Abstract
Introduction
Approximately the same percentage of male high school students in the United States currently uses conventional smokeless tobacco as smokes cigarettes, resulting in toxin exposure.
Methods
This study assessed tobacco product use (smokeless, combustible, and electronic cigarettes) and nicotine and carcinogen exposures in a sample of 594 male rural high school baseball players—a population traditionally at risk for smokeless tobacco use. Salivary specimens were assayed for cotinine (a biomarker of nicotine exposure) and urine specimens for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, a biomarker of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) using liquid chromatography–tandem mass spectrometry.
Results
The prevalence of past 30-day use of any tobacco product was 29%. Past 7-day smokeless tobacco use (prevalence: 13%) was associated with the highest levels of cotinine and NNAL observed in the sample, whether smokeless tobacco was used exclusively (geometric means: cotinine 11.1 ng/mL; NNAL 31.9 pg/mg-creatinine) or in combination with combustible products (geometric means: cotinine 31.6 ng/mL; NNAL 50.0 pg/mg creatinine). Cotinine and NNAL levels were incrementally higher in each increasing category of smokeless tobacco use frequency. However, observed levels were lower than previously reported for adults, likely reflecting less smokeless use per day among adolescents.
Conclusions
Based on these biomarker observations, adolescents who use conventional smokeless tobacco products are exposed to substantial levels of nicotine and NNK. Although exposed to lower levels than adult smokeless users, the findings are concerning given the young age of the sample and tendency for smokeless tobacco users to increase use intensity over time.
Implications
This study demonstrates that adolescents using smokeless tobacco are exposed to levels of nicotine and NNK that increase with use frequency and that exceed exposures among peers using other tobacco products. Youth smokeless tobacco use in the United States has not declined along with youth smoking prevalence, giving greater importance to this health concern. To reduce youth (and adult) exposures, needed actions include effective smokeless tobacco use prevention, potentially in combination with reducing the levels of harmful and potentially harmful chemicals in smokeless tobacco products currently popular among adolescents.
Introduction
Approximately 8%–9% of male high school students in the United States currently use conventional smokeless tobacco (oral moist snuff and chewing tobacco), nearly equal to the prevalence of cigarette smoking in this group.1,2 Smokeless tobacco use is associated with dental problems3 and with oral and pancreatic cancer,4,5 posing a significant health risk. Two potent carcinogens specific to tobacco and found in smokeless tobacco products are N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), believed to be among the primary causative agents in oral and lung cancer, respectively, when considering all types of tobacco.6
In January 2017, the U.S. Food and Drug Administration (FDA) proposed a tobacco product standard that would limit the amount of NNN in finished smokeless tobacco sold in the United States to no more than 1 μg/g tobacco (dry weight), estimating that such a restriction would prevent more than 12 000 cases of oral cancer over 20 years.7 While such a standard, if implemented, would presumably reduce health risks for smokeless tobacco users of all ages, younger populations, who have not yet accumulated substantial lifetime exposure, might particularly benefit. Higher risk groups for smokeless tobacco use include young, rural, White, and low-income males, especially those engaged in certain outdoor activities and sports, such as baseball.8,9 Existing studies have measured smokeless tobacco use behaviors among adolescents and young adults engaged in amateur and professional baseball,8,10,11 but none, to our knowledge, have assessed this high-risk population for actual exposure to potential carcinogens related to their smokeless tobacco use.
For measuring human carcinogen exposure related to tobacco use, the urinary NNK metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) serves as a biomarker of NNK exposure in humans.12,13 Compared to cotinine, a nicotine metabolite with a mean half-life of 16–24 hours,14 NNAL has a longer half-life of 10–16 days, making it sensitive to tobacco intake over the preceding month or longer.15 Relatively long half-life biomarkers may be of particular importance for assessing tobacco-related exposures among adolescents, who are less likely than adult tobacco users to consume tobacco products on a daily basis.16 While NNK is not thought to be a cause of oral cancer, levels of NNK and NNN in smokeless tobacco products are highly correlated, so that NNK can be viewed as an indicator of NNN exposure and related oral cancer risk.
In a previous pooled analysis of clinical studies, median levels of NNAL found in adult smokeless tobacco users exceeded those found among adult cigarette smokers.13 Similarly, using nationally representative data from the U.S. National Health and Nutrition Examination Survey (NHANES), NNAL levels were substantially higher among adult exclusive smokeless tobacco users compared to exclusive cigarette smokers.17 To our knowledge, no study has assessed NNK exposure (as measured by NNAL) among adolescents who use smokeless tobacco products, including in combination with nicotine assessment.
The goal of the present study is to evaluate urinary NNAL levels among rural male adolescents to provide insight into the levels of carcinogen exposure among young smokeless tobacco users, both compared to users of other tobacco products (e.g., combustible tobacco and e-cigarettes) and according to their reported frequency of smokeless tobacco use. Additionally, different types, brands, and varieties of smokeless tobacco differ in their levels of bioavailable nicotine, NNN, and, NNK.18–20 Therefore, as a hypothesis-generating exploratory aim, we examined differences in biomarker levels according to participants’ report of the characteristics of the smokeless tobacco products they used.
Methods
Participants
Study participants were male baseball players at rural California high schools, as detailed elsewhere.21 Baseball athletes were specifically recruited based on elevated levels of smokeless tobacco use in this population.10,22 Briefly, 36 high schools located in rural areas (county population density <1000 persons/square mile and municipality size <50 000 residents)23 were recruited through purposeful sampling over 3 years (2014–2016). During a school visit, baseball athletes completed confidential computer-based surveys to record their tobacco-related perceptions, attitudes, and behaviors.
All participants either returned signed parental consent and granted assent (ages 14–17) or provided self-consent (age 18). Approximately 1060 consent forms were distributed and 762 were returned (72%). Of returned forms, 642 (84%) indicated positive, active consent, and 594 students completed the in-person survey. An institutional review board at the University of California San Francisco approved all study procedures. Participants received $10 credit to an online retailer. Schools received $150–300 according to the percentage of returned consent forms, regardless whether parents provided or declined consent.
Survey Items
Questions were reproduced or slightly modified from existing national surveys.2,24 Representative images and brief descriptions were shown for seven tobacco products: cigarettes, cigars, e-cigarettes (including cigarette-like disposable, rechargeable, and larger refillable devices), waterpipe (hookah), snus, dissolvable tobacco, and conventional smokeless tobacco (moist snuff and chewing tobacco, listed in surveys as dip and chew, respectively). Dissolvable tobacco was excluded from analysis due to leaving the market and low reported use (past 30-day use: 0.3%). For each tobacco product, questions included: “Have you ever tried [tobacco product]?”; “During the past 30 days, on how many days did you use [tobacco product]?”; and “During the past 7 days, on how many days did you use [tobacco product]?” Individuals reporting past 30-day use of conventional smokeless tobacco were asked to indicate the type (moist snuff, chewing tobacco, or both), the “brand of smokeless tobacco you use most often” (choose from a list or “other”), and the flavor (if any) of the smokeless tobacco usually used (choose from a list or “other”).
Biomarker Collection and Analysis
Coincident with the survey, saliva and urine specimens were collected from each participant to measure cotinine and NNAL, respectively. Participants rinsed their mouths with water, chewed a small piece of wax to stimulate saliva, and provided approximately 3 mL of saliva into a barcode-labeled plastic screw-top vial. Participants each provided a mid-stream urine sample into a wide-mouth plastic cup, which study staff transferred to barcode-labeled vials. All saliva and urine samples were placed on ice and transported to the analysis facility within 48 hours. Valid samples were available for 589 (saliva) and 582 (urine) participants.
Laboratory Procedures
Saliva samples were analyzed for cotinine using liquid chromatography–tandem mass spectrometry (LC–MS/MS).25 Urine samples were analyzed for total (free plus conjugated) NNAL by LC–MS/MS.26 Values below the lower limit of quantification (LOQ, 1.0 ng/mL for cotinine and 0.25 pg/mL for NNAL) were assumed to be 0. Values were standardized for urine concentration by dividing by measured creatinine (mg/mL).
Categorizing Tobacco Product Use
Adolescent tobacco users are less likely than adults to use tobacco daily,16 but approximately 40%–50% of adolescent tobacco users use multiple tobacco product types,2,16 precluding simple categorization into “smokeless tobacco users” and “smokers.” Thus, we grouped participants to account for nondaily and dual or poly use. Based on reported tobacco use in the 7 days prior to the survey, we categorized participants as past 7-day nonusers, smokeless tobacco users (only moist snuff, chewing tobacco, and/or snus), combustible tobacco users (only cigarettes, cigars, and/or hookah), smokeless and combustible dual users (used both smokeless and combustible tobacco and e-cigarette users excluded), e-cigarette users (only e-cigarettes), and e-cigarette dual users (used e-cigarettes together with smokeless and/or combustible tobacco). As a secondary analysis, we created another version of 7-day use categories that accounted for reported tobacco use in the past 30 days: specifically, an “exclusive” smokeless tobacco category that omitted past 30-day users of any combustible tobacco, an exclusive combustible tobacco category that removed past 30-day users of any smokeless tobacco, and an exclusive e-cigarette category that removed past 30-day users of any combustible or smokeless tobacco. All categories excluded individuals with incomplete data for ≥1 tobacco product (n = 6).
We also created categories based on the frequency of smokeless use (including moist snuff, chewing tobacco, and/or snus) in the past 7 and 30 days: no use in the past 30 days, use in the past 30 days but not the past week, used 1–6 days in the past week, and use all 7 days in the past week. We examined these smokeless tobacco frequency categories both including and excluding past 30-day users of combustible tobacco and/or e-cigarettes.
Smokeless Tobacco Characteristics
To visualize the correlation between carcinogen and nicotine exposure, we plotted log-scale NNAL values against log-scale cotinine (for past 7-day smokeless tobacco users). We separately plotted different smokeless tobacco types (moist snuff, chewing tobacco, and snus), brands (Copenhagen vs. all other brands; Copenhagen was the preferred usual brand for 65% of the sample compared to no more than 10% for any other brand), and self-reported “usual” flavor used in the past 30 days (unflavored or tobacco flavor vs. mint, wintergreen, or fruit).
Statistical Analysis
Due to the asymmetric (nonnormal) distributions of cotinine and NNAL values, with a large number of observations near 0 and a smaller number of observations at higher values, descriptive statistics included the arithmetic mean, geometric mean, and median. Whereas the arithmetic mean is the sum of n numbers divided by n, the geometric mean is the nth root of the product of n numbers. Box plots values were displayed on the log scale. Differences were assessed according to past 7-day tobacco use categories using Kruskal–Wallis (multiple categories) and Mann–Whitney U (pairwise) tests, with p < .05 considered statistically significant. Differences by product characteristics were considered exploratory.
Results
Participant Characteristics
Among participants with complete survey and biomarker data (n = 583), the mean age was 15.8 years and about half (49%) identified as non-Hispanic White (Table 1). Past 7-day use of smokeless tobacco (13%) was more common than use of combustible tobacco (9%) or e-cigarettes (5%). Compared to the total sample, participants who used only smokeless tobacco in the past 7 days were older (mean age: 16.5 years) and more likely to identify as non-Hispanic White (65%); most (83%) had used moist snuff in the past week (Table 1).
Table 1.
Participant characteristics and recent tobacco behaviors by tobacco use group
| Tobacco use groupsa | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total n = 583b |
Nonuse n = 467 |
Smokeless n = 52 |
Combustible n = 20 |
Dual smokeless combustible n = 16 |
E-cigarettes n = 12 |
Mixed use n = 16 |
| Age, years (mean) | 15.8 | 15.7 | 16.5 | 16.0 | 16.3 | 14.8 | 16.3 |
| Race/ethnicity (%): | |||||||
| Non-Hispanic White | 49 | 46 | 65 | 35 | 56 | 58 | 63 |
| Hispanic/Latino | 40 | 41 | 29 | 45 | 38 | 33 | 25 |
| Other | 12 | 12 | 6 | 20 | 6 | 8 | 13 |
| Past 7-day use (%): | |||||||
| Any smokeless tobacco | 13 | 0 | 100 | 0 | 100 | 0 | 44 |
| Moist snuff | 11 | 0 | 83 | 0 | 81 | 0 | 38 |
| Chewing tobacco | 8 | 0 | 56 | 0 | 69 | 0 | 25 |
| Snus | 3 | 0 | 19 | 0 | 44 | 0 | 19 |
| Any combustible tobacco | 9 | 0 | 0 | 100 | 100 | 0 | 88 |
| Cigarettes | 2 | 0 | 0 | 20 | 19 | 0 | 19 |
| Cigars | 5 | 0 | 0 | 40 | 69 | 0 | 75 |
| Hookah | 4 | 0 | 0 | 60 | 50 | 0 | 38 |
| E-cigarettes | 5 | 0 | 0 | 0 | 0 | 100 | 100 |
| Daily use (%): | |||||||
| Any smokeless tobacco | 4 | 0 | 27 | 0 | 50 | 0 | 19 |
| Moist snuff | 4 | 0 | 25 | 0 | 50 | 0 | 19 |
| Chewing tobacco | 1 | 0 | 8 | 0 | 0 | 0 | 13 |
| Snus | 1 | 0 | 4 | 0 | 0 | 0 | 6 |
| Any combustible tobacco | <1 | 0 | 0 | 10 | 0 | 0 | 0 |
| Cigarettes | <1 | 0 | 0 | 5 | 0 | 0 | 0 |
| Cigars | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hookah | <1 | 0 | 0 | 5 | 0 | 0 | 0 |
| E-cigarettes | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
aDefined according to self-reported tobacco product use in the past 7 days (see text).
bSample limited to those with no missing data for past 7-day tobacco use and salivary and/or urine biomarker analysis.
Cotinine and NNAL Levels by Tobacco Product
Levels of salivary cotinine and urinary NNAL were lowest among past 7-day tobacco nonusers, e-cigarette users, and combustible tobacco users (Table 2). At least three-fourths of the e-cigarette use category and combustible tobacco use category. In each of these categories, three-fourths of all participants in the category had salivary cotinine levels below the quantification limit (Table 2), likely reflecting infrequent use of tobacco and nicotine products by the individuals in these categories (Table 1). Cotinine and NNAL levels were highest among past 7-day smokeless tobacco users and dual smokeless/combustible users (Table 2). All pairwise differences within the past 7-day use categories in cotinine and NNAL levels were each statistically significant (Mann–Whitney U-tests, p < .05) with the following exceptions: cotinine and NNAL levels were not statistically significantly different (p > .05) between tobacco nonusers and e-cigarette users or between e-cigarette users and combustible tobacco users; cotinine levels were not statistically significantly different between smokeless-only users and mixed users; and NNAL levels were not statistically significantly different between smokeless-only users and smokeless/combustible dual users.
Table 2.
Markers of nicotine and nitrosamine exposure by tobacco use group
| Salivary cotinine, ng/mL | Urinary NNAL, pg/mg creatinine | |||||||
|---|---|---|---|---|---|---|---|---|
| Past 7-day use categorya | N | Arithmetic mean (SD) | Median (IQR) | Geometricb,c mean (95% CI) | n | Arithmetic mean (SD) | Median (IQR) | Geometricb,c mean (95% CI) |
| Nonuse (past 7 days) | 467 | 0.69 (5.26) |
0 (0, 0) |
0.78 (0.74, 0.82) |
460 | 1.13 (8.65) |
0 (0, 0.32) |
0.23 (0.21, 0.26) |
| Nonuse (past 30 days) | 413 | 0.59 (4.58) |
0 (0, 0) |
0.77 (0.73, 0.81) |
409 | 0.97 (8.31) |
0 (0, 0.26) |
0.21 (0.19, 0.24) |
| Smokeless tobacco | 52 | 58.04 (119.9) |
12.97 (0, 70.06) |
9.35 (4.98, 17.57) |
52 | 110.9 (193.6) |
35.13 (5.90, 120.8) |
27.43 (16.08, 46.80) |
| Smokeless tobacco (exclusive)d | 46 | 64.67 (126.0) |
18.09 (0, 74.12) |
11.14 (5.64, 21.99) |
46 | 123.4 (202.6) |
41.54 (6.67, 157.7) |
31.92 (17.88, 57.01) |
| Combustible tobacco | 20 | 6.36 (18.91) |
0 (0, 0) |
1.21 (0.63, 2.33) |
20 | 5.29 (17.66) |
0.30 (0.17, 0.95) |
0.63 (0.28, 1.39) |
| Combustible tobacco (exclusive)d | 19 | 6.70 (19.37) |
0 (0, 0) |
1.24 (0.62, 2.49) |
19 | 5.55 (18.10) |
0.25 (0.15, 0.95) |
0.64 (0.28, 1.49) |
| Dual smokeless/combustible | 16 | 100.4 (104.7) |
69.88 (4.99, 181.4) |
31.58 (10.22, 97.54) |
16 | 183.6 (185.7) |
118.4 (11.33, 296.6) |
49.95 (13.63, 183.1) |
| E-cigarettes | 12 | 0.24 (0.85) |
0 (0, 0) |
0.80 (0.61, 1.03) |
12 | 1.72 (4.05) |
0.26 (0, 1.10) |
0.44 (0.16, 1.20) |
| E-cigarettes (exclusive)d | 9 | 0 (0) |
0 (0, 0) |
BLQ | 9 | 0.68 (0.95) |
0.32 (0, 1.09) |
0.36 (0.13, 1.00) |
| Mixed usee | 16 | 19.70 (35.88) |
3.09 (0, 21.39) |
4.24 (1.53, 11.70) |
16 | 30.0 (63.65) |
2.37 (1.04, 12.16) |
4.18 (1.38, 12.62) |
BLQ = below limit of quantification; CI = confidence interval; IQR = interquartile range; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; SD = standard deviation.
aReporting use ≥1 days in past 7 days of smokeless tobacco (moist snuff, chewing tobacco, and/or snus), combustible tobacco (cigarettes, cigars, and/or hookah), or e-cigarettes.
bGeometric mean defined as the nth root of the product of n observations.
cValues below limit of quantification imputed as the quantification limit divided by the square root of 2 (i.e., rather than 0) to allow calculation of a geometric mean that includes all observations.
dExclusive smokeless tobacco use in past 7 days excludes past 30-day combustible tobacco users; exclusive combustible tobacco use in past 7 days excludes past 30-day smokeless tobacco users; exclusive e-cigarette use excludes past 30-day smokeless and/or combustible tobacco users.
eAll remaining participants, which included e-cigarette use in combination with smokeless and/or combustible tobacco.
Cotinine and NNAL Levels by Smokeless Tobacco Use Frequency
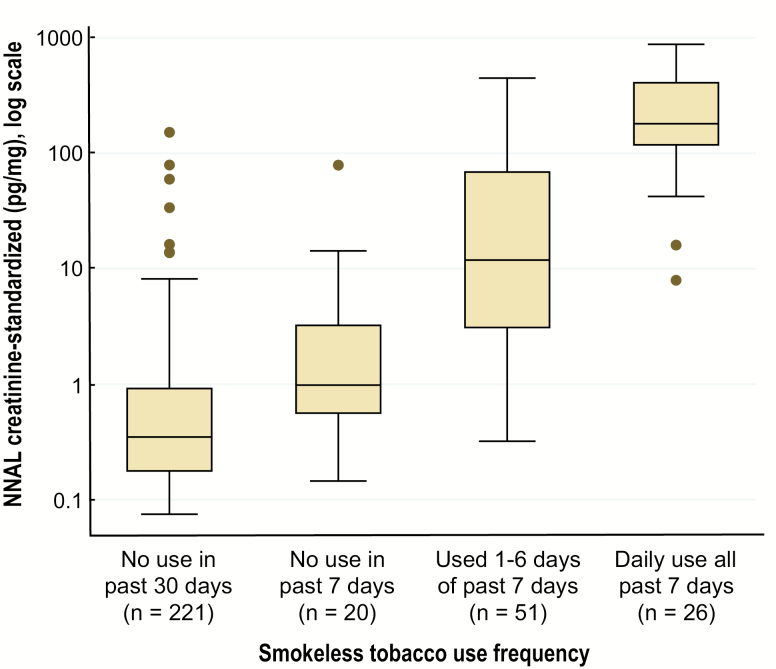
The majority of individuals who had not used smokeless tobacco in the past 30 days or the past 7 days had salivary cotinine levels below the quantification limit (Table 3), with no statistically significant difference in cotinine levels between these two groups. All other pairwise differences within the four smokeless tobacco use frequency categories in cotinine and NNAL levels were each statistically significant (Mann–Whitney U-tests, all p < .05), whether or not past 30-day users of other tobacco products were excluded from analysis. Levels of urinary NNAL were incrementally higher in each increasing level of smokeless tobacco use frequency (Table 3; Figure 1). Among participants who reported using smokeless tobacco daily, most (73%) reported using 2–5 “dips or chews” per day; the remainder reported using more.
Table 3.
Marker of nitrosamine exposure by smokeless tobacco use frequency
| Smokeless tobacco use categorya | Salivary cotinine, ng/mL | Urinary NNAL, pg/mg creatinine | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Arithmetic mean (SD) | Median (IQR) |
Geometricb,c mean (95% CI) | n | Arithmetic mean (SD) | Median (IQR) |
Geometricb,c mean (95% CI) | |
| All participants | ||||||||
| Smokeless nonuse (past 30 days) | 484 | 0.85 (5.88) |
0 (0, 0) |
0.80 (0.75, 0.84) |
478 | 1.14 (8.51) |
0 (0, 0.32) |
0.23 (0.21, 0.26) |
| Use in past 30 days (none in past 7) | 27 | 2.84 (12.56) |
0 (0, 0) |
0.97 (0.65, 1.44) |
26 | 4.83 (15.46) |
0.67 (0.15, 2.84) |
0.85 (0.43, 1.67) |
| Used 1–6 days of the past 7 days | 52 | 30.30 (54.29) |
3.60 (0, 31.20) |
5.75 (3.29, 10.04) |
52 | 50.45 (87.74) |
11.53 (3.08, 64.91) |
13.09 (7.87, 21.75) |
| Used daily over the past 7 days | 26 | 137.4 (154.8) |
127.3 (51.35, 169.5) |
71.12 (37.30, 135.6) |
26 | 272.7 (240.1) |
180.7 (118.3, 408.9) |
174.8 (111.6, 273.8) |
| Other tobacco users excludedd | ||||||||
| Smokeless nonuse (past 30 days) | 413 | 0.59 (4.58) |
0 (0, 0) |
0.77 (0.73, 0.81) |
409 | 0.97 (8.31) |
0 (0, 0.26) |
0.21 (0.19, 0.24) |
| Use in past 30 days (none in past 7) | 16 | 0.54 (2.15) |
0 (0, 0) |
0.83 (0.59, 1.15) |
15 | 1.25 (1.65) |
0.60 (0, 2.09) |
0.60 (0.28, 1.29) |
| Used 1–6 days of the past 7 days | 33 | 26.27 (43.15) |
3.24 (0, 30.89) |
5.11 (2.48, 10.50) |
33 | 54.01 (92.13) |
13.74 (3.41, 72.76) |
15.37 (8.23, 28.73) |
| Used daily over the past 7 days | 13 | 171.5 (196.6) |
140.7 (58.85, 176.1) |
91.97 (34.46, 245.5) |
13 | 296.4 (292.9) |
169.4 (100.5, 296.1) |
197.7 (113.0, 345.9) |
CI = confidence interval; IQR = interquartile range; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; SD = standard deviation.
aReported use of moist snuff, chewing tobacco, and/or snus.
bGeometric mean defined as the nth root of the product of n observations.
cValues below limit of quantification imputed as the quantification limit divided by the square root of 2 (i.e., rather than 0) to allow calculation of a geometric mean that includes all observations.
dExcludes individuals using combustible tobacco (cigarettes, cigars, and/or hookah) and/or e-cigarettes ≥1 day in the past 30 days.
Figure 1.
Nitrosamine (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol [NNAL]) exposure by smokeless tobacco use frequency. Box plots of urinary NNAL according to self-reported use frequency of smokeless tobacco (moist snuff, chewing tobacco, and/or snus). Box widths represent interquartile range on the logarithmic scale. Only plotted are values for individuals with measured NNAL levels above the lower limit of quantification (0.25 pg/mL urine), which excludes, by category: no use in the past 30 days (n = 257), no use in the past 7 days (n = 6), and used 1–6 days of the past 7 days (n = 1). Mean and median NNAL values that include these individuals (with assigned NNAL values of 0) are given in Table 3.
Cotinine and NNAL Levels by Smokeless Tobacco Characteristics
Among past 7-day smokeless tobacco users, urinary NNAL levels increased linearly with salivary cotinine levels on the log scale (Supplementary Figure). Log-scale cotinine and NNAL were strongly correlated (Pearson correlation: 0.74, p < .001; Spearman correlation: 0.76, p < .001). There were no statistically significant differences in NNAL or cotinine between subgroups defined by dual use with other tobacco products, type of smokeless tobacco used (i.e., combinations of moist snuff, chewing tobacco, or snus), or whether the product currently used was flavored (Supplementary Table); although not statistically significant, median values were higher among dual users, users of multiple types of smokeless tobacco, and users of flavored varieties. Individuals who reported that their usual brand was Copenhagen had higher levels of salivary cotinine (p = .02) and urinary NNAL (p = .05) than individuals reporting use of other smokeless tobacco brands (Supplementary Table).
Discussion
Use of conventional smokeless tobacco was more common and more frequent than use of combustible tobacco or e-cigarettes in this population of rural male baseball athletes. Those participants who used smokeless tobacco demonstrated levels of exposure to the tobacco-specific human carcinogen NNK (as measured by its metabolite, NNAL) that were higher than those observed among peers using other tobacco products and increased with the frequency of smokeless tobacco use. Comparisons between users of different tobacco product types and smokeless products of different characteristics should be cautiously interpreted due to sample size constraints. Nonetheless, exposures observed represent a health concern among adolescents who have been using smokeless tobacco for a relatively short duration of time and at lower intensity than typical for adults.
Levels of urinary NNAL observed among male adolescent past 7-day smokeless tobacco users in the present study (median: 181 pg/mg; interquartile range [IQR]: 118–409 pg/mg) exceeded those recently observed in a sample of urban male adolescent (age: 13–19) active cigarette smokers (median: 45 pg/mg; IQR: 39–55 pg/mg), in which active smoking was defined as urinary cotinine >30 ng/mL.27 Higher NNAL levels among smokeless users relative to same-aged cigarette smokers is consistent with a previous pooled analysis of clinical studies involving adult male smokeless users and cigarette smokers.13 However, the urinary NNAL levels observed among the adolescent daily smokeless tobacco users in the present study (geometric mean: 175 pg/mg) were lower than those reported in the pooled analysis of adult smokeless (geometric mean: 532 pg/mg) and cigarette (geometric mean: 488 pg/mg) users,13 as well as lower than NNAL levels reported for adults in NHANES (geometric mean smokeless: 583 pg/mg; geometric mean cigarettes: 218 pg/mg).17 Inclusion criteria for the adult clinical studies included using smokeless tobacco ≥6 dips/day13—a level of use intensity most adolescents in the present study did not reach. Thus, lower average NNAL levels among the adolescents likely relates to less frequent smokeless use throughout the day. In both adult and adolescent studies, NNAL and cotinine levels have been shown to be strongly correlated13,27 and, in the present study, adolescent smokeless users at the higher range of measured cotinine did reach NNAL levels comparable to the average values previously reported for adults.
In a different analysis of NHANES study data, an NNAL threshold of 34 pg/mL was proposed as a cutoff point to differentiate adult smokeless tobacco users from nonusers.28 NNAL values for most daily smokeless tobacco users in the present study exceeded that cutoff point (92%), but only 35% of adolescents using smokeless tobacco 1–6 days in the prior week had biomarker levels reaching the proposed threshold. Thus, lower thresholds should be considered for capturing nondaily smokeless tobacco use among younger populations.
Despite lower exposures than observed among adult tobacco users, the levels of urinary NNAL seen among male adolescents using smokeless tobacco represent a health concern. Even at low doses, NNK has been shown to induce tumors of the lung, pancreas, and nasal cavity in rats.6,29 Oral cavity tumors are induced in rats when NNK is administered together with NNN, another tobacco-specific nitrosamine (TSNA) found in smokeless tobacco products.30 The International Agency for Research on Cancer classifies smokeless tobacco as carcinogenic to humans and a cause of pancreatic and oral cancers.4 Additionally, the exposure levels observed in the present study may be prelude to higher exposures as use behaviors evolve throughout adulthood. Among male adult smokeless tobacco users, levels of cotinine and NNAL were found to correlate positively with duration of use: more experienced smokeless tobacco users exhibited greater exposure to nicotine and NNK, suggesting that individuals increase their use intensity, and subsequent TSNA exposure, over years of use.31
Our exploratory results suggest that there may be differences in TSNA exposure according to flavor, type, and brand used, but this hypothesis-generating finding should be examined in larger studies with more precise categorization of use behaviors and product characteristics. In this study, there were too few regular users of snus, brands other than Copenhagen, and unflavored products to draw firm conclusions. Previous analyses of the TSNA and nicotine content of commercially available smokeless tobacco products suggest extensive variation by type, brand, and variety.32,33 While several factors, including use frequency, must be considered, TSNA levels in the smokeless products used independently predict biological exposures in users.33 A clinical study that encouraged adult smokeless tobacco users to switch to sequentially lower nicotine- and TSNA-content brands demonstrated comparable proportionate reductions in measured cotinine and NNAL.34 This finding is notable in the case of adolescents, because industry documents describe positioning certain smokeless tobacco brands as “starter products” for novice and inexperienced users who are later graduated to higher-nicotine and higher-TSNA brands.35
While the proposed FDA product standard to limit NNN in finished smokeless tobacco did not set an allowable limit for NNK, the FDA noted the correlation between NNN and NNK in existing smokeless tobacco products and surmised that the proposed NNN standard, if implemented, would likely lead to lower NNK levels as well.7,20,36 If that correlation holds, such a standard would presumably yield a substantial reduction in both NNN and NNK exposure among adolescent and adult smokeless tobacco users. In making the case for its proposed standard, the FDA cited large reductions in NNN and NNK in snus products sold in Sweden following adoption of the GothiaTek voluntary manufacturing standard that set limits on combined NNN and NNK to 0.95 μg/g wet weight in Swedish snus.36 While not carcinogen free, a meta-analysis of epidemiological studies from Nordic countries suggests no oral cancer risk from use of low-nitrosamine snus products, unlike the findings of elevated oral cancer risk associated with smokeless tobacco products in the United States and Asia.37 After more than 2 years since issuing its proposed standard, the FDA has not publicly taken action toward implementation.
Participants in many high school sports, particularly boys playing baseball, are at elevated risk of smokeless tobacco use. A review of six studies found that high school athletes were at 60% greater odds of using smokeless tobacco than were nonathletes, despite lower risk of cigarette smoking.38 Young players and fans may view tobacco use by admired coaches and professional players as an implicit product endorsement.39 In the same population studied presently, high school baseball players who perceived that their favorite Major League Baseball (MLB) player used smokeless tobacco were more likely to use smokeless tobacco or be susceptible to future use themselves.40 After several U.S. cities passed local ordinances to ban tobacco use in their MLB stadia beginning in 2015, MLB implemented rules preventing players newly entering the league in 2017 or after from carrying or using smokeless tobacco on the field of play.41
A recent report highlights the global burden of smokeless tobacco use and documents the incomplete implementation and commitment to WHO Framework Convention on Tobacco Control measures for smokeless tobacco products relative to cigarettes.42 The report recommends that regulation be implemented to monitor and control the levels of toxicants in smokeless tobacco products, including setting an upper limit for TSNAs. Only six Framework Parties regulate the chemical composition of smokeless tobacco products.42 Relevant to youth, the report also recommends implementation of graphic warning labels, public education and use prevention campaigns, and comprehensive provisions to prevent the sale of smokeless tobacco products to minors.42
Several study limitations should be taken into consideration. This study population included only male rural high school baseball athletes from California. This population was selected because of expected high levels of smokeless tobacco use, but it is not necessarily representative of all rural adolescents, participants in other athletic activities, or other geographic regions. The relatively small number of individuals in some tobacco behavior categories did not allow for precise estimates of exposure in those groups. Also, in this population of athletes, use of combustible tobacco products and e-cigarettes was infrequent. Data were collected prior to the sharp rise in e-cigarette use seen in the United States from 2017 to 2018.43 The lower prevalence of use on nonsmokeless products limited the ability to compare exposure levels by product type. To compare exposure levels with more precision, larger, population-based studies, such as NHANES or the Population Assessment of Tobacco and Health Study, should consider adding biomarker assessments to adolescent study procedures.
Conclusions
The present study showed substantial exposure to nicotine and NNK among male adolescents who use conventional smokeless tobacco products. Although exposure levels were lower than those observed among adults, they represent a health concern, particularly given the tendency for smokeless tobacco users to increase use intensity over time. The present findings suggest that efforts to reduce the appeal of high-nicotine and high-nitrosamine smokeless tobacco brands to youth, as well as a product standard that sets limits for levels of known carcinogens in finished smokeless tobacco products, would have favorable public health implications for this population. However, successful prevention of tobacco use in all forms would have the greatest impact on reducing exposures.
Supplementary Material
Acknowledgments
The authors acknowledge the late Prof. Margaret Walsh, who played a central role in study planning and design. We also thank Victoria Campbell, Dr. Gwen Essex, Joanna Hill, Catherine Kavanagh, Jana Murray, Dr. Archnaa Rajasekaran, and Janelle Urata for their support in data collection, Lawrence Chan, Yvonne Lei, and Trisha Mao for LC–MS/MS analysis of biofluid samples, and Lisa Yu for supervising the analytical chemistry laboratory and preparation of reports.
Funding
This research received support from the US National Institutes of Health and Food and Drug Administration (P50CA180890 and U54HL180890) and the National Institutes of Health (NIH; KL2TR000143). Instrumentation and other analytical chemistry laboratory resources were supported by the National Institutes of Health (P30DA012393 and S10RR026437. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or FDA.
Declaration of Interests
N.L. Benowitz is a consultant/advisory board member for Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has served as a paid expert witness in litigation against tobacco companies. The other authors report no potential conflicts of interest.
References
- 1. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67(8):1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco product use among middle and high school students—United States, 2011–2017. MMWR Morb Mortal Wkly Rep. 2018;67(22):629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warnakulasuriya S, Dietrich T, Bornstein MM, et al. Oral health risks of tobacco use and effects of cessation. Int Dent J. 2010;60(1):7–30. [PubMed] [Google Scholar]
- 4. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2007;89:1–592. [PMC free article] [PubMed] [Google Scholar]
- 5. Wyss AB, Hashibe M, Lee YA, et al. Smokeless tobacco use and the risk of head and neck cancer: pooled analysis of US Studies in the INHANCE consortium. Am J Epidemiol. 2016;184(10):703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res. 2016;49(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food Drug Administration. Tobacco Product Standard for N-Nitrosonornicotine Level in Finished Smokeless Tobacco Products (proposed Rule). Washington, DC: FDA; 82 Federal Register 8004. Docket No. FDA-2016-N-2527; 2017. [Google Scholar]
- 8. Walsh MM, Hilton JF, Ernster VL, Masouredis CM, Grady DG. Prevalence, patterns, and correlates of spit tobacco use in a college athlete population. Addict Behav. 1994;19(4):411–427. [DOI] [PubMed] [Google Scholar]
- 9. Roberts ME, Doogan NJ, Stanton CA, et al. Rural versus urban use of traditional and emerging tobacco products in the United States, 2013-2014. Am J Public Health. 2017;107(10):1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Severson HH, Klein K, Lichtensein E, Kaufman N, Orleans CT. Smokeless tobacco use among professional baseball players: survey results, 1998 to 2003. Tob Control. 2005;14(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh MM, Ellison J, Hilton JF, Chesney M, Ernster VL. Spit (smokeless) tobacco use by high school baseball athletes in California. Tob Control. 2000;9 (suppl 2):Ii32–Ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1257–1261. [PubMed] [Google Scholar]
- 13. Hecht SS, Carmella SG, Murphy SE, et al. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1567–1572. [DOI] [PubMed] [Google Scholar]
- 14. Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 15. Goniewicz ML, Havel CM, Peng MW, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3421–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rostron BL, Chang CM, van Bemmel DM, Xia Y, Blount BC. Nicotine and toxicant exposure among U.S. smokeless tobacco users: Results from 1999 to 2012 National Health and Nutrition Examination Survey Data. Cancer Epidemiol Biomarkers Prev. 2015;24(12):1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richter P, Hodge K, Stanfill S, Zhang L, Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob Res. 2008;10(11):1645–1652. [DOI] [PubMed] [Google Scholar]
- 19. Stepanov I, Biener L, Knezevich A, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel marlboro and camel smokeless tobacco products: findings from round 1 of the new product watch. Nicotine Tob Res. 2012;14(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem Toxicol. 2013;57:380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaffee BW, Cheng J. Cigarette and smokeless tobacco perception differences of rural male youth. Tob Regul Sci. 2018;4(4):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gansky SA, Ellison JA, Kavanagh C, Isong U, Walsh MM. Patterns and correlates of spit tobacco use among high school males in rural California. J Public Health Dent.. 2009;69(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hewitt ME. Defining “Rural” Areas: Impact on Health Care Policy and Research. Washington, DC, USA: DIANE Publishing; 1989. [Google Scholar]
- 24. Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacob P III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(3–4):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob P III, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(21):8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benowitz NL, Nardone N, Jain S, et al. Comparison of urine 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol and cotinine for assessment of active and passive amoke exposure in Urban Adolescents. Cancer Epidemiol Biomarkers Prev. 2018;27(3):254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agaku IT, Vardavas CI, Connolly G. Proposed cutoff for identifying adult smokeless tobacco users with urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol: an aggregated analysis of NHANES 2007-2010 data. Nicotine Tob Res. 2013;15(11):1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):559–603. [DOI] [PubMed] [Google Scholar]
- 30. Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46(8):4162–4166. [PubMed] [Google Scholar]
- 31. Hecht SS, Carmella SG, Edmonds A, et al. Exposure to nicotine and a tobacco-specific carcinogen increase with duration of use of smokeless tobacco. Tob Control. 2008;17(2):128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8(2):309–313. [DOI] [PubMed] [Google Scholar]
- 33. Hatsukami DK, Stepanov I, Severson H, et al. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev Res (Phila). 2015;8(1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hatsukami DK, Ebbert JO, Anderson A, Lin H, Le C, Hecht SS. Smokeless tobacco brand switching: a means to reduce toxicant exposure? Drug Alcohol Depend. 2007;87(2–3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Connolly GN. The marketing of nicotine addiction by one oral snuff manufacturer. Tob Control. 1995;4(1):73–79. [Google Scholar]
- 36. Osterdahl BG, Jansson C, Paccou A. Decreased levels of tobacco-specific N-nitrosamines in moist snuff on the Swedish market. J Agric Food Chem. 2004;52(16):5085–5088. [DOI] [PubMed] [Google Scholar]
- 37. Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9(7):667–675. [DOI] [PubMed] [Google Scholar]
- 38. Diehl K, Thiel A, Zipfel S, Mayer J, Litaker DG, Schneider S. How healthy is the behavior of young athletes? A systematic literature review and meta-analyses. J Sports Sci Med. 2012;11(2):201–220. [PMC free article] [PubMed] [Google Scholar]
- 39. Connolly GN, Orleans CT, Blum A. Snuffing tobacco out of sport. Am J Public Health. 1992;82(3):351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaffee BW, Couch ET, Gansky SA. Adolescents’ smokeless tobacco susceptibility by perceived professional baseball players’ use. J Public Health Dent. 2018;78(1):5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lacques G. Can Major League Baseball really rid itself of smokeless tobacco? USA Today December 1, 2016. https://www.usatoday.com/story/sports/mlb/2016/12/01/baseball-smokeless-tobacco-cba-mlb/94721720/. Accessed June 28, 2019.
- 42. Mehrotra R, Yadav A, Sinha DN, et al. Smokeless tobacco control in 180 countries across the globe: call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019;20(4):e208–e217. [DOI] [PubMed] [Google Scholar]
- 43. Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: tobacco product use among middle and high school students—United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2019;68(6):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.