Abstract
Transposable temperate phages randomly insert into bacterial genomes, providing increased supply and altered spectra of mutations available to selection, thus opening alternative evolutionary trajectories. Transposable phages accelerate bacterial adaptation to new environments, but their effect on adaptation to the social environment is unclear. Using experimental evolution of Pseudomonas aeruginosa in iron-limited and iron-rich environments, where the cost of producing cooperative iron-chelating siderophores is high and low, respectively, we show that transposable phages promote divergence into extreme siderophore production phenotypes. Iron-limited populations with transposable phages evolved siderophore overproducing clones alongside siderophore non-producing cheats. Low siderophore production was associated with parallel mutations in pvd genes, encoding pyoverdine biosynthesis, and pqs genes, encoding quinolone signalling, while high siderophore production was associated with parallel mutations in phenazine-associated gene clusters. Notably, some of these parallel mutations were caused by phage insertional inactivation. These data suggest that transposable phages, which are widespread in microbial communities, can mediate the evolutionary divergence of social strategies.
Keywords: siderophore, temperate phage, cooperation, Pseudomonas aeruginosa, experimental evolution
1. Introduction
Bacteriophages (phages) are viruses of bacteria, outnumbering eukaryotic viruses in abundance and diversity [1]. Phages have been found in almost all environments studied so far, including human gastrointestinal [2,3] and respiratory tracts [4]. Phages can undergo lytic (killing the infected host cell by lysis to transmit horizontally) or lysogenic cycles (integrating into the host chromosome as a prophage to transmit vertically) [5,6], and are defined as either lytic or temperate depending on the absence or presence of a lysogenic cycle, respectively. While lytic phages have been shown to have wide-ranging ecological and evolutionary effects on bacterial populations [7], the influence of temperate phages on bacterial evolution is less well understood.
Although temperate phages are capable of killing their bacterial host cell, integration into the bacterial chromosome as a prophage can establish long-lasting relationships between phage and bacterium [8]. Integration events may benefit the host by fuelling bacterial evolutionary innovation in two ways: First, accessory gene functions encoded on the phage genome can provide or be co-opted to form new bacterial traits (e.g. phage tail-derived bacteriocins [9]). Second, integration events per se can increase the supply of mutations available to selection via insertional inactivation of bacterial genes [10]. For example, mu-like phages insert at random sites, generating inversions, deletions and integration between copies of itself, the chromosome and other mobile elements [8,11]. Infection of Pseudomonas aeruginosa PAO1 with a mu-like phage (D3112) enhances the emergence of antibiotic-resistant colonies through insertional activation of ami genes [12]. The temperate phage LESϕ4 (closely related to mu-like D3112) was isolated from the lung of a cystic fibrosis (CF) patient and allows P. aeruginosa to adapt faster to a sputum-like environment, by increasing the supply of mutations available to P. aeruginosa through prophage insertion into existing chromosomal genes [13].
Studies showing that an elevated bacterial mutation rate can accelerate adaptation to the social environment [14–16] suggest that mu-like phages could have a similar effect. Microbes produce a range of metabolically costly public goods that increase their growth and survival, but which are open to exploitation by non-producing ‘cheats’. Siderophores, secreted by many bacterial species in response to iron starvation [17], are one example of a public good. Siderophore production can be cooperative under nutrient-limited conditions, since it is individually costly to make but the benefits are shared with neighbouring cells [18–20]. Consequently, producing populations are vulnerable to invasion by de novo non-producing cheats, who lose the ability to produce siderophores but still retain the ability to use siderophores produced by others [14–16,19,21,22]. Since cheating confers a fitness advantage in this context, an increased supply of mutations available to selection can increase the rate at which cheats evolve [14,15]. We hypothesize that random integration events by the mu-like temperate phage LESϕ4 will similarly enhance the supply of mutations, increasing the probability of cheats evolving.
Here, we examine the impact of a mu-like transposable temperate phage, LESϕ4, on the evolutionary dynamics of siderophore cooperation in P. aeruginosa. Populations were experimentally evolved in either iron-limited or iron-rich culture conditions where cooperative siderophore production is, respectively, required or not required for growth. We observed that, contrary to our prediction, transposable phage did not drive a greater breakdown of mean siderophore cooperation at the population level. By contrast, transposable phages did promote greater divergence of siderophore production among bacterial clones, and thus led to the coexistence of extreme social strategies within populations under iron limitation.
2. Material and methods
(a). Strains and culturing conditions
We used P. aeruginosa PAO1 as our siderophore-producing wild-type ancestor, and the temperate transposable phage LESϕ4. LESϕ4 inserts randomly into the host genome [13] and displays high rates of lytic activity in chronic CF lung infections, including being induced into the lytic cycle by clinically relevant antibiotics [23].
We confirmed that our PAO1 strain was susceptible to LESϕ4 using a plaque assay: susceptibility was confirmed by a clear plaque on a bacterial lawn formed by phage-mediated lysis. Evolution experiments were performed in casamino acids medium (CAA; 5 g casamino acids, 1.18 g K2HPO4 · 3H2O, 0.25 g MgSO4 · 7H2O per litre). Media was made iron-limited through the addition of sodium bicarbonate to a final concentration of 20 mM, and 100 µg ml−1 of human apotransferrin. Iron-replete media was established by the addition of 20 µM FeSO4. We confirmed that our iron-rich conditions negated the fitness cost experienced by non-producers under iron limitation, by growing wild-type and a siderophore non-producing mutant PAO1ΔpvdDΔpchEF (harbouring loss of function mutations in the two major siderophores pyoverdine and pyochelin; [24] in iron-rich and limited media in a 96-well plate, and measuring optical density (OD) after 24 h (iron-rich: non-producers reach higher densities than wild-type, t-test: t10 = 3.7476, p = 0.004; iron-limited: wild-type reach higher densities than non-producers: Kolmogorov–Smirnov test: D = 1, p = 0.005). Wild-type per capita pyoverdine production (see §2c) was also repressed under iron-rich compared with iron-limited conditions (Welch t-test: t5.13 = 236.84, p < 0.0001). The fitness advantage experienced by non-producers in iron-rich environments has been attributed to the cost of harbouring the siderophore-producing machinery [25].
(b). Evolution experiment
We followed real-time evolutionary changes in the production of the most costly and efficient siderophore, pyoverdine, over time [26–28], in response to the present and absence of LESϕ4, in iron-rich and iron-limited CAA. To ensure that each population was colonized by a single colony (i.e. relatedness = 1 at the first transfer), PAO1 was cultured in 6 ml King's Medium B (KB); (10 g glycerol, 20 g protease peptone no. 3, 1.5 g K2HPO4 · 3H2O, 1.5 g MgSO4 · 7H2O, per litre) for 24 h at 37°C, after which it was diluted with M9 minimal salt solution and grown for 24 h on KB agar at 37°C. A single colony was then selected and grown in KB medium at 37°C for 24 h and subsequently used to establish populations.
To initiate the experiment, twelve 30 ml universal glass tubes were filled with 6 ml iron-limited CAA and 12 with iron-rich CAA. PAO1 was inoculated to a density of 5 × 106 CFU ml−1. In a full factorial design, 106 ml−1 plaque-forming units of LESϕ4 phage was added to six iron-limited and six iron-rich populations (MOI = 1). Cultures were grown shaken at 180 rpm with slightly loose caps, at 37°C.
Every 24 h, 1% of each population was transferred to fresh tubes (iron-rich or limited as appropriate). The experiment was conducted for 30 transfers, and every second transfer populations were mixed with 20% glycerol and frozen at −80°C for further analysis. At transfers 10, 15, 20, 25 and 30, populations were (1) plated on KB agar to assess density (colony forming units (CFU)/ml) and (2) tested for the presence of free phage.
(c). Pyoverdine assays
Every 10 transfers, populations were diluted and plated on the KB agar. Thirty colonies from each population were selected at random, and pyoverdine production quantified for each colony (2160 colonies total). Using sterile toothpicks, individual colonies were transferred to 120 µl KB medium in 96 well plates. Plates were incubated statically for 24 h at 37°C to approximately equalize densities across cultures. 1% of each culture was then transferred to 180 μl iron-limited CAA (siderophore-stimulating conditions) and incubated statically for 24 h at 37°C. Colonies were analysed for pyoverdine production (RFU) using a pyoverdine-specific emission assay [29,30]. Briefly, fluorescence emission of each culture was measured at 460 nm following excitation at 400 nm, using a Tecan infinite M200 pro spectrophotometer. OD was also measured at 600 nm, and the ratio RFU/OD was employed as a quantitative measure of per capita pyoverdine production ([31]). Non-producers were classified as those colonies producing < 5% ancestral pyoverdine levels (less than 6069.25 RFU). Overproducing colonies were in the 95th percentile (greater than 146 590 RFU) for per capita pyoverdine production. Ancestral per capita pyoverdine was 121 385 RFU.
(d). Sequencing evolved clones
To identify the underlying genetic drivers of siderophore cheating in the iron-limited phage treatment, we specifically selected the two highest pyoverdine producers and two lowest producers from each population evolving in this treatment. Since the proportion of siderophore producers relative to non-producers did not always permit this at the final transfer, clones were isolated from the iron-limited phage treatment at either the 10th (Populations 2, 5 and 6) or 30th (Populations 1, 3 and 4) transfer. To test whether observed parallel mutations in these clones are treatment-specific, we next selected four clones at random from every population in the remaining treatments at the final time point (72 clones from 18 populations).
The Wizard Genomic DNA Purification kit (Promega) was used to isolated genomic DNA from overnight cultures, according to the manufacturer's instructions. The quality and quantity of the isolated gDNA was assessed using Nanodrop (Thermo Scientific) and Qubit, respectively. Illumina Nextera XT genomic libraries were prepared by the Centre for Genomic Research, University of Liverpool, and 2 × 250 bp paired-end reads generated on an Illumina MiSeq platform.
Reads were aligned to the PA01 reference (NC_002516.2) using BWA-MEM [32], duplicates removed, and variants detected and filtered using the Genome Analysis Toolkit (GATK) HaplotypeCaller and VariantFiltration tools [33] based on GATK filtering recommendations. Mutations common to all clones were removed from the dataset, as these probably represented divergence between our PAO1 stock and the reference genome. Only variants passing filtering were included in the final dataset (196 mutations across all clones). A further 75 were mutations associated with pf1 bacteriophage. Mutations in Pf1 were present in all 96 sequenced genomes and are therefore likely to be a response to the laboratory environment, rather than selection imposed by phage or iron in our experiment. Hence, these mutations were removed from further analysis. Of the remaining 121 mutations, 12 were synonymous and were excluded from analysis. The remaining 109 mutations comprised our final dataset, consisting of frameshifts (33%), missense mutations (49%), gene deletions (13%) and stop-gained (6%). Evolved clones acquired between 15 and 32 non-synonymous mutations each, with a mean of 16.47 mutations per clone. We further analysed the data to pinpoint the genes in which phage had inserted (SNP calling does not detect phage-mediated mutations [13]). All reads were mapped to the PA01 and phi4 genomes and reads retained where one member of a read pair mapped to PA01 and the other to phi4. From these, reads mapping to the PA01 genome were counted within 1 kbp moving windows and potential insertion sites further inspected in IGV manually [34]. Phage insertion sites were identified in all 48 phage-evolved clones isolated from 12 populations.
(e). Statistical analysis
All data were analysed using R v. 2.15.1 [35]. We used a t-test (for normally distributed data and equal variances) and Kolmogorov–Smirnov test (non-normal distribution and unequal variances) to compare Malthusian growth rates (m) of each strain under iron-rich and iron-limited conditions, respectively. Malthusian growth rate (m) was quantified as ln(final density/starting density) [36]. We used linear mixed-effects models to investigate how iron limitation and phage presence influenced (a) per capita pyoverdine production, (b) variance in pyoverdine production, (c) population density, and (d) numbers of overproducers, over the course of the experiment (electronic supplementary material, table S4). The effect of phages and iron on population density after 24 h growth was tested using a 2-way ANOVA. Finally, after ensuring high and low producers did not differ in the variance of non-synonymous mutations (permutational ANOVA, permutation test: F1,10 = 0.5373, p = 0.491), we performed a permutational multivariate analysis of variance using the adonis function in R to assess mutational differences between phenotypes [37].
3. Results
(a). No effect of temperate phages on population densities
While free phage particles were detected in all populations from phage treatments throughout the experiment, the presence of phages had no effect on bacterial densities, either over the course of the experiment (LMER; p = 0.68, electronic supplementary material figure S4) or after 24 h growth with or without phages (F = 3 × 10−4, p < 0.98). This suggests that any observed differences between treatments propagated with or without phages were unlikely to have been primarily driven by the ecological effects of phage killing [21].
(b). The evolutionary dynamics of pyoverdine production
To obtain a quantitative measure of siderophore production, we calculated per capita pyoverdine production for 30 colonies per population every tenth transfer for 30 transfers. Iron limitation reduced per capita pyoverdine production, but this effect weakened over time (LMER; iron × transfer interaction, p = 0.004; figure 1; electronic supplementary material, figure S3). To a lesser extent, transposable phage presence also reduced per capita pyoverdine production, but again, this effect became weaker over time (LMER; phage × transfer interaction, p = 0.03; figure 1; electronic supplementary material, figure S3). The degree to which iron limitation affected pyoverdine production was not influenced by the presence of phage over the course of the experiment or vice versa (LMER; non-significant iron × phage × transfer interaction, p = 0.9; figure 1; electronic supplementary material, figure S3).
Figure 1.
Changes in per capita pyoverdine production over the course of 30 transfers for 24 populations assigned to one of the following treatments (a) iron-rich, (b) iron-limited, (c) iron-rich and phage, and (d) iron-limited and phage. Iron limitation reduced per capita pyoverdine, but this effect was less strong over time (LMER; iron × transfer interaction, p = 0.004). To a lesser extent, phage presence also reduced per capita pyoverdine production, but again, this effect was reduced over the course of the experiment (LMER; phage × transfer interaction, p = 0.03). The extent to which iron limitation influences pyoverdine production was not influenced by the presence of phage over the course of the experiment, and vice versa (LMER; non-significant iron × phage × transfer interaction, p = 0.9). Note that one population (green) in the iron-limited and phage treatment (d) went extinct by time point 20. Data show means 30 isolated colonies per population ±SEM's. Colours represent different evolving populations (1–6) that can be cross-referenced with other figures in this manuscript. (Online version in colour.)
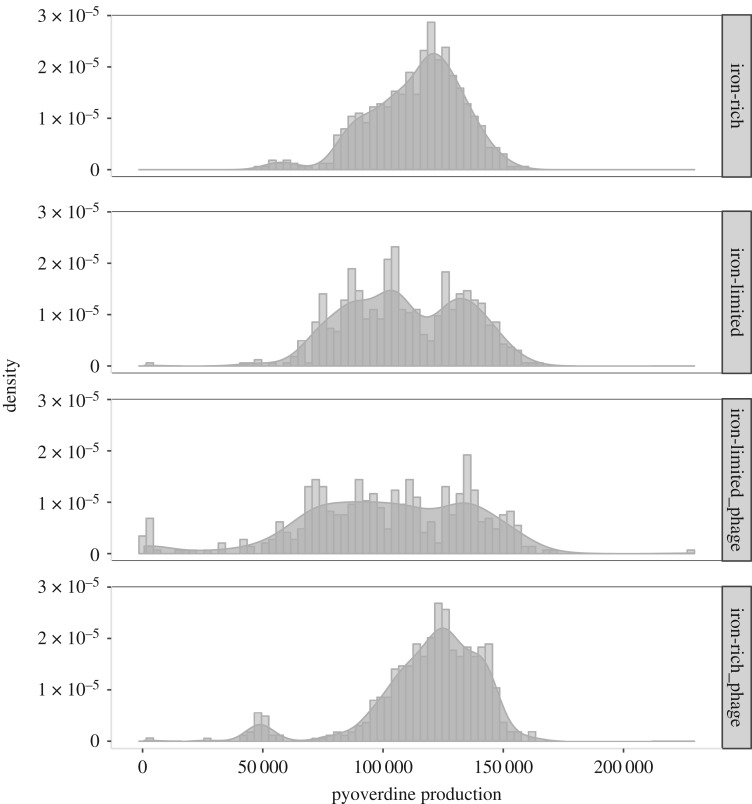
Within-population variance in pyoverdine production was highest in iron-limited populations evolving with phages (LMER; phage × iron interaction, p = 0.0004; figure 2; electronic supplementary material, figures S1–S3), irrespective of time (LMER non-significant phage × iron × transfer interaction p = 0.4981; figure 2; electronic supplementary material, figures S1–S3). Neither iron limitation nor phage presence alone influenced within-population variance (LMER; iron effect p = 0.27; phage effect p = 0.08; figure 2; electronic supplementary material, figures S1–S3), indicating that variation is maximized by social exploitation in the presence of phage.
Figure 2.
Density histogram illustrating variation in pyoverdine production within each treatment. Data show per capita pyoverdine production for 30 colonies isolated per population at each time point (pooling all replicates and time points within a treatment). Within-population variance in pyoverdine production increased only under iron limitation and in the presence of phage (LMER; phage × iron interaction, p = 0.0004), irrespective of time (LMER non-significant phage × iron × transfer interaction p = 0.4981). Neither iron limitation nor phage presence alone influenced within-population variance (LMER; iron effect p = 0.27; phage effect p = 0.08).
To determine whether this higher variance was driven by increased numbers of pyoverdine overproducers, non-producers, or coexistence of both, we analysed the number of non- and overproducing clones in each treatment. Numbers of overproducing clones increased in the presence of phage over time (phage × time point interaction: p = 0.022), and this was not significantly influenced by iron availability (effect of iron: p = 0.4). Equivalent results were obtained using different cut-off values for over-production (phage × time point interaction, 75th: p = 0.01, 80th: p < 0.05, 85th: p < 0.05 and 90th: p < 0.05) suggesting that our observation that the frequency of overproducers increased over time in the presence of phage is robust.
Non-producing clones (n = 17) were identified throughout the experiment, predominantly (in 15/17 cases) in iron-limited populations evolving with phages (electronic supplementary material, table S1). By the final transfer, non-producing mutants had been observed at least once in 5/6 populations from the iron-limited treatment with phages. Together, this suggests that the increased variation in pyoverdine production observed in the iron-limited populations evolved with phages was driven by increased numbers of pyoverdine non-producers in this treatment in addition to increased numbers of overproducers driven by the presence of phage per se.
(c). Genome sequencing of evolved clones
To examine the genetic bases of the observed divergence in pyoverdine production phenotypes observed in iron-limited populations evolved with phages, we obtained whole genome sequences for the two highest- and the two lowest-pyoverdine-producing clones from each population (i.e. 24 clones; 4 clones each from 6 populations). Comparing the highest- (n = 12) and lowest-producing clones (n = 12), we sought to identify mutations distinguishing these classes. First, we analysed all non-synonymous mutations, including SNPs and indels together with genes affected by phage insertional inactivation mutations. High and low producers did not differ in the variance of non-synonymous mutations (permutational ANOVA, permutation test: F1,10 = 0.5373, p = 0.491), but the loci affected by non-synonymous mutations did differ between high and low producers (permutational ANOVA, permutation test: F1,10 = 2.1429, p = 0.014; figure 3; electronic supplementary material, table S2).
Figure 3.
Genetic loci under positive selection in (a) high and (b) low pyoverdine-producing clones evolving with phages in iron-limited media. Each concentric circle represents a replicate population in either (a) high or (b) low producers. Concentric circles correspond to populations 1–6, from the innermost to outermost circle, respectively. Colours represent different evolving populations (1–6) that can be cross-referenced with other figures in this manuscript. Positions around each concentric circle correspond to positions around the PAO1 published and annotated chromosome. Small black dots around these circles indicate the occurrence of an indel or SNP, grey dots represent phage integration events in those regions, and white dots indicate both. Four colonies were selected in total per population: Dot size corresponds to the number of colonies in which a given mutation was observed. When two genes are mentioned, the mutation is intergenic. A complete list of mutations can be found in tables S2 and S3. (Online version in colour.)
To identify loci likely to have been under divergent positive selection between high and low producers, we looked for evidence of phenotype-specific parallel evolution, i.e. pathways targeted by mutation in multiple clones isolated from independently evolving replicate populations that occurred exclusively in either high or low pyoverdine producers. In low producing clones, we observed parallel mutations in quorum-sensing associated loci (pqsA, pqsR; 3/6 populations; 5 clones; figures 3a,b and 4; electronic supplementary material, table S2) and pyoverdine biosynthesis-associated loci (pvdA, pvdD, pvdI, pvdS binding site; 4/6 populations; 8 clones; figures 3a,b and figure 4; electronic supplementary material, table S2). Notably, two of these parallel mutations were caused by prophage-mediated insertional inactivation into pqsA and pvdD genes, respectively. Furthermore, while clones with pqs mutations produced less pyoverdine than the ancestor (figure 4), clones harbouring both pvd and pqs mutations did not produce any detectable pyoverdine (figure 4).
Figure 4.

Pyoverdine production relative to ancestor for all clones harbouring mutations in phz, pvd or pqs associated loci in iron-limited population evolving with phage. Clones are colour coded based on the population from which they originate. phz mutants produce higher pyoverdine relative to the ancestor (output >1), while pvd and pqs mutations are associated with reduced pyoverdine production (output <1). (Online version in colour.)
Exclusive to the highest-producing clones, we observed parallel mutation of a phenazine (phz)-associated intergenic region in 2/6 populations (3 clones), all of which were caused by prophage insertion (figures 3a,b and 4; electronic supplementary material, table S2). These patterns of distinct parallel evolution in high and low producers are suggestive of divergent selection in the iron-limited populations evolving with phages, and, furthermore, shows that prophage-mediated insertional inactivation mutations contributed to the response to this divergent selection. In addition, we observed shared targets of parallel evolution in flagella-associated genes (flgE, flgF, flgG, flgJ, fliF, fliI) in both highest- (2/6 populations; 3 clones) and lowest-producer clones (4/6 populations; 7 clones). This suggests that impairment of flagellar motility was beneficial per se in our experimental setup, and, moreover, since all but one of these mutations were caused by prophage insertional inactivation, that transposable phage mediated this response to selection.
Next, to determine whether the identified targets of parallel evolution were specific to the iron-limited populations evolved with phages, we obtained whole genome sequences for four randomly chosen evolved colonies from each of the replicate populations from the other treatments (electronic supplementary material, table S3). Because, in these treatments, variation among clones in siderophore production was less extreme, colonies from these populations were chosen at random (instead of selecting high and low producers). Importantly, we never observed mutations in pqs, pvd or phz associated loci in any of these other treatments. This suggests that mutations in pqs, pvd or phz associated loci predominantly occurred in iron-limited populations evolved with phages, indicating that these populations followed an evolutionary direction distinct from both iron-rich populations and iron-limited populations evolved without phages.
Populations evolved in iron-rich environments underwent highly parallel evolution of the quorum-sensing master regulator lasR, with 12/12 populations acquiring non-synonymous SNPs or indels irrespective of phage presence or absence. lasR mutations were never caused by prophage insertional inactivation and were never observed in the iron-limited environment, suggesting that loss of lasR was an adaptation to iron-rich conditions and was unaffected by the presence or absence of phages. Mutation of flagella-associated loci was observed in all iron-rich populations irrespective of whether phages were present or absent, confirming that loss of flagellar motility is likely to be adaptive per se in this well-mixed laboratory environment. When phage was present, prophage-mediated insertional inactivation was the primary mode of flagella-associated mutation, occurring in all six replicate populations. Interestingly, under iron limitation, the increased mutational supply provided by transposable phage insertion appears to have promoted loss of flagellar motility: mutation of flagella-associated loci was not observed in iron-limited populations evolved without phage, in contrast to iron-limited populations evolved with phage where such mutations were common and frequently associated with prophage insertional inactivation (described above).
4. Discussion
Transposable phages increase the supply of mutations and thereby can accelerate bacterial adaptation to a new environment [13]. Here, we show that as well as enhancing the response to abiotic environmental selection, transposable phages can also mediate evolutionary responses to the social environment. Populations evolving with phages in an iron-limited environment requiring cooperative siderophore production followed a distinct evolutionary trajectory compared both to populations in the same environment evolved without phages and to populations evolved with or without phage in an iron-rich environment not requiring cooperation. Specifically, within iron-limited populations evolved with phage we observed greater variation in pyoverdine production among bacterial clones. This was caused by a combination of more overproducing mutants evolved in populations with phage (irrespective of iron availability), and the evolution of more under-producing and non-producing mutants in the iron-limited populations with phage, specifically. Our results suggest therefore that transposable phage promoted the evolutionary divergence of the population into more extreme social strategies.
We examined the genetic basis of divergence between the highest- and lowest-producing clones that evolved in the iron-limited treatment with phage. In the lowest-producer class, we observed that pyoverdine synthesis (pvd) and regulators of PQS quorum-sensing (pqs) associated loci were repeatedly targeted by parallel mutations in independent replicate lines. Mutation of pvd-loci encoding pyoverdine synthesis can cause reduced pyoverdine production [38–40] (this study; figure 4) and are likely to explain low pyoverdine production in some clones. However, pvd mutations were not universal among low pyoverdine producers, and one of the evolved clones from the highest-producer class also carried a pvd mutation and maintained ancestral levels of pyoverdine production (electronic supplementary material, table S2). It is likely, therefore, that pqs mutations also played a role in the evolution of reduced pyoverdine production. Consistent with this, P. aeruginosa pqsR null mutants produce reduced pyoverdine compared with wild-type [41] (this study; figure 4), and we could not detect any pyoverdine production in clones harbouring both pqs and pvd mutations (figure 4). Interestingly, the PQS signal molecule acts as an iron-chelator, so the loss of PQS quorum sensing would increase the relative availability of iron, reducing the need for siderophore production [41–43].
Exclusive to the highest-producing clones, we observed parallel mutation of a phenazine (phz)-associated intergenic region, mediated by prophage insertion between phzG2-PA1906, and phzG1-phzS. P. aeruginosa operons phzA1-G1 and phzA2-G2 are involved in biosynthesis of the pyocyanin precursor, phenazine-1-carboxylic acid (PCA). The intergenic region phzG2-PA1906 contains a putative transcriptional terminator 30 bp downstream of the phzG2 stop codon, and the intergenic region directly downstream of the phz1 cluster (phzG1-phzS) contains a ribosome binding site [44]. Hence, it is likely that these mutations impede both stability and efficiency of the translated phenazine product. Since pyocyanin increases iron availability by reducing Fe3+ to bioavailable Fe2+ [45] it is possible that high pyoverdine production in these mutants is a response to reduced iron availability caused by reduced levels of the pyocyanin precursor PCA [40]. Interestingly, pqs mutations in low pyoverdine producers were always found in the same populations as clones with phz mutations—hence, it is possible that pqs mutations in under-producing clones are a response to, or a driver of, phz mutations in coexisting overproducing clones.
Adaptation to the iron-rich environment was associated with acquisition of non-synonymous. SNPs or indels in LasR, the master regulator of acyl-homoserine lactone (AHL) quorum sensing. This pattern was irrespective of phage, occurring both in their presence or absence, and the mutations were not mediated by prophage insertion. Loss of AHL quorum sensing is a common adaptation to the CF lung [13,18,46,47], the Caenorhabditis elegans gut [40,48] as well as laboratory media [40,49]. Similarly, the loss of lasR in CAA is unsurprising—lasR is costly and not required in CAA [42,43,50–52]. Ghoul et al. [52] demonstrated that a lasR mutant grows faster than the wild-type producer in CAA, both when grown alone and in direct competition—showing that mutations in this region are beneficial per se. However, while we found lasR mutations in 12/12 populations evolving under iron-rich conditions, this was never observed under iron limitation. This may be because lasR expression is enhanced by iron limitation [53], suggesting that functions regulated by LasR are beneficial in this environment. An alternative explanation is that iron-limited populations failed to undergo lasR mutations because their densities were lower and their evolutionary potential was correspondingly decreased compared with iron rich (electronic supplementary material, figure S4). However, even under iron-limited conditions, populations reached 108–109 CFU's ml−1, which is roughly tenfold higher than that observed in CF artificial sputum—conditions under which lasR mutations are commonly observed [13]. Hence, density alone is unlikely to explain the lack of lasR mutation under iron-limited conditions.
Previous studies suggest that the ecological effects of phage killing can influence siderophore production (e.g. [16,21]). Vasse et al. [21] reported that LKD16 lytic phages of P. aeruginosa select for pyoverdine non-producers, both in the presence and absence of iron. Similarly, O'Brien et al. [16] found that Pseudomonas fluorescens SBW25 non-producers grow better than producers in the presence of the lytic bacteriophage φ2 under iron-limited conditions. This latter finding was explained by the ‘kill the winner’ hypothesis—the rate of killing is enhanced for the most prevalent genotype; so, there is a fitness advantage to being rare. Although LESφ4 is capable of both lytic and lysogenic infection [23,54], and we found evidence of lysis throughout our experiment, similarly to Davies et al. [13] we observed no effect of LESφ4 on bacterial densities either in the short-term or during the long-term experiment. This suggests that the ecological effect of phage killing is unlikely to explain our finding that transposable phage promote the evolution of both higher and lower siderophore production under iron limitation.
Flagella-associated mutations were common in our shaken liquid cultures frequently caused by prophage-mediated insertional inactivation mutations. Flagella-associated mutations were observed in every (12/12) iron-rich population (irrespective of phage) and were mediated by phage insertional inactivation in every case where phage was present. Under iron-limited conditions, flagella impairment was less common (4/12 populations; 10 clones) and only observed in the presence of phages, where in 9/10 cases this was mediated by prophage insertional inactivation, suggesting that transposable phage promoted the loss of flagellar motility by increasing mutational supply. Lim et al. [55] found that in a closely related species, P. fluorescens, flagella expression was reduced under iron limitation. Hence, it is possible that the selective benefit of losing flagella is reduced in iron-limited compared with iron-rich conditions. However, mutations in flagella-associated loci have been previously observed in P. aeruginosa evolving in an iron-limited liquid culture environment after 150 generations [39] and in iron-limited artificial sputum medium [13]. In our study, population densities were lower under iron starvation so the reduced mutation supply may explain the lack of flagella mutations under iron limitation, which was alleviated by the increased mutational supply afforded by transposable phage (electronic supplementary material, figure S4).
Our results also have applied relevance: P. aeruginosa is a common cause of chronic lung infections in CF and bronchiectasis, where diverse communities of temperate phages also reside and are associated with disease progression and antimicrobial resistance [56]. Analysis of the sputum of CF patients shows that even within a single sample, the coexistence of overproducers alongside non-producers is common for a variety of P. aeruginosa virulence-associated phenotypes, such as pyoverdine, LasA protease and pyocyanin [49,57]. Crucially, temperate phages, including LESϕ4 used in this study, are active in the CF lung [23,56], suggesting that transposable phage may play a role in the generation of genetic diversity in chronic infections.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to acknowledge support from staff at the Centre for Genomic Research, University of Liverpool, and Alexandre Figueiredo for assistance with pyoverdine assays.
Data accessibility
Data are provided as electronic supplementary material.
Authors' contributions
S.O. carried out experimental work and wrote the manuscript. S.O., M.A.B., C.W. and S.P. designed the study and M.A.B., C.W., S.P. and R.K. helped interpret results and provided comments on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by a fellowship from the Centre for Chronic Diseases and Disorders at the University of York to S.O. (2015–2017), a Phillip Leverhulme Prize to MAB, the European Research Council under the grant agreement no. 681295 (R.K.) and the Swiss National Science Foundation no. 31003A_182499 (R.K.).
References
- 1.Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. 2012. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat. Rev. Microbiol. 10, 607–617. ( 10.1038/nrmicro2853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. 2003. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 185, 6220–6223. ( 10.1128/jb.185.20.6220-6223.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M-S, Park E-J, Roh SW, Bae J-W. 2011. Diversity and abundance of single-stranded DNA viruses in human feces. Appl. Environ. Microbiol. 77, 8062–8070. ( 10.1128/aem.06331-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willner D, et al. 2009. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS ONE 4, e7370 ( 10.1371/journal.pone.0007370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little JW. 2005. Lysogeny, prophage induction and lysogenic conversion. In Phages (eds Waldor MK, Friedman A, Adhya S), pp. 37–54. Washington, DC: ASM Press. [Google Scholar]
- 6.Ghosh D, Roy K, Williamson KE, Srinivasiah S, Wommack KE, Radosevich M. 2009. Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl. Environ. Microbiol. 75, 7142–7152. ( 10.1128/aem.00950-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koskella B, Brockhurst MA. 2014. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 38, 916–931. ( 10.1111/1574-6976.12072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison E, Brockhurst MA. 2017. Ecological and evolutionary benefits of temperate phage: what does or doesn't kill you makes you stronger. Bioessays 39, 1700112 ( 10.1002/bies.201700112) [DOI] [PubMed] [Google Scholar]
- 9.Strauch E, Kaspar H, Schaudinn C, Dersch P, Madela K, Gewinner C, Hertwig S, Wecke J, Appel B. 2001. Characterization of enterocoliticin, a phage tail-like bacteriocin, and its effect on pathogenic Yersinia enterocolitica strains. Appl. Environ. Microbiol. 67, 5634–5642. ( 10.1128/aem.67.12.5634-5642.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulo C, Masson P, Le Mercie P, Toussaint A. 2015. A structured annotation frame for the transposable phages: a new proposed family ‘Saltoviridae’ within the caudovirales. Virology 477, 155–163. ( 10.1016/j.virol.2014.10.009) [DOI] [PubMed] [Google Scholar]
- 11.Toussaint A, Rice PA. 2017. Transposable phages, DNA reorganization and transfer. Curr. Opin. Microbiol. 38, 88–94. ( 10.1016/j.mib.2017.04.009) [DOI] [PubMed] [Google Scholar]
- 12.Rehmat S, Shapiro JA. 1983. Insertion and replication of the Pseudomonas aeruginosa mutator phage D3112. Mol. Gen. Genet. 192, 416–423. ( 10.1007/BF00392184) [DOI] [PubMed] [Google Scholar]
- 13.Davies EV, James CE, Williams D, O'Brien S, Fothergill JL, Haldenby S, Paterson S, Winstanley C, Brockhurst MA. 2016. Temperate phages both mediate and drive adaptive evolution in pathogen biofilms. Proc. Natl Acad. Sci. USA 113, 8266–8271. ( 10.1073/pnas.1520056113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison F, Buckling A. 2005. Hypermutability impedes cooperation in pathogenic bacteria. Curr. Biol. 15, 1968–1971. ( 10.1016/j.cub.2005.09.048) [DOI] [PubMed] [Google Scholar]
- 15.Harrison F, Buckling A. 2007. High relatedness selects against hypermutability in bacterial metapopulations. Proc. R. Soc. B 274, 1341–1347. ( 10.1098/rspb.2006.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien S, Rodrigues AMM, Buckling A. 2013. The evolution of bacterial mutation rates under simultaneous selection by interspecific and social parasitism. Proc. R. Soc. B 280, 20131913 ( 10.1098/rspb.2013.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holden VI, Bachman MA. 2015. Diverging roles of bacterial siderophores during infection. Metallomics 7, 986–995. ( 10.1039/c4mt00333k) [DOI] [PubMed] [Google Scholar]
- 18.Fothergill JL, Panagea S, Hart CA, Walshaw MJ, Pitt TL, Winstanley C. 2007. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 7, 45 ( 10.1186/1471-2180-7-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butaitė E, Baumgartner M, Wyder S, Kümmerli R. 2017. Siderophore cheating and cheating resistance shape competition for iron in soil and freshwater Pseudomonas communities. Nat. Commun. 8, 414 ( 10.1038/s41467-017-00509-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sexton DJ, Schuster M. 2017. Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nat. Commun. 8, 230 ( 10.1038/s41467-017-00222-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasse M, Torres-Barceló C, Hochberg ME. 2015. Phage selection for bacterial cheats leads to population decline. Proc. R. Soc. B 282, 20152207 ( 10.1098/rspb.2015.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasse M, Noble RJ, Akhmetzhanov AR, Torres-Barceló C, Gurney J, Benateau S, Gougat-Barbera C, Kaltz O, Hochberg ME. 2017. Antibiotic stress selects against cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 114, 546–551. ( 10.1073/pnas.1612522114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James CE, Davies EV, Fothergill JL, Walshaw MJ, Beale CM, Brockhurst MA, Winstanley C. 2014. Lytic activity by temperate phages of Pseudomonas aeruginosa in long-term cystic fibrosis chronic lung infections. ISME J. 9, 1391–1398. ( 10.1038/ismej.2014.223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghysels B, Dieu BTM, Beatson SA, Pirnay JP, Ochsner UA, Vasil ML, Cornelis P. 2004. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150, 1671–1680. ( 10.1099/mic.0.27035-0) [DOI] [PubMed] [Google Scholar]
- 25.Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027. ( 10.1038/nature02744) [DOI] [PubMed] [Google Scholar]
- 26.Visca P, Imperi F, Lamont IL. 2007. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 15, 22–30. ( 10.1016/j.tim.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 27.Youard ZA, Wenner N, Reimmann C. 2011. Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. Biometals 24, 513–522. ( 10.1007/s10534-010-9399-9) [DOI] [PubMed] [Google Scholar]
- 28.Dumas Z, Ross-Gillespie A, Kümmerli R. 2013. Switching between apparently redundant iron-uptake mechanisms benefits bacteria in changeable environments. Proc. R. Soc. B 280, 20131055 ( 10.1098/rspb.2013.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ankenbauer R, Sriyosachati S, Cox CD. 1985. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect. Immun. 49, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox CD, Adams P. 1985. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 48,130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kümmerli R, Jiricny N, Clarke LS, West SA, Griffin AS. 2009. Phenotypic plasticity of a cooperative behaviour in bacteria. J. Evol. Biol. 22, 589–598. ( 10.1111/j.1420-9101.2008.01666.x) [DOI] [PubMed] [Google Scholar]
- 32.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv: 1303.3997v2.
- 33.McKenna A, et al. 2010. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303. ( 10.1101/gr.107524.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorvaldsdottir H, Robinson JT, Mesirov JP. 2012. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192. ( 10.1093/bib/bbs017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 36.Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138, 1315–1341. ( 10.1086/285289) [DOI] [Google Scholar]
- 37.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. [Google Scholar]
- 38.Lamont IL. 2003. Identification and characterization of novel pyoverdine synthesis genes in Pseudomonas aeruginosa. Microbiology 149, 833–842. ( 10.1099/mic.0.26085-0) [DOI] [PubMed] [Google Scholar]
- 39.Kümmerli R, Santorelli LA, Granato ET, Dumas Z, Dobay A, Griffin AS, West SA. 2015. Co-evolutionary dynamics between public good producers and cheats in the bacterium Pseudomonas aeruginosa. J. Evol. Biol. 28, 2264–2274. ( 10.1111/jeb.12751) [DOI] [PubMed] [Google Scholar]
- 40.Granato ET, Ziegenhain C, Marvig RL, Kümmerli R. 2018. Low spatial structure and selection against secreted virulence factors attenuates pathogenicity in Pseudomonas aeruginosa. ISME J. 12, 2907–2918. ( 10.1038/s41396-018-0231-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popat R, Harrison F, da Silva AC, Easton SAS, McNally L, Williams P, Diggle SP. 2017. Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. Proc. R. Soc. B 284, 20170200 ( 10.1098/rspb.2017.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414. ( 10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- 43.Diggle SP, et al. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 14, 87–96. ( 10.1016/j.chembiol.2006.11.014) [DOI] [PubMed] [Google Scholar]
- 44.Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. 2001. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183, 6454–6465. ( 10.1128/jb.183.21.6454-6465.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox CD. 1986. Role of pyocyanin in the acquisition of iron from transferrin. Infect. Immun. 52,263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith EE, et al. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 103, 8487–8492. ( 10.1073/pnas.0602138103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaFayette SL, et al. 2015. Cystic fibrosis-adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci. Adv. 1, e1500199 ( 10.1126/sciadv.1500199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen G, Crummenerl LL, Gilbert F, Mohr T, Pfefferkorn R, Thänert R, Rosenstiel P, Schulenburg H. 2015. Evolutionary transition from pathogenicity to commensalism: global regulator mutations mediate fitness gains through virulence attenuation. Mol. Biol. Evol. 32, 2883–2896. ( 10.1093/molbev/msv160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien S, Williams D, Fothergill JL, Paterson S, Winstanley C, Brockhurst MA. 2017. High virulence sub-populations in Pseudomonas aeruginosa long-term cystic fibrosis airway infections. BMC Microbiol. 17, 30 ( 10.1186/s12866-017-0941-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandoz KM, Mitzimberg SM, Schuster M. 2007. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc. Natl Acad. Sci. USA 104, 15 876–15 881. ( 10.1073/pnas.0705653104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darch SE, West SA, Winzer K, Diggle SP. 2012. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc. Natl Acad. Sci. USA 109, 8259–8263. ( 10.1073/pnas.1118131109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghoul M, West SA, Diggle SP, Griffin AS. 2014. An experimental test of whether cheating is context dependent. J. Evol. Biol. 27, 551–556. ( 10.1111/jeb.12319) [DOI] [PubMed] [Google Scholar]
- 53.Bollinger N, Hassett DJ, Iglewski BH, Costerton JW, McDermott TR. 2001. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 183, 1990–1996. ( 10.1128/jb.183.6.1990-1996.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James CE, et al. 2012. Differential infection properties of three inducible prophages from an epidemic strain of Pseudomonas aeruginosa. BMC Microbiol. 12, 216 ( 10.1186/1471-2180-12-216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim CK, Hassan KA, Tetu SG, Loper JE, Paulsen IT. 2012. The effect of iron limitation on the transcriptome and proteome of Pseudomonas fluorescens Pf-5. PLoS ONE 7, e39139 ( 10.1371/journal.pone.0039139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tariq MA, et al. 2019. Temperate bacteriophages from chronic Pseudomonas aeruginosa lung infections show disease-specific changes in host range and modulate antimicrobial susceptibility. mSystems 4, e00191-18 ( 10.1128/msystems.00191-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mowat E, Paterson S, Fothergill JL, Wright EA, Ledson MJ, Walshaw MJ, Brockhurst MA, Winstanley C. 2011. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am. J. Respir. Crit. Care Med. 183, 1674–1679. ( 10.1164/rccm.201009-1430OC) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided as electronic supplementary material.