Abstract
Objective
Assessment of results of repairing vesicovaginal fistula (VVF) with or without the use of interposition flaps.
Material and methods
This prospective randomized study was conducted between January 2012 to December 2017 in the Department of Urology, King George’s Medical University, Lucknow, India. Obstetric and gynecological simple fistula of ≤4 cm were included for evaluation. Those with complex or complicated fistula or fistula due to malignancy were excluded. Patients were divided into two groups (group 1 and group 2) depending upon route of repair i.e., transvaginal or transabdominal, respectively, as per the characteristics and location of the fistula. These two groups of patients were randomized into two subgroups (1A, 1B and 2A, 2B) based on the inclusion or omission of the interposition flap during fistula repair. Perioperative and postoperative parameters (blood loss, mean operating time, hospital stay, and requirement of analgesics) and success rates of fistula repair were compared. All complications that occurred in the postoperative period till the last follow-up appointment were recorded. The Clavien-Dindo Classification was used to stratify the complications.
Results
Fifty-seven patients underwent transvaginal repair in group 1 (29 with Martius flap: group 1A; 28 without Martius flap: group 1B), while 69 patients underwent transabdominal repair in group 2 (35 with interposition flap: group 2A; 34 without flap: group 2B). Blood loss, mean operating time, hospital stay, and the requirement of analgesics were comparable between each subgroup-1A versus 1B and 2A versus 2B, respectively. The overall success rate of repair across all groups was 96.04% (121/126). The success rate was 93.1% in transvaginal repair with Martius flap versus 96.43% in transvaginal repair with no flap (p=1.0). Success rate was 97.1% in transabdominal repair with an omental flap versus 97.06% in without an omental flap (p=1.0). Mean follow-up period was 39.6 months (range: 6–68 months). Out of 29 patients with Martius flap interposition, 9 (31.03%) of them reported a significantly reduced sensation on the labia majora. Of these 9 patients, 5 reported numbness while the remaining 4 experienced pain as compared to the patients in subgroup IB, who did not report any altered sensation in the labia. (p=0.0019).
Conclusion
The success rates are similar in simple VVF repair (fistula size less than 4 cm) irrespective of the use of interposition flaps. However, overall morbidities following repair with the interposition flap are higher when compared with repair without interposition flap, either by the transvaginal or by the transabdominal route.
Keywords: Omentum, martius flap, vesicovaginal fistula repair
Introduction
The interposition flap is traditionally used in transabdominal and transvaginal repair of vesicovaginal fistula (VVF). The purpose of using this flap is to prevent the apposition of suture lines, to increase the success rate of fistula repair, and decrease postoperative dyspareunia.[1] The overall success rate of simple VVF repair varies from 90% to 95%.[1,2] In cases with complex and complicated fistula, interposition flaps are almost always required to prevent recurrence, but for simple VVF repair, there are non-randomized studies that have shown similar results, irrespective of the use of the interposition flap.[3–5] Till date, to the best of our knowledge, there is no prospective, English-language, randomized study in the scientific literature that compares the success rate and postoperative morbidities following simple VVF repair, with or without the use of interposition flap.
The present study was executed as a prospective randomized a study with primary objective of comparing the success rates of simple VVF (size of fistula ≤4 m) repair with or without the use of interposition flap. Our study also compares the postoperative morbidities in both the subgroups.
Material and methods
This study was conducted at a tertiary care apex hospital in Northern India between January 2012 and December 2017. Ethical approval was obtained from institutional ethical review board (DRC/38/Uro/KGMU) and the practices are in accordance with the Declaration of Helsinki. The Consort diagram for randomization is depicted in the Figure 1. A total of 2114 female patients with all types of urinary incontinence attended the Urology outpatient department during the study period. Out of these, 149 patients were diagnosed with VVF. A total of 18 patients did not fulfill the selection criteria, and 5 did not give consent for randomization, so they were excluded from this study. Finally, 126 patients were randomized following their verbal and written informed consent. Inclusion criteria were fistula size of 4 cm or less and number of fistulae as 2 or less (defined as simple VVF). Those with complex or complicated fistula or fistula due to malignancy were excluded from this study. A complex fistula was defined as fistula greater than 4 cm with or without the involvement of the continence mechanism, having multiple openings (>2), and with history of previous failed repair or vesico-cervico-vaginal fistula.[5] A complicated fistula was defined as fistula greater than 6 cm, with an associated bladder stone or reduced bladder capacity, complete loss of the urethral function, and associated uretero-vaginal fistula or post-radiation fistula.[5]
Figure 1.
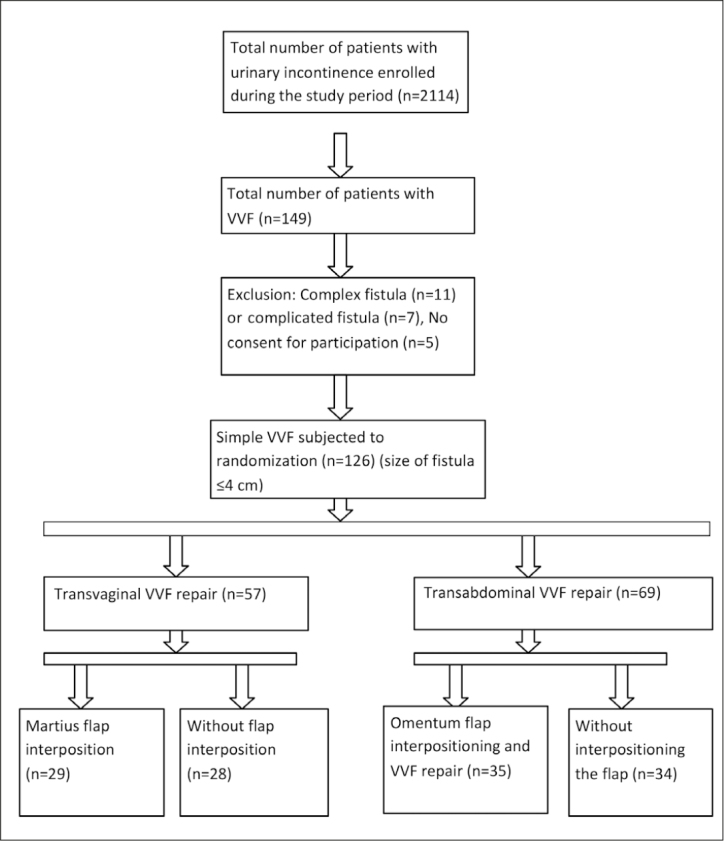
Consort diagram
Cystoscopy was performed to determine the site, size, and number of fistulae along with the assessment of bladder mucosa around the fistulous opening. Examination using a vaginal speculum was done to assess the vaginal capacity and mucosal integrity. Depending upon the route of repair, patients were divided into two groups (group 1 and group 2), i.e., transvaginal or transabdominal, respectively, as per the characteristics of the fistula and associated findings.
Transvaginal route was preferred where vaginal architecture allowed adequate exposure of the fistula (2 fingers inserted easily in vagina), with single fistula, ureteric orifices at least 1 cm away from the edge of fistula, and no concurrent abdominal pathology. If two fistulae were present adjacent to each other with a thin intervening septum, they were converted into a single fistula by excising the septum and were repaired through the transvaginal route. Transabdominal route was preferred if there were two fistulae lying apart, ureteric orifices were at the edge of fistula requiring reimplantation (within 5 mm from the edge of fistula), vaginal architecture was hindering adequate exposure, and concurrent pelvic and abdominal pathology required intervention during the same operation.
These two groups were randomized further into two subgroups (1A, 1B and 2A, 2B), based on the use or absence of the interposition flap. The randomization was performed using a computer-generated randomization chart. The subgroups were as follows:
Subgroup 1A-Transvaginal repair with Martius flap interposition,
Subgroup 1B-Transvaginal repair without Martius flap interposition,
Subgroup 2A-Transabdominal repair with an interposition flap,
Subgroup 2B-Transabdominal repair without an interposition flap.
Data were recorded on a pre-designed proforma. The parameters evaluated were age, an antecedent event leading to fistula formation, educational status, parity, duration of incontinence, and the size, location, and number of fistulae present. Perioperative and postoperative parameters (blood loss, mean operating time, hospital stay, requirement of analgesics) were also recorded. Patients were followed-up for 3 weeks, 3 months, 6 months, and annually thereafter. All complications that occurred in the postoperative period till their last follow-up were recorded; the Clavien-Dindo Classification was used to stratify the complications.[6] Sexual intercourse was advised to be safe after 3 months from the date of the surgery. Dyspareunia was defined as difficult or painful sexual intercourse after 3 months of fistula repair.
Surgical technique
Transvaginal repair with or without Martius flap
Following cystoscopy, the ureteric orifices were catheterized with 5 French (F) ureteric catheters if they were close to the fistula to prevent inadvertent intraoperative injury. Another 6F ureteric catheter was brought out of the vagina (over a guidewire) following insertion through the fistulous tract. The 18F Suprapubic Foley catheter (SPC) and 16F per urethral Foley catheters (PUC) were placed. Gentle traction was placed on the 6F ureteric catheter (within the fistula tract) to view the margins of the comparatively higher-placed fistula. The fistula was incised circumferentially with a margin of approximately 0.5–1.0 cm, and a plane was created by performing sharp dissection between the urinary bladder and the vagina. Full-thickness closure of bladder wall was performed with interrupted 3-0 polygalactin sutures. The perivesical fascia was closed with interrupted 3-0 polygalactin sutures.
In group 1A patients, the Martius flap was harvested from the labia majora and tunneled under the vaginal mucosa to be fixed as an interposition flap over the bladder’s suture line. The vaginal incision was closed in a single layer with interrupted 3-0 polyglactin sutures. The incision on labia majora was closed in 2 layers with absorbable sutures after insertion of a Penrose drain, which was removed after 48 hours.
Transabdominal repair with or without interposition flap
Transabdominal repair was performed by the O’Conor technique.[7] Urinary bladder was exposed via the transperitoneal route through the midline via an infraumbilical or Pfannenstiel incision. The bladder was bivalved from the anterior wall (including the dome) to the fistulous site. Bilateral ureteric orifices were identified, and 5F soft silastic tubes were inserted within them. A plane was created between the bladder and the vagina after sharp encircling of the fistula. The vaginal opening was closed in single layer with interrupted stitches using 3-0 polygalactin sutures, while the urinary bladder was closed in two layers in a continuous manner with the same suture. The 18F SPC and 16F PUC were inserted. Following urinary bladder closure, the urinary bladder was gently distended to check for watertight closure; if there was any leak at any point, an additional suture was applied at that site. The omentum flap was fixed over the vaginal suture line as an interposition flap in group 2A.
The patients were discharged after 3–7 days with postoperative advice regarding the intake of anticholinergics (Tolterodine 2 mg, twice a day), antibiotics (prophylactic dose), and stool softeners. Continuous urinary drainage was maintained for three weeks with SPC and PUC in situ. A voiding trial was given at 3 weeks. Failure of VVF repair was defined as urine leakage per vagina either before or after catheter removal. The patients in whom the procedure failed were called after 3 months for reassessment and repeat fistula repair.
Statistical analysis
The unpaired t-test was used to compare continuous data. Fischer’s exact test was used to analyze categorical data using Graph Pad Prism version 6.00 for Windows software (Graph Pad Software, La Jolla California USA). Statistical significance was defined as a p-value of <0.05.
Results
Please refer to the Consort chart (Figure 1) for a comprehensive view of the results. A total of 126 patients were subjected to surgical VVF repair. Transvaginal repair was performed in 57 patients, i.e., group 1 (29 with Martius flap=group 1A; 28 without flap=group 1B), while 69 patients underwent transabdominal repair, i.e., group 2 (35 with interposition flap=group 2A; 34 without flap=group 2B). The reasons for transabdominal approach were as follows. The number of fistulae was more than one (n=12), ureteric orifices at the edge of the fistula required ureteric reimplantation (n=14), vaginal architecture hindered adequate exposure (n=19), concurrent abdominal surgery, such as bilateral fallopian tube ligation (n=9), oophrectomy (n=11), hysterectomy (n=4), and ventral hernia repair (n=4). The baseline demographic parameters are summarized in Table 1. The abovementioned associated causes were mostly present in combination with another condition or set of conditions.
Table 1.
Comparison of baseline parameters
| Variables | Subgroup 1A (n=29) | Subgroup 1B (n=28) | p | Subgroup 2A (n=35) | Subgroup 2B (n=34) | p |
|---|---|---|---|---|---|---|
| Age in years (mean+SD) | 34.35±8.22 | 32.15±7.61 | 0.30 | 35.7±6.60 | 34.85±7.21 | 0.61 |
|
| ||||||
| Education (n) | ||||||
| Uneducated | 12 | 09 | 0.89 | 15 | 12 | 0.62 |
| Educated | 17 | 19 | 20 | 22 | ||
|
| ||||||
| Parity (n) | ||||||
| Up to 2 | 16 | 19 | 0.41 | 16 | 19 | 0.47 |
| More than 2 | 13 | 09 | 19 | 15 | ||
|
| ||||||
| Antecedent event (n) | ||||||
| Obstructed labor | 12 | 15 | 11 | 13 | 0.83 | |
| Vaginal hysterectomy | 09 | 05 | 0.63 | 08 | 06 | |
| Abdominal hysterectomy | 05 | 04 | 08 | 10 | ||
| Laparoscopic hysterectomy | 03 | 04 | 07 | 05 | ||
|
| ||||||
| Duration of incontinence (n) | ||||||
| <12 months | 12 | 15 | 0.43 | 20 | 18 | 0.81 |
| >12 months | 17 | 13 | 15 | 16 | ||
|
| ||||||
| Size of fistula (cm) | 2.7+1.7 | 3.2+1.5 | 0.24 | 2.9+1.4 | 3.1+1.2 | 0.53 |
|
| ||||||
| Location of fistula (n) | ||||||
| Trigonal | 18 | 17 | 1.0 | 18 | 16 | 0.81 |
| Supratrigonal | 11 | 11 | 17 | 18 | ||
|
| ||||||
| Number of fistula (n) | ||||||
| One | 29 | 28 | - | 25 | 24 | 1.0 |
| Two | 0 | 0 | 10 | 10 | ||
|
| ||||||
| Flap used (n) | ||||||
| Martius flap | 29 | - | - | - | - | |
| Omental flap | - | - | - | 35 | - | - |
The perioperative and postoperative parameters are summarized in Table 2. The amount of blood loss, mean operating time, hospital stay, and the requirement of analgesics were comparable between subgroup 1A versus 1B and 2A versus 2B. The overall success rate of fistula repair across all the groups was 96.04% (121/126). The success rate was 93.1% (n=27/29) in transvaginal repair with Martius flap versus 96.34% (n=27/28) in transvaginal repair without flap (p=1.0). Successful repair was 97.1% (n=34/35) in transabdominal repair with interposition flap versus 97.06% (33/34) in those without flap (p=1.0).
Table 2.
Comparison of perioperative and postoperative parameters
| Parameters | Sub-group 1A (n=29) | Sub-group 1B (n=28) | p | Sub-group 2A (n=35) | Sub-group 2B (n=34) | p |
|---|---|---|---|---|---|---|
| Blood loss (mL) (mean+SD) | 141.3±42.7 | 122.3 ± 38.3 | 0.08 | 217±50.2 | 198.3± 46.3 | 0.11 |
|
| ||||||
| Mean operative time (min) (mean+SD) | 117±27.3 | 102.3±32.3 | 0.07 | 168±29.4 | 154±36.3 | 0.08 |
|
| ||||||
| Hospital stay (days) (mean+SD) | 6.7±3.4 | 6.2±3.2 | 0.06 | 9.3±2.6 | 9.8±4.1 | 0.54 |
|
| ||||||
| Requirement of analgesics | ||||||
| Mean tramadol hydrochloride (mg) (mean+SD) | 229±36 | 218±34 | 0.24 | 354±40 | 339±23 | 0.06 |
| Success (n) | 27/29 | 27/28 | 1.0 | 34/35 | 33/34 | 1.0 |
The mean follow-up period was 39.6 months (range: 6–68 months). The Clavien-Dindo classification was used to stratify perioperative and postoperative complications (Table 3).[6] Out of 29 patients, 9 (31.03%) underwent repair with Martius flap interposition. They reported significantly reduced sensation at labia majora; 5 of these 9 cases had numbness, while the other 4 experienced pain at the labia majora. In comparison, none of the patients in group IB (without Martius flap interposition) reported any pain or altered sensation in the labia majora (p=0.0019).
Table 3.
Perioperative and postoperative complications according to the Clavien-Dindo grading of surgical complications
| Complications | Subgroup 1A | Subgroup 1B | p | Subgroup 2A | Subgroup 2B | p |
|---|---|---|---|---|---|---|
| Clavien grade I | ||||||
| Vaginal bleeding (n) | 2 | 1 | 1.0 | 0 | 0 | - |
| Abdominal pain (n) | 1 | 2 | 0.61 | 3 | 0 | 0.24 |
| Constipation (n) | 3 | 2 | 1.0 | 4 | 3 | 1.0 |
| Pelvic collection (n) | 0 | 0 | - | 2 | 1 | 1.0 |
|
| ||||||
| Clavien grade II | ||||||
| Urinary tract infection | 2 | 1 | 1.0 | 3 | 2 | 1.0 |
| Irritative voiding symptoms | 5 | 4 | 1.0 | 5 | 3 | 0.71 |
| Temporary paralytic ileus (n) | 0 | 0 | -- | 2 | 0 | 0.49 |
| Seroma or hematoma at Martius Flap harvest site (n) | 2 | 0 | 0.49 | - | - | - |
| Suprapubic catheter issue | 2 | 3 | 1.0 | 4 | 3 | 1.0 |
| Distortion of labia majora (n) | 2 | 0 | 0.49 | - | - | - |
| Numbness or pain at Martius flap | 9 | 0 | 0.0019 | - | - | |
| harvest site (n) | - | |||||
| Dyspareunia (n) | 1 | 2 | 1.0 | 0 | 0 | - |
| Urinary incontinence (urge or stress) 3 | 2 | 1.0 | 1 | 2 | 1.0 | |
|
| ||||||
| Clavien grade III | ||||||
| Failure (n) | 2 | 1 | 1.0 | 1 | 1 | 1.0 |
Note: Each individual patient may have more than one complication
Discussion
Vesicovaginal fistula occurs due to complications following obstructed labor and/or gynecological procedures. Transvaginal and transabdominal route of repair depends mainly on the etiology of the fistula, associated pathology, vaginal architecture, and the preference of the operating surgeon.[8] Transabdominal route is preferred in patients with large defects or fistulae involving the ureters, in those with concurrent abdominal or pelvic pathology, or where the vaginal architecture hinders adequate exposure.[9,10] Transvaginal route is preferred where vaginal space is adequate or in those with a comparatively low-lying VVF.
Various interposition flaps have been described in the literature. These include omentum, peritoneum, tinea epiploica, labial, and rarely myocutaneous or gracilis muscle flaps.[10–14] Of these the omentum is the most commonly preferred interposition flap in transabdominal repair, while the labial fibro-fatty flap (Martius flap) is the most common interposition flap for transvaginal repair.
In our study, the success rate of VVF repair via transvaginal route did not differ whether the interposition (Martius) flap was used or not (93.1% vs. 96.43%, p=1.0). Similar results were also observed by Browning et al.[15] in 413 cases of obstetric fistula repair; 207 patients underwent repair with Martius flap interposition with a success rate of 96.6% vs. 99% (203/206) (p=0.18). Pshak et al.[16] reported a 100% success rate in 49 patients who underwent transvaginal repair without interposition flap.
Contrary to the abovementioned studies, Rangnekar et al.[17] reported superior outcomes of transvaginal repair using the Martius flap as compared to regular anatomic repair. The failed cases in this study were mainly patients who had multiple and recurrent fistulae.
The major drawback of the abovementioned studies is their retrospective nature. Based on the findings of these studies, it can be concluded that the interposition flap can be omitted in transvaginal repair of simple VVF, however, this has never been proved in a prospective randomized study, which is a better scientific method to prove the superiority of one surgical technique over another.
Harvesting the Martius flap leads to complications like seroma, hematoma, numbness, pain, or labial distortion, which can be avoided by repairing the wound without using the Martius flap. In subgroup 1A, seroma or hematoma formation at Martius flap harvest site occurred in 6.9% of patients (n=2/29); both these patients showed distortion of the labia majora.
In our experience, complete hemostasis at the site of harvest along with insertion of a Penrose drain may decrease the risk of seroma, hematoma, and labial distortion. However, these complications can only be completely averted by omitting the Martius flap altogether.
Although rare, Martius flap interposition may lead to pain at the harvest site. Further, the clitoris has rich sensory nerve supply that may get damaged during flap harvest leading to persistent pain. In this study, 17.24% (n=5/29) of patients had numbness, and 13.8% (n=4/29) of patients had pain at the Martius flap harvest site in subgroup 1A. These complications can be prevented by omitting the Martius flap.
Martius flap interposition may lessen scarring because of the vascularity of the flap and enhanced lymphatic drainage at the site of fistula repair. This subsequently caused less vaginal discomfort or dyspareunia. In our study, dyspareunia was present in 3.45% of patients (n=1/29) in subgroup 1A and in 6.9% of patients (n=2/29) in subgroup 1B. Rangnekar et al.[17] in their series of 38 patients who underwent successful fistula repair, reported dyspareunia in 6 out of 18 patients (33%) who did not receive a Martius flap, and no dyspareunia in 20 patients with Martius flap interposition. Therefore, one of the advantages of Martius flap interposition is to reduce postoperative morbidity. Mean blood loss and operative time were comparatively higher in the Martius flap interposition group but were statistically insignificant (141.3±42.7 versus 120.3±38.3 ml; 117±27.3 versus 101.3±32.3 minutes, respectively).
In transabdominal repair, the omentum remains the first choice for placing an interposition flap. In 1967, Turner-Warwick et al.[18] described the principles of omental flap mobilization based on the right gastroepiploic artery emerging from the greater curvature of the stomach. Omentum has excellent vascularity and when it is placed between the bladder and the vagina, it prevents contact between the two suture lines. For this reason, omentum flaps are used in malignant, complex, and complicated fistulae. However, in cases of a simple fistula, one can safely omit interposition flap since the surrounding tissues are well-vascularized and healthy. There were two failures out of 69 patients in both subgroups in transabdominal repair (2A and 2B). Postoperative abdominal pain and temporary ileus were present in 8.6% (n=3/35) and 5.7% (n=2/35) of patients in subgroup 2A but were completely absent in patients in subgroup 2B. The probable reason for pain could be increased gut motility following enteral feed, which in turn increased omental motility, leading to tension on the fixed omental flap at the vaginal suture line. This phenomenon may be prevented by omitting flap interposition. Mean blood loss and operating time were comparatively higher if the omental flap was used, although this was not statistically significant (217±50.2 versus 198.3±46.3 mL; 168±29.4 versus 154±36.3 minutes, respectively). Evans et al.[19] reported a 100% success rate with the interposition flap in a transabdominal approach for both benign and malignant fistula, 63% success rate without interposition flap in benign fistula, and 67% success rate without interposition flap in malignant fistula. In this study, the low success rate of benign fistula repair was probably due to the history of previous failed repairs in 21% of patients.
To the best of our knowledge, our study is the first English-language, prospective, randomized study in the scientific literature that compares the success rate and complications of simple VVF repair either by transvaginal or transabdominal route, with or without the use of interposition flap.
In conclusion, the success rates are similar in simple VVF repair, irrespective of the use of the interposition flap. However, the overall morbidity following repair with an interposition flap is higher as compared to the repair without interposition flap, either by transvaginal or transabdominal route.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of King George’s Medical University (DRC/38/Uro/KGMU).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.S., R.J.S.; Design - A.A.; Supervision - V.S., S.M.; Resources - V.S., S.M., A.B.; Materials - V.S., A.B.; Data Collection and/or Processing - V.S., A.B.; Analysis and/or Interpretation - V.S., A.A., A.B.; Literature Search - V.S., S.M., R.J.S., A.B.; Writing Manuscript - V.S., R.J.S., A.B.; Critical Review - V.S., R.J.S., S.M., A.A.; Other - R.J.S., V.S., S.M., A.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Sinha HH, Mishra M. A review of urinary fistulae. J Obstet Gynecol India. 2000;50:79–80. [Google Scholar]
- 2.Kapoor R, Ansari MS, Singh P, Gupta P, Khurana N, Mandhani A, et al. Management of Vesicovaginal fistula: An experience of 52 cases with a rationalized algorithm of choosing the transvaginal or transabdominal approach. Indian J Urol. 2007;23:372–6. doi: 10.4103/0970-1591.36709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach G, Trockman B. Surgery for vesicovaginal and urethrovaginal fistula and urethral diverticulum. In: Walsh PC, Retik AB, Vaughan ED Jr, et al., editors. Campbell’s Urology. 7th ed. Philadelphia: WB Saunders; 1998. pp. 1135–53. [Google Scholar]
- 4.Kursh E, Morse R, Resnik M, Persky L. Prevention and development of vesicovaginal fistula. Surg Gynecol Obstet. 1988;166:409–12. [PubMed] [Google Scholar]
- 5.Singh V, Sinha RJ, Sankhwar SN, Sinha SM, Vatsal P, Jain V. Transvaginal repair of complex and complicated vesicovaginal fistulae. Int J Gynaecol Obstet. 2011;144:51–5. doi: 10.1016/j.ijgo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 7.O’Conor VJ. Review of experience with vesicovaginal fistula repair. J Urol. 1980;123:367–9. doi: 10.1016/S0022-5347(17)55939-X. [DOI] [PubMed] [Google Scholar]
- 8.Blaivis JG, Heritz DM, Romanzi LJ. Early versus late repair of vesicovaginal fistulas: vaginal and abdominal approaches. J Urol. 1995;153:1110–1. doi: 10.1097/00005392-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Raz S, Little NA, Juma S. Female urology. In: Walsh PC, Retik AB, Stamey TA, et al., editors. Campbell’s Urology. 6th ed. Philadelphia: WB Saunders; 1992. pp. 2820–5. [Google Scholar]
- 10.Singh V, Sinha RJ, Mehrotra S, Sankhwar SN, Bhatt S. Repair of vesicovaginal fistula by the transabdominal route: outcome at a north Indian tertiary hospital. Int Urogynecol J. 2012;23:411–6. doi: 10.1007/s00192-011-1544-7. [DOI] [PubMed] [Google Scholar]
- 11.Raz S, Bregg KJ, Nitti VW, Sussman E. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol. 1993;150:56–9. doi: 10.1016/S0022-5347(17)35396-X. [DOI] [PubMed] [Google Scholar]
- 12.Margolis T, Elkins TE, Seffah J, Oparo-Addo HS, Fort D. Full-thickness Martius grafts to preserve vaginal depth as an adjunct in the repair of large obstetric fistulas. Obstet Gynecol. 1994;84:148–52. [PubMed] [Google Scholar]
- 13.Elkins TE, DeLancey JO, McGuire EJ. The use of modified Martius graft as an adjunctive technique in vesicovaginal and rectovaginal fistula repair. Obstet Gynecol. 1990;75:727–33. [PubMed] [Google Scholar]
- 14.Ingelmann-Sundberg AGI. Pathogenesis and operative treatment of urinary fistulae in irradiated tissue. In: Youssef AF, editor. Gynaecological Urology. Springfield, Ill: Charles C Thomas; 1960. pp. 263–79. [Google Scholar]
- 15.Browning A. Lack of value of the Martius fibrofatty graft in obstetric fistula repair. Int J Gynecol Obstet. 2006;93:33–7. doi: 10.1016/j.ijgo.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Pshak R, Nikolavsky D, Terlecki R, Flynn BJ. Is tissue interposition always necessary in transvaginal repair of benign, recurrent vesicovaginal fistula. Urology. 2013;82:707–12. doi: 10.1016/j.urology.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 17.Rangnekar NP, Imdad Ali N, Kaul SA, Pathak HR. Role of the martius procedure in the management of urinary-vaginal fistulas fistulas. J Am Coll Surg. 2000;191:259–63. doi: 10.1016/S1072-7515(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 18.Turner-Warwick RT, Wynne EJC, Handley-Ashken M. The use of the omental pedicle graft in the repair and reconstruction of the urinary tract. Br J Surg. 1967;54:849–53. doi: 10.1002/bjs.1800541013. [DOI] [PubMed] [Google Scholar]
- 19.Evans D, Madjar S, Politano VA, Bejany DE, Lynne CM, Gousse AE. Interposition flap in trans-abdominal vesicovaginal fistula repairs: are they really necessary? Urology. 2001;57:670–4. doi: 10.1016/S0090-4295(01)00933-5. [DOI] [PubMed] [Google Scholar]


