Abstract
Responsive neurostimulation (RNS) has emerged as an adjunctive treatment modality for patients with intractable focal epilepsy who are not surgical candidates or have more than one ictal onset focus. We report a 34‐year‐old patient with intractable, childhood‐onset, genetic generalized epilepsy (GGE) with tonic, atonic, myoclonic and absence seizures treated with RNS. Strip electrodes over the right posterior frontal cortex and depth electrodes placed in the right anterior nucleus were used for event detection and responsive stimulation. Two‐year follow‐up revealed 90–95% clinical seizure reduction. This case suggests that refractory GGE may be effectively treated with RNS targeting thalamocortical networks.
Introduction
Over the past decade, neuromodulation modalities have evolved and become an increasingly attractive treatment alternative for patients with neurological and psychiatric disorders. Neurostimulation has emerged as a promising treatment modality in patients with medication resistant epilepsy, especially those who are not surgical candidates or had prior resective surgeries with an incomplete response. Responsive neurostimulation (RNS) delivers stimulation to a defined focal target and its associated circuitry after detecting specific electrographic patterns. In a pivotal randomized blinded controlled trial of RNS treatment for focal seizures, the active treatment group had a 37.9% decrease in reported seizures whereas the sham treatment group had a 17.3% decrease during the 12 week blinded assessment.1 Long‐term open label follow‐up studies showed seizure reduction by 53% at 2 years and up to 66% over 3 to 6 years time.2 The utility of RNS in generalized epilepsies arising from cortico‐thalamic network dysfunction, especially those with a known or suspected genetic basis, has not previously been described or systematically studied. Here, we report the index case of genetic (idiopathic in the previous classification) generalized epilepsy (GGE) treated with cortico‐thalamic RNS.
Case Report
The patient was a 34‐year‐old right‐handed man with epilepsy beginning at age 3 characterized by mixed seizures including absence, atonic, tonic, and myoclonic seizures with frequent progression to generalized tonic‐clonic seizures. Patient had no family history of epilepsy. Gestation, birth, development, and cognition were all normal. Multiple EEGs showed 3–6 Hz generalized, bi‐frontally predominant, polyspike/spike‐and‐wave (PSW) discharges and asynchronous left and right hemispheric discharges. Structural brain imaging including computed tomography and MRI have been negative throughout his life. At the age of 12 years, he was evaluated elsewhere for epilepsy surgery using bilateral 64 electrode grids over the frontal convexities placed in a symmetric fashion along with interhemispheric strips to test the hypothesis that his pharmaco‐resistance was secondary to cryptic frontal lobe epilepsy (FLE) with rapid secondary bilateral synchrony. Interictally, he had synchronous bi‐hemispheric discharges. Seizures had broad onset with alternating hemispheric lead‐in. He underwent an anterior corpus callosotomy (CC). Postoperatively, atonic seizures resolved with transient reduction in other seizure types. Throughout adolescence, his cumulative seizure frequency worsened from 3–5 per week to 15–20 per day. His longest seizure free interval was a few weeks on the ketogenic diet, but abrupt cessation resulted in status epilepticus without regaining its initial efficacy. Five years after his CC, he underwent a second surgical evaluation at a second facility. MEG revealed asynchronous left more than right supplementary premotor and primary motor area discharges with trans‐callosal propagation especially from the left to right. To retest the hypothesis of cryptic FLE, he had a second invasive surgical evaluation with placement of bi‐frontal grids. Ictal EEG revealed rapid secondary generalization and electrodecrement response without focal onset. He declined the proposed bi‐frontal multiple subpial transactions and opted to try other AEDs. At the age of 24 years, he had a vagal nerve stimulator placed; however, there was little improvement and it was eventually turned off due to inefficacy.
Thirteen years after his CC, he presented to Massachusetts General Hospital on valproic acid, rufinamide, and lamotrigine. At time of presentation, the tonic and hypermotoric seizures were the most frequent and disabling manifestations. They consisted of upward elevation of either upper extremities often followed by flapping movement of that arm or the ipsilateral lower limb. Myoclonic seizures were frequent, yet nondisabling, whilst the absence seizures were occasional. Repeat MRI brain showed changes consistent with CC. PET scan showed antero‐medial bi‐frontal hypometabolism. Repeat scalp EEG disclosed 1–4 sec bursts of 3–5 Hz generalized polyspike and wave (PSW) discharges with a shifting hemispheric predominance and left frontal spikes. Hypermotoric seizures were associated with diffuse fast frequency activity (FFA) at onset; while tonic seizures were associated with generalized either bi‐frontally or centrally predominant PSW discharges followed by generalized attenuation and terminated with generalized FFA (Fig. 1). Due to lack of evidence to distinguish between GGE and a non‐lesional FLE with rapid bilateral propagation, he had his third invasive surgical evaluation with stereotactic placement of 14 orthogonal depth electrodes. Seven depths in each hemisphere targeted the orbitofrontal, anterior/middle/posterior frontal, and anterior/middle/posterior temporal areas. We captured all his seizures types with consistent ictal onset in the lateral contacts of the frontal depths with shifting hemispheric predominance followed by rapid contralateral hemispheric propagation (Fig. 1). His epilepsy was deemed GGE with asymmetric thalamocortical propagation, particularly to the lateral aspect of the posterior frontal area. He had RNS placement targeting left and right lateral posterior frontal cortex (PFC) with four contact strips and bilateral anterior thalamic nucleus (ATN) 4‐contacts depth electrodes (Fig. 2). Due to semiological predominance of seizures on the left hemibody, right sided contacts were connected to the device. One‐month detection from both right PFC (rPFC) and right ATN (rATN) revealed near‐simultaneous ictal onset from both regions. Various detection and stimulation sites and paradigms were tested empirically over time (Fig. 3). Ultimately, while clinical responses varied, based on patient reports and recorded detections and stimulations we found the best response came with event detection triggered from the rPFC electrode strip and triggered stimulation delivered through the rATN depth (Fig. 3). There was no difference in the ictal patterns detected on the RNS electrocorticography during all reported seizure types (myoclonic, absence, tonic, hypertonic). At 2 years’ follow‐up visit, he had 90–95% self‐reported seizure reduction (15–20/day to 2–3/day) in his tonic seizures along with hypermotoric manifestation. Clinical seizure duration decreased from 10–40 sec to 5–15 sec. He remained on the same dose and number of AEDs. There was no subjective change in cognition after RNS implantation and stimulation.
Figure 1.
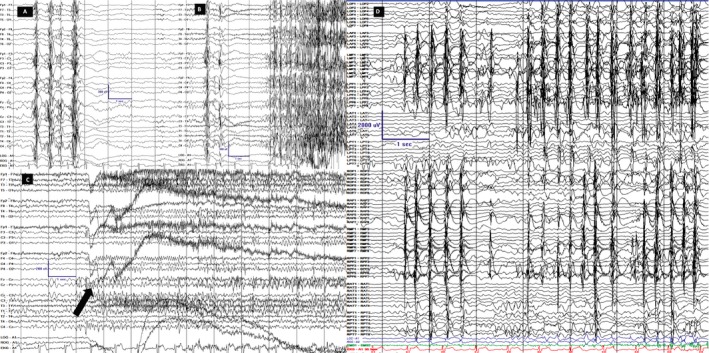
Scalp and invasive EEG recordings. LFF 1Hz; HFF 70Hz, Notch OFF; Sensitivity 7 uV/mm Timebase 30mm/sec. Scale is displayed in all images; y‐axis is amplitude in uV and x‐axis is time in seconds. Scalp (A–C) and intracranial (D) recordings are on longitudinal bipolar montage along with coronal ring on scalp. A. Interictal background displayed generalized PSSW (polyspike, spike and slow wave) discharges without hemispheric predominance B. Tonic seizure. The first unequivocal ictal electrographic change occurred as burst of generalized PSSW followed by electro‐decrement and generalized FFA (faster frequency activity). The first ictal behavior occurred 2‐3 seconds after the first EEG change. C. Hypermotoric seizure. The first unequivocal electrographic change occurred as generalized, centrally predominant, medium amplitude FFA (arrow) and clinically evidenced by eyelid myoclonia followed by myoclonus of legs and flailing of left hand. D. Typical brief hypermotoric and myoclonic seizure evidenced at onset by synchronous periodic discharges from the RPF (right posterior frontal)/ RMF (right middle frontal)/ RAF (right anterior frontal)/ ROF (right orbitofrontal)/ LMF (left middle frontal)/ LAF (left anterior frontal)/ LPF (left posterior frontal) highest in amplitude on the lateral contacts.
Figure 2.
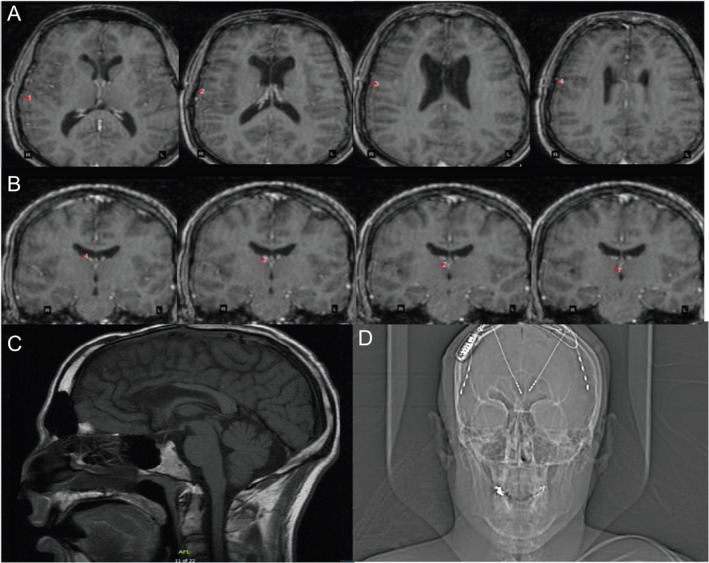
(A–B) 3D reconstruction of the T1‐MRI brain sequences after RNS implantation (A) Axial T1‐sequences revealing the annotations of the strip placed on the right posterior frontal cortex (rPFC) contact with the first image revealing first contact while the last sequence shows the last contact on the strip # 4. (B) Coronal T1‐sequences revealing the depth electrode to the right anterior thalamic nucleus (rATN) with the first image revealing contact # 4 that is the most lateral contact and the last image is contact # 1 that is the deepest contact (C) MRI T1‐sagittal view displaying anterior corpus callosotomy changes. (D) An X‐ray of head displaying the implantation of RNS device with 2‐depth electrodes each with four contacts targeting the ATN and 2‐strips at the PFC. Only the rATN depth and rPFC strip were connected to the RNS device.
Figure 3.
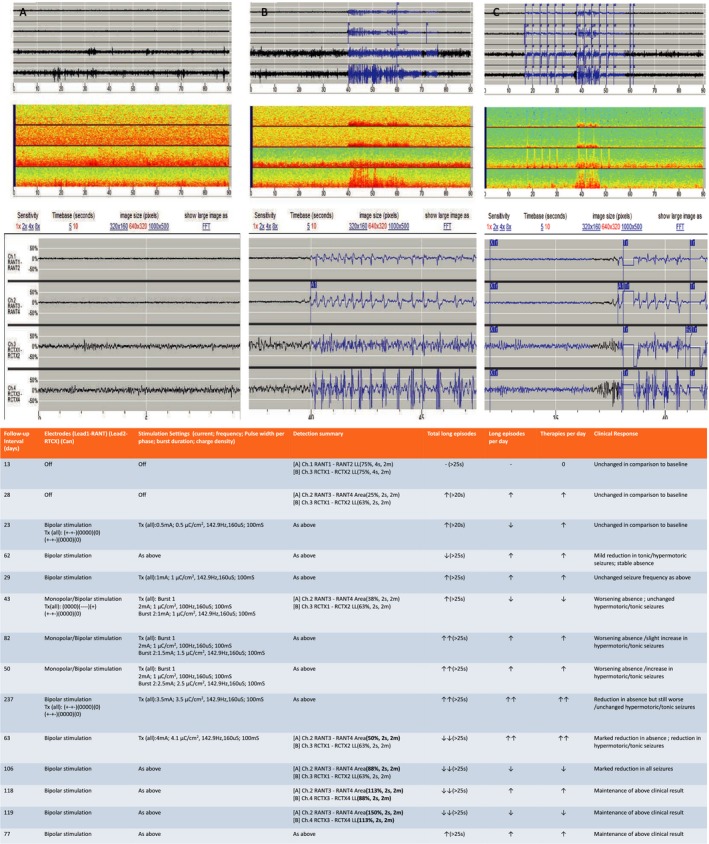
Data from the responsive neurostimulation (RNS). (1) (A–C) Electrocorticography (ECOG) from the RNS device and (2) table depicting various stimulation/detection parameters with corresponding ECOG and clinical response. (A–C) Channel (Ch) 1 and 2 are the deepest contacts of the depth electrode targeting the right anterior thalamic nucleus (rATN). Channel (Ch) 3 and 4 are the most posterior contacts on the right posterior frontal cortex (rPFC) strip. A1:Prestimulation baseline background recording from both rATN and rPFC. A2 is a magnification of A1. A3 represents the spectral analysis during prestimulation interictal phase. B1:Prestimulation baseline ictal recording from both rATN and rPFC. The seizures start synchronously from both rATN and rPFC. B2 is a magnified ECOG of the ictal activity. B3. Represents the spectral analysis during prestimulation ictal phase. C1. Stimulation parameters were turned on delivering burst therapy to both rATN and rPFC contacts to abort the seizure. Stimulation was delivered in bipolar mode. C2 is a magnified display of the treatment bursts delivered. Note the ictal onset occuring in rPFC 0.5 seconds prior to recruitment of rATN. C3. Represents the spectral analysis during ictal phase stimulation. Table Legend: TX: Treatment; ↓:mild decrease; ↓↓: marked decreased; ↑:mild increase; ↑↑: marked increase.
Discussion
We provide evidence that RNS targeting the ATN can be utilized successfully in patients with refractory GGE. Evidence derived from repeated scalp and invasive EEG recordings over years was most consistent with the diagnosis of a generalized epilepsy phenotype arising from cortico‐thalamic network dysfunction. Semiology and the lack of neuroimaging findings were concordant with electrodiagnostic evidence of what has now been classified as GGE. Detection of some seizures apparently starting from cortex with rapid recruitment of ATN emphasizes the idea that this epilepsy type is not truly generalized, but rather localized to a distributed but specific functional and anatomic network. Although the current RNS technology only allows sparse sampling of cortical and thalamic activity, demonstration of consistent relationships between cortical and thalamic activity across patients would advance our understanding of the “generalized” epilepsy phenotype and physiology. The exponential increase in absence seizures during simultaneous stimulation of both the cortex and thalamus led to stimulation of thalamus alone in response to cortical and/or thalamic detections. This resulted in marked reduction of the absence seizure frequency. One plausible hypothesis for worsening of absence seizures could be attributable to the “cortical focus theory” from rodent models of epilepsy.3 It suggests the necessity of a “cortical focus” to generate a rapidly propagating bilateral activity and oscillations within aberrant thalamocortical networks (Fig. 3).4 It is possible that the overall seizure reduction is due to responsive stimulation interfering with interhemispheric spread via diencephalic commissural pathways. It is important to note that our patient previously had an anterior callosotomy, depriving him of important alternative interhemispheric connections, thus his response to stimulation may not be fully predictive of how other patients would respond.
Both the ATN and centromedian nucleus of the thalamus (CMN) have been suggested as targets for modulation in generalized epilepsy syndromes.5, 6 The ATN plays an integral role in ictogenesis and propagation pathway receiving projections from the mamilllothalamic tracts projecting to the cingulate, orbitofrontal, and mesial prefrontal cortex.7 The CMN receives afferent projections from spinal cord, brainstem basal ganglia, and amygdala and projects to lateral, rostral, and mesial frontal cortex.8
Several animal and human studies with primary and secondary generalized seizures demonstrated that the ATN stimulation results in notable seizure frequency reduction.9, 10 Stimulation of ATN has recently been shown effective in treating focal epilepsy as well.9 Several clinical studies with limited number of patients studied the effect of stimulation via DBS on the CMN, particularly in patients with primary and secondary generalized epilepsies were unable to demonstrate a robust effect on seizure frequency.5, 11 Nonetheless, our decision to choose ATN as a target was somewhat arbitrary and future studies are needed to determine whether one or the other thalamic target should be preferred.
Animal models, depth recordings, and neuroimaging studies have long implicated the cortico‐thalamo‐cortical circuitry in generalized epilepsies.12, 13 This circuitry generates thalamic oscillations through intrinsic burst and rhythmic burst discharges that lead to the spike‐and‐wave discharges seen in absence epilepsy while intrinsic thalamic tonic firing occurs in seizure free periods.14, 15 The availability of implanted devices to chronically record, detect and modulate this circuitry in humans offers and exciting opportunity to learn more about the basic mechanisms of interictal‐ictal transition, cortico‐thalamic relationships, and the biology of spike and wave syndromes. Based on our patient’s initial response to responsive stimulation, a clinical trial to identify the optimal target(s) and stimulation parameters are necessary to understand the specific role and utility of stimulation in generalized epilepsy that is medication resistant.
Author Contributions
A. Herlopian has contributed in gathering the data, reviewing of the literature, writing, reviewing and finalizing the manuscript. A.J. Cole conceptualized the study, gathered the data, reviewed, edited and finalized the manuscript. T. Jennings has reviewed and finalized the manuscript. E. Eskandar has reviewed and finalized the manuscript. Cash SS has reviewed and finalized the manuscript.
Conflict of Interest
Dr Andrew Cole received coverage for travel expenses but no honorarium for a Neuropace advisory meeting. Dr Aline Herlopian received travel funding during fellowship to attend a training conference on responsive neurostimulation sponsored by Neuropace. None of the other authors has any conflict of interest to disclose pertinent to this article.
Funding Information
No funding information provided.
References
- 1. Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. [DOI] [PubMed] [Google Scholar]
- 2. Bergey GK, Morrell MJ, Mizrahi EM, et al. Long‐term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015;84:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol 2005;62:371–376. [DOI] [PubMed] [Google Scholar]
- 4. Pinault D, O’Brien TJ. Cellular and network mechanisms of genetically‐determined absence seizures. Thalamus Relat Syst 2005;3:181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res 1997;28:89–100. [DOI] [PubMed] [Google Scholar]
- 6. Fisher RS, Uematsu S, Krauss GL, et al. Placebo‐controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 1992;33:841–851. [DOI] [PubMed] [Google Scholar]
- 7. Child ND, Benarroch EE. Anterior nucleus of thalamus: functional organization and clinical implications. Neurology 2013;81:1869–1876. [DOI] [PubMed] [Google Scholar]
- 8. Royce GJ, Bromley S, Gracco C. Subcortical projections to the centromedian and parafascicular thalamic nuclei in the cat. J Comp Neurol 1991;306:129–155. [DOI] [PubMed] [Google Scholar]
- 9. Salanova V, Witt T, Worth R, et al. Long‐term efficacy and safety of thalamic stimulation for drug‐resistant partial epilepsy. Neurology 2015;84:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KJ, Shon YM, Cho CB. Long‐term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact Funct Neurosurg 2012;90:379–385. [DOI] [PubMed] [Google Scholar]
- 11. Velasco AL, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox‐Gastaut Syndrome. Epilepsia 2006;47:1203–1212. [DOI] [PubMed] [Google Scholar]
- 12. Zhang CH, Sha Z, Mundahl J, et al. Thalamocortical relationship in epileptic patients with generalized spike and wave discharges ‐ A multimodal neuroimaging study. Neuroimage Clin 2015;9:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorokin JM, Davidson TJ, Frechette E, et al. Bidirectional Control of Generalized epilepsy networks via rapid real‐time switching of firing mode. Neuron 2017;93:194–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorji A, Mittag C, Shahabi P, et al. Seizure‐related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiol Dis 2011;43:266–274. [DOI] [PubMed] [Google Scholar]
- 15. Lee SE, Lee J, Latchoumane C, et al. Rebound burst firing in the reticular thalamus is not essential for pharmacological absence seizures in mice. Proc Natl Acad Sci USA 2014;111:11828–11833. [DOI] [PMC free article] [PubMed] [Google Scholar]


