% ethanol followed by xylene and paraffin infiltration (Shandon Excelsior, Thermo Fisher, Waltham, MA) and embedded in paraffin blocks. Sections of 4 μm were cut using a microtome (RM2125, Leica) and mounted on slides (Superfrost Plus Slides, Thermo Scientific). The slides were baked at 60°C for 1 h and then placed in xylene and ethanol for dewaxing. The in situ hybridization was performed by following the manufacturers’ manual of a commercially available in situ hybridization kit, RNAscope 2.5 HD detection (cat. no. 322360, Advanced Cell Diagnostics, Milan, Italy), in combination with immunofluorescence. After the in situ hybridization procedure, the slides were incubated with blocking buffer, followed by incubation with the primary GLP-1 (1:2,500, in house, 2135–8) or SS (1:2,500, Santa Cruz, sc-7819; for overview of antibody, see Supplemental Table S1) antibody in blocking buffer overnight at 4°C. The next day, the slides were washed and incubated with AF488 conjugated secondary antibodies for 1 h at room temperature. Coverslips were then mounted with ProLong Gold Antifade Mountant with DAPI (P-36931, Thermo Fisher). The slides were analyzed using a Zeiss wide field fluorescence microscope, and images were captured using a Zeiss Axiocam 506 mono camera.
Transfection of COS cells and inositol (1,4,5)-trisphosphate assay.
COS-7 cells were transfected as described by Toräng et al. (53). In short, the cells were transfected with 10 μg DNA of mouse SSTr (mSSTr) subtypes 1–5 together with 10 μg of the chimeric G protein GαΔ6qi4myr using the calcium phosphate precipitation method with the addition of chloroquine (23, 30). The chimeric G protein GαΔ6qi4myr was a kind gift from Evi Kostenis (University of Bonn, Germany) This transfection results in Gαq-mediated pathways being engaged when Gαi-coupled receptors are activated (28, 53) and allows receptor activation to be assessed by measuring the formation of inositol (1,4,5)-trisphosphate instead of the inhibition of cAMP. The following day, the cells were seeded in white 96-well plates at a density of 3.5 × 105 cells/well and incubated with myo-[3H]inositol (5 μL/mL, 2 μCi/mL, PerkinElmer, Skovlunde, Denmark) overnight. The next day, the cells were washed once in PBS and twice in HBSS (Invitrogen) with 10 mM LiCl. Receptor activation by SS-14, SS-28, SSTr2a, or SSTr5a was determined by adding increasing concentrations of the peptide, followed by incubation at 37°C for 90 min. To test the antagonistic effect of SSTr5a and SSTr2a, the cells were preincubated for 15 min with the antagonists, after which a fixed concentration of SS-14 (and SS-28 for SSTr5) corresponding to 50%–80% of maximal functional response (Emax) was added and incubated for an additional 75 min. Cells were placed on ice, and assay buffer was removed. Subsequently, the cells were lysed by addition of 40 μL of 10 mM formic acid to each well, followed by incubation on ice for 30–60 min. Quantification of the [3H]inositol phosphate in the formic acid lysates was carried out by adding yttrium silicate-poly-d-Lys-coated scintillation proximity assay beads. Hereafter, the plates with cells were sealed and shaken on a table shaker for at least 30 min. After the scintillation proximity assay beads had been allowed to settle and react with the extract for at least 8 h, the radioactivity was measured using a Packard Top Count NXTTM scintillation counter (PerkinElmer Life Sciences).
Perfusions of the proximal small intestine in mice.
Male C57BL/6JRj mice (9–12 wk, n = 6–10) were anesthetized with intraperitoneal injection of ketamine-xylazine (same mixture as used for autoradiography studies). The perfusion setup is a single-pass perfusion system (52). Briefly, the abdominal cavity of the mice was opened, and the colon and the distal part of the small intestine were tied off and removed, leaving 12–15 cm of the proximal small intestine intact. A tube was placed in the lumen of the intestine and perfused with saline (0.9%; 0.31 osmol/l NaCl) at a flow rate of 0.035 mL/min. The spleen and stomach were additionally removed, and the kidneys were tied off, thereby preventing perfusion of these organs. A catheter (BD Insyte Autoguard, 24 gage 0.75 IN, 0.7 × 19 mm, Becton Dickinson, Lyngby, Denmark) was placed in the abdominal part of the aorta to perfuse the intestine through the superior mesenteric artery, and a similar catheter was placed in the portal vein to collect the venous effluent (1-min fractions). Hereafter, the mice were euthanized by perforating the diaphragm. The intestine was perfused at 2.5 mL/min with a modified Krebs-Ringer bicarbonate buffer containing 0.1% BSA (Merck, Darmstadt, Germany), 5% Dextran T-70 (Dextran Products, Scarborough, Canada), 3.5 mmol/L glucose, and 5 mmol/L each of pyruvate, fumarate, and glutamate, 10 μmol/L IBMX (Sigma-Aldrich, cat. no. 5879) and 5 mmol/L Vamin (a mixture of essential and nonessential amino acids; Fresenius Kabi, Copenhagen, Denmark). The perfusion buffer was gassed with a 95% O2-5% CO2 mixture to achieve pH ~7.5 and maintained at 37°C during the experiment.
Hormone analysis by RIA.
In-house RIAs were used to determine hormone concentrations of GLP-1, SS, and enteroglucagon. GLP-1 (7–36amide) was measured with an assay based on a COOH terminally directed antiserum specific for the amidated GLP-1 form (code no. 89390), which has been validated according to the Clinical and Laboratory Standards Institute Protocols (1). The standard was synthetic GLP-1 (7–36)NH2 (cat. no. H-6795-GMP, 4081700, Bachem) and the tracer was monoiodinated 125I-labeled GLP-1(7–36)NH2 (a gift from Novo Nordisk, Bagsværd, Denmark). Free and bound peptides were separated with plasma-coated charcoal (cat. no. 1.02186.0250, Merck). SS was measured using a well-characterized in-house RIA (antibody code no. 1758), described in Ref. 24. It uses a rabbit polyclonal antiserum raised against synthetic cyclic SS, recognizing equally both isoforms of SS. SS-14 (cat. no. H-1490, Bachem) was used as standard and 125I-labeled Tyr11-SS (NEX389, PerkinElmer) was used as tracer. Enteroglucagon was measured with a cross-reacting glucagon RIA (in-house code no. 4304). This antibody recognizes a midsequence of the glucagon moiety that is present in glicentin and oxyntomodulin, which are secreted from the L-cell in parallel with GLP-1 (2, 26). The tracer used was a 125I-labeled glucagon tracer (gift from Novo Nordisk).
Statistics.
Data are presented as means ± SE. Calculation of statistical analysis in the in vitro experiments was carried out using an unpaired t test based on the effect observed at each single concentration used (from 10−11 to 10−5 M of SS-14 vs. SS-28 or from 10−8 to 10−5 M for SSTr2a vs. SSTr5a). Calculation of statistical significance in the perfusion data was based on output calculated by the mean hormone output (concentration × perfusate flow of 2.5 mL/min) during the stimulation period; 10 min was included for SS-14/SS-28 stimulation, and for the antagonist stimulations, a 5-min post stimulation period was included because of the often-delayed response when applying antagonists compared with the average of 5 min preceding baseline. The output during stimulation (10 or 15 min) was calculated by subtracting the mean output of 5 min baseline (prestimulation) period. Outputs were evaluated by one-way ANOVA for repeated measurements, followed by Bonferroni post hoc analysis. In the case of nonrelated experiments, for example, the effect of SS-14 versus SS-28 on GLP-1 and SS secretion, the effects were compared by unpaired t test based on the average outputs. Statistical calculations and graphs were made using GraphPad Prism 6 (GraphPad, La Jolla, CA).
RESULTS
Spatial relationship between L- and D-cells, showing the GLP-1r is expressed in D-cells and labeled SS is seen on surfaces of both cell types.
The spatial relationship between small intestinal D- and L-cells was visualized by immunohistochemical staining of SS and GLP-1-positive cells in the proximal small intestine from mice. Five random fields of view with mucosa for each intestinal section were analyzed, and between 10 and 20 L-cells were identified. L-cells were categorized as either being in the proximity of D-cells (defined as no more than 3 cells in the epithelium between the L-cell and the closest D-cell) or not in the proximity (more than 4 cells in the epithelium between the L-cell and closest D-cells). Fifteen of 206 analyzed L-cells were in the vicinity of a D-cell (15/206) (Fig. 1A). Furthermore, we observed that many SS-positive cells possess basolateral cytoplasmic projection, and that staining for SS and the GLP-1r overlapped (Fig. 1B).
Fig. 1.
Mouse small intestinal mucosa: glucagon-like peptide (GLP)-1 and somatostatin (SS) cells are found in close proximity, GLP-1 receptors are present on D-cells, and both D- and L-cells show SS binding. A: representative images showing immunohistochemical staining of GLP-1 (green) and SS (red) positive cells in the proximal small intestine, female mice, n = 17. B: immunohistochemical staining of the GLP-1 receptor (black arrow) and immunohistochemical staining of SS-positive cells (white arrow) on the same slide, female mice, n = 8. C: left: autoradiography before immunohistochemical staining showing grains representing 125I-labeled SS-28, male mice, n = 3. Right: immunohistochemical staining of GLP-1 (solid black arrow) in combination with autoradiography showing grains of 125I-labeled SS-28 (punctuated black arrow). D: left: the same as C, but with immunohistochemical staining of SS-positive cells, male mice. Right: The same as C but with immunohistochemical staining of SS-positive cells. ab, antibody. n = 3. Bar = 50 µm.
Autoradiography was carried out on the proximal part of the intestine to visualize binding of I-125-labeled SS-28 to surfaces of L- and D-cells, with I-125-labeled SS-28 being administered systemically before euthanasia. It revealed that around 70% of the GLP-1 immunoreactive cells were also labeled with I-125-labeled SS-28 on their surfaces (Fig. 1C). The incidence of overlap between SS immunoreactive cells and I-125-labeled SS-28 revealed that ~30% of the I-125 SS-28-labeled cells were SS immunoreactive cells (Fig. 1D). Specific binding to the SSTrs was verified by adding an excess of unlabeled SS-28 to the I-125-labeled SS-28, which abolished binding of I-125-labeled SS-28 to the surfaces of the cells (data not shown). We also observed many other cells showing I-125-labeled SS-28 surface binding, for which the secretory products were, however, not identified in this study.
SSTr5 expression is high and enriched in the intestinal L-cells.
To characterize which SSTrs are expressed on L-cells, intestinal tissue samples from the GLU-Venus reporter mouse were used to distinguish the L-cells from the other cell types in the intestinal segment. The cells were purified by FACS, which gave rise to two populations of cells, one representing the GLP-1 (proglucagon)-positive cells in the intestine, and the other the GLP-1 (proglucagon)-negative cells, consisting of neighboring cells in the particular intestinal segment. Expression levels of SSTrs were analyzed by real-time quantitative qPCR.
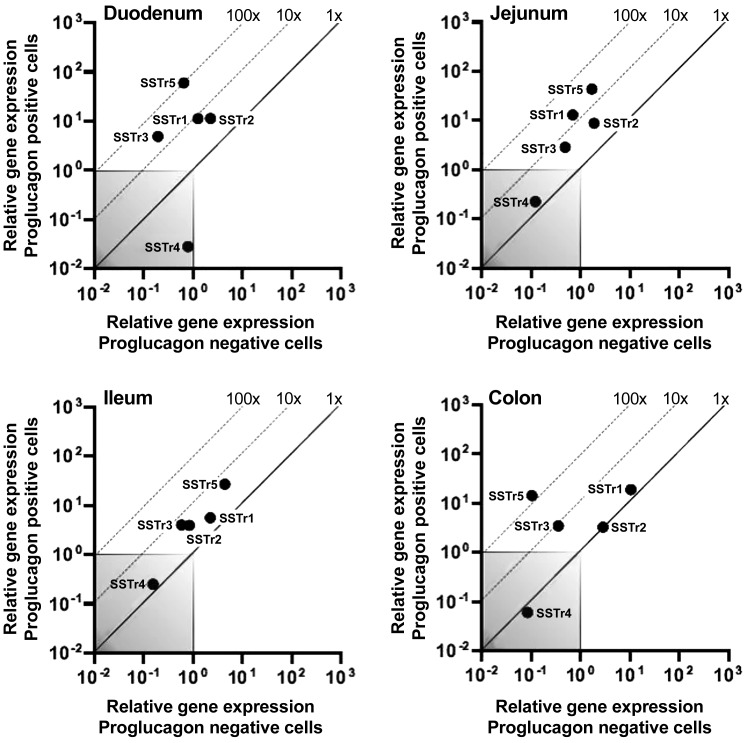
SSTr5 was highly expressed and enriched in GLP-1-positive cells of the duodenum, jejunum, ileum, and colon (i.e., 92-, 26-, 6-, and 138-fold enriched, respectively) compared with neighboring cells (Fig. 2). SSTr2 was expressed in GLP-1-positive cells and displayed a minor enrichment compared with neighboring cells (~5-fold enrichment in duodenum, jejunum, and ileum; Fig. 2, A–C). SSTr3 was also enriched in GLP-1-positive cells in the intestine; however, the expression levels were very low compared with, for example, SSTr5. Expression of SSTr1 was also observed in GLP-1-positive cells, with up to ~10-fold enrichment in the more proximal parts of the intestine. However, inspired by previous studies by Moss et al. (37) and Sprecher et al. (51) investigating the SSTrs and certain antagonists, as well as the commercial availability of selective SSTr2 and SSTr5 antagonists, we focused on the physiological properties of these two receptors in the following experiments.
Fig. 2.
The somatostatin receptor SSTr5 is the most expressed and enriched SSTr in the proglucagon-like positive cells of the mouse intestine. SSTr expression in proglucagon-positive cells (y-axis) compared with their surrounding proglucagon-negative cells (x-axis) from duodenum, jejunum, ileum, and colon. The dotted line represents fold change enrichment in the positive vs. the negative cells, and the gray box is defined as noise (threshold cycle level 35 < cycles). Male mice, n = 3, consisting of cells from 10 mice in each run pooled together (total 30 male mice).
L-cells express more SSTr5 compared with SSTr2, whereas expression of both SSTr5 and SSTr2 is low in D-cells.
Expression of SSTr2 and SSTr5 in the L-cells was visualized by in situ hybridization with subtype-specific probes targeting SSTr2 or SSTr5 mRNA. This was combined with immunohistochemical staining of GLP-1 cells in mouse jejunum and proximal ileum (Fig. 3A). Staining of SS cells was also included to investigate the possibility of an autocrine loop involving SSTr2 and SSTr5 (Fig. 3B). As a positive control, probes targeting mRNA from a housekeeping gene (Mus musculus peptidylpropyl isomerase B) showed strong mRNA staining covering the cytosol of GLP-1 and SS-stained cells as well as the surrounding tissue. By contrast, the negative control probes specific for Bacillus subtilis dihydrodipicolinate reductase did not show any staining of mRNA in D- or L-cells, as expected (Supplemental Fig. S2, https://doi.org/10.6084/m9.figshare.8307368). In situ hybridization is a semiquantitative method, as beyond a certain level of expression, it is impossible to quantify the number of stained mRNA transcripts with certainty as individual dots begin to merge together. The term “high expression” was therefore defined as mRNA staining equal to or greater than the positive control probe staining (targeting the housekeeping gene Mus musculus peptidylpropyl isomerase B mRNA). “Low expression,” on the other hand, was defined as observing only a few stained transcripts within the cytosol of a cell. GLP-1 cells expressed low levels of SStr2 mRNA, whereas a high expression was observed for SStr5 in both jejunum and proximal ileum. SS cells had low levels of both SSTr2 and SSTr5 mRNA.
Fig. 3.
Somatostatin (SS) receptor SSTr5 mRNA is more expressed in GLP-1 positive cells than SSTr2 mRNA, and none of them show high mRNA levels in the SS-positive cells. Dual in situ hybridization and immunohistochemical staining of SSTr2 and SSTr5 mRNA in small intestinal D- and L-cells. A: representative images of immunohistochemically stained GLP-1 cells (green) and in situ hybridization stained SSTr2 or SSTr5 mRNA (red dots) and a merge image (third picture) performed on mouse jejunum or proximal ileum sections. B: the same as above, however, with SS immunolabeled cells. Each red dot represents a single stained mRNA transcript. Dashed line outlines SS cell borders. Nuclei were visualized with DAPI counterstaining (blue). Ab, antibody; Pb, probe; bar = 5 µm. Male mice, n = 4.
SS-14 and SS-28 inhibit GLP-1 secretion in the perfused mouse intestine.
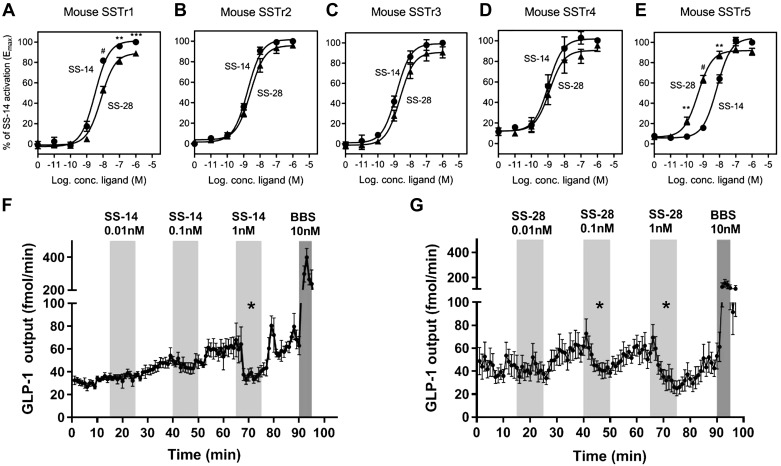
Since SS is found in two isoforms, their binding affinity toward the five mSSTrs as well as their ability to inhibit GLP-1 secretion in the perfused mouse intestine were investigated. In vitro activation studies of SS-14 and SS-28 showed similar potencies toward SSTr1–4, whereas for SSTr5, there was a significant difference using 10−10, 10−9, and 10−8 M of SS-14 or SS-28 (P < 0.01, P < 0.0001, and P < 0.01, respectively). This corresponds to a 14-fold lower potency for SS-14 compared with SS-28 (Fig. 4, A–E; for SSTr5, EC50 of 6.92 nM for SS-14 and 0.48 nM for SS-28. For more details on the other receptors, see Supplemental Table S3, https://doi.org/10.6084/m9.figshare.8307401). To confirm that the chimeric GαΔ6qi construct was activated in the same way as the native Gαi, the potencies of ligands in the SSTr system (using SS-14 and the five human SSTrs) were obtained by measuring Gαi-coupling (determined as inhibition of forskolin-induced cAMP production), and compared with the potencies measured using cotransfection with the chimeric Gαq/Gαi-subunit (and subsequent measurement of inositol (1,4,5)-trisphosphate formation). We found a match between the two pathways that was consistently similar for all five receptor subtypes; i.e., no subtype-specific alterations in potency depending on the chosen pathway (Supplemental Fig. S5, https://doi.org/10.6084/m9.figshare.9752357). A similar comparison, but using other receptors, also found overlapping potencies for the ligands in the two pathways (29).
Fig. 4.
Somatostatin (SS)-14 has a lower potency for SS receptor (SSTr) 5, but both SS-14 and SS-28 inhibit glucagon-like peptide-1 (GLP-1) secretion from the perfused mouse intestine. Mouse somatostatin receptor (mSSTr) subtypes 1 (A), 2 (B), 3 (C), 4 (D), and 5 (E) were transiently transfected in COS-7 cells, and activation of the receptors by SS-14 (●) and SS-28 (▲) was tested with increasing concentrations (100 pM to 1 μM) and analyzed with the inositol (1,4,5)-trisphosphate (IP3) formation assay (A–E). Data are shown as means ± SE of independent experiments performed in duplicates, n ≥ 3. Statistical significance of the in vitro data are calculated by an unpaired t test based on percentage of activation by SS-14 or SS-28 comparing each concentration applied. Effect of SS-14 on GLP-1 output (F); data shown as means ± SE. Effect of SS-28 on GLP-1 output (G); data shown as means ± SE. Bombesin (BBS) was included as a positive control at the end of every experiment. One-way ANOVA for repeated measurements followed by Bonferroni post hoc analysis was used for statistical evaluation of the effect on GLP-1 secretion compared with baseline; *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.0001. SS-14 data n = 6; SS-28, male mice, n = 8–10.
Since SSTr5 is highly expressed and enriched in GLP-1 cells, we sought to investigate whether the differing potencies of SS-14 and SS-28 toward SSTr5 would translate to differences in their effects on GLP-1 secretion in the perfused mouse intestine. Intra-arterial infusions of 0.01 nM, 0.1 nM, or 1 nM of SS-14 (Fig. 4F) or SS-28 (Fig. 4G) were given to the proximal small intestine. The GLP-1 output after SS-14 application was only significantly lower compared with the decrease from the preceding baseline at 1 nM, in which a ~30% average decrease was observed (baseline 62.8 ± 8.9 fmol/min to 41.7 ± 4.3 fmol/min, P = 0.016). SS-28 significantly decreased GLP-1 secretion by ~20% at 0.1 nM and ~30% 1 nM (Fig. 4G 0.01 nM baseline 60 ± 8 fmol/min to 48 ± 5 fmol/min, P = 0.029 and 1 nM baseline 57 ± 8 fmol/min to 40 ± 7 fmol/min, P = 0.014, respectively). At 0.01 nM, neither SS-14 nor SS-28 significantly decreased GLP-1 output compared with baseline (P = 0.7 and 0.1, respectively). The ability of SS-14 and SS-28 to inhibit GLP-1 secretion was compared based on the fractional decrease from the preceding baseline of average GLP-1 output. It was found that SS-28 inhibited GLP-1 secretion more potently at 0.1 nM compared with SS-14 (SS-14 decreased GLP-1 secretion by an average of ~6%, whereas SS-28 decreased the GLP-1 secretion by ~20%, P = 0.02). At 1 nM, SS-14 and SS-28 inhibited GLP-1 secretion equally by ~30% from baseline (P = 0.016 and P = 0.014, respectively).
SSTr5a is a strong and specific antagonist for SSTr5.
The potencies of the two SSTr2 and SSTr5 antagonists were determined in transfected cell cultures (Fig. 5, A–J, and Supplemental Table S3). A comparison of the effects of SSTr5a and SSTr2a clearly showed that SSTr5a is a highly potent antagonist of mSSTr5 with weak potency inhibition of only one other receptor: SSTr4 (only observed at the higher concentrations of 10−5 and 10−6 M (P < 0.001 and P < 0.05, respectively). The same picture was observed when inhibiting SS-28-activated receptors (Supplemental Fig. S3, https://doi.org/10.6084/m9.figshare.8307377). However, since SSTr4 is barely expressed in the intestine, it was considered unlikely that the effects of the SSTr5a in the following experiments could have been mediated through SSTr4. The SSTr2a, on the other hand, was shown to be a partial agonist to SSTr3–5 at concentrations ≥1 μM (Fig. 5, C–E). With regard to SSTr3 and SSTr4, we considered that this partial agonism would only have a minor influence in the intestine because of the low expression levels of these two subtypes. However, SSTr2a had intrinsic agonistic activity on SSTr5, amounting to 40% of the Emax (the maximum possible effect) of SS-14 at concentrations of 1 μM, which could confound the interpretation of the results. The SSTr2a was also capable of inhibiting SSTr1 and SSTr3, though with a much lower potency compared with SSTr2 (interference was observed only for concentrations above 1 μM; Fig. 5, F and H, and Supplemental Table S3).
Fig. 5.
Somatostatin (SS) receptor (SSTr) 5 agonist (SSTr5a) is a highly specific antagonist for the SSTr5, whereas the SSTr2 agonist (SSTr2a) is less specific. Both of the antagonists increase glucagon-like peptide-1 (GLP-1) and SS in the perfused mouse intestine. Mouse somatostatin receptor (mSSTr) subtypes 1 (A, F), 2 (B, G), 3 (C, H), 4 (D, I), and 5 (E, J) were transiently transfected in COS-7 cells, and the properties were analyzed with the inositol (1,4,5)-trisphosphate (IP3) formation assay. To test the potential agonism of the receptors by the antagonists, increasing concentrations of the antagonists (1 nM to 10 μM) SSTr5 agonist (SSTr5a) (♦) and SSTr2a (■) were added (A–E). The antagonistic properties of SSTr2a and SSTr5a on the five receptors were tested by adding increasing concentrations (10 μM to 10 nM) together with a fixed concentration of SS-14 (F–J; ◊ for SSTr5a and □ for SSTr2a). Data are shown as means ± SE; independent experiments were performed in duplicates, n ≥ 3. Statistical significance of the in vitro data are calculated by unpaired t test based on percentage of activation by SSTr2a or SSTr5a comparing each concentration applied. Intra-arterial infusion of 100 nM, 1 μM, and 10 μM SSTr2a and the effect on GLP-1 and SS outputs shown as means ± SE (K, L). SSTr5a’s effect on GLP-1 and SS outputs shown as means ± SE (M, N). Bombesin (BBS) was included as a positive control at the end of each experiment. Statistical evaluation was carried out by one-way ANOVA followed by Bonferroni post hoc analysis for evaluation of the effect on GLP-1 secretion compared with baseline; *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.0001; male mice, n = 6–7.
Antagonizing SSTr2 or SSTr5 increases GLP-1 and SS secretion from the perfused proximal small intestine in mice.
The effect of antagonizing SSTr2 and SSTr5 on intestinal GLP-1 and SS secretion was investigated in the isolated proximal perfused mouse intestine. The antagonists were applied by intra-arterial infusion of 100 nM, 1 μM, or 10 μM of SSTr2a (Fig. 5, K and L) or SSTr5a (Fig. 5, M and N), and GLP-1 and SS levels in the venous effluent were measured by RIA. Cross reactivity was tested for the SSTr2 and SSTr5 antagonists, which showed no cross reactivity with the SS RIA for either of the antagonists (Supplemental Table S4, https://doi.org/10.6084/m9.figshare.9747410).
Infusion of SSTr2a resulted in a dose-dependent increase in GLP-1 secretion compared with the preceding baseline (Fig. 5K, 100 nM: from 23 ± 2.7 fmol/min to 32.4 ± 3.6 fmol/min, P < 0.05, 1 μM: 37.3 ± 6.3 fmol/min to 62 ± 12.4 fmol/min, P < 0.05 and 10 μM: 66.8 ± 15 fmol/min to 98.7 ± 15.9 fmol/min, P < 0.001). However, the fractional increases are approximately the same, especially at 1 and 10 μM (~60%), because the preceding baseline increased during the experiment. SS secretion showed a similar pattern to that of GLP-1, with dose-dependent increases in response to SSTr2a infusions (Fig. 5L, SS output from preceding baseline at 100 nM; baseline 97.6 ± 20 fmol/min to 173.7 ± 32.4 fmol/min, 1 μM 158.6 ± 43 fmol/min to 281 ± 73.8 fmol/min, P < 0.05, 10 μM; 214.8 ± 51.7 fmol/min to 490 ± 88.6 fmol/min, P < 0.01).
SSTr5a also increased GLP-1 secretion dose dependently after intra-arterial infusions compared with the preceding baseline (Fig. 5M, GLP-1 100 nM: baseline 30.3 ± 6 fmol/min to 44.3 ± 5.6 fmol/min P < 0.05, 1 μM: 52 ± 5 fmol/min to 88.9 ± 3.9 fmol/min P < 0.001, 10 μM: 87.7 ± 4.2 fmol/min to 185 ± 8.3 fmol/min, P < 0.001). SS secretion likewise showed a dose-dependent increase in secretion (Fig. 5N, GLP-1 100 nM: 55 ± 7.6 fmol/min to 76.4 ± 10.7 fmol/min, P < 0.01, 1 μM: 77.8 ± 7.1 fmol/min to 141.4 ± 19.2 fmol/min P < 0.01, 10 μM: 110.6 ± 13.9 fmol/min to 251 ± 31.9 fmol/min, P < 0.001).
The ability of SSTr2a and SSTr5a to increase GLP-1 secretion was compared based on the fractional increase from the preceding baseline of average GLP-1 output. SSTr5a was significantly more potent to increase GLP-1 secretion at 10 µM compared with SSTr2a (SSTr2a increased GLP-1 secretion by an average of ~60%, whereas SSTr5a increased the GLP-1 secretion by ~110%, P = 0.008). At 100 nM, no significant difference was observed, in which SSTr2 on average increased the secretion by ~45% and SSTr5a increased GLP-1 secretion on average by ~60% (P = 0.6). Likewise at 1 µM, SSTr5a on average increased GLP-1 secretion more than SSTr2a, however without any significant difference (SSTr2a increased secretion by ~65%, SSTr5 ~80%, P = 0.6). No difference was observed in SS secretion based on the fractional increase from the preceding baseline comparing the two antagonists.
SS is secreted in response to increased GLP-1 levels via a mechanism involving the GLP-1r.
To investigate the paracrine interaction between L- and D-cells in the perfused intestine, we used the SSTr5a and the GLP-1r antagonist, exendin-(9–39). To ensure that no agonistic effect at high concentrations of exendin-(9–39) would occur we tested the effect of exendin-(9–39) on the GLP-1R in COS-7 cells expressing mouse GLP-1R (mGLP-1R; Supplemental Fig. S4 https://doi.org/10.6084/m9.figshare.9752354). Only GLP-1 activated the mGLP-1R, whereas exendin-(9–39) had no intrinsic activity, not even at the highest given concentration of 1 µM. Furthermore exendin-(9–39) was able to inhibit a GLP-1 mediated cAMP response, confirming that the peptide was active. Whether exogenous GLP-1 together with endogenous GLP-1 would have an additive effect on SS secretion was investigated by intra-arterial infusion of exogenous GLP-1 and the SSTr5a. Since the GLP-1 assay does not distinguish endogenously released GLP-1 from the exogenously infused peptide, we used an assay for another fragment of the precursor for GLP-1, proglucagon (here designated enteroglucagon, which is the sum of glicentin and oxyntomodulin which are simultaneously released with GLP-1) as a surrogate for endogenous GLP-1. Exogenous GLP-1 infusion had no effect on endogenous GLP-1 secretion (Fig. 6, A and B, 22.6 ± 2.9 fmol/min to 19.8 ± 1.6 fmol/min, P > 0.99) and a minor increase in SS secretion from preceding baseline was seen (Fig. 6, C and D, average baseline 42.4 ± 11.55 fmol/min increased to 62.3 ± 11.23, P < 0.05). Enhancing endogenous GLP-1 secretion with the SSTr5a (as shown in Fig. 6, A and B, by measuring enteroglucagon) led to an average increase of SS secretion from the preceding baseline, although this was not significant (Fig. 6, C and D, baseline 44.5 ± 14.2 fmol/min to 93 ± 24.5 fmol/min, P = 0.1). Combining SSTr5a with exogenous GLP-1 had a significant additive effect on the SS output when comparing output during SSTr5 and GLP-1 infusions separately (Fig. 6D, SS output during GLP-1 infusion: 318.9 ± 78.12 fmol/15 min, SS output during SSTr5a: 710.8 ± 227.8 fmol/15 min, combination: 1,796 ± 419 fmol/15 min, P < 0.01 for both).
Fig. 6.
Increased glucagon-like peptide-1 (GLP-1) levels increase somatostatin (SS) secretion. Enteroglucagon secretion (the sum of glicentin and oxyntomodulin) was used as a surrogate end point for secretion of proglucagon-derived products, including GLP-1. A: intra-arterial infusion of 500 pM GLP-1, 1 µM SS receptor (SSTr) 5 agonist (SSTr5a) or the two in combination shown as mean output ± SE. B: output of enteroglucagon during the three different stimulations. C: the same as A but showing the SS levels. D: the same as C but for SS output. E: GLP-1 output shown as mean ± SE after intra-arterial infusions of 1 µM SSTr5a, 1 µM exendin (9–39) (Ex9–39), or the two in combination. F: GLP-1 output during the three different stimulations based on output. G: the same as E but showing SS levels. H: the same as F but for SS levels. Statistical analysis of the output response from baseline was calculated by one-way ANOVA for repeated measurements followed by Bonferroni post hoc analysis. Bombesin (BBS) was included as a positive control at the end of each experiment. *P < 0.05, **P < 0.01; male mice, n = 5–6.
Whether the increases in SS secretion were due to a direct effect of GLP-1 binding to a stimulatory GLP-1r on the surface of D-cells or were mediated indirectly via blocking an autocrine restraining pathway involving SSTr5 was investigated using the GLP-1r antagonist exendin-(9–39) and the SSTr5a. The two antagonists were infused intra-arterially to the perfused mouse intestine, either alone or in combination (Fig. 6, E–H). As observed before, the SSTr5a increased both GLP-1 and SS secretion from the preceding baseline (Fig. 6, E and G, GLP-1 baseline: 25.6 ± 5.9 fmol/min to 65.9 ± 16.7, P = 0.06 and SS baseline: 48.6 ± 10.4 fmol/min to 92.9 ± 20.1 fmol/min, P = 0.059). In contrast, blocking the GLP-1r using exendin-(9–39) decreased SS secretion (Fig. 6, G and H, baseline 71 ± 15.7 fmol/min to 53 ± 11 fmol/min, P = 0.08), whereas GLP-1 secretion was similar as seen during SSTr5a infusion alone (Fig. 6F, mean difference between SSTr5a and exendin-(9–39) infusion 10.3 ± 143.8 fmol/15 min, P > 0.99). Simultaneous blocking of both SSTr5 and GLP-1r further increased the GLP-1 output compared with the individual SSTr5a and exendin-(9–39) infusions [Fig. 6F, GLP-1 output during SSTr5a infusion: 587.1 ± 169.4 fmol/15 min, GLP-1 output during exendin-(9–39): 576.8 ± 98.31 fmol/15 min and combination: 1,329 ± 268.7 fmol/min P < 0.05 for both], whereas SS secretion still remained completely inhibited despite the greatly enhanced GLP-1 secretion [Fig. 6H, SS output during SSTr5a infusion: 647.5 ± 189 fmol/15 min, during exendin-(9–39) infusion: 35.23 ± 12.9 fmol/15 min and combination: 130.3 ± 52.7 fmol/15 min, P < 0.05 and P = 0.053, respectively]. To rule out the possibility of any cross reactivity confounding interpretation, the GLP-1r antagonist exendin9–39 was measured in the GLP-1 RIA, which revealed no cross reactivity at concentrations up to 1 μM (data not shown).
DISCUSSION
Previous studies have shown that infusions of DPP-4 inhibitors decrease levels of total GLP-1 (9, 22), suggesting that the inhibition leads to a decrease in L-cell secretion, and it was hypothesized that a paracrine loop involving SS was a possible explanation (9). In support of the finding that SS restrains GLP-1 secretion (19), we found SS-positive cells with pronounced cytoplasmic elongations that could serve to increase the available area for paracrine regulation of neighboring cells as originally described for gastric SS cells (32). Furthermore, we confirmed the presence of GLP-1rs on the surface of D-cells, and, by combining autoradiography and immunohistochemistry, we were able to show clear SS binding to the surfaces of L-cells in ~70% of the GLP-1-positive cells. Based on this method, we cannot exclude that additional L-cells would bind SS to their surfaces since the mice were injected with I125 radiolabeled SS-28 and tissues fixed after either 5 or 10 min. SS-28 has a very short half-life of 2–3 min (42), and, therefore, the exposure time may have been insufficient, and some of the SS binding sites may have escaped detection.
In our investigation of the expression of the five different SSTrs, we found that the Sstr5 was enriched and had the highest expression in proglucagon-positive cells (L-cells) in the intestine compared with the negative cells. This observation was also verified by in situ hybridization, in which we observed that GLP-1-immunoreactive cells did indeed express Sstr5, whereas the expression of SSTr2 was much lower. Although we also found enrichment of SSTr1, this was not to the same extent as the SSTr5. Studies of SSTr1 have mainly focused on its role in certain types of cancers (36, 44, 59), and its role, if any, in GLP-1 secretion is unknown. An involvement of SSTr2 and SSTr5 in GLP-1 secretion has been suggested in a few studies. Thus, Chisholm and Greenberg (7) showed that an SSTr2-specific agonist was capable of decreasing GLP-1 secretion from rat primary cells in culture, although the effect was much less compared with that of an SSTr5 agonist. Moss et al. (37) also speculated that SSTr2 could be involved in regulating GLP-1 secretion, since they observed that adding an SSTr5-specific antagonist to primary small intestinal cells did not completely reverse the inhibitory effect of SS on GLP-1 secretion, suggesting a role for other receptors, such as SSTr2.
Functional effect of SS-14 and SS-28 and blocking of SSTr2 and SSTr5 in the perfusion model.
Since SS-14 and SS-28 have different potencies toward the SSTr5 in vitro (SS-28 being the most potent), we investigated whether this actually translated into differential effects on GLP-1 secretion in the perfused mouse intestine. We did see a minor difference at 0.1 nM, in which SS-28 had a greater effect to inhibit GLP-1 secretion, in line with the enriched expression of SSTr5 on L-cells. When we blocked the SSTr2 and SSTr5 by infusing subtype-specific antagonists, the two antagonists increased the secretion of GLP-1 in a dose-dependent manner, with SSTr5a clearly eliciting a greater stimulatory effect. This suggests, therefore, that GLP-1 secretion is under tonic restraint from SS, in line with previous studies (19, 20), and allows the conclusion that the tonic inhibition is mainly mediated through SSTr5 but that SSTr2 also plays a role.
Paracrine relationship between the L- and D-cell.
Infusion of the SSTr2 and SSTr5 antagonists could, in theory, reveal an autocrine effect of SS on the D-cell itself, potentially explaining their ability to increase SS secretion, also observed previously in the stomach (11). We detected binding of I-125-labeled SS on the surface of some D-cells, indicating expression of SSTrs. However, the in situ hybridization, using specific probes, showed that D-cells expressed low levels of both SSTr2 and SSTr5 mRNA, thereby arguing against SSTr2 or SSTr5 as primary target receptors for an autocrine loop on the intestinal D-cells. That it is, instead, GLP-1 that stimulates SS secretion was supported by the experiments in the perfused mouse intestine. In these studies, exogenous GLP-1 increased SS secretion, and an even greater increase was seen when exogenous GLP-1 was combined with SSTr5a (thereby eliminating the SS-mediated inhibition of GLP-1 release as well). Furthermore, blocking the GLP-1r with exendin-(9–39) completely terminated SS secretion, suggesting that activation of the GLP-1r on the D-cells is essential for the stimulation of SS secretion. Indeed, this also provides an explanation for the increase in endogenous GLP-1 release, which was associated with exendin-(9–39): lack of GLP-1 signaling to the D-cells reduces SS secretion, lifting the tonic suppression of the L-cell and allowing GLP-1 secretion to rise, again illustrating the powerful paracrine interaction between the L- and D-cells.
That SS is a powerful regulator of hormone secretion has already been exploited in the clinic today in the form of targeting the receptors with agonists, but the use of an antagonist has to our knowledge not been applied yet in physiologically relevant doses in the clinic. The antagonists have so far been applied as a diagnostic tool for certain types of gastroenteropancreatic neuroendocrine tumors in micro-dosing (38), which should not affect the endocrine systems. Agonists of the SS system are used in the treatment of acromegaly, Cushing disease and certain types of cancers (17, 18, 54). In particular, treatment with pasireotide (having high affinity for SSTr5) causes glucose disorders by decreasing insulin and/or GLP-1 secretion (21), which both may lead to hyperglycemia and diabetes, and it has been suggested that pasireotide may act directly on the pancreatic, insulin-secreting beta cells, in which Sstr5 has been found to be expressed (14, 21, 31). However the high expression of Sstr5 on the L-cell, as shown in this study, could also explain the observed decrease in GLP-1. This hypothesis is supported by a study by Liu et al. (33) showing that a specific SSTr5 antagonist alone and in combination with a DPP-4 inhibitor increased circulating GLP-1 levels and thus improved glucose-dependent insulin secretion. Whether antagonizing the pancreatic or the intestinal SSTr is more powerful is still unclear.
Concluding remarks.
In this study, we focused on characterizing the paracrine relationship between the L- and D-cell as well as elucidating the underlying molecular mechanism by combining a wide range of experimental techniques to corroborate our observations. A limitation of the study is the less-than-perfect selectivity of the SSTr2a, which may have influenced the results. An approach to circumvent the use of unspecific antagonists could, therefore, be to include SSTr subtype-specific knockout mice or search for more selective antagonists. Accordingly, although the unspecific properties of the SSTr2a preclude a definitive conclusion of the specific role of SSTr2, the data did show the following: 1) other SS receptors, in addition to SSTr5, to a greater or lesser extent, are involved in regulating GLP-1 secretion, 2) SSTr5 exhibits a strong tonic inhibition of L-cell secretion, and 3) the secretion of SS in the intestine is GLP-1r dependent.
GRANTS
The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unconditional grant from the Novo Nordisk Foundation to the University of Copenhagen (NNF10CC1016515).
DISCLOSURES
A. D. Christ and R. E. Martin (F. Hoffmann-La Roche Ltd.) hold a patent on the SSTr5 antagonist (Compound B), patent no. WO 2008019967A3.
AUTHOR CONTRIBUTIONS
S.L.J., N.J.W.A., J.P., C.F.D., and J.J.H. conceived and designed research; S.L.J., K.V.G., M.S.E., M.B.N.G., E.P.J., and C.Ø. performed experiments; S.L.J., K.V.G., M.S.E., M.B.N.G., E.P.J., C.Ø., S.S.P., M.M.R., F.M.G., F.R., and T.W.S. analyzed data; S.L.J., K.V.G., N.J.W.A., M.S.E., M.B.N.G., C.Ø., S.S.P., M.M.R., A.D.C., R.E.M., and J.J.H. interpreted results of experiments; S.L.J., K.V.G., M.B.N.G., S.S.P., and R.E.M. prepared figures; S.L.J., N.J.W.A., M.B.N.G., and E.P.J. drafted manuscript; S.L.J., K.V.G., N.J.W.A., M.S.E., M.B.N.G., E.P.J., C.Ø., S.S.P., M.M.R., J.P., F.M.G., F.R., C.F.D., T.W.S., A.D.C., R.E.M., and J.J.H. edited and revised manuscript; S.L.J., K.V.G., N.J.W.A., M.S.E., M.B.N.G., E.P.J., C.Ø., S.S.P., M.M.R., J.P., F.M.G., F.R., C.F.D., T.W.S., A.D.C., R.E.M., and J.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Barbara Thaysen (Novo Nordisk Foundation Center for Basic Metabolic Research) for excellent technical support and Mari Lilith Lund for graphic design of the expression data (Novo Nordisk Foundation Center for Basic Metabolic Research).
REFERENCES
- 1.Bak MJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK, Vilsbøll T, Jørgensen NB, Hartmann B, Deacon CF, Dragsted LO, Holst JJ. Specificity and sensitivity of commercially available assays for glucagon-like peptide-1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes Obes Metab 16: 1155–1164, 2014. doi: 10.1111/dom.12352. [DOI] [PubMed] [Google Scholar]
- 2.Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV. Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept 21: 151–166, 1988. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 3.Baldissera FG, Nielsen OV, Holst JJ. The intestinal mucosa preferentially releases somatostatin-28 in pigs. Regul Pept 11: 251–262, 1985. doi: 10.1016/0167-0115(85)90057-6. [DOI] [PubMed] [Google Scholar]
- 4.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker PL, Efendic S, Greenberg GR. Truncated and full-length glucagon-like peptide-1 (GLP-1) differentially stimulate intestinal somatostatin release. Endocrine 6: 91–95, 1997. doi: 10.1007/BF02738808. [DOI] [PubMed] [Google Scholar]
- 6.Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci USA 89: 11151–11155, 1992. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisholm C, Greenberg GR. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab 283: E311–E317, 2002. doi: 10.1152/ajpendo.00434.2001. [DOI] [PubMed] [Google Scholar]
- 8.Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother 14: 2047–2058, 2013. doi: 10.1517/14656566.2013.824966. [DOI] [PubMed] [Google Scholar]
- 9.Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. J Endocrinol 172: 355–362, 2002. doi: 10.1677/joe.0.1720355. [DOI] [PubMed] [Google Scholar]
- 10.Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer EM, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153: 5782–5795, 2012. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egerod KL, Engelstoft MS, Lund ML, Grunddal KV, Zhao M, Barir-Jensen D, Nygaard EB, Petersen N, Holst JJ, Schwartz TW. Transcriptional and functional characterization of the g protein-coupled receptor repertoire of gastric somatostatin cells. Endocrinology 156: 3909–3923, 2015. doi: 10.1210/EN.2015-1388. [DOI] [PubMed] [Google Scholar]
- 12.Eissele R, Koop H, Arnold R. Effect of glucagon-like peptide-1 on gastric somatostatin and gastrin secretion in the rat. Scand J Gastroenterol 25: 449–454, 1990. doi: 10.3109/00365529009095514. [DOI] [PubMed] [Google Scholar]
- 13.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2: 376–392, 2013. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagan SP, Azizzadeh A, Moldovan S, Ray MK, Adrian TE, Ding X, Coy DH, Brunicardi FC. Insulin secretion is inhibited by subtype five somatostatin receptor in the mouse. Surgery 124: 254–259, 1998. doi: 10.1016/S0039-6060(98)70128-X. [DOI] [PubMed] [Google Scholar]
- 15.Fam BC, Sgambellone R, Ruan Z, Proietto J, Andrikopoulos S. Contribution of the hypothalamus and gut to weight gain susceptibility and resistance in mice. J Endocrinol 225: 191–204, 2015. doi: 10.1530/JOE-15-0131. [DOI] [PubMed] [Google Scholar]
- 16.Gluschankof P, Morel A, Gomez S, Nicolas P, Fahy C, Cohen P. Enzymes processing somatostatin precursors: an Arg-Lys esteropeptidase from the rat brain cortex converting somatostatin-28 into somatostatin-14. Proc Natl Acad Sci USA 81: 6662–6666, 1984. doi: 10.1073/pnas.81.21.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon MB, Spiller KL. Pasireotide in an insulin-requiring diabetic acromegalic patient without worsening of hyperglycemia. Endocrinol Diabetes Metab Case Rep 2017: 17-0003, 2017. doi: 10.1530/EDM-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarnotta V, Pizzolanti G, Ciresi A, Giordano C. Insulin sensitivity and secretion and adipokine profile in patients with Cushing’s disease treated with pasireotide. J Endocrinol Invest 41: 1137–1147, 2018. doi: 10.1007/s40618-018-0839-7. [DOI] [PubMed] [Google Scholar]
- 19.Hansen L, Hartmann B, Bisgaard T, Mineo H, Jørgensen PN, Holst JJ. Somatostatin restrains the secretion of glucagon-like peptide-1 and -2 from isolated perfused porcine ileum. Am J Physiol Endocrinol Metab 278: E1010–E1018, 2000. doi: 10.1152/ajpendo.2000.278.6.E1010. [DOI] [PubMed] [Google Scholar]
- 20.Hansen L, Hartmann B, Mineo H, Holst JJ. Glucagon-like peptide-1 secretion is influenced by perfusate glucose concentration and by a feedback mechanism involving somatostatin in isolated perfused porcine ileum. Regul Pept 118: 11–18, 2004. doi: 10.1016/j.regpep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab 98: 3446–3453, 2013. doi: 10.1210/jc.2013-1771. [DOI] [PubMed] [Google Scholar]
- 22.Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de Lepeleire I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 91: 4612–4619, 2006. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 23.Heydorn A, Ward RJ, Jorgensen R, Rosenkilde MM, Frimurer TM, Milligan G, Kostenis E. Identification of a novel site within G protein alpha subunits important for specificity of receptor-G protein interaction. Mol Pharmacol 66: 250–259, 2004. doi: 10.1124/mol.66.2.250. [DOI] [PubMed] [Google Scholar]
- 24.Hilsted L, Holst JJ. On the accuracy of radioimmunological determination of somatostatin in plasma. Regul Pept 4: 13–31, 1982. doi: 10.1016/0167-0115(82)90105-7. [DOI] [PubMed] [Google Scholar]
- 25.Hocart SJ, Jain R, Murphy WA, Taylor JE, Coy DH. Highly potent cyclic disulfide antagonists of somatostatin. J Med Chem 42: 1863–1871, 1999. doi: 10.1021/jm9806289. [DOI] [PubMed] [Google Scholar]
- 26.Holst JJ. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J 207: 381–388, 1982. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol 308: F867–F877, 2015. doi: 10.1152/ajprenal.00527.2014. [DOI] [PubMed] [Google Scholar]
- 28.Jensen PC, Thiele S, Ulven T, Schwartz TW, Rosenkilde MM. Positive versus negative modulation of different endogenous chemokines for CC-chemokine receptor 1 by small molecule agonists through allosteric versus orthosteric binding. J Biol Chem 283: 23121–23128, 2008. doi: 10.1074/jbc.M803458200. [DOI] [PubMed] [Google Scholar]
- 29.Karlshøj S, Amarandi RM, Larsen O, Daugvilaite V, Steen A, Brvar M, Pui A, Frimurer TM, Ulven T, Rosenkilde MM. Molecular mechanism of action for allosteric modulators and agonists in CC-chemokine receptor 5 (CCR5). J Biol Chem 291: 26860–26874, 2016. doi: 10.1074/jbc.M116.740183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kissow H, Hartmann B, Holst JJ, Viby NE, Hansen LS, Rosenkilde MM, Hare KJ, Poulsen SS. Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept 179: 91–100, 2012. doi: 10.1016/j.regpep.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Kumar U, Sasi R, Suresh S, Patel A, Thangaraju M, Metrakos P, Patel SC, Patel YC. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48: 77–85, 1999. doi: 10.2337/diabetes.48.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science 205: 1393–1395, 1979. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Shao PP, Liang GB, Bawiec J, He J, Aster SD, Wu M, Chicchi G, Wang J, Tsao KL, Shang J, Salituro G, Zhou YP, Li C, Akiyama TE, Metzger DE, Murphy BA, Howard AD, Weber AE, Duffy JL. Discovery and pharmacology of a novel somatostatin subtype 5 (SSTR5) antagonist: synergy with DPP-4 inhibition. ACS Med Chem Lett 9: 1082–1087, 2018. doi: 10.1021/acsmedchemlett.8b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh CH. Gastrointestinal somatostatin: distribution, secretion and physiological significance. Life Sci 37: 2043–2058, 1985. doi: 10.1016/0024-3205(85)90576-4. [DOI] [PubMed] [Google Scholar]
- 36.Modarai SR, Opdenaker LM, Viswanathan V, Fields JZ, Boman BM. Somatostatin signaling via SSTR1 contributes to the quiescence of colon cancer stem cells. BMC Cancer 16: 941, 2016. doi: 10.1186/s12885-016-2969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss CE, Marsh WJ, Parker HE, Ogunnowo-Bada E, Riches CH, Habib AM, Evans ML, Gribble FM, Reimann F. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia 55: 3094–3103, 2012. doi: 10.1007/s00125-012-2663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J, Erlanger TE, Cathomas R, Christ E, Fani M, Wild D. Comparison of 68Ga-OPS202 68Ga-NODAGA-JR11 and 68Ga-DOTATOC 68Ga-Edotreotide PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: evaluation of sensitivity in a prospective phase ii imaging study. J Nucl Med 59: 915–921, 2017. doi: 10.2967/jnumed.117.199760. [DOI] [PubMed] [Google Scholar]
- 39.O’Carroll AM, Lolait SJ, König M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol 42: 939–946, 1992. [PubMed] [Google Scholar]
- 40.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 41.Patel YC, Greenwood M, Panetta R, Hukovic N, Grigorakis S, Robertson LA, Srikant CB. Molecular biology of somatostatin receptor subtypes. Metabolism 45, Suppl 1: 31–38, 1996. doi: 10.1016/S0026-0495(96)90076-1. [DOI] [PubMed] [Google Scholar]
- 42.Patel YC, Wheatley T. In vivo and in vitro plasma disappearance and metabolism of somatostatin-28 and somatostatin-14 in the rat. Endocrinology 112: 220–225, 1983. doi: 10.1210/endo-112-1-220. [DOI] [PubMed] [Google Scholar]
- 43.Patel YC, Wheatley T, Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology 109: 1943–1949, 1981. doi: 10.1210/endo-109-6-1943. [DOI] [PubMed] [Google Scholar]
- 44.Pedraza-Arévalo S, Hormaechea-Agulla D, Gómez-Gómez E, Requena MJ, Selth LA, Gahete MD, Castaño JP, Luque RM. Somatostatin receptor subtype 1 as a potential diagnostic marker and therapeutic target in prostate cancer. Prostate 77: 1499–1511, 2017. doi: 10.1002/pros.23426. [DOI] [PubMed] [Google Scholar]
- 45.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532–539, 2008. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rorsman P, Huising MO. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat Rev Endocrinol 14: 404–414, 2018. doi: 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept 155: 91–98, 2009. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 101: 1421–1430, 1998. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schonbrunn A, Tashjian H Jr. Characterization of functional receptors for somatostatin in rat pituitary cells in culture. J Biol Chem 253: 6473–6483, 1978. [PubMed] [Google Scholar]
- 50.Skak-Nielsen T, Holst JJ, Baldissera FG, Poulsen SS. Localization in the gastrointestinal tract of immunoreactive prosomatostatin. Regul Pept 19: 183–195, 1987. doi: 10.1016/0167-0115(87)90275-8. [DOI] [PubMed] [Google Scholar]
- 51.Sprecher U, Mohr P, Martin RE, Maerki HP, Sanchez RA, Binggeli A, Künnecke B, Christ AD. Novel, non-peptidic somatostatin receptor subtype 5 antagonists improve glucose tolerance in rodents. Regul Pept 159: 19–27, 2010. doi: 10.1016/j.regpep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Svendsen B, Pais R, Engelstoft MS, Milev NB, Richards P, Christiansen CB, Egerod KL, Jensen SM, Habib AM, Gribble FM, Schwartz TW, Reimann F, Holst JJ. GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J Endocrinol 228: 39–48, 2016. doi: 10.1530/JOE-15-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toräng S, Bojsen-Møller KN, Svane MS, Hartmann B, Rosenkilde MM, Madsbad S, Holst JJ. In vivo and in vitro degradation of peptide YY3-36 to inactive peptide YY3-34 in humans. Am J Physiol Regul Integr Comp Physiol 310: R866–R874, 2016. doi: 10.1152/ajpregu.00394.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vergès B. Effects of anti-somatostatin agents on glucose metabolism. Diabetes Metab 43: 411–415, 2017. doi: 10.1016/j.diabet.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Wang J, Liu D, Su Y. Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal Biochem 399: 211–217, 2010. doi: 10.1016/j.ab.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 56.Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA 89: 251–255, 1992. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, Kagimoto S, Seino M, Seino Y, Bell GI, Seino S. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol 6: 2136–2142, 1992. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]
- 58.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830, 2002. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhao J, Liang Q, Cheung KF, Kang W, Dong Y, Lung RW, Tong JH, To KF, Sung JJ, Yu J. Somatostatin receptor 1, a novel EBV-associated CpG hypermethylated gene, contributes to the pathogenesis of EBV-associated gastric cancer. Br J Cancer 108: 2557–2564, 2013. doi: 10.1038/bjc.2013.263. [DOI] [PMC free article] [PubMed] [Google Scholar]