Abstract
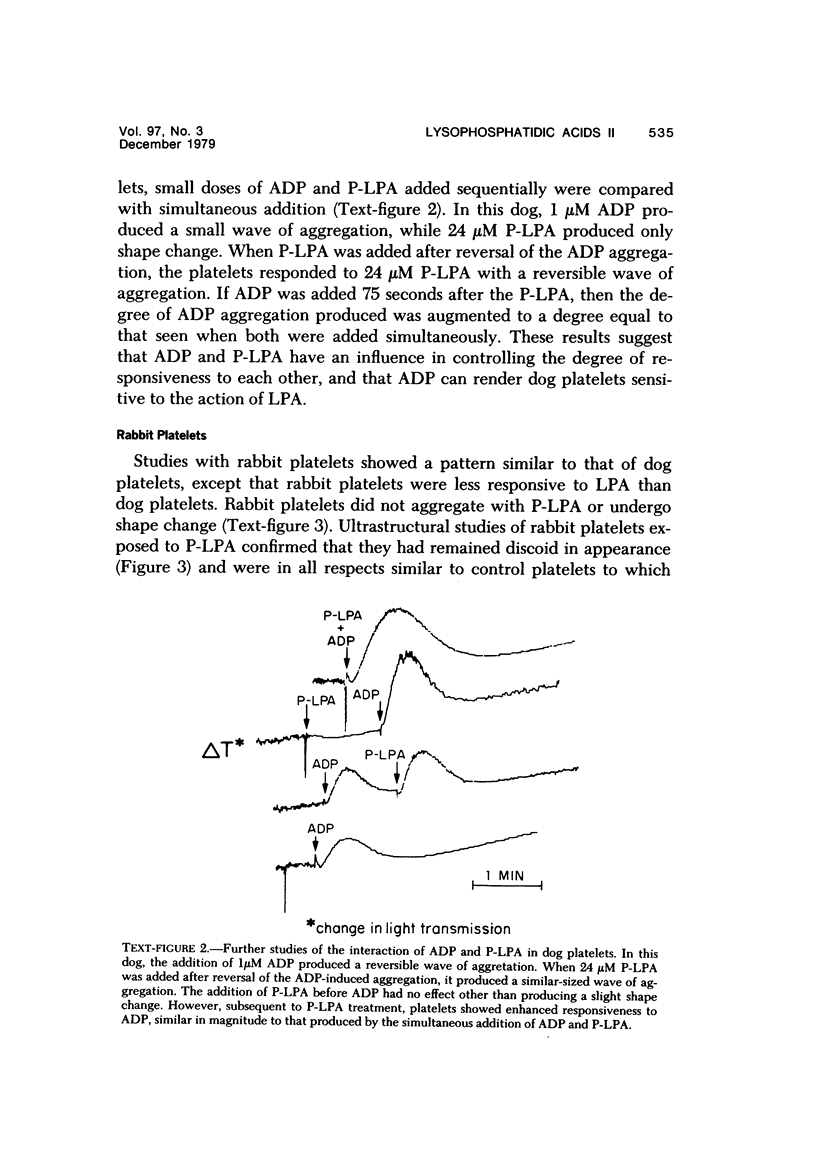
In order to explore a possible relationship between platelet aggregation induced by lysophosphatidic acid (LPA) and that induced by adenosine diphosphate (ADP), we have studied the influence of palmitoyl-LPA (P-LPA) on platelets from dogs and rabbits and on human platelets made refractory to LPA. Dog platelets did not aggregate with P-LPA alone, but P-LPA enhanced ADP aggregation, and after a small dose of ADP, P-LPA was itself effective in causing aggregation and internal contraction in dog platelets. Rabbit platelets showed no response to P-LPA alone, but, as with dog platelets, P-LPA enhanced ADP aggregation. In addition, when P-LPA was added during or immediately after ADP aggregation, it caused a contraction within the platelets and a small wave of aggregation by itself. P-LPA added to human platelets caused aggregation without the need for ADP. However, when a small dose of P-LPA was added to human platelets and the wave of aggregation was allowed to reverse, these platelets subsequently were unresponsive to P-LPA, although they showed an enhanced response to ADP. The addition of a small dose of ADP to the P-LPA refractory platelets partially reversed the refractory state, and the platelets then showed aggregation with P-LPA. The results demonstrate that ADP and P-LPA have significant interactions in their effects on platelets. These interactions are discussed in terms of a two-component mechanism for the ADP-induced intracellular calcium flux, LPA, or possibly phosphatidic acid, being one component.
Full text
PDF












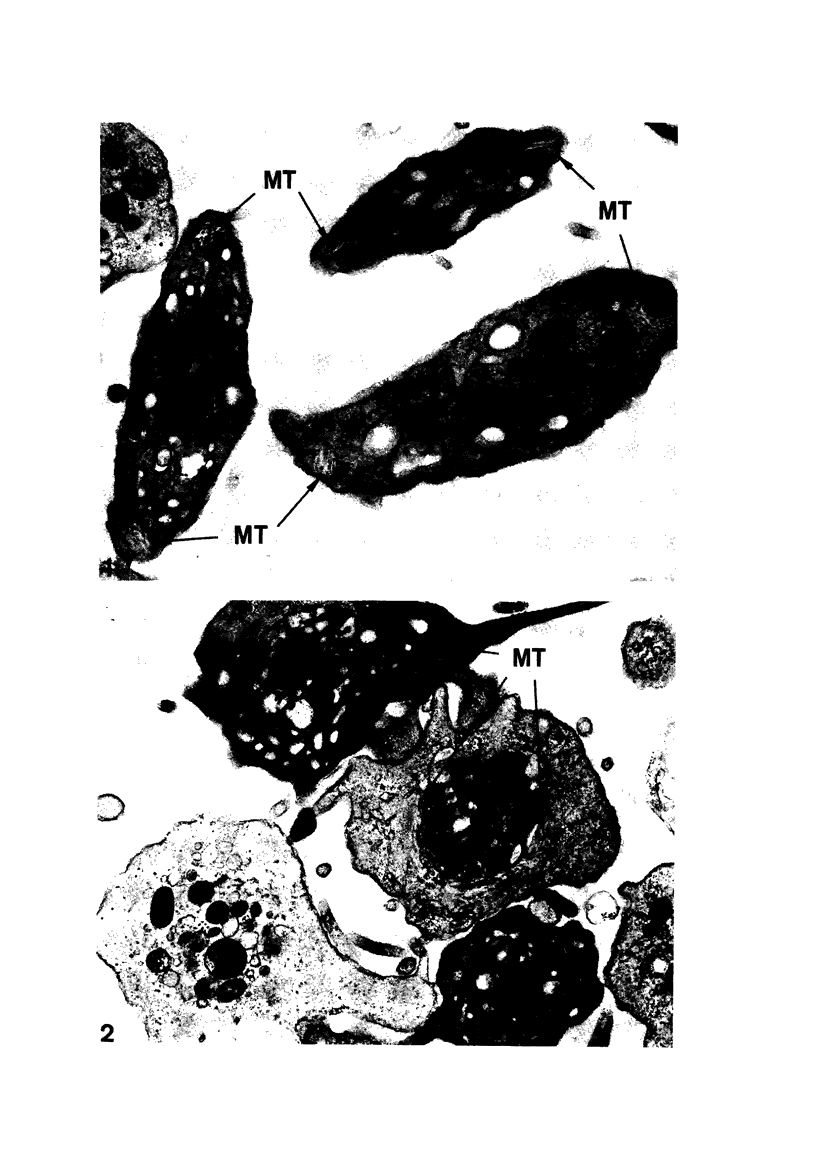


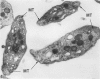
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Benner K. U., Schumacher K. A., Classen H. G. Platelet aggregation induced by DAS in vitro: some investigations on its mechanism of action. Thromb Diath Haemorrh. 1974 May 15;31(2):354–362. [PubMed] [Google Scholar]
- GAARDER A., JONSEN J., LALAND S., HELLEM A., OWREN P. A. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961 Nov 11;192:531–532. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Butler A. M., Peterson D. A., White J. G. Phosphatidic acid releases calcium from a platelet membrane fraction in vitro. Prostaglandins Med. 1978 Nov;1(5):387–396. doi: 10.1016/0161-4630(78)90125-8. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Kindom S. E., Peterson D. A., Peller J., Krantz K. E., White J. G. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am J Pathol. 1979 Aug;96(2):423–438. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G. Prostaglandins and thromboxanes: "middlemen" modulating platelet function in hemostasis and thrombosis. Prog Hemost Thromb. 1978;4:87–125. [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Rao G. H. Effects of the lonophore A23187 on the blood platelets II. Influence on ultrastructure. Am J Pathol. 1974 Nov;77(2):151–166. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G. The influence of aspirin and indomethacin on the platelet contractile wave. Am J Pathol. 1976 Mar;82(3):513–526. [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G. The structure and function of platelets, with emphasis on their contractile nature. Pathobiol Annu. 1976;6:31–59. [PubMed] [Google Scholar]
- HELLEM A. J. The adhesiveness of human blood platelets in vitro. Scand J Clin Lab Invest. 1960;12 (Suppl):1–117. [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim Biophys Acta. 1955 Sep;18(1):102–110. doi: 10.1016/0006-3002(55)90013-5. [DOI] [PubMed] [Google Scholar]
- HOVIG T. The ultrastructure of rabbit blood platelet aggregates. Thromb Diath Haemorrh. 1962 Dec 20;8:455–471. [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Le Breton G. C., Dinerstein R. J. Effect of the calcium antagonist TMB-6 on intracellular calcium redistribution associated with platelet shape change. Thromb Res. 1977 Mar;10(3):521–523. doi: 10.1016/0049-3848(77)90161-x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Haldar J., Mustard J. F. Changes in 32 p-labelling of platelet phospholipids in response to ADP. Br J Haematol. 1972 Nov;23(5):571–585. doi: 10.1111/j.1365-2141.1972.tb07092.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Joist J. H., Mustard J. F. Effect of ADP-induced aggregation on 32 PO 4 incorporation into phosphatidic acid and the phosphoinositides of rabbit platelets. Br J Haematol. 1973 May;24(5):589–604. doi: 10.1111/j.1365-2141.1973.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Mustard J. F. Effect of ADP-induced shape change on incorporation of 32P into platelet phosphatidic acid and mono-, di- and triphosphatidyl inositol. Br J Haematol. 1973 Jul;25(1):77–99. doi: 10.1111/j.1365-2141.1973.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Mauco G., Chap H., Simon M. F., Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978 Sep 29;60(6-7):653–661. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- OLLGAARD E. Macroscopic studies of platelet aggregation. Nature of an aggregating factor in red blood cells and platelets. Thromb Diath Haemorrh. 1961 Jul 15;6:86–97. [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- RODMAN N. F., Jr, MASON R. G., BRINKHOUS K. M. SOME PATHOGENETIC MECHANISMS OF WHITE THROMBUS FORMATION: AGGLUTINATION AND SELF-DESTRUCTION OF THE PLATELET. Fed Proc. 1963 Nov-Dec;22:1356–1365. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K. A., Classen H. G. Platelet aggregation and increased pulmonary vascular resistance in cats induced by DAS and ADP. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(4):373–381. doi: 10.1007/BF00501126. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Vande Zande H., Green D. E. Phospholipids as ionophores. J Biol Chem. 1976 Mar 10;251(5):1326–1332. [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]
- ØLLGAARD E. Clot retraction as a quantitative test of the function and agglutinability of the blood platelets. Acta Haematol. 1951 Oct;6(4):220–230. doi: 10.1159/000203924. [DOI] [PubMed] [Google Scholar]