Abstract
Previous research has shown a link between eye contact and interpersonal motor resonance, indicating that the mirroring of observed movements is enhanced when accompanied with mutual eye contact between actor and observer. Here, we further explored the role of eye contact within a naturalistic two-person action context. Twenty-two participants observed simple hand movements combined with direct or averted gaze presented via a live model in a two-person setting or via video recordings, while transcranial magnetic stimulation was applied over the primary motor cortex (M1) to measure changes in M1 excitability. Skin conductance responses and gaze behavior were also measured to investigate the role of arousal and visual attention herein. Eye contact significantly enhanced excitability of the observer’s M1 during movement observation within a two-person setting. Notably, participants with higher social responsiveness (Social Communication subscale of the Social Responsiveness Scale) displayed a more pronounced modulation of M1 excitability by eye gaze. Gaze-related modulations in M1 excitability were, however, not associated with differences in visual attention or autonomic arousal. In summary, the current study highlights the effectiveness and feasibility of adopting paradigms with high ecological validity for studying the modulation of mirror system processes by subtle social cues, such as eye gaze.
Keywords: action observation, eye contact, mirror neuron system, skin conductance, transcranial magnetic stimulation (TMS)
Introduction
In humans, observation of others’ actions has been shown to activate similar brain regions as those involved when executing that action. At the basis of this mechanism are neural cells, known as ‘mirror neurons’, which respond to action observation, imagination and execution (Cattaneo & Rizzolatti, 2009). By means of several neuroimaging techniques, distinct frontal and parietal regions in the human brain (i.e. the inferior frontal gyrus, inferior parietal lobule and the ventral premotor cortex) have been identified with mirror-like properties (Caspers et al., 2010; Molenberghs et al., 2012), together constituting the action observation network or ‘mirror system’.
According to the embodied simulation account (Gallese & Sinigaglia, 2011), the automatic simulation of observed actions in the mind of the observer, a process also known as ‘mirror-motor mapping’ or ‘interpersonal motor resonance’, is anticipated to constitute the core neural mechanism for recognizing and understanding others’ actions, intentions and emotional states (but see De Bruin & Gallagher, 2012; Press & Cook, 2015; Schilbach, 2010 for critical appraisals of a pure mirror neuron account of social cognition). In this view, the human mirror system plays an important role in facilitating everyday social functioning in general, and interpersonal reciprocity during social interactions in specific, by synchronizing the own behavior to others’ actions (Feldman, 2017).
One of the most salient cues to initiate interpersonal synchrony is eye contact. Indeed, previous research showed that observed gaze cues form an important modulator of autonomic mimicry and interpersonal motor resonance. Wang et al. (2011a) studied the effect of perceived eye contact on automatic motor mimicry using a stimulus-response compatibility paradigm, in which participants were asked to perform the same movement or the opposite movement as viewed in a video clip. A clear congruency effect was found, indicating that responses were significantly faster when the same movement was performed, compared to trials in which the opposite hand movement was performed. Notably, it was shown that this mimicry congruency effect was further enlarged when direct eye contact was established between the observer and the observed actor.
These initial behavioral observations were recently extended by our laboratory by investigating the neurophysiological mechanism underlying the enhancing effect of eye gaze on automatic motor simulation, using transcranial magnetic stimulation (TMS) (Prinsen et al., 2017; Prinsen et al., 2018). Transcranial magnetic stimulation is a noninvasive method for stimulating cortical neurons via the administration of a brief magnetic pulse to the scalp. A single TMS pulse delivered to the somatotopically organized primary motor cortex (M1) produces a muscle contraction or motor-evoked potential (MEP) in the corresponding peripheral muscles, as measured with electromyography (EMG). Importantly, Fadiga et al. (1995) demonstrated that when TMS is applied to M1 during the mere observation of others’ actions, MEP amplitudes within the stimulated muscles are enhanced, indicating a muscle-specific and observation-induced facilitation of corticospinal excitability at the level of M1. Adopting this TMS technique, we have demonstrated that MEP amplitudes are significantly enhanced when participants observe actions accompanied with direct versus averted gaze from the model, indicating increased observation-induced M1 excitability upon mutual eye contact (Prinsen et al., 2017, 2018).
Previous mirror system research predominantly adopted video presentations of dynamic (i.e. involving biological motion to recruit mirror system regions) but simplified action contexts, such as point light displays (e.g. Ulloa & Pineda, 2007) or videos of isolated limb movements (e.g. Alaerts et al., 2009a). This allows for a strict control over the stimuli and accurate time locking of behavioral (e.g. mimicry) or neurophysiological (e.g. M1 excitability) responses. While these screen-based paradigms have been highly instrumental in uncovering several important properties of the human action observation network, the gap between the highly controlled laboratory setting and everyday social interactions remains high. Indeed, screen-based presentations have been argued to lack the ‘richness’ of real life aspects of social interactions, which may be of particular relevance when examining the effect of salient social cues such as eye contact (Reader & Holmes, 2016; Risko et al., 2012). Consequently, researchers have advocated for a more naturalistic, second-person approach to investigate social cognition and its neurobiological bases (Schilbach et al., 2013).
To date, however, studies investigating interpersonal motor resonance upon observation of real life versus videotaped motor actions are sparse. One previous magnetoencephalography (MEG) study by Järveläinen et al. (2001) reported significantly stronger M1 activity upon the observation of live versus videotaped hand movements, thereby underlining the relevance of adopting naturalistic action contexts in mirror system research. With the present study, we specifically aim to further address these concerns about ecological validity by re-exploring the enhancing effect of perceived eye contact on M1 excitability (as conveyed with video recordings in previous studies) when gaze and movement cues are presented in a naturalistic two-person action context (as suggested by Reader & Holmes, 2016). To do so, TMS was applied and MEPs were recorded while participants observed a live or videotaped model performing a simple index finger movement accompanied by direct or averted gaze. In line with Järveläinen et al. (2001), we hypothesized naturalistic gaze cues to be more salient for facilitating M1 excitability compared to video presentations. Furthermore, and in accordance to previous mirror system research adopting TMS (Fadiga et al., 1995; Strafella & Paus, 2000), we expected the facilitatory effect of eye gaze on M1 excitability to be specific to the muscle implicated in the observed movement.
In addition to the TMS-based assessments, the current study also performed measurements of skin conductance responses (SCRs) to assess whether gaze-related effects on interpersonal motor resonance are potentially modulated by variations in autonomic arousal. Indeed, research has shown that—especially within ecologically salient social contexts—the experienced level of arousal can influence action readiness and motor flexibility (for a review, see Frijda, 2010). Furthermore, a series of previous studies robustly demonstrated that perceived direct gaze from a live model induces significantly higher states of arousal (Helminen et al., 2011; Hietanen et al., 2008; Pönkänen et al., 2011). Considering that a similar live action context is adopted in the current study, the combined assessment of SCRs allowed to re-explore the effect of eye contact on autonomic arousal, as well as to examine for the first time whether eye contact–induced changes in M1 excitability are related to eye contact–induced changes in autonomic arousal.
Finally, considering the hypothesized link between motor resonance and high-level social skills (Iacoboni & Dapretto, 2006), we aimed to explore the association between gaze-related modulations in the recorded measures and a self-report measure of social responsiveness (Social Responsiveness Scale [SRS]; Constantino & Todd, 2005).
Materials and methods
Participants
A total of 23 right-handed individuals (8 men and 15 women) aged between 21 and 30 years (mean ± SD: 25;3 ± 2;5 years;months) participated in this study. Right-hand dominance was assessed with the Edinburgh Handedness Questionnaire (Oldfield, 1971). Exclusion criteria comprised medication use, any diagnosed psychiatric (e.g. autism, attention-deficit/hyperactivity disorder) or neurological disorder (e.g. stroke, epilepsy, concussion), left handedness or any contraindication to TMS (Rossi et al., 2012). Written informed consent was obtained from all participants. Consent forms and study design were approved by the Ethics Committee for Biomedical Research at the University of Leuven in accordance to the Declaration of Helsinki (World Medical Association, 2013). One female subject did not complete the full experimental procedure because of intolerability to TMS and was excluded from the analyses.
Experimental procedure and stimuli
Prior to the experimental procedure, subjects completed the Dutch self-report version of the SRS for adults (Constantino & Todd, 2005). The SRS is a widely used screening tool to identify the presence and extent of any social impairments using a four-point Likert scale. The Dutch version (Noens et al., 2012) consists of 64 items encompassing four subscales: Social Awareness, Social Communication, Social Motivation and Rigidity/Repetitiveness. Higher scores indicate more social impairments.
Afterward, participants were seated at a distance of approximately 80 cm from a computer screen or panel and were instructed to observe and pay close attention to the presented stimuli and to stay as motionless and relaxed as possible. The presented stimuli comprised the face of a female experimenter (J.P.) gazing either toward the participant (i.e. showing direct gaze and engaging in mutual eye contact) or gazing 30° to the right (i.e. showing averted gaze). In half of the trials, the model performed a simple index finger abduction movement (movement observation condition). In the other half of the trials, only the gaze cues were conveyed, without hand movements (control condition).
Stimuli were presented to the observing participant in two separate modes: by means of a live model or by means of videos of the same model presented on a computer screen (Fig. 1A). In the live condition, the stimulus person’s face was presented through a 20 × 30-cm voltage-sensitive liquid crystal (LC) shutter screen (DreamGlass Group, Spain) attached to a black frame between the stimulus person and the participant (similar setup as used by Hietanen et al., 2008). The stimulus person was sitting at a distance of 15 cm from the panel. Signal software (version 6.02; Cambridge Electronic Design, UK) was used to trigger the LC window to shift from an opaque to a transparent state within milliseconds. In the video condition, videos (frame rate 29 Hz) of the previously described stimuli were displayed on a widescreen computer screen (resolution 1920 × 1080 pixels, refresh frequency 60 Hz). Video presentation timing was controlled by LabVIEW software (version 14.0; National Instruments, UK) and triggered by Signal software. The order of the presentation mode (live, video) was counterbalanced across participants. An illustration of the stimuli is provided in Fig. 1B.
Fig. 1.
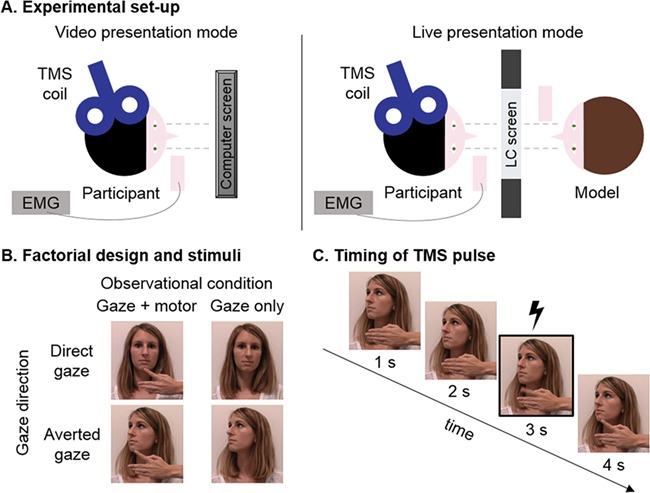
(A) Schematic overview of the experimental set-up in the video (left panel) and live (right panel) presentation mode. (B) Factorial design and stimuli showing the stimulus person (experimenter J.P.) engaging in mutual eye contact (direct gaze) or not (averted gaze) while performing either a simple finger abduction movement or no movement. The last still of each condition is depicted. (C) Single-pulse TMS was delivered 3 seconds after the onset of each video clip (in the video presentation mode) or after the opening of the LC screen (in the live presentation mode), which corresponded to the execution phase of the observed movement.
Per presentation mode, each of the four conditions was presented five times in blocks of four (i.e. total of 20 trials per condition for each presentation mode). This resulted in a 2 × 2 × 2 factorial design with the factors ‘presentation mode’ (live or video), ‘observed movement’ (present or not) and ‘observed gaze direction’ (direct or averted). Presentation order of blocks was pseudo-random (no more than three consecutive blocks of the same type). The duration of a single trial in each presentation format was 4 seconds. The interstimulus interval between trials was 2 seconds, during which the shutter remained opaque and the computer screen had a black background.
During all trials, single-pulse TMS was administered, and dependent measures of M1 excitability (i.e. MEPs) were recorded via EMG. Simultaneous recordings of autonomic arousal were performed by assessing SCRs. Additionally, eye tracking was performed to ascertain that participants were attentive toward the presented stimuli.
Electromyography recordings and TMS
During observation of the experimental stimuli, single-pulse TMS (Magstim-200 stimulator; Magstim Company Ltd, UK) was applied over the left primary motor cortex (M1) using a handheld 70-mm figure-of-eight coil. The coil was positioned over left M1, tangentially to the scalp and 45° away from the midsagittal line. Optimal coil location for TMS stimulation was determined as the site that produced maximal responses while at rest (‘hotspotting’) in the contralateral first dorsal interosseous (FDI) muscle, a muscle implicated in the to-be-observed finger movement. Motor-evoked potentials were also collected from the abductor minimi digiti (ADM), which is not implicated in the movement and therefore serves as a control muscle. Although parameter setting procedures were prioritized for the FDI, they are assumed to be effective for assessing condition-specific modulations simultaneously for both muscles due to overlapping hand muscle representations in M1 (Gentner & Classen, 2006; Krings et al., 1998). Motor-evoked potentials were measured by means of surface EMG recorded from electrodes attached to the muscle bellies of the investigated muscles, with referential electrodes attached at the wrist.
Experimental stimulation intensity was defined according to the resting motor threshold (rMT) for each participant, i.e. the lowest stimulation intensity that produced a peak-to-peak MEP of at least 50 μV in 5 of 10 consecutive trials (Rossini et al., 1994), and set at a suprathreshold of 130% of the subject’s rMT. In both presentation modes, a single TMS pulse was delivered on the third second of stimulus presentation (with 6-second interpulse interval), which coincided with the execution of the index finger abduction movement of the model (Fig. 1C). Electromyography recordings were sampled (2000 Hz), amplified and bandpass filtered (5–1000 Hz) via a CED Power 1401 analog-to-digital converting unit (Cambridge Electronic Design) and stored on a PC for offline analysis. Signal software was used for EMG recordings and triggering of the TMS stimulator.
Based on the recorded EMG data, peak-to-peak amplitudes of the TMS-evoked MEPs were determined to assess condition-induced changes in M1 excitability. Additionally, background EMG was quantified by calculating the root mean square across the 110- to 10-millisecond interval prior to TMS stimulation to ensure that subjects were completely relaxed during stimulation. Trials with excessive tonic muscle activity prior to TMS stimulation (i.e. background EMG > mean ± 2.5 SD) were not included in the final analysis. Further, extreme MEP amplitudes (exceeding 1.5 interquartile distance from mean) were removed from the analysis. This procedure omitted 11.42% of all trials for FDI and 10.43% of all trials for the ADM.
Skin conductance recordings
The Nexus-32 multimodal acquisition system and BioTrace+ software (version 2015a; Mind Media, the Netherlands) were used to collect stimuli-specific SCRs with a sampling rate of 128 Hz. Two Ag/AgCl Velcro snap-on electrodes were attached to the palmar surface of the distal phalanxes of the index and middle fingers on the participant’s nondominant hand. Before electrode attachment, the skin was prepared with TD-246 Skin Conductance Electrode Paste (0.5% saline in a neutral base).
Typically, stimuli-specific SCRs are characterized by a rise from initial level to a peak within 1 to 4 seconds after stimulus onset, followed by a relatively long recovery period of 20 to 30 seconds (Boucsein et al., 2012). Since our experimental design was optimized to measure TMS-evoked MEP responses, incorporating a short ISI of 2 seconds, superimposed SCRs were likely to be observed within blocks. Therefore, only SCRs to the first stimulus of a block were analyzed. A standard peak-detection method was used, defining the SCR as the maximum change in amplitude relative to baseline during the 4 seconds after the start of stimulus onset. The baseline was determined as the average of the 2 seconds right before stimulus onset. Maximum changes from baseline amplitude scores below 0 μS were set to zero. The SCR data of one participant were not recorded (video session only) because of the malfunctioning of the acquisition hardware.
Eye tracking
Gaze behavior was recorded with a sampling rate of 30 Hz by means of head-mounted SMI eye tracking glasses and SMI iView acquisition software (SensoMotoric Instruments, Germany). The glasses were adjusted to the participant’s comfort, and a three-point calibration procedure was performed before recording. The ‘semantic gaze mapping’ procedure incorporated in SMI BeGaze analysis software (version 3.0; SensoMotoric Instruments) was used, whereby fixations were mapped into a prespecified areas of interest (AOIs) positioned over the eye region of the stimulus person. For each condition, the number of fixations and the average gaze time (in milliseconds) per fixation toward the eye AOI were assessed across trials.
Data analysis and statistics
All statistics were calculated with Statistica 10 (StatSoft, USA), and results were considered significant with a P value lower than 0.05. To investigate condition-specific modulations of M1 excitability, a four-way repeated-measures analysis of variance (RM-ANOVA) with the within-subject factors ‘muscle’ (experimental FDI, control ADM), ‘presentation mode’ (video, live), ‘observed gaze’ (averted, direct) and ‘observation condition’ (movement, no movement) was performed on the recorded MEPs. Significant effects were further investigated by means of Fisher least significant difference (LSD) post hoc tests. The partial η2 value was calculated as an estimate of effect size. Similar RM-ANOVAs were conducted for the other outcome measures (SCRs and gaze time) and were adapted when necessary (e.g. no inclusion of the ‘muscle’ factor in these analyses).
In order to explore the potential relationship between the effect of eye contact on M1 excitability (MEPs) and autonomic arousal (SCRs), a Pearson correlation analysis was performed. To capture the ‘eye contact effect’, the difference between responses recorded during the direct and averted gaze conditions was calculated (i.e. MEP amplitudedirect gaze − MEP amplitudeaverted gaze and SCR amplitudedirect gaze − SCR amplitudeaverted gaze difference score), with positive scores indicating a higher MEP or SC response during the direct versus the averted gaze condition. Pearson correlation coefficients were also calculated between the eye contact effect on MEPs/SCRs and the (sub) scores of the SRS questionnaire. However, to restrict the number of performed correlations, difference scores and Pearson correlation coefficients were computed only for those conditions in which a significant eye contact effect was encountered. For all performed correlations, Cook’s distance metric was used to identify influential outliers, but none were detected (all Cook’s D < 0.86).
Results
The effect of eye contact on M1 excitability (TMS-induced MEPs)
A four-way RM-ANOVA was performed. The mean MEP peak-to-peak amplitude values for each condition and muscle are presented in Fig. 2. The analysis demonstrated a significant main effect of observed eye gaze [F(1,21) = 10.97, P = 0.003, η2 = 0.34], indicating that, on average, MEP responses were higher during conditions with direct eye gaze, compared to conditions with averted gaze. However, the identification of a significant four-way interaction of perceived eye gaze with the factors ‘muscle’, ‘presentation mode’ and ‘movement condition’ indicated that the effect was modulated by these factors (F(1,21) = 4.33, P = 0.04, η2 = 0.17).
Fig. 2.

The effect of perceived eye gaze (direct, averted) and presentation mode (live, video) on MEP peak-to-peak amplitude, per observational condition (upper panels: gaze and motor cues; lower panels: gaze cues only) and muscle (left panels: experimental FDI muscle; right panels: control ADM muscle). **P < 0.001, error bars denote mean ± SE.
Indeed, post hoc investigations of this four-way interaction, directly assessing the effect of eye gaze separately for each muscle and movement condition, showed that, for MEPs recorded from the target FDI muscle, the effect of eye gaze was most pronounced during the observation of movements performed by the live stimulus person (Fisher LSD: P < 0.001). The effect was only evident at trend level during the video presentation mode (P = 0.07), and absent when no movement was observed (live: P = 0.11; video: P = 0.41). As expected, further post hoc explorations showed no significant effects of observed eye gaze for MEPs recorded from the control ADM muscle, for any of the four observational conditions (Fisher LSD: all P > 0.19).
Also, a significant main effect of presentation mode [F(1,21) = 5.77, P = 0.03, η2 = 0.22], as well as a significant presentation mode by muscle interaction [F(1,21) = 7.48, P = 0.01, η2 = 0.27] were encountered. Post hoc investigation of this two-way interaction showed that, for the FDI muscle, MEPs were generally higher during presentation of the live model than during the video presentations (irrespective of eye gaze or movement observation condition; Fisher LSD: P < 0.001), whereas for the ADM muscle, MEPs were on average not significantly modulated by presentation mode (P = 0.70).
The overall ANOVA model also revealed a significant main effect of muscle [F(1, 21) = 13.98, P = 0.001, η2 = 0.40] indicating that on average, MEP responses recorded from the FDI muscle were larger compared to MEP responses recorded from the ADM muscle. The main effect of observed movement was however not significant [F(1,21) = 0.17, P = 0.68, η2 = 0.008], nor was any of the two- or three-way interactions with this factor (all P > 0.42).
Together, these results indicate that observed eye contact significantly augmented M1 excitability upon movement observation, especially when eye contact was conveyed by a live model. Furthermore, these effects were specific for the muscle that was implicated in the observed index finger movement (i.e. the FDI muscle of the index finger).
The effect of eye contact on autonomic arousal (SCRs)
Similar to the analysis on TMS-induced MEPs, we performed a three-way RM-ANOVA on the recorded SCRs (note that there is no muscle factor for the SC data). The analysis revealed a significant main effect of eye gaze [F(1,20) = 16.39, P < 0.001, η2 = 0.45] and presentation mode [F(1,20) = 7.94, P = 0.01, η2 = 0.28], as well as a two-way interaction between these two factors [F(1,20) = 5.24, P = 0.03, η2 = 0.21]. Post hoc tests showed that although direct eye contact generally yielded higher SCRs, the differential response was more pronounced when gaze cues were conveyed by the live model (Fisher LSD: P < 0.001), compared to video presentations (P = 0.23). The main and interaction effects of the ‘observed movement’ factor were not significant (all P > 0.17). Fig. 3A shows the average SC response magnitude as a function of gaze direction and presentation mode.
Fig. 3.

(A) Magnitude of the average skin conductance response per presentation mode and observed gaze direction (averaged across movement observation conditions). **P < 0.001, error bars denote mean ± SE. (B) The relationship between the eye contact effect on M1 excitability and the eye contact effect on autonomic arousal was not significant. (C) Opposite modulatory effects were noted for the association between the SRS Social Communication score (higher scores denote more impairments) and the eye contact effect on M1 excitability (left panel; negative correlation) and autonomic arousal (right panel; positive correlation). Dotted lines denote 95% CI.
Gaze behavior
Eye tracking was performed to ascertain that participants were attentive toward the presented stimuli. A RM-ANOVA with within-subject factors presentation mode, observed gaze direction and observation condition showed no significant main or interaction effects (all P > 0.06) for the average fixation duration data, indicating that the average gaze time per fixation toward the eye region of the stimulus person’s face was overall similar across conditions. A similar RM-ANOVA on the fixation count data revealed a significant main effect of observed gaze [F(1,21) = 9.27, P = 0.006, η2 = 0.31] and movement condition [F(1,21) = 19.79, P < 0.001, η2 = 0.49], indicating that participants made more eye movements toward the eyes AOI during direct gaze conditions and during conditions in which only the gaze cues were conveyed (without the inclusion of a hand movement) (Fig. 4).
Fig. 4.

Effect of observed gaze direction and presentation mode on average fixation duration (upper panels) and number of fixations (lower panels) to the ‘eyes’ area of interest (AOI). *P < 0.05, error bars denote mean ± SE.
Relationship between M1 excitability, autonomic arousal and person-dependent factors
As outlined in the previous sections, direct gaze was shown to significantly enhance M1 excitability (MEP responses), as well as autonomic arousal (SC responses) upon movement observation, especially when conveyed by a live model. In order to explore the possibility that the observed eye contact–induced increases in autonomic arousal were potentially related to the eye contact–induced enhancements of M1 excitability during the observation of movements in a live two-person setting, a Pearson correlation analysis was performed taking into account the ‘eye contact effect’ score for each measure (i.e. direct minus averted difference scores, see Methods section). No significant relationship was revealed (r = −0.14, P = 0.53; Fig. 3B).
It was also explored whether the eye contact effect on M1 excitability and/or autonomic arousal (i.e. direct-averted difference scores for MEPs and SCRs) was associated with self-reported social responsiveness (as measured by the SRS). The analysis identified a trend toward a negative correlation between the eye contact effect on M1 excitability and the SRS total score (r = −0.38, P = 0.08). Correlation analyses performed separately for each SRS subscale revealed that the relationship was significant for the subscale assessing Social Communication (r = −0.46, P = 0.03; Fig. 3C) and at trend level for the subscales assessing Social Awareness and Social Motivation (both P < 0.1; Table 1). Notably, opposite modulatory effects were evident for autonomic arousal; i.e. a positive association between the eye contact effect on autonomic arousal and SRS Total Score was found (r = 0.43, P = 0.04; Table 1). Also here, the association was most pronounced for the SRS subscale assessing Social Communication (r = 0.57, P = 0.006; Fig. 3C).
Table 1.
Means, standard deviations and Pearson correlations coefficients of self-report scores on the SRS (n = 22). Correlation coefficients assess the relationship between the SRS (subscale) scores and the ‘eye contact effect’ for M1 excitability (MEP difference score: MEPdirect − MEPaverted) and autonomic arousal (SCR difference score: SCRdirect − SCRaverted) in the live movement observation condition
| SRS subscale | Mean | SD |
MEP eye contact effect
Pearson r (P) |
SCR eye contact effect
Pearson r (P) |
|---|---|---|---|---|
| Total score | 30.86 | 13.48 | −0.38 (0.08) | 0.43 (0.04) |
| Social Awareness | 8.82 | 4.87 | −0.39 (0.07) | 0.27 (0.27) |
| Social Communication | 9.18 | 4.78 | −0.46 (0.03) | 0.57 (0.006) |
| Social Motivation | 5.82 | 2.75 | −0.37 (0.09) | 0.40 (0.07) |
| Rigidity and Repetitiveness | 7.09 | 3.60 | −0.008 (0.97) | 0.19 (0.40) |
Discussion
In the present study, single-pulse TMS was applied over the primary motor cortex (M1) to assess changes in M1 excitability (interpersonal motor resonance), while participants observed a live or videotaped model performing simple hand movements accompanied by direct or averted gaze. Additionally, stimuli-specific SCRs and gaze behavior were recorded to obtain a measure of autonomic arousal and visual attention.
Compared to averted gaze, direct eye gaze conveyed within a live two-person action context was shown to enhance M1 excitability during movement observation, but not during nonmovement-related trials. For the screen-based video presentations, the eye contact effect was only evident at trend level. Furthermore, and in accordance to previous mirror system research adopting TMS (Fadiga et al., 1995; Strafella & Paus, 2000), the facilitatory effect of eye gaze on M1 excitability was shown to be specific to the muscle implicated in the observed movement. These findings extend results from previous behavioral studies (Wang & Hamilton, 2013; Wang, Newport, et al., 2011) and TMS studies from our laboratory (Prinsen et al., 2017, 2018) showing that eye contact conveyed by video stimuli facilitates automatic mirror-motor mapping of others’ actions.
While previous studies have shown that impoverished motion stimuli such as point light displays (Ulloa & Pineda, 2007) or ‘pictorial’ movement features such as seen in shadow motions (Alaerts et al., 2009b) are sufficient to trigger mirror-motor system activation, findings from the present study, as well as a previous MEG study (Järveläinen et al., 2001), highlight that the use of ecological valid stimuli may provide a more salient context for inducing interpersonal motor resonance. In this context, Järveläinen et al. (2001) argued that real life action paradigms are more likely to increase participant interest, attention or motivation, as they are more representative of the way in which actions are observed in daily life. This notion was also discussed in a recent review by Reader & Holmes (2016), who identified the visual fidelity or quality of the observed stimuli as one potential source of variability that might drive encountered differences between naturalistic and experimental responses. Relating to the present study, it can be envisaged that—although great care was taken to ensure that the visual information during both presentation formats was similar—larger variability in head positioning, hand and wrist stabilization movements and eye blinks might have occurred in the live presentation format compared to the video presentations. Although these sources of variability can be regarded as ‘nuisance’ factors, they are actually in favor of the ecological appearance of the action.
Another critical factor outlined by Reader & Holmes (2016), but also by Risko et al. (2012), is the social potential of the stimuli, i.e. the ability of the stimuli to provide actual interactions. They argued that whereas the use of video stimuli may reduce social interaction to the level of observation only, the mere potential for social interaction in a live two-person paradigm may already increase its social validity (see for example the study by Laidlaw et al., 2011). In the particular case of eye contact research, it has initially been suggested by Conty et al. (2016)) and more recently also by Hietanen (2018) that responses to directly looking eyes might additionally reflect the awareness of another individual’s attention directed to the self, rather than the basic processing of visual information from the eyes of the sender (also known as the ‘watching eyes’ effect). Or, as elegantly phrased by Hietanen (2018), ‘images do not look back’.
Indeed, while in the current study the purely visuomotor information in both presentation formats was overall similar, the knowledge that the self is attended to by the other person in the live presentation format might have been the decisive component for driving the enhancement of M1 excitability. Evidence in support of this view was provided by a study from Myllyneva & Hietanen (2015) investigating autonomic arousal in response to a live individual’s gaze direction in two conditions. Either the participant believed that he/she and the model were able to see each other normally, or the participant was led to believe that a half-silvered mirror was placed between him/her and the model and that the model could not see the participant. Crucially, greater arousal responses to direct compared to averted gaze were observed only when the participants believed that the model was able to see them.
Taken together, the current results are in line with the recent proposal of a ‘second-person neuroscience’ (Risko et al., 2012; Schilbach et al., 2013; Reader & Holmes, 2016) and promote the use of ecologically valid stimuli for investigating naturalistic social cognition. Here, the employment of realistic, contextually embedded motor acts demonstrated that mirror system engagement can be modulated by observed gaze cues. According to the STORM (‘social top-down response modulation’) framework by Wang & Hamilton (2012)), this adjusting property of motor resonance at the level of M1 is grounded in a top-down control presumably originating from the mentalizing system and driven by an integrative evaluation of all social features in the current interaction (Wang et al., 2011b). This framework also fits recent theoretical proposals (Yang et al., 2015; Vogeley, 2017) and meta-analytic findings (Arioli & Canessa, 2019), suggesting the joint involvement of two neural systems when processing interpersonal actions: the mirror system, responsible for the automatic processing of biological motion, and the mentalizing system, involved in inferring others’ mental states and intentions.
For the first time, the present study combined a TMS-based assessment of M1 excitability with recordings of autonomic arousal based on SCRs. Similar to previous studies, direct gaze was shown to elicit significantly higher autonomic arousal responses (Hietanen et al., 2008; Helminen et al., 2011; Pönkänen et al., 2011). Also in the present study, the eye contact effect on autonomic arousal was shown to be more salient when eye gaze was conveyed by a live model, compared to video recordings of gaze cues. Critically however, while both SCRs and MEP responses were shown to be significantly enhanced during the direct compared to the averted gaze condition, no apparent association was revealed between the two measures. This result implies that the eye contact–induced enhancements in M1 excitability were not driven by or dependent on eye contact–induced increments in autonomic arousal, a finding that is also supported by the observation that eye contact–induced changes in MEPs were only evident in trials in which the live model performed a movement, but not during trials in which the model was presented without performing a movement or in the video presentation conditions. Lastly, the absence of any significant modulatory effects in the control ADM muscle also precludes a nonspecific arousal effect affecting all muscles. Broadening this notion to other measures of arousal, one other study has investigated whether the modulation of corticospinal excitability by a salient social context (moral vs. immoral actions) could be explained by nonspecific pupil dilation responses. In summary, pupillary responses showed a different pattern of results and did not correlate with MEP amplitudes (Liuzza et al., 2014).
While no direct relationship was evident between MEPs and SCRs, opposite associations were revealed with interindividual differences in social responsiveness (assessed with the SRS). Specifically, participants with higher self-reported social responsiveness (lower scores on the Social Communication subscale) were shown to display stronger enhancements in M1 excitability (interpersonal motor resonance), but fewer changes in autonomic arousal upon direct eye gaze. Together, these observations suggest a complex interplay between person- and context-dependent factors, indicating that the presented social context (i.e. mutual gaze) may be experienced differently depending on individual characteristics. In line with this notion, a series of studies has linked self-report measures of empathy to mirror system activation, suggesting that participants with greater empathy may show greater motor resonance during observation of others’ actions (for a review, see Baird et al., 2011, specific studies by Borgomaneri et al., 2015; Cheng et al., 2008; Gazzola et al., 2006). Furthermore, previous studies adopting naturalistic gaze cues conveyed by a live model identified similar associations between heightened autonomic arousal and person-dependent factors, such as the level of social impairments in children with ASD (Kaartinen et al., 2012), and the extent of social anxiety in individuals with social anxiety disorders (Myllyneva et al., 2015). Considering these associations, we encourage future investigations to explore how these person-dependent factors shape the effect of contextual (social) factors on adaptive mirror system functioning.
Limitations of the study should be noted. First, we did not reveal a significant main effect of ‘observation condition’ (movement, no movement), indicating that recorded MEP responses were not significantly higher during trials in which the movement was displayed, compared to trials in which only the model was presented without performing a movement. Since movement and nonmovement trials were presented in a random order and since participants were unaware of the nature of the upcoming trials, the possibility has to be considered that the use of a within-subjects protocol induced a ‘carryover’ or ‘priming’ effect, leading to similar (anticipatory) motor resonance processes during both movement and nonmovement trials. One way to avoid this anticipation of movement is to adopt a between-subjects design, testing condition-specific modulations of M1 excitability in separate groups of participants (as adopted in Lagravinese et al., 2017). Nonetheless, the observation that the effect of eye gaze was only evident in terms of M1 excitability of the FDI muscle (not for the control ADM muscle) and only during movement observation trials (not for nonmovement trials), affirms that gaze-related modulations of M1 excitability are condition- and muscle-specific. Further, the same female model was presented to every participant, but previous studies have shown that both the participant’s and the model’s gender may influence gaze processing (Jones et al., 2010; Slepian et al., 2011). Also in terms of autonomic arousal, Pönkänen et al. (2011) showed that for female participants, a significant effect of eye contact was evident for viewing female, but not male faces. Since the investigation of gender differences was beyond the scope of the present study, future research is warranted to systematically assess whether gender impacts the eye contact effect.
Conclusion
With the present study, we show that interpersonal motor resonance is modulated by the broader social context in which movement observation is embedded. More specifically, using a naturalistic two-person action context, we revealed that mutual eye contact significantly augmented M1 excitability during movement observation, particularly in individuals with higher self-reported social responsiveness. Importantly, the eye contact effect encompassed a muscle-specific increase in M1 excitability and was not driven by or dependent on differences in autonomic arousal or visual attention. The current findings highlight the importance and feasibility of employing stimuli with high ecological validity to investigate modulations of interpersonal motor resonance processes by subtle social cues, such as eye contact.
Acknowledgments
We are thankful for all participating subjects. Furthermore, we would like to thank Jolien Bergsma and Josien Kosse for their help in conducting the experiment; Esther Shin Hyun Kang and Emma García López for their help with the semantic gaze mapping analysis and Paul Meugens, Dr. Sharissa Corporaal and Prof. Stephan P. Swinnen for their methodological and technical support.
Funding
This research was supported by grants from the Flanders Fund for Scientific Research [FWO (KAN 1506716 N, KAN 1521313 N and G.0401.12)] and the Branco Weiss fellowship of the Society in Science—ETH Zurich granted to KA. JP is supported by an internal fund of the KU Leuven [STG/14/001] and the Marguerite–Marie Delacroix foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alaerts K., Swinnen S.P., Wenderoth N. (2009a). Is the human primary motor cortex activated by muscular or direction-dependent features of observed movements? Cortex, 45(10), 1148–55. [DOI] [PubMed] [Google Scholar]
- Alaerts K., Van Aggelpoel T., Swinnen S.P., Wenderoth N. (2009b). Observing shadow motions: resonant activity within the observer’s motor system. Neuroscience Letters, 461(3), 240–4. [DOI] [PubMed] [Google Scholar]
- Arioli M., Canessa N. (2019). Neural processing of social interaction: coordinate-based meta-analytic evidence from human neuroimaging studies. Human Brain Mapping, 40, 3712–3737. 10.1002/hbm.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A. D., Scheffer I. E., and Wilson S. J. (2011). Mirror neuron system involvement in empathy: A critical look at the evidence. Social Neuroscience, 6(4), 327–335. 10.1080/17470919.2010.547085 [DOI] [PubMed] [Google Scholar]
- Borgomaneri S., Gazzola V., and Avenanti A. (2015). Transcranial magnetic stimulation reveals two functionally distinct stages of motor cortex involvement during perception of emotional body language. Brain Structure and Function, 220(5), 2765–2781. 10.1007/s00429-014-0825-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W., Fowles D.C., Grimnes S., et al. (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49(8), 1017–34. [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Rizzolatti G. (2009). The mirror neuron system. Archives of Neurology, 66(5), 557–60. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Lee P.-L., Yang C.-Y., Lin C.-P., Hung D., and Decety J. (2008). Gender differences in the mu rhythm of the human mirror-neuron system. PloS One, 3(5), e2113 10.1371/journal.pone.0002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J.N., Todd R.D. (2005). Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry, 57(6), 655–60. [DOI] [PubMed] [Google Scholar]
- Conty L., George N., Hietanen J.K. (2016). Watching eyes effects: when others meet the self. Consciousness and Cognition, 45, 184–97. [DOI] [PubMed] [Google Scholar]
- De Bruin L., Gallagher S. (2012). Embodied simulation, an unproductive explanation: comment on Gallese and Sinigaglia. Trends in Cognitive Sciences, 16(2), 98–9. [DOI] [PubMed] [Google Scholar]
- Fadiga L., Fogassi L., Pavesi G., Rizzolatti G. (1995). Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology, 73(6), 2608–11http://jn.physiology.org/content/jn/73/6/2608.full.pdf. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21(2), 80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Frijda N.H. (2010). Impulsive action and motivation. Biological Psychology, 84(3), 570–9. [DOI] [PubMed] [Google Scholar]
- Gallese V., Sinigaglia C. (2011). What is so special about embodied simulation? Trends in Cognitive Sciences, 15(11), 512–9. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Aziz-Zadeh L., and Keysers C. (2006). Empathy and the Somatotopic Auditory Mirror System in Humans. Current Biology, 16(18), 1824–1829. 10.1016/J.CUB.2006.07.072 [DOI] [PubMed] [Google Scholar]
- Gentner R., Classen J. (2006). Modular organization of finger movements by the human central nervous system. Neuron, 52(4), 731–42. [DOI] [PubMed] [Google Scholar]
- Helminen T.M., Kaasinen S.M., Hietanen J.K. (2011). Eye contact and arousal: the effects of stimulus duration. Biological Psychology, 88(1), 124–30. [DOI] [PubMed] [Google Scholar]
- Hietanen J.K. (2018). Affective eye contact: an integrative review. Frontiers in Psychology, 9(1587), 1–15. 10.3389/fpsyg.2018.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen J.K., Leppänen J.M., Peltola M.J., Linna-Aho K., Ruuhiala H.J. (2008). Seeing direct and averted gaze activates the approach-avoidance motivational brain systems. Neuropsychologia, 46(9), 2423–30. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Dapretto M. (2006). The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience, 7(12), 942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Järveläinen J., Schürmann M., Avikainen S., Hari R. (2001). Stronger reactivity of the human primary motor cortex during observation of live rather than video motor acts. Neuroreport, 12(16), 3493–5. [DOI] [PubMed] [Google Scholar]
- Jones B.C., Debruine L.M., Main J.C., et al. (2010). Facial cues of dominance modulate the short-term gaze-cuing effect in human observers. Proceedings of the Royal Society B: Biological Sciences, 277(1681), 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M., Puura K., Mäkelä T., et al. (2012). Autonomic arousal to direct gaze correlates with social impairments among children with ASD. Journal of Autism and Developmental Disorders, 42, 1917–27. [DOI] [PubMed] [Google Scholar]
- Krings T., Naujokat C., Keyserlingk D.G. (1998). Representation of cortical motor function as revealed by stereotactic transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 109(2), 85–93. [DOI] [PubMed] [Google Scholar]
- Lagravinese G., Bisio A., De Ferrari A.R., et al. (2017). An emotion-enriched context influences the effect of action observation on cortical excitability. Frontiers in Human Neuroscience, 11(504), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw K.E.W., Foulsham T., Kuhn G., Kingstone A. (2011). Potential social interactions are important to social attention. Proceedings of the National Academy of Sciences of the United States of America, 108(14), 5548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzza M.T., Candidi M., Sforza A.L., Aglioti S.M. (2014). Harm avoiders suppress motor resonance to observed immoral actions. Social Cognitive and Affective Neuroscience, 10(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews, 36(1), 341–9. [DOI] [PubMed] [Google Scholar]
- Myllyneva A., Hietanen J.K. (2015). There is more to eye contact than meets the eye. Cognition, 134, 100–9. [DOI] [PubMed] [Google Scholar]
- Myllyneva A., Ranta K., Hietanen J.K. (2015). Psychophysiological responses to eye contact in adolescents with social anxiety disorder. Biological Psychology, 109, 151–8. [DOI] [PubMed] [Google Scholar]
- Noens I., De la Marche W., Scholte E. (2012). SRS-A handleiding voor volwassenen.pdf.
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pönkänen L.M., Peltola M.J., Hietanen J.K. (2011). The observer observed: frontal EEG asymmetry and autonomic responses differentiate between another person’s direct and averted gaze when the face is seen live. International Journal of Psychophysiology, 82(2), 180–7. [DOI] [PubMed] [Google Scholar]
- Press C., Cook R. (2015). Beyond action-specific simulation: domain-general motor contributions to perception. Trends in Cognitive Sciences, 19(4), 176–8. [DOI] [PubMed] [Google Scholar]
- Prinsen J., Bernaerts S., Wang Y., et al. (2017). Direct eye contact enhances mirroring of others’ movements: a transcranial magnetic stimulation study. Neuropsychologia, 95, 111–8. [DOI] [PubMed] [Google Scholar]
- Prinsen J., Brams S., Alaerts K. (2018). To mirror or not to mirror upon mutual gaze, oxytocin can pave the way: a cross-over randomized placebo-controlled trial. Psychoneuroendocrinology, 90, 148–56. [DOI] [PubMed] [Google Scholar]
- Reader A.T., Holmes N.P. (2016). Examining ecological validity in social interaction: problems of visual fidelity, gaze, and social potential. Culture and Brain, 4(2), 134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risko E.F., Laidlaw K., Freeth M., Foulsham T., Kingstone A. (2012). Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience, 6, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. (2012). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Barker A.T., Berardelli A., et al. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. [DOI] [PubMed] [Google Scholar]
- Schilbach L. (2010). A second-person approach to other minds. Nature Reviews Neuroscience, 11(6), 449–9. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., et al. (2013). Toward a second-person neuroscience. Behavioral and Brain Sciences, 36(04), 393–414. [DOI] [PubMed] [Google Scholar]
- Slepian M.L., Weisbuch M., Adams R.B., Ambady N. (2011). Gender moderates the relationship between emotion and perceived gaze. Emotion, 11(6), 1439–44. [DOI] [PubMed] [Google Scholar]
- Strafella A.P., Paus T. (2000). Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport, 11(10), 2289–92. [DOI] [PubMed] [Google Scholar]
- Ulloa E.R., Pineda J.A. (2007). Recognition of point-light biological motion: mu rhythms and mirror neuron activity. Behavioural Brain Research, 183, 188–94. [DOI] [PubMed] [Google Scholar]
- Vogeley K. (2017). Two social brains: neural mechanisms of intersubjectivity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 372(1727), 20160245. doi: 10.1098/rstb.2016.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hamilton A.F. (2012). Social top-down response modulation (STORM): a model of the control of mimicry in social interaction. Frontiers in Human Neuroscience, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hamilton A.F. (2013). Why does gaze enhance mimicry? Placing gaze-mimicry effects in relation to other gaze phenomena. The Quarterly Journal of Experimental Psychology, 67(4), 747–762. [DOI] [PubMed] [Google Scholar]
- Wang Y., Newport R., Hamilton A.F. (2011a). Eye contact enhances mimicry of intransitive hand movements. Biology Letters, 7(1), 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ramsey R., Hamilton A.F. (2011b). The control of mimicry by eye contact is mediated by medial prefrontal cortex. Journal of Neuroscience, 31(33), 12001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association (2013). Declaration of Helsinki. Ethical principles for medical research involving human subjects. Journal of the American Medical Association, 310(20),2191–4. [DOI] [PubMed] [Google Scholar]
- Yang D.Y.-J., Rosenblau G., Keifer C., Pelphrey K.A., et al. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience and Biobehavioral Reviews, 51, 263–275. 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


