Abstract
Ewing sarcoma (EWS) is the second most common and aggressive type of metastatic bone tumor in adolescents and young adults. There is unmet medical need to develop and test novel pharmacological targets and novel therapies to treat EWS. Here, we found that EWS expresses high levels of a p53 isoform, delta133p53. We further determined that aberrant expression of delta133p53 induced HGF secretion resulting in tumor growth and metastasis. Thereafter, we evaluated targeting EWS tumors with HGF receptor neutralizing antibody (AMG102) in preclinical studies. Surprisingly, we found that targeting EWS tumors with HGF receptor neutralizing antibody (AMG102) in combination with GD2-specific, CAR-reengineered T cell therapy synergistically inhibited primary tumor growth and establishment of metastatic disease in preclinical models. Furthermore, our data suggested that AMG102 treatment alone might increase leukocyte infiltration including efficient CAR-T access into tumor mass and thereby improves its antitumor activity. Together, our findings warrant the development of novel CAR-T-cell therapies that incorporate HGF receptor neutralizing antibody to improve therapeutic potency, not only in EWS but also in tumors with aberrant activation of the HGF/c-MET pathway.
Keywords: Ewing Sarcoma, Metastasis, delta133p53, HGF, AMG102, CAR-T Cell Therapy, Preclinical Studies
Introduction
Ewing sarcoma (EWS) develops most commonly in the midportion of long bones or in soft tissue locations, and is the second most common solid bone malignancy diagnosed in pediatric and young adolescent populations 1. Outcomes in patients with EWS who present with localized disease have improved over the past several decades, largely due to advances in multimodal therapy and supportive care 1. However long-term outcomes in patients with metastatic, relapsed, or treatment-refractory EWS are dismal, with survival rates less than 20% 2. Consequently, new therapies for this disease are critically needed.
Hepatocyte growth factor (HGF) is a multifunctional cytokine composed of an amino-terminal domain and four kringle domains in the alpha chain and a serine protease homology domain in the beta chain 3. The HGF is produced by mesenchymal cells, while its receptor c-MET is primarily expressed in epithelial cells 4. HGF/c-MET-mediated cross-talk between the epithelial and stromal compartments is required for normal physiological processes, and it is tightly regulated 5. In particular, the role of HGF as mediator of the interactions between cancerous cells and adjacent stroma seems to be fundamental to create a microenvironment that promotes the further development and invasiveness of cancer 6. For example, upregulation of HGF and the overexpression and activation of cMET are observed in breast, head and neck, lung, prostate, renal, colorectal, and hepatocellular carcinomas as well as myeloma, glioblastoma and ovarian cancer 7–10. Activation of the HGF/c-MET axis in these tumors induces different phenotypes depending on tumor stage, inducing proliferation and angiogenesis in primary tumors, stimulating motility to form micrometastases, and regaining the proliferation phenotype to form overt metastases 6, 11, 12. Thus, both HGF and cMET are promising therapeutic targets 13.
The tumor suppressor protein p53 plays a pivotal role in the prevention of oncogenic transformation. Cancers frequently evade the potent antitumor surveillance mechanisms of p53 through mutation of the TP53 gene, with approximately 50% of all human malignancies expressing dysfunctional, mutated p53 proteins. Remarkably, the TP53 gene encodes 12 isoforms that moderate p53 activities as well as having independent functions 14. Several isoforms are dysregulated in human tumors leading to the suggestion that they promote tumor formation 15. Therefore, specific efforts focused on mechanistic understanding and biologic consequences of dysregulation of these isoforms in cancer will help develop new therapeutic approaches.
Chimeric antigen receptors (CARs) are synthetic receptors that target and reprogram T-cells to acquire augmented antitumor properties 16 and have demonstrated potent clinical efficacy in patients with B-cell malignancies 17. For instance, chimeric antigen receptor-engineered GD2-specific T cells efficiently interact with GD2-expressing neuroblastoma cells in vitro, resulting in specific tumor cytolysis 18. Further studies revealed that GD2-specific T-cell transfer was well tolerated and demonstrated antitumor activity in a clinical trial in patients with refractory and relapsed disease 19, 20. Nevertheless, solid tumors present several barriers to T-Cell–Based Immuno-Oncology therapies that are largely absent in B-cell malignancies including heterogeneous antigen expression, a hostile immunosuppressive microenvironment, and sites that are difficult for the infused T-cells to track to and infiltrate 21. In addition, the range of antigens expressed in solid tumors poses problems for both TIL and CAR-T cell therapies 21. Together, it is well known that solid tumors can create a complex microenvironment that hinders immunotherapies 22, 23. We thus sought to determine if inhibition of HGF signaling by neutralizing antibody (AMG102) modifies the tumor microenvironment and improves the therapeutic efficacy of CAR-transduced T-cells in a preclinical model of EWS.
Materials and Methods
Cell lines and media
Patient derived xenografts (PDX) and all Ewing sarcoma cell lines were described 24. All EWS cell lines [ES1 (RRID: CVCL_1198), ES2 (RRID: CVCL_AX39), ES4 (RRID: CVCL_1200), ES6 (RRID: CVCL_1202), ES7 (RRID: CVCL_1203), ES8 (RRID: CVCL_1204), SKNEP1 (RRID: CVCL_0631), TC71 (RRID: CVCL_2213), CHLA258 (RRID: CVCL_A058), and EW8 (RRID: CVCL_V618)] were maintained in RPMI supplemented with 10% FBS, 0.1 mM non-essential amino acids, sodium pyruvate, penicillin, streptomycin, L-glutamine (Thermo Fisher Scientific, Waltham, MA). GD2 negative A204 (RRID: CVCL_1058) rhabdomyosarcoma cells were purchased from ATCC (American Type Culture Collection, Manassas, VA) and were transduced with a gammaretroviral cassette co-expressing GD2 and GD3 synthases (Addgene, Plasmid #75013) to drive the biosynthesis of GD2 as described 25. A204 cells were cultivated in IMDM supplemented with 10% FCS and L-Glutamine. Immortalized human bone marrow mesenchymal cells (hMSC-TERT, Cat. No. T0523) cells were purchased from Applied Biological Materials Inc. (Richmond, BC, CANADA) and maintained in Prigrow II medium (Applied Biological Materials Inc.) supplemented with 10% FBS, hydrocortisone to 10–6 mol/L and Penicillin/Streptomycin Solution to a final concentration of 1%. To generate EWS metastatic cell lines, EWS cells (ES2, ES4, SKNEP1 and TC71) were injected intravenously into NSG mice (The Jackson Laboratory, Bar Harbor, ME). Mice were observed regularly and euthanized upon meeting endpoint criteria as described in below. Formed metastases were extracted during necropsy under sterile conditions. Tumors were minced using scalpels and digested with 0.25% Trypsin-EDTA. The resulting cell suspension was subsequently transferred into T175 tissue culture flasks and cells were kept in RPMI, 10% FBS, Pen-Strep. Upon reaching confluence cell cultures were cleaned of possible mouse fibroblast contaminations using a mouse cell depletion kit (Miltenyi Biotech, Bergisch Gladbach, Germany). All cell lines have been authenticated using STR profiling by Genetica DNA Laboratories, Burlington, NC (05–29-2019). Cell cultures were not used beyond 2 months from initial thawing with culture supernatants tested yearly for mycoplasma infection using a PCR based detection kit (Southern Biotech, Birmingham, AL). All experiments were performed with mycoplasma-free cells.
Quantitative reverse transcription PCR (RT-qPCR) and western blot
RNA isolation and reverse transcription were performed as described 26. RT-qPCR was performed on an ABI Prism 7900HD Sequence Detection System (Applied Biosystems) using the TaqMan Universal Mastermix (Applied Biosystems). Gene expression was normalized to the internal housekeeping gene GAPDH. Western blot analysis performed as described in 27. Nitrocellulose membranes were incubated overnight with the following primary antibodies: β-Actin (Santa Cruz), anti-human HGF (Abcam, cat. no. ab83760), FLI-1 (Abcam, cat. no ab15289). For the detection of delta133p53 protein expression in EWS, anti-p53 antibody from BD Pharmingen™ (cat. no. 55147) was used.
Quantitative ELISA, shRNA generation and immunohistochemical (IHC) staining
For ELISA, supernatants were removed from the indicated cell cultures after 48 hours and HGF secretion was quantified by using Quantikine Human HGF ELISA Kit according to the manufacturer’s instructions (R&D Systems). shRNAs sequences for the targeting human HGF, Δ133p53 or scrambled (shCtr) were cloned into the lentiviral expression vector pLKO.1 or Tet-pLKO.1 (Addgene). Following target sequences were used: sh1HGF: ACCAATGTGCTAATAGATGTA, sh2HGF: AGGACTTCCATTCACTTGCAA, shΔ133p53: GCTCCTGAGGTGTAGACGCTT, shCtr: CCTAAGGTTAAGTCGCCCTCG. Production of lentiviral particles, lentiviral infections and selection of transduced tumor cells were described in Addgene’s pLKO.1 protocol. For the immunohistochemistry, formalin-fixed, paraffin-embedded sections were dewaxed by heating at 60°C followed by incubation in Xylene and subsequent lowering concentrations of ethanol. Antigen retrieval was performed by microwaving in VECTOR Antigen unmasking solution. Endogenous peroxide activity was quenched by a 30min incubation 3% hydrogen peroxide. Slides were then stained using the Vectastain Elite ABC kit and ImmPACT DAB Peroxidase Substrate following the manufacturer protocol (Vector Laboratories). Primary antibodies against anti-human CD3 (dilution,1:30; NCL-L-CD3–565) and anti-human CD8 (dilution,1:30; cat. no. NCL-L-CD8–4B11) were purchased from Leica BIOSYSTEMS. Primary antibody against CD34 were purchased from Abcam (cat. no. ab81289).
Lentivirus design and T-cell transduction
GD2 antibody producing hybridoma cells were generated as described in 28. The genes encoding the VH and the VL domains were subsequently amplified from cDNA prepared from isolated hybridoma cells using a set of murine variable domain-specific primers 18. Primers were modified to generate specific restriction sites at one end and complementary linker fragments at the other end of the amplified VH and VL molecules. Subsequently, combinatorial scFv genes were generated by splicing-by-overlap PCR. cDNA coding for the chimeric CD28-OX40-CD3ζ signaling domain were generated as described in 29. Amplified DNAs were inserted into the lentiviral expression vector pHR-SFFV (Addgene, plasmid #79121) to generate pHR- scFvGD2-CAR vector. Lentivirus was produced by HEK293FT cells transfected with pMD2.G, pCMV-dR8.91 and pHR-scFvGD2-CAR using TransIT-293 Transfection Reagent (Mirus Bio LLC). The media on the HEK293FT cells was replaced with fresh media 24 hr post transfection to remove transfection reagent. At 72 hr post-transfection, the lentiviral media was filtered through a 0.45 μm filter. The supernatant was then concentrated using amicon viral concentration ultrafiltration 100 kDa tubes (Merck Milipore) with centrifugation at 1200 × g for 15 min at 4°C. For T-cell transduction, T-cells were isolated from consented research participants (healthy donors, under protocols approved by the Nationwide Children’s Hospital Review Board) by using RosetteSep Human T-Cell Enrichment Cocktail (Stemcell Technologies). The isolated T-cells (4×106) were re-suspended in 4 ml medium per well of a 6-well plate and stimulated with Dynabeads Human T-Activator CD3/CD28 (Life Technologies) and IL-2 (Peprotech, 100 unit/ml) for 24 h. Next, the media (3 ml/well) was gently removed without disturbing clustering T-cells, and pHR- scFvGD2-CAR fresh vector supernatants was added (final 3 ml/well). T-cells were then cultured with the lentiviral vector for 24 h in RPMI1640 containing 10% FBS and IL-2 (100 unit/ml). Three days after transduction of pHR-GD2-CAR, CAR-T-cells were analyzed by biotin-SP-AffiniPure F(ab’)2 fragment-specific goat anti-mouse IgG (Jackson Immuno Research Laboratories) and streptavidin-Phycoerythrin (PE) (BD Pharmingen). CAR-T-cells were enriched using the biotin-SP-AffiniPureF (ab’)2 fragment-specific goat anti-mouse IgG and Streptavidin Micro Beads (Miltenyi Biotec Inc.) according to the manufacturer’s instructions. The purity of the isolated CAR-T-cells was > 90% (Supplementary Figure 1).
Proliferation assay
In order to measure CAR-mediated T-cell proliferation, 2.5 × 104 CAR-T cells were stimulated either with irradiated 2.5 × 104 GD2-transduced A204 rhabdomyosarcoma cells at a 1:1 ratio or anti-CD3/CD28 beads similar in 30. Briefly, CAR-T cell populations were normalized to equivalent percentages of CAR+ cells before plating in 96-well microtiter plate. CAR-T cell expansion was calculated on indicated days, and cells were re-stimulated with rhabdomyosarcoma cells on days 8 and 17. CAR-T cell expansion was calculated using flow cytometry with counting beads (CountBright fluorescent beads, Invitrogen) according to manufacturer’s instructions.
Flow cytometry analysis
Surface expression of the mouse scFv GD2 on CAR-T cells was determined by staining with a biotinylated goat antimouse mAb specific for IgG F(ab’)2 fragment (Jackson Immuno-Research) for 20 min at 4°C and then washed with 1× PBS before staining with secondary phycoerythrin labeled streptavidin (BD Biosciences). To determine GD2 expression on ES2 and ES4 Ewing sarcoma cells were stained with FITC-labeled 14.G2a antibody (BD Biosciences). All samples were analyzed with BD-LSR II (BD Biosciences) and all data were analyzed with FlowJo software. Relative fluorescence intensities (RFI) were calculated by dividing mean fluorescence intensities of mAb-stained cells by those obtained with isotype antibodies or in the absence of antibody.
Flow cytometric cytokine release assay
To assess target-induced cytokine production, CAR-transduced or untransduced (UTD) T cells were seeded at 5×105 cells per well in a 24-well plate and stimulated with 5×105 irradiated ES2 or ES4 tumor target cells (effector: target ratio of 1:1) for 48h. Subsequently, culture supernatants were assessed for IFN-γ, IL-2, and TNF-α by using flow cytometry with Cytometric Bead Array (CBA, Flex Sets; BD Biosciences). The manufacturer’s protocol was followed by measuring the above cytokines in undiluted samples against the standards provided. Cytokine concentrations are expressed as picograms or nanograms 1×106 cells per mL, the data were expressed as the mean of triplicate wells ± SE.
ELISpot assay and target cell lysis
For quantitative determination of the frequency of cells releasing IFN-γ, a commercial kit (ELISPOT human IFN-γ; R&D Systems) was utilized. Briefly, 1×104 T cells per well were plated in triplicate and stimulated overnight with 5×104 tumor cells per well on IFN-γ pre-coated 96-well plate, then incubated with the respective capture antibodies and analyzed following the instructions of ELISpot kits. Spots were counted using an automated reader (ImmunoSpot® image analyzer). The susceptibility of ES2 and ES4 cells to cytolysis by the CAR-T-cells was evaluated using a 4-h 51Cr-release assay as described in 30. Briefly, CAR-T cells were co-incubated with indicated ratios of 51Cr-labeled target cells [effector-to-target ratio (E:T)] in RPMI1640 medium in 96-well U-bottom plates at 37°C for 4 hr. Lysis was measured by 51Cr release in the medium: percent lysis = (sample release – minimum release)/(maximum release – minimum release) x100, average of duplicate samples. For all experiments, untransduced (UTD) T cells served as negative controls.
Animal Studies
All mouse experiments were conducted according to Nationwide Children’s Hospital’s Animal Care and Use Committee (IACUC)–approved protocols. For the in vivo metastasis studies, 6–8 week old NSG mice (Jackson) were inoculated via tail vein with 1 × 106 luciferase-labeled tumor cells. Luciferase-labeled tumor cells were generated by infecting of luciferase expressing lentivirus (Cellomics Technology) and following selection with 1 and 0.5μg/mL puromycin (Thermo Fisher Scientific). In single treatments, mice received treatment for twice a week with a single dose of 300 μg AMG102 (provided by AMGEN) for six weeks or a single dose 2 × 106 CAR-T or UTD were injected intravenously via tail vein in the first week. In combination therapies, similarly, mice received treatment for twice a week with a single dose of 300 μg AMG102 for six weeks with the exception that the same group of mice also received a single dose 2 × 106 CAR-T or UTD in the first week after tumor cell inoculations. Ten mice for each group were used. All mice were monitored with twice weekly weights and enhanced body condition scoring (eBCS) as described in 31. Mice demonstrating >10% weight loss or eBCS <8 were euthanized and lungs harvested, insufflated and fixed in 10% neutral buffered formalin, then embedded and processed for the routine histological examination. Mice not demonstrating metastatic disease burden (presumably dying from other causes) are censored in the survival analysis. To evaluate the effect of drug treatments on primary tumor growth in the bone environment, we utilized intratibial injection mouse model. Briefly, 1×105 luciferase-labeled tumor cells were inoculated into the tibia of NSG mice. After tumor volumes reached 0.5cm3, animals were treated. In these experiments, AMG102 was administrated twice a week with a single dose of 300 μg for four weeks alone or in combination with a single dose 2 × 106 CAR-T or UTD injected intravenously via tail vein in the first week of inoculation. Tumor growth was monitored by measuring width (W) and length (L) of the proximal tibia and the stifle joint weekly using calipers, as well as by in vivo imaging as described in 32. Bioluminescence imaging (Xenogen/IVIS spectrum, Caliper Life Sciences, Hopkinton, MA) was done after injection of D-Luciferin (Gold Biotechnology) following isoflurane inhalant anesthesia and analyzed with Living Image Software (Caliper Life Sciences). For the survival analysis, mice were observed for up to 100 days or until tumor endpoint criteria were reached (ill, tumor reaching 1 cm in the largest diameter, visible lameness, pain or severe weight loss), at which time they were humanely euthanized. Primary bone tumors were decalcified and fixed in 10% neutral-buffered formalin, and processed for routine histological examination.
Statistical analysis
Data were graphed and analyzed using Graphpad Prism 7 (GraphPad Software). Error bars represent mean ± SD from triplicate measurements from one experiment. One representative experiment is showing. Most of the experiments were repeated at least two times with similar results. Differences between two groups were analyzed by unpaired Student’s t-test. For experiments involving comparisons between more than 2 groups, data were subjected to analysis of variance (ANOVA) followed by Tukey’s post-hoc testing. The cytokine secretions between two groups (UTD vs CAR-T) were compared with ordinary Two-way Anova analysis. Survival curves were plotted using the Kaplan–Meier method, and log-rank tests were used to compare curves between groups.
Data Availability
Plasmids, generated cell lines during the current study, and other data are available from the corresponding author on request.
Results
Ewing Sarcoma aberrantly expresses Hepatocyte Growth Factor (HGF)
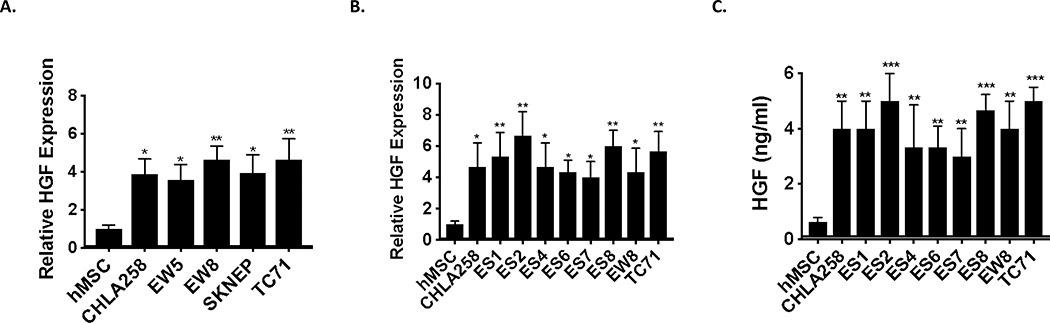
Aberrant activation of hepatocyte growth factor (HGF) and its receptor, cMET, triggers cell proliferation, angiogenesis, invasion and metastasis in a wide variety of cancers 33, 34. We therefore examined whether deregulation of this pathway contributes to EWS tumorigenesis. We first assessed the expression of HGF levels in EWS and found that HGF is transcriptionally overexpressed in the majority of pediatric xenograft EWS tumors and cell lines when compared to control tissue (Figures 1A-B). Additionally, we performed ELISA to determine secreted levels of HGF in EWS cell lines. As shown in Figure 1C, in contrast to control cells, EWS cell lines secrete high levels of HGF, which correlated with the mRNA expression. Together, our data showed that EWS xenografts and cells overexpress HGF suggesting high HGF levels play essential role in EWS tumorigenesis.
Figure 1. Ewing sarcoma tumors and cells aberrantly express and secret high level of HGF.
A-B. RT-qPCR was used to assay HGF mRNA levels in the indicated pediatric xenograft EWS tumors (A) and cell lines (B) and compared to human Mesenchymal Stem Cells, hMSC (putative cell of origin). Total RNA was reverse-transcribed and subjected to real-time PCR with probes specific for HGF. Results were normalized to GAPDH. C. ELISA assay for HGF protein secretion in EWS cells. Indicated EWS cell lines were cultured in media containing 1% FBS for 48 hr, and used for human HGF ELISA assays as described in materials and methods. Unpaired Student’s t-tests (vs hMSC), * p <0.05, ** p <0.01, *** p <0.001.
The p53 isoform delta133p53 drives high level of HGF secretion in Ewing Sarcoma
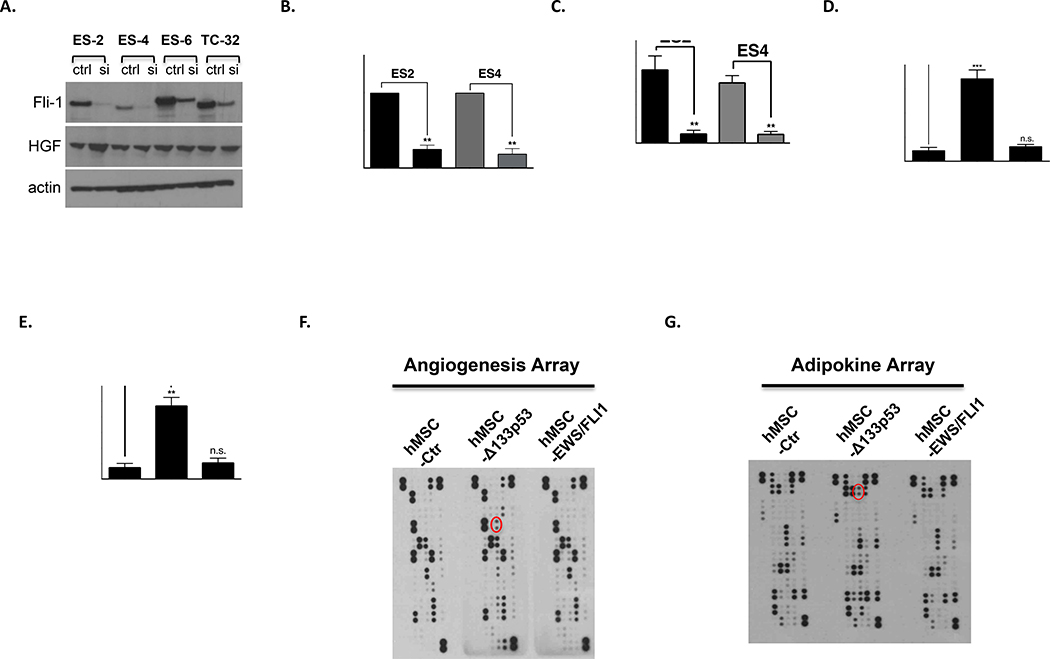
We next examined the underlying molecular mechanism of high levels of HGF expression in EWS. One key pathologic feature of EWS that distinguishes it from other pediatric sarcomas is the expression of the EWS/FLI fusion protein. EWS/FLI is the critical driver mutation in Ewing sarcoma, and functions as an aberrant transcription factor to dysregulate gene targets involved in the oncogenic phenotype 35. Based on this disease-specific chromosomal translocation, we hypothesized that high levels of HGF expression and secretion result from downstream effects of EWS/FLI. To test this hypothesis, we knocked down EWS/FLI1 expression by small interfering RNA (siRNA) and analyzed HGF expression in EWS cell lines. As shown in Figure 2A, HGF expression surprisingly was not changed following EWS/FLI knockdown, suggesting deregulation of HGF expression is independent of the EWS/FLI chromosomal translocation.
Figure 2. Δ133p53 expression drives HGF expression and secretion.
A. HGF expression does not correlate with EWS/FLI1 expression in EWS cell lines. Indicated cell lines were transfected either scrambled control (ctrl) or siRNA targeting Fli-1 (Dharmacon). After 72h transfection, knockdown efficiency of Fli-1 and the expression of HGF were analyzed by western blot. B. HGF expression from indicated cell lines was analyzed by RT-qPCR. Results were first normalized to GAPDH and subsequently data were normalized to the control. Data shown are mean ± SD of triplicate measurements from one representative experiment. C. Indicated EWS cell lines were cultured in media containing 1% FBS for 48 hr, and serum were used for human HGF ELISA assay according to the manufacturer’s protocol (R&D Systems). D. Immortalized hMSCs were transduced separately with empty vector, Δ133p53 or EWS/FLI expressing lentiviruses and recombinant protein expression was verified by western blot analysis (Supplementary Figure 1F). After results were normalized to GAPDH, HGF expression from indicated cell lines is analyzed as in Fig. 1A-B. E. HGF ELISA assay from indicated hMSCs were performed similar in Fig. 1C. F-G. Cell extracts from hMSC transduced with lentiviral expression vectors were analyzed using indicated profiler antibody arrays according to the manufacturer’s instructions (R&D Systems). Red circle marks HGF. (B-E) Data sets were analyzed with unpaired student’s t-test and significance between two groups is shown **p < 0.01, *** p <0.001, n.s. (not significant).
Interestingly, genetic lesions in the TP53 gene are only observed in 10% of Ewing sarcomas, with the majority of these sarcomas expressing a functional wild-type p53 36. In addition, the p53 downstream signaling pathways and DNA-damage cell cycle checkpoints remain functionally intact in these sarcomas 36. Remarkably, we found that an oncogenic p53 isoform, Δ133p53, is abnormally expressed in EWS (Supplementary Figure 2A-D). We then investigated whether Δ133p53 is driving HGF expression in EWS. Ewing cell lines, ES2 and ES4 were stably transduced with either control short hairpin (shCtr) or short hairpin targeting Δ133p53 (shΔ133p53) containing doxycycline-inducible Lentiviral vectors (Supplementary Figure 2E). Cells were maintained in doxycycline (200ng/ml) to turn-off Δ133p53 expression in media containing 1% FBS for 48 hr. Subsequently, cells were harvested for HGF expression analysis, whereas cell culture supernatant was collected for the HGF specific ELISA. As shown in Figures 2B-C, inhibition of Δ133p53 expression in EWS cells significantly reduced HGF expression and secretion. To confirm our findings further, we genetically engineered human mesenchymal stem cell (hMSC), thought to be the cellular origin of EWS, by overexpressing Δ133p53 or EWS/FLI (Supplementary Figure 2F). As shown in Figures 2D-E, in contrast to EWS/FLI-1 expression, only overexpression of Δ133p53 in hMSC significantly increased both HGF expression and secretion. Furthermore, we confirmed by two different profiler antibody arrays that only Δ133p53 expression altered HGF expression in hMSC (Figures 2F-G) and induced the activity of the HGF promoter (Supplementary Figure 3). Taken together, our data demonstrate that high level of HGF expression and secretion are driven by Δ133p53 and independent of EWS/FLI1 expression.
Elevated HGF secretion promotes Ewing Sarcoma tumor growth and metastasis
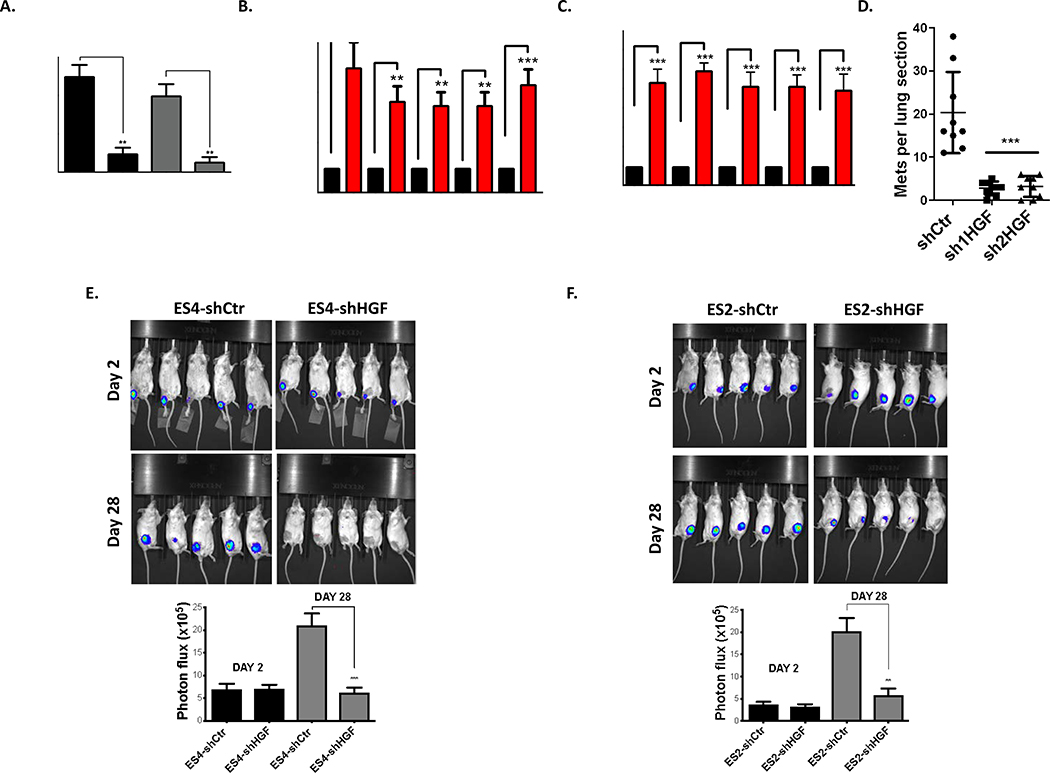
We next examined the biologic consequence of high levels of HGF expression in EWS. We first evaluated whether high levels of HGF secretion play any role in angiogenesis, cellular invasion and metastasis in EWS. To explore this possibility, ES2 and ES4 cells were transduced with lentivirus expressing shRNA against HGF and cells were selected with hygromycin. Subsequently, HGF knockdown efficiency was verified by ELISA (Supplementary Figure 4A). Depletion of HGF expression in EWS cells abolished both cellular angiogenesis (Supplementary Figure 4B), and invasion (Figure 4A). Next, we evaluated whether elevated HGF secretion plays any role in EWS metastasis. Comparative investigations of paired cell line clones that differ in metastatic potential have been particularly helpful in defining both metastasis-associated and metastasis-suppressing genes in several cancer histologies 37, 38. Consequently, EWS cells (ES2, ES4, SKNEP1 and TC71) were injected into the tail veins of NSG mice (5 mice per group). Mice were euthanized at endpoints as described in materials and methods. We were able to observe metastatic spread of injected cells into the different organs (e.g. bone, lungs, intraperitoneal, as well as kidney). Subsequently, metastatic tumors were isolated and metastatic cell lines were generated as described in materials and methods. We found that HGF transcript levels are highly enriched in metastasis models compared to their parental cell lines (Figure 3B). In addition, we identified that these cells also highly secrete HGF in contrast to parental cells (Figure 3C). To interrogate the role of HGF in EWS metastasis further, highly metastatic MAN020E cells (derived from ES4 cells) were stably transduced with either control short hairpin (shCtr) or two different short hairpin targeting HGF (sh1HGF and sh2HGF, Supplementary Figure 4C). Afterwards, cells were injected into the tail vein of NSG mice (10/group). After 50 days, lungs were harvested, insufflated, fixed, sectioned, and stained, and number of metastases per section was quantified. As shown in Figure 3D, inhibition of HGF abolished metastatic potential of the MAN020E cells to the lung suggesting inhibition of HGF signaling presents a valuable target to prevent EWS metastasis. In addition, we demonstrated that inhibition of HGF in EWS cell lines significantly reduced primary tumor growth in an orthotopic mouse model (Figure 3E-F). Taken together, our data established elevated HGF secretion driven by Δ133p53 in EWS stimulates primary tumor growth in the bone and metastasis to the lung.
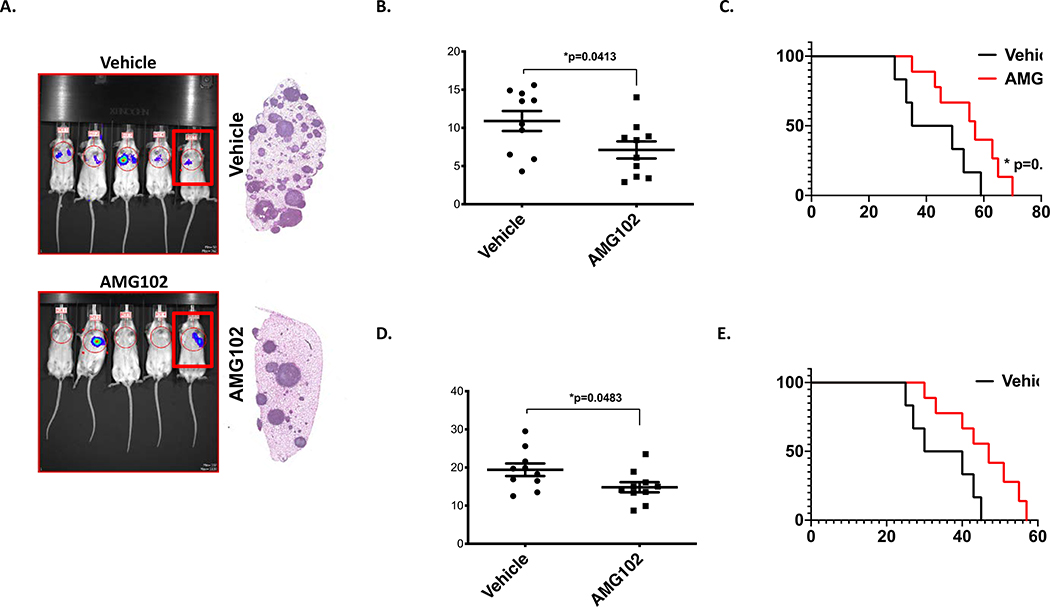
Figure 4. Pharmacologic targeting c-Met/HGF signaling moderately reduces metastatic lung colonization and primary tumor growth.
A. Mice inoculated with 1×106 MAN020E -luc cells were treated with AMG102 or Vehicle (n=10, for each group). Bioluminescent imaging completed at 28 days post-inoculation. Some mice in the AMG102 treatment group, showing no visible luminescence, exhibited response at early timepoints, but all subsequently developed metastasis. A representative cage from each group is shown. Right: gross appearance of representative lung blocks taken from the mice identified with box in the image at the time of euthanasia (endpoint criteria as described in 46. B. Quantification of the lung-field bioluminescence from day 28 (n=10 mice per group), Data sets were analyzed with unpaired student’s t-test and significance between two groups is * p=0.0413). C. Kaplan- Meier survival analysis of the mice. As noted, treatment continued until day 42, and then was stopped. Mice that received single AMG102 therapy experienced moderately better outcomes than those who received no treatment (n=10 mice per group, Mantel-Cox log-rank test, comparison to vehicle). * p=0.0494. D. Quantification of bioluminescence from the tibia at day 28 was performed similar as Figures 3E-F. Data sets were analyzed with unpaired student’s t-test and significance between two groups is * p=0.0483. Ten mice per group were used. E. Kaplan- Meier survival analysis of the mice. As noted, treatments were started after tumor volumes reached 0.5cm3 (around two weeks) continued for four weeks. Mice that received single AMG102 therapy experienced moderately better outcomes than those who received no treatment (n=10 mice per group, Mantel-Cox log-rank test, comparison to vehicle).
Figure 3. High-level of HGF secretion drives primary tumor growth in the bone environment and metastasis to the lung.
A. Invasion assays were carried out using Matrigel precoated inserts (Corning) as described in 45. Representative fields of stained cells were counted. Assays were performed in triplicate for each group. Results are expressed as relative number of cells migrating compared to total number of cells loaded onto the inserts. B. HGF expression is enriched in EWS cell lines developed for high metastatic potential compared to their parental cells. RT-qPCR was used to assay HGF mRNA levels in primary versus matched metastatic cell lines. Experimental data were first normalized to the parental cells group and presented as fold change. C. HGF ELISA assays from indicated EWS cells were performed similar in Fig. 1C. Experimental data were first normalized to the parental cells group and presented as fold change. D. HGF inhibition revealed significantly lower metastatic burden. NSG mice were inoculated via tail vein with highly metastatic MAN020E cells (MAN020E-shCtr, MAN020E-sh1HGF and MAN020E-sh2HGF). The numbers of lung sections with metastatic nodules were compared with the 1-way ANOVA with Tukey’s post hoc test. *** p <0.001 relative to MAN020E-shCtr. E-F. Significant reduction of the tumor growth following HGF inhibition. 1×105 Luciferase expressing EWS cells were injected into the tibia of NSG mice (n=10 for each group). A representative cage from each group is shown. Bioluminescence imaging studies were conducted using the Xenogen IVIS Spectrum at days 2 and 28. The below graph shows the quantitation of bioluminescence between two groups at indicated days. The bioluminescence intensity was quantified using Living Image Software. Signal intensity was quantified as the sum of detected photons per second within the region of interest. (A-C, E-F) Data sets were analyzed with Living Image Software (Caliper Life Sciences) unpaired student’s t-test and significance between two groups is shown * p <0.05, ** p <0.01, *** p <0.001.
Targeting of c-Met/HGF signaling by a HGF neutralizing antibody (AMG102) decreased lung metastasis and tumor growth rates without substantial tumor regression
To evaluate the functional importance of c-Met/HGF signaling pathway to EWS lung metastasis and primary tumor growth, we turned to our xenograft models of EWS metastasis and tibia injection model of EWS for the primary tumor growth. NSG mice, inoculated via tail vein with 1×106 luciferase-labeled MAN020E cells, received treatment twice a week either with 300 μg with a HGF neutralizing antibody (AMG102) or Vehicle (PBS) by intraperitoneal injection (i.p.) for six weeks, after which treatment stopped. As shown in Figure 4, AMG102 treatment only moderately decreased tumor burden in the lungs and increased the survival of mice receiving AMG102 relative to control treatment groups (Figure 4C). To evaluate the effect of AMG102 treatment on primary tumor growth in the bone, using an intratibial injection mouse model, 1×105 luciferase-labeled ES2 cells were inoculated into the tibia of NSG mice. After tumor volumes reached 0.5cm3, animals were treated with vehicle control or AMG102 alone for 4 weeks. Similar to the drug effects on lung metastasis, AMG102 treatment only moderately decreased tumor burden in the bone and increased the survival of mice receiving AMG102 relative to control treatment groups (Figure 4D) suggesting targeting of c-Met/HGF signaling extends survival rate without tumor regression (Figure 4E).
GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevents primary tumor growth and metastasis in Ewing Sarcoma
As we demonstrated above, we were able to retard primary tumor growth, reduce metastasis and extend the survival of mice by single-agent treatment in our orthotopic and EWS metastatic models. Although we observed significant reduction of primary tumor vascularization in the AMG102 treated group (Supplementary Figure 4D), we could not achieve complete tumor regression after treatment. We next considered that targeting of residual disease by novel treatment strategies might sustain remission and improve outcome. In order to test the hypotheses, we selected GD2 immune targeting in combination with AMG102 in this cancer. Importantly, we speculated that decreased tumor mass that we observed by AMG102 treatment alone might increase leukocyte infiltration, including efficient CAR-T access into tumor mass, and thereby improve its antitumor activity. Of note, the antitumor activity of T-cells expressing a chimeric antigen receptor specific for GD2 against Ewing sarcoma has been tested in a subcutaneous model 39. We thus sought to test GD2-targeting in combination with AMG102 in our orthotopic and EWS metastatic models.
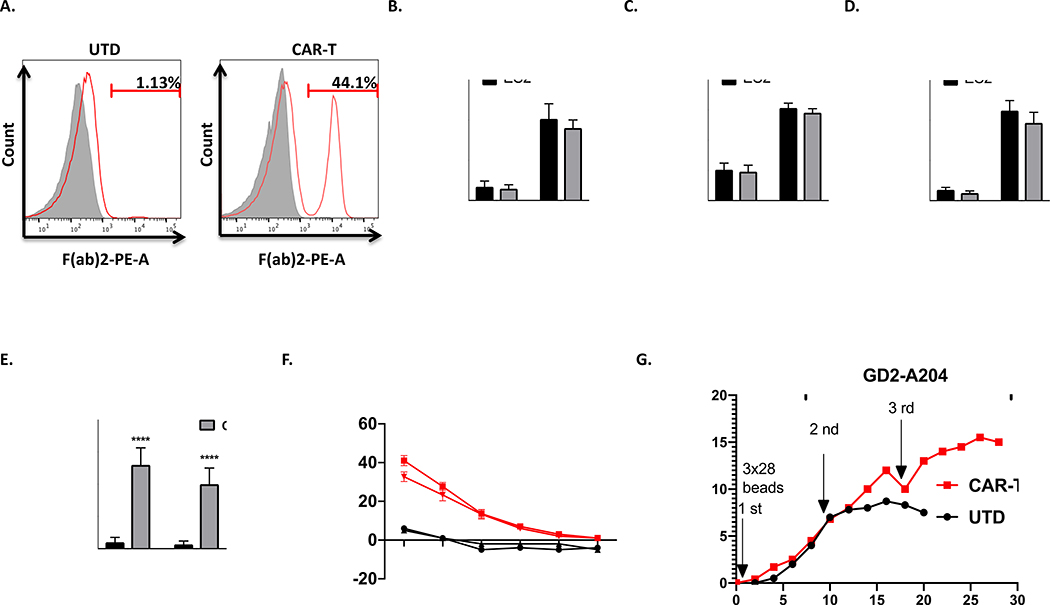
To generate GD2 specific-CAR-T-cells (herein, CAR-T-cells), CD3+ T-cells were isolated from healthy donors and transduced with pHR-scFvGD2-CAR vector as described in materials methods. Subsequently, we evaluated the expression levels of the transgene. Using anti-mouse F(ab’)2 Ab, we detected nearly 45% of the T-cells expressing the GD2-derived scFv on their surface (Figure 5A). Before we determined the cytotoxicity of chimeric antigen receptor (CAR)-expressing T cells, GD2 expression on ES2 and ES4 cells were first verified by FACS analysis (Supplementary Figures 5A-B). Next, CAR-T or UTD cells were incubated with irradiated GD2-positive Ewing sarcoma cell lines ES2 and ES4. As shown in Figure 5B-D, co-incubation of CAR-T cells with the GD2-positive Ewing sarcoma cell lines ES2 and ES4 induced significant production of interferon γ (IFN-γ), interleukin 2 (IL-2) and tumor necrosis factor (TNF-α). Furthermore, for the quantitative determination of the frequency of cells releasing IFN-γ, we performed ELISpot assay. As demonstrated in Figure 5E, the GD2 specific CAR-T cells proved their ability to specifically produce IFN-γ and lyse the tumor target cells (Figure 5F). In addition, the cytokine release and cytotoxicity experiments with antigen-negative cells conformed that our CAR-T cells are highly GD2 specific (Supplementary Figures 6A-B). Lastly, we determined whether repeated antigen stimulation could specifically enhance CAR-T cell expansion and persistence. Thus, GD2-directed CAR-T cells were stimulated with GD2-positive A204 rhabdomyosarcoma cells over the course of 4 weeks in vitro. The timing of restimulation was based on prior studies 30, 40. As shown in Figure 5G, repeated stimulation with GD2-expressing cells allowed CAR-T cells to continue to expand, whereas lack of antigen stimulation on UTDs resulted in culture growth arrest. Together, our GD2-directed CAR-T cells demonstrated cytolytic activity, cytokine production, and specific proliferation in response to GD2 stimulation in vitro.
Figure 5. The in vitro activity of GD2 directed CAR-T cells to EWS cells.
A. Expression of GD2 specific mouse scFv on transduced T-cells was evaluated by flow cytometry as described in material and methods. Histograms were gated with the percentage of positive cells. Untransduced (UTD) T cells served as negative controls. B-D. Results of cytokine release assay. The level of different cytokines, including IL-2, TNF-α, and IFN-γ, were assayed as described in materials and methods. The cytokine secretions between two groups (UTD vs CAR-T) were compared with ordinary Two-way Anova analysis, ***, P < 0.001. ****, P < 0.0001. E. The quantitation of the number of IFN-γ reactive spots. (UTD vs CAR-T) were compared with ordinary Two-way Anova analysis, ****, P < 0.0001. F. Chromium release assays of cytotoxicity of CAR-T or UTD T cells at ratios from 40:1 to 0.6:1 with either ES2 or ES4 targets. Plots indicate means ± SEM of triplicate wells. G. Long-term proliferation of CAR or UTD T cells in response to repetitive stimulation with either antigen (GD2-transduced A204 cells, 2nd and 3rd stimulation) or anti-CD3/CD28 beads (1st stimulation).
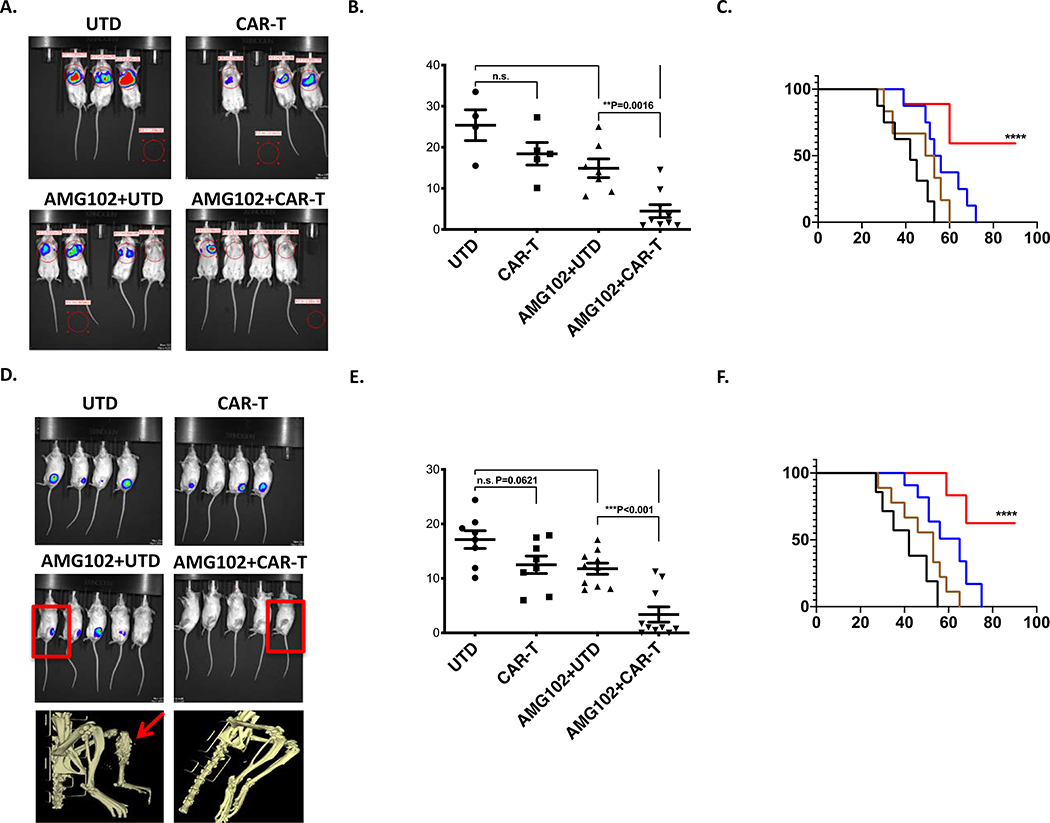
We next examined GD2 directed CAR-T efficacy alone or in combination with AMG102 in our orthotopic and metastatic mouse models. To evaluate the efficacy of the combinational therapy on EWS lung metastasis, NSG mice were inoculated via tail vein with 1×106 luciferase-labeled MAN020E cells received treatment with UTD or CAR-T alone or in combination with AMG102. In single treatments, mice received treatment for twice a week with 300 μg AMG102 for six weeks as described in previous experiments above or a single dose 2 × 106 CAR-T or UTD were injected intravenously via tail vein in the first week following tail vein injections. In combination therapies, similarly, mice received treatment for twice a week with 300 μg AMG102 for six weeks with a single administration of 2 × 106 CAR-T or UTD in the first week. Intravital imaging for in vivo assessment of tumor burden performed at 50 days post-inoculation suggested markedly decreased tumor burden in the lungs of mice receiving combined therapy relative to those receiving no treatment or single-agent therapy (Figure 6A-B). Following treatments, mice were then observed until demonstrating signs of clinical deterioration, either weight loss >10% or enhanced body condition score (eBCS) <8. Survival analysis revealed that nearly all mice receiving single agent therapy developed lethal lung metastasis by 60–70 days, whereas most mice receiving combined therapy (CAR-T + AMG102) remained healthy until end of the study (at 100 days, Figure 6C). The effect of CAR-T cells or AMG102 as single agents had modest and not statistically significant antitumor activity in this disseminated lung metastasis model. Next, we determined the effect of single or combinational therapy on EWS primary tumor growth in the bone environment. By using an intratibial injection mouse model, 1×105 luciferase-labeled ES2 cells were inoculated into the tibia of NSG mice. After tumor volumes reached 0.5cm3, animals were treated with CAR-T or UTD alone or in combination with AMG102, administrated twice a week (300 μg/mouse) for four weeks. A single administration of 2 × 106 T cells was injected intravenously via tail vein in the first week following tibia injections. As shown in Figures 6D-F, in contrast to all mice receiving single agent therapy, most of the mice treated with CAR-T in combination with AMG102 showed no sign of primary tumor development in the bone, and disease free survival was dramatically extended. In our models where CAR-T cells were given in combination with AMG102 there was a significant delay in tumor progression and combination treatment appeared to control/eradicate tumor effectively. Moreover, we compared T cells that accumulated within the tumor sections. As shown in Supplementary Figure 7, both CD3 and CD8 staining were higher in CAR-T + AMG102 treatment group. These data can be integrated into a coherent model in which inhibition of c-Met/HGF signaling in tumor environment facilitates CAR-T infiltration into the tumor mass, thereby improving its antitumor activity (Supplementary Figure 8).
Figure 6. Combined therapy with AMG102 and CAR-T treatment prevents both metastatic lung colonization and growth of primary orthotopic tumors.
A. Bioluminescent imaging completed at 42 days post-inoculation. A representative cage from each group is shown. B. Quantification of the lung-field bioluminescence from day 50. C. Survival analysis of the mice shown. Mice that received combination therapy experienced significantly better outcomes than those who received no treatment or treatment with only one inhibitor (n=10 mice per group, Mantel-Cox log-rank test, comparison to UTD). D-E. Quantification of bioluminescence from the tibia at day 28 was performed as in Figures 3E-F. Data sets were analyzed with unpaired student’s t-test and significance between the groups is shown. Ten mice per group were used. Below, representative MicroCT scans taken from the mice identified with box in the image. AMG102+UTD mouse showing osteolytic lesion (arrow) in contrast to AMG102+CAR-T treated one. F. Survival analysis of the mice shown. As noted, treatments were started after tumor volumes reached 0.5cm3 (around two weeks) continued for four weeks as described above. In contrast to UTD treated group, mice that received AMG102+UTD therapy experienced moderately better outcomes. However, combined therapy with AMG102 and CAR-T treatment significant results survival increase at ****P level vs UTD and ***P vs AMG102+UTD. (n=10 mice per group, Mantel-Cox log-rank test, comparison to UTD).
Discussion
Survival rates for patients with relapsed and metastatic EWS showed only minor improvements in overall survival over the past 40 years, emphasizing the need to move away from traditional nonspecific agents to drugs that specifically target the underlying drivers of Ewing tumorigenesis. Our data demonstrate that EWS cell lines and PDX tumors express elevated levels of HGF, with shRNA depletion abolishing tumorigenic properties of EWS cells in vivo. Importantly, it is well documented that elevated HGF expression was associated with poor overall survival, consistent with several other type of cancers 13. Thus, in addition to EWS/FLI, high levels of HGF secretion may represent a crucial secondary “hit” required to drive EWS tumorigenesis, which unlike EWS/FLI can be easily targeted through small-molecule or antibody blockade.
Abrogation of the p53 pathway through mutation is typically associated with enhanced tumor invasive and metastatic capabilities, and poorer patient survival rates. EWS is an aggressive malignancy with the lowest patient survival rates of all primary musculoskeletal tumors, traits rarely possessed by cancers that retain wild-type p53. We discovered that an oncogenic p53 isoform delta133p53 is aberrantly expressed in EWS by a yet to be determined mechanism. Our data showed that aberrant expression of Δ133p53 drives HGF gene expression resulting in angiogenesis, invasion and metastasis. The HGF promoter has been extensively studied and the binding of several transcription factors confirmed. Though we cannot formally rule out post-transcriptional changes, our data imply that Δ133p53 functions as a transcriptional activator on the HGF promoter by interacting or recruiting transcriptional factors. While Δ133p53 lacks a transcriptional activation domain and is therefore transcriptionally incompetent, it still harbors a DNA binding domain. It is conceivable that Δ133p53 recruits another transcription factor to the p53 binding site in the HGF promoter. Together, the precise molecular mechanism of Δ133p53 induced HGF expression warrants further investigation.
Our preclinical studies showed that when administered as a single agent, CAR-T cells were not able to eliminate either metastatic or orthotopically-injected EWS tumors, but deeper regression was observed when CAR-T cells were administered with adjuvant AMG102. Remarkably, CAR-T cells also seemed to traffic to the site of antigen and infiltrate into the tumors more effectively with adjuvant AMG102, indicating that intravenous administration is likely a suitable route to target EWS tumors with T cells. Of note, Kailayangiri et al. demonstrated that GD2-targeted CAR-T cells reduced tumor progression in subcutaneous EWS tumors 39. It is reasonable that this discrepancy between our results and published data are due to selection of different models (orthotopic vs subcutaneous), T cells injection route (i.v. vs intratumoral injection) and both dose and frequency of T cell administration (single dose, 2 × 106 in the first week vs five administrations totaling 1 × 107 cells over 2 weeks). Taken together, our in vivo data demonstrate that the therapeutic efficacy of CAR-T cells can be achieved only in combination with AMG102 treatment. We speculate that the major effect of AMG102 therapy is a reduction in tumor burden causing more favorable tumor microenvironment for immunotherapy, which is consistent with previous studies that showing malignant transformation and growth of tumor mass influence surrounding tumor microenvironment 41, 42. However, we do not exclude other possibilities that AMG102 could enhance the efficacy of CAR-T cell therapy through other mechanisms. For example, previous reports demonstrated that HGF limits effective murine CTL (Cytotoxic T Lymphocyte) responses via antigen-presenting cells 43 or directly down-modulates the cytolytic function of c-Met+CTLs through regulation of cytotoxic effector molecule expression 44. Whether AMG102 treatment in EWS patients will abolish these limitations on CTLs warrants further investigation.
Supplementary Material
Novelty & Impact Statements.
We identified that an oncogenic p53 isoform, delta133p53, drives high level of HGF secretion in Ewing Sarcoma. In an effort to favorably affect patient outcome, the premise of this study was to reveal a novel therapeutic strategy to treat EWS. Our preclinical studies demonstrated that targeting EWS tumors with HGF receptor neutralizing antibody (AMG102) in combination with GD2-specific, CAR-reengineered T cell therapy synergistically inhibited primary tumor growth and establishment of metastatic disease, thereby laying the groundwork for future clinical trials in children affected with this devastating disease.
Acknowledgments
We like to thank Nationwide Children’s Hospital’s Morphology Core for the preparation of lung and bone tissue slides, Flow Cytometry and Animal Core. This work was supported by grants from the Sunbeam Foundation, CancerFree KIDS, P01CA165995 and K08CA201638 (R.D. Roberts) from the National Cancer Institute and by start-up funds from the Nationwide Children’s Hospital Research Institute.
Abbreviations
- EWS
Ewing Sarcoma
- delta133p53
an oncogenic p53 isoform
- HGF
Hepatocyte growth factor
- AMG102
a HGF neutralizing antibody
- cMET
tyrosine-protein kinase Met, hepatocyte growth factor receptor
- GD2
disialoganglioside, a sialic acid-containing glycosphingolipid
- CAR-T
Chimeric Antigen Receptor T cells
- TIL
tumor infiltrating lymphocytes
- IL-2
Interleukin 2
- IFN-γ
Interferon gamma
- TNF-α
Tumor Necrosis Factor alpha
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Grohar PJ, Helman LJ. Prospects and challenges for the development of new therapies for Ewing sarcoma. Pharmacol Ther 2013;137: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esiashvili N, Goodman M, Marcus RB, Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 2008;30: 425–430. [DOI] [PubMed] [Google Scholar]
- 3.Lokker NA, Mark MR, Luis EA, Bennett GL, Robbins KA, Baker JB, Godowski PJ. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J 1992;11: 2503–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, Vande Woude GF. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A 1997;94: 11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2002;2: 289–300. [DOI] [PubMed] [Google Scholar]
- 6.Spina A, De Pasquale V, Cerulo G, Cocchiaro P, Della Morte R, Avallone L, Pavone LM. HGF/c-MET Axis in Tumor Microenvironment and Metastasis Formation. Biomedicines 2015;3: 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol Int 2001;51: 172–178. [DOI] [PubMed] [Google Scholar]
- 8.Arnold L, Enders J, Thomas SM. Activated HGF-c-Met Axis in Head and Neck Cancer. Cancers (Basel) 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maemura M, Iino Y, Yokoe T, Horiguchi J, Takei H, Koibuchi Y, Horii Y, Takeyoshi I, Ohwada S, Morishita Y. Serum concentration of hepatocyte growth factor in patients with metastatic breast cancer. Cancer Lett 1998;126: 215–220. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12: 89–103. [DOI] [PubMed] [Google Scholar]
- 11.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011;3: S7–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11: 834–848. [DOI] [PubMed] [Google Scholar]
- 13.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16: 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 isoforms can regulate p53 transcriptional activity. Genes Dev 2005;19: 2122–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourdon JC. p53 and its isoforms in cancer. Br J Cancer 2007;97: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature 2017;545: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5: 177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer 2001;94: 228–236. [DOI] [PubMed] [Google Scholar]
- 19.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118: 6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Wang Y, Han W. Chimeric Antigen Receptor-Modified T Cells for Solid Tumors: Challenges and Prospects. J Immunol Res 2016;2016: 3850839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 2018;11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell 2016;164: 1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurmasheva RT, Houghton PJ. Identifying novel therapeutic agents using xenograft models of pediatric cancer. Cancer Chemother Pharmacol 2016;78: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas S, Straathof K, Himoudi N, Anderson J, Pule M. An Optimized GD2-Targeting Retroviral Cassette for More Potent and Safer Cellular Therapy of Neuroblastoma and Other Cancers. PLoS One 2016;11: e0152196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bid HK, Roberts RD, Cam M, Audino A, Kurmasheva RT, Lin J, Houghton PJ, Cam H. DeltaNp63 promotes pediatric neuroblastoma and osteosarcoma by regulating tumor angiogenesis. Cancer Res 2014;74: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cam M, Bid HK, Xiao L, Zambetti GP, Houghton PJ, Cam H. p53/TAp63 and AKT regulate mammalian target of rapamycin complex 1 (mTORC1) signaling through two independent parallel pathways in the presence of DNA damage. J Biol Chem 2014;289: 4083–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res 1987;47: 1098–1104. [PubMed] [Google Scholar]
- 29.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer 2011;129: 2935–2944. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A, Boesteanu AC, Cogdill AP, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 2015;7: 275ra222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: refinement of current criteria. Comp Med 2009;59: 234–241. [PMC free article] [PubMed] [Google Scholar]
- 32.Scott MC, Tomiyasu H, Garbe JR, Cornax I, Amaya C, O’Sullivan MG, Subramanian S, Bryan BA, Modiano JF. Heterotypic mouse models of canine osteosarcoma recapitulate tumor heterogeneity and biological behavior. Dis Model Mech 2016;9: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh-Kaw P, Zarnegar R, Siegfried JM. Stimulatory effects of hepatocyte growth factor on normal and neoplastic human bronchial epithelial cells. Am J Physiol 1995;268: L1012–1020. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem 2003;88: 408–417. [DOI] [PubMed] [Google Scholar]
- 35.Sankar S, Lessnick SL. Promiscuous partnerships in Ewing’s sarcoma. Cancer Genet 2011;204: 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neilsen PM, Pishas KI, Callen DF, Thomas DM. Targeting the p53 Pathway in Ewing Sarcoma. Sarcoma 2011;2011: 746939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977;197: 893–895. [DOI] [PubMed] [Google Scholar]
- 38.Poste G, Doll J, Hart IR, Fidler IJ. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res 1980;40: 1636–1644. [PubMed] [Google Scholar]
- 39.Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, Titze U, Leuchte K, Landmeier S, Hotfilder M, Dirksen U, Hardes J, et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer 2012;106: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol 1997;159: 5921–5930. [PubMed] [Google Scholar]
- 41.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125: 5591–5596. [DOI] [PubMed] [Google Scholar]
- 42.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 2015;201: 78–89. [DOI] [PubMed] [Google Scholar]
- 43.Benkhoucha M, Molnarfi N, Schneiter G, Walker PR, Lalive PH. The neurotrophic hepatocyte growth factor attenuates CD8+ cytotoxic T-lymphocyte activity. J Neuroinflammation 2013;10: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benkhoucha M, Molnarfi N, Kaya G, Belnoue E, Bjarnadottir K, Dietrich PY, Walker PR, Martinvalet D, Derouazi M, Lalive PH. Identification of a novel population of highly cytotoxic c-Met-expressing CD8(+) T lymphocytes. EMBO Rep 2017;18: 1545–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cam M, Gardner HL, Roberts RD, Fenger JM, Guttridge DC, London CA, Cam H. DeltaNp63 mediates cellular survival and metastasis in canine osteosarcoma. Oncotarget 2016;7: 48533–48546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross AC, Cam H, Phelps DA, Saraf AJ, Bid HK, Cam M, London CA, Winget SA, Arnold MA, Brandolini L, Mo X, Hinckley JM, et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids, generated cell lines during the current study, and other data are available from the corresponding author on request.