Abstract
Background
Recent literature supports the removal of myomas during cesarean section, which traditionally was considered a relative contraindication, given a higher complication rate. This study is to share our experience of cesarean myomectomy in the last decade.
Methods
This study is a retrospective review of our prospectively maintained database, from January 2008 to December 2017, at a tertiary care level teaching institution. All patients who underwent myomectomy during cesarean section were included. There were no exclusions.
Results
A total of twenty patients underwent myoma removal along with the cesarean operation during this period with a mean age of 30 years. Majority of patients were nulliparous (70%). Common comorbidities were diabetes mellitus (40%) and hypothyroidism (20%). Mean size of myomas were 5.33 cm (± 2.08), and the number varied from one to three. The most common location was the posterior surface of the uterus with the commonest variety being subserous. Most patients were discharged on the fifth postoperative day.
Conclusion
This study demonstrates that cesarean myomectomy to be a safe and feasible procedure in experienced hands. It offers the advantage of avoiding a second surgery in selected patients.
Keywords: Cesarean myomectomy, Myoma uterus, Cesarean delivery, Myomectomy
Introduction
The myomas are the most common benign tumors of the uterus. The highest reported incidence of myomas during pregnancy is 10.7% [1]. The myomas are common during the reproductive period due to an influence of higher estrogen levels. Their increased incidence during pregnancy can be partly explained by the late age of conception [2]. Myomas in pregnancy are associated with a significantly higher risk for cesarean section (CS).
Conventionally, concomitant myomectomy during CS was considered a relative contraindication, given perceived higher complication rate. Last decade has witnessed emerging literature evidence supporting myoma excision along with CS, with acceptable results [3]. This study aims to share our experience of cesarean myomectomy during the last decade.
Materials and Methods
This study is a retrospective review of our prospectively maintained database of the last decade, from January 2008 to December 2017, at a tertiary care teaching hospital. All consecutive patients who underwent myomectomy during cesarean section, in this mentioned duration, were included. This formed a small subset, among the patients, having uterine myomas and pregnancy, a majority of those underwent vaginal delivery or had LSCS, without myomas removal. Those not matching the inclusion were not considered. There were no exclusions. The institutional ethical committee approved the study, and waiver of consent was obtained, in view of retrospective nature.
Data were collected from outpatient and inpatient records, operative and discharge notes. Data included maternal demographics such as age, parity, medical diseases, prior surgery, gestational age at the time of CS, type of anesthesia, indication for CS, elective/emergency and number of previous operations including CS. Myoma characteristics such as size, number, location and variety were noted. Other parameters like an indication for myomectomy, the number of incisions made and the presence of adhesions were observed. The total duration of surgery was taken as the time from the initial skin incision to skin closure. Approximate intraoperative blood loss was measured, and record of transfusion if any was noted. Postoperative drop in hemoglobin was checked at 48 h after surgery, and blood transfusion was given if the fall was more than 3 gm/dl. Postoperative complications like pyrexia (> 38-degree C) and wound infection were analyzed. Duration of hospital stay was noted, and patients were followed up after 2 weeks in the outpatient department.
Patients were counseled during the early third trimester regarding the need for myomectomy depending on the mode of delivery and consent obtained for the same.
Experienced gynecologists performed surgery with minimum of 10 years of experience. Preoperatively, half an hour before skin incision Inj. Tranexamic acid 1 gm in 100 ml isotonic saline was infused over 20 min. Except in four cases, myomas were situated at the incision site, and in other instances, CS preceded myomectomy. When myomas were located at the lower uterine segment, myomectomy was performed before delivery of the fetus. During CS, myomas were inspected for their size, number, type and location after the closure of the uterine incision (Fig. 1).
Fig. 1.

Multiple fibroids over the anterior surface of uterus postdelivery of the baby
Though myomectomy was tried with a single incision, in few cases where multiple fibroids were present, more than one incision over uterus was needed. An incision was made over myoma by using monopolar cautery, and the tumor was enucleated. To achieve hemostasis, bipolar cautery was used. The defect in the myometrium was closed with 1-0 vicryl suture (Fig. 2). High-dose oxytocin infusion was continued postoperatively for 24 h to prevent bleeding.
Fig. 2.
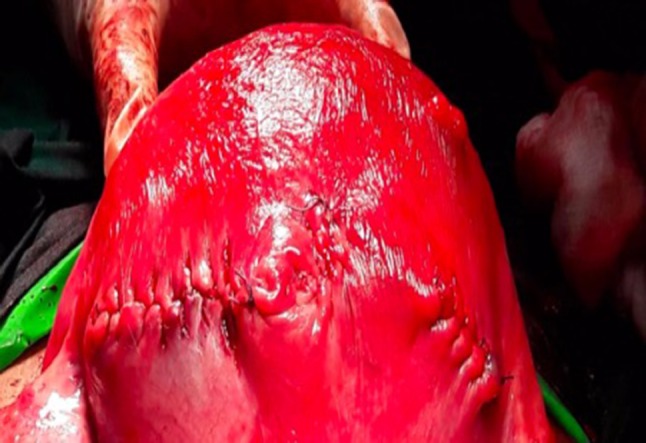
Postmyomectomy, closure by baseball sutures
Results
A total of twenty patients underwent myoma removal along with the cesarean operation during this period with a mean age of 30 years. Majority of patients were nulliparous (70%). Common comorbidities were diabetes mellitus (40%) and hypothyroidism (20%). One except all patients received regional anesthesia (spinal/epidural); the only case needed general anesthesia due to the presence of idiopathic intracranial hypertension. Sixty-five percent of surgeries were elective operations. The details of various preoperative characteristics are shown in Table 1.
Table 1.
Demographic and preoperative characteristic of patients (n = 20)
| Sr. No. | Variable | Value (mean ± SD) |
|---|---|---|
| 1. | Age (in years) | 30.85 ± 3.84 |
| 2. | Parity | |
| (a) Nulliparity | 14 | |
| (b) Multiparity | 06 | |
| 3. | Gestational Age | 37.3 ± 1.08 |
| 4. | Number of prior surgeries | |
| (a) Nil | 11 | |
| (b) LSCS | 06 | |
| (c) Myomectomy | 03 |
SD standard deviation, LSCS lower segment cesarean section
Intraoperatively, two patients had small bowel and omental adhesions at the operative site. Two patients (10%) received a blood transfusion during the procedure. The mean hemoglobin drop reported 24 h postoperative was 1.23 (± 1.16) grams. No patient warranted blood transfusion in the postoperative period (Table 2).
Table 2.
Intraoperative and follow-up variables (n = 20)
| Sr. No. | Variable | Value (mean ± SD) |
|---|---|---|
| 1. | Size of myoma (cm) | 5.33 ± 2.08 |
| 2. | Number of myoma (mean and range) | 1 (1–3) |
| 3. | Location of tumor | |
| (a) Posterior | 07 | |
| (b) Anterior | 06 | |
| (c) Right lateral wall | 04 | |
| (d) Fundal | 04 | |
| (e) Left lateral wall | 03 | |
| (f) Lower uterine segment | 04 | |
| 4. | Type of Myoma | |
| (a) Subserous | 11 | |
| (b) Intramural | 09 | |
| (c) Submucosal | 02 | |
| 5. | Operating time (minutes) | 79 ± 35.35 |
| 6. | Blood loss (mL) | 876 ± 386.9 |
| 7. | Hospital stay (days) | 5.5 ± 1.43 |
SD standard deviation
Majority of patients got the discharge on the fifth day of surgery. There were no incidences of postpartum pyrexia or surgical site infections. The patients were regularly followed in the outpatient department.
Discussion
Fibroid excision along with caesarian section was considered difficult until recently. Fibroids with pregnancy might cause miscarriage, preterm delivery, premature rupture of membranes, early separation of the placenta, labor dystocia, postpartum hemorrhage and increased rate of operative deliveries. Fibroids might also undergo red degeneration and associated complications. Myomectomy during CS has not been done routinely due to the risk of uncontrolled bleeding and the possibility of hysterectomy [4]. If myomectomy is not performed along with CS patient might need another surgery to remove it, which amounts to two operations, two anesthetic exposures and increasing cost, and there is also an enhanced risk of complications caused by fibroids in a subsequent pregnancy.
The average age of mothers in our study was 30 years. Most studies have shown myomas to be more common in nulliparous women [5]. We too observed a higher incidence of myomas among nulliparous women (70%) [6]. This could be explained due to hormonal changes and reduced sensitivity of leiomyoma to estrogen receptor [7].
Similar to many other studies, the average gestational age at the time of CS was 37 to 38 weeks in our study [4, 6, 8]. We had four patients delivering at 35 to 36 weeks of gestation. Among these four patients who had delivered prematurely, three deliveries can be attributed to the presence of fibroids. Two out of these patients have already undergone fibroid removal before becoming pregnant. The possible explanation for preterm labor could be decreased distensibility of uterine myometrium due to the presence of fibroids as the pregnancy advances [9].
Though CM is to be avoided in cases of the previous myomectomy due to more chances of complications [10], we had the opportunity to do repeat CS for one of our patient who underwent CS myomectomy 3 years ago. The indication for primary CS was a transverse lie. The malpresentation was suspected to be due to a single large (8 cm), intramural fibroid located in the lower uterine segment. Incidentally, this was the largest myoma we had come across in our study. As the fibroid was situated in the incision site, myomectomy was followed by CS. During repeat CS, which was done 3 years later, there was no fibroid detected intraoperatively. There were three more cases of lower uterine segment (LUS) fibroids; if they were less than 5 cm, then myomectomy were done following the delivery of the baby. All fibroids present in the LUS were removed through the same CS incision, without any additional incision on the uterus. The same technique was suggested in several other studies [11, 12].
Three of our patients had undergone myomectomy previously in the non-pregnant state. One of the women had undergone myomectomy twice before becoming pregnant. This confirms the well-established fact that fibroids are recurrent [13]. Among the study group, the patients who had undergone previous myomectomies had a high incidence of intraoperative adhesions as expected. However, in all three patients, the surgery was uneventful with minimal blood loss and no added morbidity.
Eight of our study subjects had multiple fibroids. Six patients had fibroids of size 8 cm (largest dimension). 55% of the myomas had size > 5 cm. The smallest dimension encountered was 2.5 cm. We had more anterior wall fibroids than posterior [4]. Traditionally myomectomy is avoided in cases of fundal and cornual sites because of excessive bleeding and possible obstruction of fallopian tubes [14, 15], but in our study, myomectomy was done regardless of the location of the myomas.
Except for a single instance of the submucosal fibroid, there was an even distribution of intramural and subserosal fibroids. Removal of submucosal fibroids during cesarean section is usually avoided as it involves resection of the full thickness of myometrium and reduced contractility might lead on to increased hemorrhage [16]. Secondary changes due to red degeneration were found in a case with single sizeable fundal fibroid. Histopathological reports of all myomectomy specimens were closely followed up, and none showed malignant change.
The same incision removed fibroids in the anterior wall and close to CS incision. Whenever possible, a minimal number of incisions were made, and adjacent ones removed through the same incision. This was done to reduce blood loss and future adhesions and improve reproductive outcome. While the operating time was more or less uniform in most of our cases, two of the cases exceeded the average operating time significantly. In these two instances, the increased operating time could be explained by multiple fibroids needing more than a single incision for removal. Other studies have also observed more than average operating time in cases of multiple fibroids [17].
Intraoperative blood transfusion was done in two cases only as they met the criteria for blood loss of more than one liter. One case had a fibroid at the incision site. The second patient who had a loss of more than two liters which was the largest in our series had three fibroids at different locations and they were removed by as many incisions. Despite intraoperative transfusion and other pharmacological measures to attain hemostasis, she had a significant drop in hemoglobin at 48 h postoperatively and needed another transfusion. She had no other postoperative morbidity like pyrexia or ileus and was discharged on the fourth day after surgery.
CM is not done routinely, because of the associated hemorrhage and the need for the hysterectomy [15]. Specific hemostatic techniques have been advocated by various authors [18, 19], but we have followed tranexamic acid infusion, vasopressin instillation, uterine artery ligation, electrocautery and high-dose oxytocin. There was not a single case which needed a hysterectomy in our study of twenty cases, though patients were counseled regarding the possibility in the prenatal period due to intractable hemorrhage. We also found no increased postoperative morbidity or expanded hospital stay in any of our patients who underwent CM as was observed by Dam Hye Kwon et al. [8].
There are a few limitations to our study. Its retrospective design coupled with proportionally small sample size limits generalization of results. This study also lacks long-term follow-up of these patients and thus to highlight effect on future pregnancies. Additionally, as this study was conducted in a tertiary level medical college setup with available expertise, the results cannot be generalized, especially in comparison with smaller installations or in the absence of an expert.
We author would like to stress this to the readers that routine use of myomectomy during CS is neither advisable nor been followed in the current study. The CM should be carefully planned for selected patients in an appropriate setup and expertise. A similar conclusion is drawn by a recent review on this topic [20], recommending standardization of best practices for CM, with the availability of more robust data and in the presence of necessary facilities.
Conclusion
Overall, our series has shown CM to be a safe and feasible procedure in well-selected patients in a tertiary care center with experienced hands. It can offer an advantage of avoiding a second intervention in selected patients. Appropriate hemostatic techniques can curtail hemorrhage which is the most severe complication. Long-term morbidity concerning future reproductive outcome should be addressed in a study with large participants and reliable design.
Dr. T. Ramya
obtained her M.B.B.S. from Coimbatore Medical College and her post-graduation M.D. in Obstetrics and Gynaecology from the Institute of Obstetrics and Gynaecology, Egmore, Chennai. Her fields of interest include Obstetrics, Ultrasound, and Fetal Medicine. She is trained in laparoscopy and Infertility at FOGSI-recognized centers. She is working as an Associate Professor at the PSG Institute of Medical Sciences and Research, Coimbatore.
Authors’ Contributions
All authors have a significant contribution for preparation of this manuscript, following ICMJE Guidelines. First and second authors share the first position.
Compliance with Ethical Standards
Conflict of interest
No author has any potential conflict of interest.
Ethical approval
The current study is approved by the responsible institutional ethics committee for human research and fully comply with the declaration of Helsinki for guideline on human research.
Footnotes
Dr. T. Ramya, Associate Professor in Department of Obstetrics and Gynaecology at PSG Institute of Medical Science and Research (PSGIMSR), Coimbatore, Tamil Nadu, India; Shraddha S. Sabnis, Assistant Professor in Department of Obstetrics and Gynaecology at PSG Institute of Medical Science and Research (PSGIMSR), Coimbatore, Tamil Nadu, India; T. V. Chitra, Professor in Department of Obstetrics and Gynaecology at PSG Institute of Medical Science and Research (PSGIMSR), Coimbatore, Tamil Nadu, India; Seetha Panicker, Professor in Department of Obstetrics and Gynaecology at PSG Institute of Medical Science and Research (PSGIMSR), Coimbatore, Tamil Nadu, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laughlin SK, Baird DD, Savitz DA, et al. Prevalence of uterine leiomyomas in the first trimester of pregnancy. Obstet Gynecol. 2009;113(3):630–635. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian J, Hu W. Cervical leiomyomas in pregnancy: report of 17 cases. Aust N Z J Obstet Gynaecol. 2012;52(3):258–261. doi: 10.1111/j.1479-828X.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 3.Sparić R, Kadija S, Stefanović A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43(5):798–804. doi: 10.1111/jog.13294. [DOI] [PubMed] [Google Scholar]
- 4.Incebiyik A, Hilali NG, Camuzcuoglu A, et al. Myomectomy during caesarean: a retrospective evaluation of 16 cases. Arch Gynecol Obstet. 2014;289(3):569–573. doi: 10.1007/s00404-013-3019-1. [DOI] [PubMed] [Google Scholar]
- 5.Sparic R, Mirkovic L, Malvasi A, et al. Epidemiology of uterine myomas: a review. Int J Fertil Steril. 2016;9(4):424–435. doi: 10.22074/ijfs.2015.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanthi JM, Sumathy S, Sreedhar S, et al. Comparative study of cesarean myomectomy with abdominal myomectomy in terms of blood loss in single fibroid. J Obstet Gynecol India. 2016;66(4):287–291. doi: 10.1007/s13224-015-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African–American women: a prospective study. Am J Epidemiol. 2004;159(2):113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon DH, Song JE, Yoon KR, et al. The safety of cesarean myomectomy in women with large myomas. Obstet Gynecol Sci. 2014;57(5):367. doi: 10.5468/ogs.2014.57.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y-H, Lin H-C, Chen S-F, et al. Increased risk of preterm births among women with uterine leiomyoma: a nationwide population-based study. Hum Reprod. 2009;24(12):3049–3056. doi: 10.1093/humrep/dep320. [DOI] [PubMed] [Google Scholar]
- 10.Sparić R, Berisavac M, Buzadzić S, et al. Complications during cesarean delivery in a patient with two previous myomectomies. Acta Chir Iugosl. 2013;60(1):99–100. doi: 10.2298/ACI1301099S. [DOI] [PubMed] [Google Scholar]
- 11.Yellamareddygari S, Chakrabarti M, Ravuri S, et al. Leaving fibroids at caesarean section, is it safe? Gynecol Surg. 2010;7(2):173–175. doi: 10.1007/s10397-008-0441-7. [DOI] [Google Scholar]
- 12.Mahendru R, Sekhon P, Gaba G, et al. At times, myomectomy is mandatory to effect delivery. Ann Surg Innov Res. 2011;5(1):9. doi: 10.1186/1750-1164-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ande ABA, Ehigiegba AE, Umeora OUJ. Repeat myomectomy at caesarean section. Arch Gynecol Obstet. 2004;270(4):296–298. doi: 10.1007/s00404-003-0528-3. [DOI] [PubMed] [Google Scholar]
- 14.Celal K, Hülya Ç. The evaluation of myomectomies performed during cesarean section in our clinic. Niger Med J. 2011;52(3):186. doi: 10.4103/0300-1652.86135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song D, Zhang W, Chames MC, Guo J. Myomectomy during cesarean delivery. Int J Gynecol Obstet. 2013;121(3):208–213. doi: 10.1016/j.ijgo.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Gbadebo AA, Charles AA, Austin O. Myomectomy at caesarean section: descriptive study of clinical outcome in a tropical setting. J Ayub Med Coll Abbottabad. 2009;21(4):7–9. [PubMed] [Google Scholar]
- 17.Ramesh Kumar R, Patil M, Sa S. The utility of caesarean myomectomy as a safe procedure: a retrospective analysis of 21 cases with review of literature. J Clin Diagn Res. 2014;8(9):OC05–OC08. doi: 10.7860/JCDR/2014/8630.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shavell VI, Thakur M, Sawant A, et al. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil Steril. 2012;97(1):107–110. doi: 10.1016/j.fertnstert.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee J-H, Cho D-H. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(S1):35–37. doi: 10.1007/s00404-010-1760-2. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68(6):432–436. doi: 10.1007/s13224-018-1114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


