Abstract
Coptisine is a natural small‐molecular compound extracted from Coptis chinensis (CC) with a history of using for thousands of years. This work aimed at summarizing coptisine's activity and providing advice for its clinical use. We analysed the online papers in the database of SciFinder, Web of Science, PubMed, Google scholar and CNKI by setting keywords as ‘coptisine’ in combination of ‘each pivotal pathway target’. Based on the existing literatures, we find (a) coptisine exerted potential to be an anti‐cancer, anti‐inflammatory, CAD ameliorating or anti‐bacterial drug through regulating the signalling transduction of pathways such as NF‐κB, MAPK, PI3K/Akt, NLRP3 inflammasome, RANKL/RANK and Beclin 1/Sirt1. However, we also (b) observe that the plasma concentration of coptisine demonstrates obvious non‐liner relationship with dosage, and even the highest dosage used in animal study actually cannot reach the minimum concentration level used in cell experiments owing to the poor absorption and low availability of coptisine. We conclude (a) further investigations can focus on coptisine's effect on caspase‐1‐involved inflammasome assembling and pyroptosis activation, as well as autophagy. (b) Under circumstance of promoting coptisine availability by pursuing nano‐ or microrods strategies or applying salt‐forming process to coptisine, can it be introduced to clinical trial.
Keywords: coptisine, crosstalk network, pharmacological mechanism, signalling pathways bioavailability
1. INTRODUCTION
Coptis chinensis (CC), known as ‘huanglian’ in Chinese, is a well‐recognized traditional herb which is widely used in food and medicinal applications.1 For centuries, CC has been one of the principle components in multiple traditional Chinese medicine prescriptions; the typical ones are Sanhuang‐Xiexin‐Tang decoction2 and Gegen‐Qinlian‐Tang decoction.3 Processed CC products are reported to exert ameliorative effect on severe skin disease, dysentery, gastroenteritis and diabetes, etc4 Structure‐activity research draw a conclusion that isoquinoline alkaloids in CC, namely berberine, coptisine, palmatine, epiberberine and jatrorrhizine, are the main constituents responsible for its bioactive properties.5, 6 By far, numerous systematic reviews have led to a summing‐up that berberine, the most in‐depth studied alkaloid of CC, exerts potent pharmacological efficacy in the treatment to mood disorders,7 tumour,8, 9 type 2 diabetes mellitus,10 nerve damage11 as well as cardiovascular, hepatic and renal disorders.12 As the second most abundant isoquinoline alkaloid in CC, coptisine shares a same parent nucleus with berberine.13 To date, many investigations have evaluated coptisine's multiple properties, and a review article released in recent year shows coptisine's considerable beneficial functions such as anti‐bacterial, gastric mucosa protection and osteoclast differentiation inhibition.14 However, the intracorporal process of coptisine was not mentioned in this review and the underlying molecular mechanism of its pharmacological activation is not fully understood. As low molecular weight compounds containing nitrogen, isoquinoline alkaloids have been widely developed into various drugs due to their high biological activity. The key findings gathered from the literature of the last two decades are scrutinized in this work, aiming to describe the implications of coptisine in multiple diseases and further provide advice for the clinical use of coptisine and CC.
2. INTRACORPORAL PROCESS
During the past decades, many pharmacokinetic studies have been focused on the intracorporal process of formulas like SHXXT and BanXia Xiexin decoctions,15, 16 the main constituents of which were all verified to include coptisine (Chemical structure showed in Figure 1). In rat model administrated with SHXXT, coptisine was not metabolized in blood and could reach hepatic and liver cells as prototype form.17 To evaluate further coptisine metabolism in liver, a study conducted in zebrafish model demonstrated that coptisine has 4 metabolites and the main metabolic ways include demethylation, hydroxylation, sulphation and glucuronidation.18 Moreover, coptisine was revealed to be the substrate of P‐gp,19 which to some extent, contributes to the low distribution of coptisine in multiple tissues.
Figure 1.

Coptis chinensis Franch. Whole plant (A), dry root (B) and chemical structure of coptisine (C)
There are several pharmacokinetic researches concerning coptisine administration apart from formulas. A study by Su et al employed rat model with two administration routes, authors reported the oral bioavailability and t1/2 of coptisine (50 mg/kg, oral, single dose, 0.083—24 hours; 10 mg/kg, i.v, single dose, 0.083—24 hours) was 8.9% and 0.71 hour, and the serum coptisine level of rat remained high for 4 hours since intravenously administrated, after which coptisine distributed at low level in multiple tissues including heart, spleen, lung, liver, kidney, brain, intestine, muscle and fat,20 the concentration of which were all within 200 ng/g, except for that of intestine for about 4000 ng/g.21
It is worth mentioning that the plasma concentration of coptisine and other CC isoquinoline alkaloids displayed explicit non‐liner relationship with the oral dosage, it exerted an obvious process of limitation, and thus, the bioavailability decreased along with the elevated dosage. More details are depicted in Table 1 and Figure 2.
Table 1.
Non‐liner relationship between dosage and plasma concentration
| Route | Ber(mg) | C max (ng/mL) | AUC(μg/Lh) | Cop(mg) | C max (ng/mL) | AUC(μg/Lh) | References |
|---|---|---|---|---|---|---|---|
| po. | 14.3 | 156.55 ± 27.85 | 571.59 ± 44.41 | 3.78 | 60.85 ± 7.34 | 309.59 ± 27.06 | 125 |
| po. | 23.76 | 12.27 ± 3.30 | 92.71 ± 15.03 | 5.16 | 1.39 ± 0.60 | 14.28 ± 2.38 | 126 |
| po. | 2.95 | 18.8 ± 4.55 | 40.7 ± 16.2 | 0.78 | 4.31 ± 0.31 | 6.2 ± 0.77 | 127 |
| po. | 3.92 | 109.40 ± 48.27 | 299.84 ± 55.27 | 1.32 | 14.13 ± 7.75 | 71.59 ± 10.72 | 128 |
| po. | 2.73 | 129.94 ± 2.56 | 38.23 ± 1.10 | 1.15 | 114.86 ± 5.89 | 45.18 ± 4.65 | 129 |
| po. | 2.31 | 42.00 ± 14.00 | 675.00 ± 270.00 | 2.24 | 31 ± 12 | 527 ± 10.00 | 16 |
| po. | 6 | 51.23 ± 7.59 | 63.24 ± 10.29 | 21 | |||
| po. | 15 | 44.15 ± 15.35 | 69.37 ± 11.92 | 21 | |||
| po. | 30 | 66.89 ± 29.66 | 87.97 ± 42.47 | 21 | |||
| po. | 10 | 210.38 ± 54.90 | 595.58 ± 123.16 | 20 | |||
| i.v | 2 | 3373.97 ± 448.92 | 167.07 ± 36.30 | 20 | |||
| i.v | 2 | 2542.03 ± 1242.16 | 1129.72 ± 289.63 | 21 |
| Route | Pal(mg) | C max (ng/mL) | AUC(μg/Lh) | Jatr(mg) | C max (ng/mL) | AUC(μg/Lh) | References |
|---|---|---|---|---|---|---|---|
| po. | 3.56 | 47.65 ± 8.69 | 204.86 ± 12.17 | 1.16 | 33.35 ± 5.82 | 96.58 ± 21.69 | 125 |
| po. | 4.02 | 0.74 ± 0.44 | 4.72 ± 0.79 | 3.12 | 0.67 ± 0.23 | 7.11 ± 0.65 | 126 |
| po. | 0.73 | 2.14 ± 0.68 | 2.1 ± 4.0 | 0.07 | 3.12 ± 0.84 | 7.5 ± 0.87 | 127 |
| po. | 1.25 | 34.07 ± 19.25 | 96.63 ± 33.22 | 128 | |||
| po. | 0.84 | 92.02 ± 9.62 | 32.19 ± 2.06 | 0.52 | 21.48 ± 0.80 | 7.85 ± 0.87 | 129 |
Figure 2.
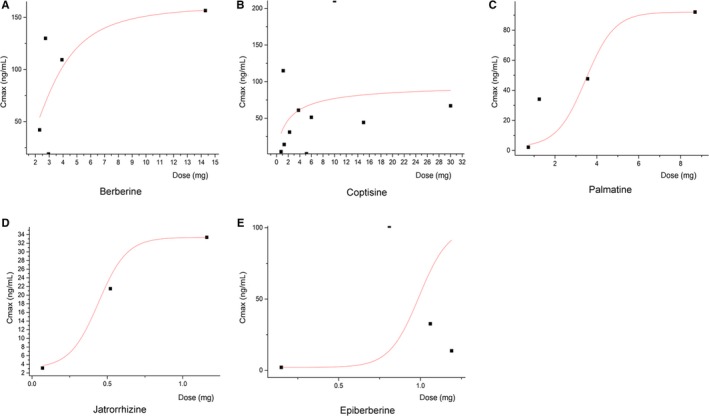
Non‐liner relationships between CC alkaloid dosages and plasma concentration (Cmax). There is an obvious process of limitation, and the bioavailability decreased along with the elevated dosage
3. ANTI‐CANCER PROPERTY
Numerous researches have verified the vital role of MMPs, MMP‐2 and MMP‐9 in particular, in promoting the membrane‐basement invasion of tumour cells.22, 23 Related to MMPs, the activation of PI3K/Akt pathway is crucial for the growth and survival of cancer cells and it plays a dominant role in regulating EMT and the following process of migration and invasion.24 As shown in Figure 3, various studies have evaluated the in vitro anti‐cancer property in multiple cancer cell lines. Coptisine (0–25 μmol/L, 24 hours) was demonstrated to inhibit the viability, adhesion and migration of HCT116 cells and the expressions of MMP‐3 and MMP‐9. It also down‐regulated PI3K and Akt expression as well as altered the downstream EMT markers such as E‐cadherin, N‐cadherin, vimentin and Snail.25 Apoptosis plays a vital role in the progression of multiple cancers and basically activated by a mitochondria‐induced intrinsic or a death receptor‐induced extrinsic pathway, both of which are correlated with mitochondria and Bcl‐2 family anti‐apoptotic proteins.26, 27, 28 Another two studies on HCT‐116 cells investigated the underlying mechanism of coptisine's anti‐apoptotic effect characterized by affecting diverse apoptosis‐associated targets including ROS, Bcl‐2/‐XL, Bid, Bax, cytochrome c, Apaf‐a, AIF, XIAP, caspase‐3 and caspase‐9. Apart from uncontrolled proliferation and apoptosis occurrence, tumour cells as well trigger impaired regulation of cell cycle. Cell circle interphase consists of G1, S and G2 phases, respectively, ensuring DNA synthesis preparation, DNA replication and mitosis preparation.29, 30 Coptisine (0‐28.11 μmol/L, 24 hours; 0‐75 μmol/L, 48 hours) induced HCT‐116 cell cycle arrest in G1 phase as well as decreased expressions of CDK4, CDK2, cyclin E and cyclin C, which were key genes of G1/S phase.31, 32
Figure 3.
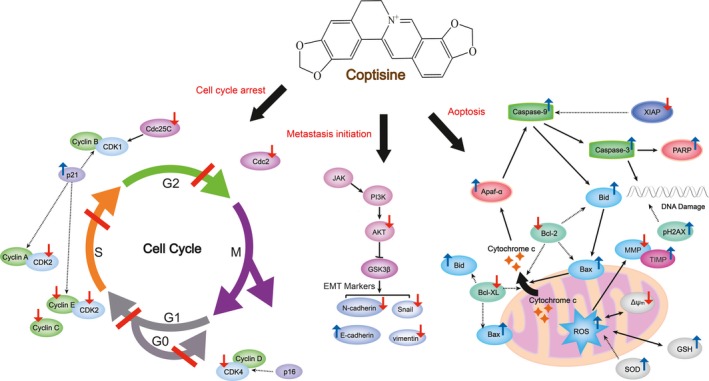
Schematic summaries of coptisine's anti‐cancer targets. Coptisine regulates cell cycle and blocks the occurrence of apoptosis and metastasis initiation by modulating the marked targets. Red arrows represent for decreased expression and/or activity; blue arrows represent for increased expression and/or activity
Similar anti‐cancer property of coptisine was observed in liver HepG2, breast MDA‐MB‐231, pancreas PANC‐1 cell and lung A549 cancer cells. To be specific, coptisine (12.5‐100 μmol/L, 24 hours; 15‐50 mg/kg, i.v; 5 days a week for 6w) functioned as reducing viability and growth of HepG2 through diminishing miR‐122 expression. Meanwhile, it induced apoptosis by enhancing transduction of 67kD laminin receptor/cGMP pathway in multiple human hepatoma cells.33, 34 In the next study, authors revealed that coptisine (0‐64 μmol/L, 24 hours) did inhibit MMP‐9 mRNA expression in MDA‐MB‐231 by elevating mRNA expression of TIMP‐1,35 a widely recognized natural inhibitor of MMPs. Coptisine (25‐150 μmol/L, 48 hours) dose‐dependently inhibited PANC‐1 cell metastasis and induced cell cyclin arrest in G1 phase as well as S phase reduction.36 In addition to G1/S phase of cell cycle, coptisine (12.5‐50 μmol/L, 48 hours) and 8‐cetylcoptisine (0‐1.25 μmol/L, 24‐48 hours) induced cell cycle arrest at G0/G1 and G2/M phases in another cell model, non‐small‐cell lung cancer cell line A549, and this effect was also accompanied by the reduced expressions of cell cycle regulatory proteins, including cyclins D/E, CDK2/4/6, Cdc2/25C and p21. Besides, coptisine (12.5‐50 μmol/L, 48 hours) not only promoted DNA damage of A594 cell through increase pH2AX protein (DNA damage marker) level but also triggered the occurrence of apoptosis by up‐regulating ROS, caspase‐3/‐9, Bax/Bcl‐2 and PARP cleavage.37 Consistent with that in A549 cell, coptisine (2.5‐40 μmol/L, 4 hours; 25‐50 μmol/L, 24 hours) as well modulate ROS caspase‐3/‐9 and Bax/Bcl‐2 in H2O2‐stimulated EA.hy926 and NCI⁃H1650 cells.38, 39, 40
Several studies have established that coptisine exerts ameliorative effect on cancer in in vivo model. Cao et al reported that coptisine (30‐90 mg/kg, i.p, once daily for 14 days) could prevent tumour development by inhibiting MFG‐E8, MMP‐2/9, N‐cadherin, vimentin and Snail expressions and increase E‐cadherin expression in HCT116‐challenged nude mouse.25 Interestingly, in the same xenograft tumour model, administration of coptisine (50‐150 mg/kg, oral, once daily for 25d) decreased serum level of tumour markers such as CEA, CA119 and CYFRA, and this effect was related to the changed mRNA expressions of TNF‐β, KRAS, ERK and p53.41 Another study, which was aimed to investigate the effect of coptisine on liver cancer‐associated acute liver failure, verified that coptisine (37.5‐150 mg/kg, oral, once daily for 7 days) down‐regulated TLR‐4 expressions and apoptotic protein level.42 Furthermore, coptisine (50 mg/kg, oral, once daily for 24 hours) suppressed aggressive osteosarcoma cell proliferation and induced cell cycle arrest at G0/G1 phase in xenograft BALB/c nude mice by subcutaneously injected with MG63 cell.43
4. ANTI‐INFLAMMATION PROPERTY
Involved in the pathogenesis of many types of cancer, inflammation is one part of protective biological response to harmful stimuli (damaged cells, pathogens, irritants, etc). Inflammatory transductions depend on cellular pathways, among which NF‐κB, MAPK and PI3K/AKT attract most attention in resent research.44 NF‐κB is a classic pathway and typically activated by LPS. Upon stimulation, TLR‐4 directly binds to LPS and then recruits adaptor protein MyD88, resulting in the primary activation of enzyme complex IKK.45, 46 In addition to that, IKK activation is also followed by RIP2/caspase‐1 of MAL/caspase‐1 interaction.47, 48 MAPK is another key inflammatory signalling pathway, and it consists of three subfamilies, namely p38, JNK and ERK. Accordingly, natural products which exert properties to block inflammatory signalling transduction by inactivating targets mentioned above are able to be regarded as potential candidates of clinical anti‐inflammation drug.
Accumulative studies have been designed to assess coptisine's anti‐inflammatory effect in vitro (Figure 4). It was observed that coptisine (1‐30 μmol/L, 15 minutes‐24 hours) inhibited LPS‐induced inflammatory response in Raw 264.7 macrophages which was attributed by coptisine‐mediated blocking of NF‐κB, MAPK and PI3K/AKT pathway signalling transduction.49 Coptisine (10‐30 μmol/L, 12 hours) was also reported to decrease productions of histamine, IL‐4 and TNF‐α in (DNP‐IgE/HSA)‐stimulated rat RBL‐2H3 cells by suppressing PI3K/Akt phosphorylation.50 The findings of Zhou et al demonstrated that coptisine treatment reversed IL‐1β‐induced p‐p65 mRNA/protein overexpression and IκBα degradation in OA chondrocyte.51 Recently, coptisine was found to exert considerable suppressing effect on NLRP3 inflammasome activation in multiple models. In the work of Ren et al, CC ameliorated renal damage of rats with early obesity‐related glomerulopathy by interfering the activation of NLRP3 inflammasome, and this effect was confirmed to be related to coptisine, the main constituent of CC.52 Coptisine (1‐30 μmol/L, 3.75 hours) was reported to inhibit caspase‐1 cleavage in LPS plus ATP/MSU/Nigericin through preventing NLRP3 inflammasome priming and assembling in stimulated Raw 264.7 macrophages.53
Figure 4.
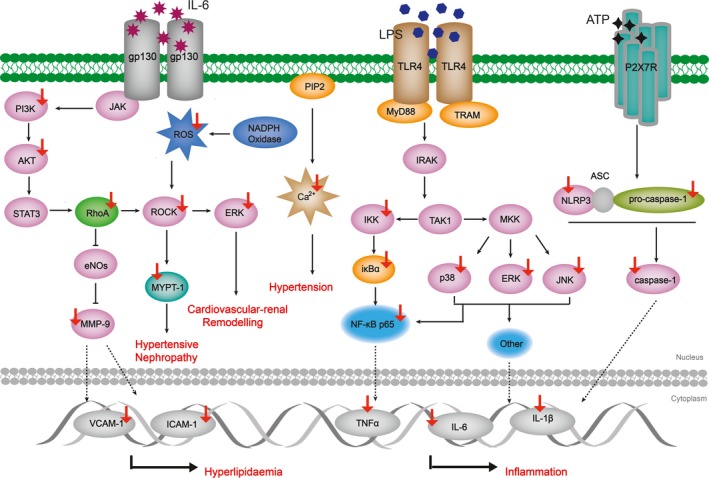
Schematic summaries of coptisine's anti‐inflammatory and CVD protection targets. Coptisine blocks inflammatory response and ameliorates CADs through modulating the marked targets. Red arrows represent for decreased expression and/or activity; blue arrows represent for increased expression and/or activity
Similarly, inhibition of inflammatory process was also observed in animal models including xylene‐induced ear oedema, carrageenan‐elicited paw oedema, LPS‐induced shock, OVA‐induced allergic rhinitis and MSU‐elicited gouty after coptisine administration.54 Mechanistically, coptisine (50‐200 mg/kg, oral, once daily for 10 days; 10‐40 mg/kg, oral, once daily for 7 days; 2.91‐11.61 mg/kg, i.v, single dose, 0‐24 hours) diminished cytokine production through blocking inflammatory transduction mediated by the phosphorylation of IKKα/β and MAPK subfamilies, the translocation and degradation of p65 in oedema tissue as well as suppression of NLRP3 inflammasome activation.50, 53, 55 Of interest, coptisine (0.97‐3.87 mg/kg, i.v; single dose, 0‐4 hours) showed no effect on the up‐regulation of oedema skin temperature induced by carrageenan subcutaneously injection in rats.49 Furthermore, there is a study investigating a pharmacokinetic‐pharmacodynamic model for coptisine challenge of inflammation in LPS‐elicited rats, and authors emphasized early stage of TNF‐α response is the key factor of the subsequent inflammatory cascade.56
5. AMELIORATIVE EFFECT ON CVDS
The typical cardiovascular diseases (CVDs) includes heart attack (or myocardial infarction), atherosclerosis, heart failure and stroke.57 In clinical, key biomarkers for dyslipidaemia and high blood pressure include TC, TG, HDL, LDL, VCAM, ICAM, Apo‐A and Apo‐B.58 ROCK pathway is another signalling pathway related to CVDs, the blocking of which leads to cardiovascular protection through decreasing NADPH oxidases and ROS level. MYPT‐1 phosphorylation is a marker of ROCK activity and plays a crucial role in cardiovascular‐renal remodelling.59 As a NADPH rate‐controlling enzyme, HMGCR is a target for atherosclerosis‐ameliorative drug because it participates in cholesterol synthesis.60 Moreover, NLRP3 inflammasome was reported to be involved in the pathological progression of multiple CVDs such as myocardial ischaemia disorders, atherosclerosis, hypertension and cardiomyopathy.61 Therefore, natural compounds targeting markers above can be regarded as potential candidates to ameliorate CVDs.
Coptisine exerted ameliorative effects on CVDs both in vitro and in vivo. A study by Wang et al reported the cardiovascular protection property of coptisine (0.3‐10μmol/L, 6 hours) in NaS2O4‐stimulated H9c2 cardiomyocyte, and the underlying mechanism was the suppressing of autophagy and apoptosis markers.62 Another study concerning coptisine's (10‐100 μmol/L, 20 minutes) vascular‐relaxing effect on isolated aortic rings of rats concluded that coptisine mediated aortic ring relaxation partially through blocking extracellular Ca2+ influx.63 VSMC proliferation is a key event during atherosclerosis progression, and analysis of gene expression showed proliferation inhibition might be related to enhanced Gadd45a and Rgs32 genes which is induced by coptisine (30 μmol/L, 72 hours).64
A recent in vivo study which conducted to examine how coptisine (150 mg/kg, oral, once daily for 12w) affects ApoE‐/‐ C57BL/6J mice demonstrated coptisine exerted lipid‐lowering property through regulating biomarkers in serum. Coptisine reduced mRNA level of p65, VCAM‐1, ICAM‐1 and IL‐6/1β in aorta and liver, and it also prevented the phosphorylation of both p38 and JNK1/2.65 Also to assess lipid‐lowering effect of coptisine, He et al announced modified serum level of TC, TG, LDL‐c, HDL‐c and TBA followed by coptisine treatment (70.05 mg/kg, oral, once daily for 4w) in obesity syrian golden hamsters, and this effect was related to the reduced HMGCR and elevated CYP7A1 gene level, which were important markers of cholesterol metabolism.66 In the model of isoproterenol‐induced myocardial infarction rats, oral pre‐treatment of coptisine (25‐100 mg/kg, oral, once daily for 21 days) displayed potent anti‐oxidative and mitochondrial respiratory dysfunction‐ameliorative activity through restraining signalling transduction of ROCK pathway.67 Furthermore, another study designed by Guo et al employed rat model of myocardial ischaemia/reperfusion injury, and the authors revealed coptisine (10‐30 mg/kg, oral, two times) narrowed infract size by preventing apoptotic progression in heart. Coptisine also negatively affected NF‐κB inflammatory response and meanwhile suppressed MYPT‐1 phosphorylation, which in turn lowered ROCK activity.68
6. ANTI‐BACTERIAL PROPERTY
The study screened the five protoberberine alkaloids by microcalorimetry and confirmed berberine and coptisine were more appropriate candidates as new anti‐infective drug.69 Berberine is now already a widely used anti‐bacterial applied in the fight against diseases such as bacteria diarrhoea and fungi infection.6, 70, 71 Similarly, coptisine (50 μmol/L, 0‐120s) was revealed to reduce S.aureus adhesion and inhibit PfDHODH, an anti‐malarial chemotherapy target.72 Coptisine (50‐200 μmol/L, 30 minutes) also exhibits anti‐H.pylori effect through the inhibition of urease activity, suggesting coptisine was a promising drug for digestive diseases.73, 74 Moreover, Zhang et al built a nude mouse model of chemotherapy‐related diarrhoea by using irinotecan, and the result showed coptisine (30 mg/kg, oral, twice a day, 4 days) was able to reduce the degree of diarrhoea and ileum mucosal injury through modulating IκBα/NF‐κB signalling pathway.75 Previous studies which evaluated coptisine's (15.6‐93.8 μmol/L, 30 minutes; 62‐468 μmol/L, 0‐13.3 hours) action group verified that the group responsible for its anti‐Ecoli and anti‐bifdobacterium adolescentis property was methylenedioxy at C2 or C3.76, 77
7. OTHER PROPERTIES
A study designed to evaluate the neuroprotection effect of coptisine discovered that coptisine (50 mg/kg, oral, once daily for one month; 10 μmol/L, 5 hours) reversed the enhanced IDO activity in AβPPswe/PS1ΔE9 mice, and it as well prevented AD pathogenesis by means of blocking microglia and astrocyte activation through inactivating CD11b and GFAP, respectively.78 In accordance with in vivo study, coptisine (0‐40 μmol/L, 24 hours) diminished IDO overexpression and GUIN overproduction induced by Aβ1‐42 in microglia cells. Coptisine was also reported to down‐regulate TXNIP protein concentration in SH‐SY5Y cells, which checked oxidative damage.79 Ge et al established an activity‐integrated strategy by performing UHPLC/Q‐TOF‐MS‐FC to screen potential α‐glucosidase inhibitor in CC, and the results demonstrated coptisine (IC50 = 25.6 μmol/L) had considerable α‐glucosidase inhibitory effect, which was further confirmed by molecular docking.80, 81 It is reported by Shi et al that coptisine (15‐50 mg/kg, oral, once daily for 20d) decreased blood‐glucose level in alloxan‐induced type 1 diabetic mice, and coptisine (1‐10 μmol/L, 24 hours) also increased AMPK phosphorylation while reducing Akt phosphorylation in both HepG2 hepatic cells and C2C12 myotubes, indicating its role as a enhancer of glucose consumption which can promote glucose metabolism.82 Kang et al developed a radioisotope detection biochip to evaluate natural small compounds’ inhibitory effect on protein kinase C and found that coptisine dose‐dependently suppress the activity of protein kinase C.83 These results showed coptisine's great potential in diabetes treatment. In addition, coptisine (10‐40 mg/kg, once daily for 7d) showed anti‐ulcer efficacy through the inhibition of p38 MAPK and the activation of Nrf2 signalling pathway.84, 85
Interestingly, the effect of coptisine (0.0025‐0.01 mg/mL, 12 hpf; 0.06‐0.25 μmol/L, 3 hours) on MAPK subfamilies ERK, JNK and p38 demonstrated to be reversed in oxidative injury model of AAPH‐challenged adult zebrafish.86 It protected zebrafish against oxidative injury through up‐regulating mRNA expression of ERK, JNK, p38, NQO1, Nrf2 and Akt, and this effect was further confirmed in anther in vitro model, AAPH‐stimulated HepG2 cells. Compared to other in vivo and in vitro experiments, the dose used in this study was much lower, which might present an explanation on the reversed effect of coptisine.
Pharmacological research in current years indicates coptisine is a multi‐targeting isoquinoline alkaloid (Tables 2 and 3), while there are several aspects need considering before its translation from bench to bedside.
Table 2.
Targets of coptisine activity
| Mechanism | Effect | Targets | References |
|---|---|---|---|
| Anti‐cancer | Elevated | Caspase‐3/8/9 | 31, 34, 37, 38, 39, 40, 42 |
| PARP | 34, 40 | ||
| 67LR | 34 | ||
| Apaf‐1 | 31, 42 | ||
| AIF | 31 | ||
| Cytochrome C | 31, 42 | ||
| Bid | 31 | ||
| Bad | 31 | ||
| Bax | 31, 40, 42 | ||
| pH2AX | 40 | ||
| ROS | 31, 40 | ||
| GSH | 42, 84 | ||
| SOD | 42, 84 | ||
| TIMP‐1 | 18 | ||
| E‐cadherin | 25 | ||
| Reduced | TLR‐4 | 42 | |
| Δψ m | 31 | ||
| XIAP | 31 | ||
| Bcl‐2 | 31, 37, 38, 39, 40, 42 | ||
| Bcl‐XL | 31, 37, 38, 39, 40, 42 | ||
| MMP‐3/9 | 25 | ||
| PI3K | 25, 31, 41 | ||
| AKT | 25, 31, 41 | ||
| Vimentin | 25 | ||
| N‐cadherin | 25 | ||
| Snail | 25 | ||
| CDK2/4/6 | 37, 41 | ||
| Cyclin D/E | 37, 41 | ||
| p21 | 40 | ||
| Cdc2/25C | 37, 40 | ||
| CEA | 41 | ||
| CA119 | 41 | ||
| CYFRA | 41 | ||
| KRAS | 41 | ||
| p53 | 41 | ||
| Anti‐inflammation | Elevated | Nrf2 | 84 |
| Reduced | p38 | 49, 55, 65 | |
| ERK | 49, 55 | ||
| JNK | 44, 47, 56 | ||
| PI3K | 50, 53 | ||
| AKT | 49, 65 | ||
| IKKα/β | 53, 55 | ||
| p65 | 51, 53, 55, 65 | ||
| iκBα | 49, 51, 53, 83 | ||
| TNF‐α | 49, 53, 65 | ||
| IL‐4/6/1β | 49, 65 | ||
| NLRP3 | 53 | ||
| Pro‐/caspase‐1 | 53 | ||
| MAL | 53 | ||
| Histamine | 65 | ||
| CAD protection | Elevated | Gadd45a | 64 |
| Rgs32 | 64 | ||
| CYP7A1 | 66 | ||
| Reduced | RhoA | 67, 68 | |
| ROCK | 67, 68 | ||
| MYPT‐1 | 68 | ||
| VCAM‐1 | 65 | ||
| ICAM‐1 | 65 | ||
| Ca2+ influx | 63 | ||
| HMGCR | 66 |
Table 3.
Signalling pathways modulated by coptisine activity
| Pathways | References |
|---|---|
| p38MAPK/Nrf2 | 84 |
| 67LR/cGMP | 34 |
| ASK1‐P58 | 79 |
| Beclin 1/Sirt1 | 62 |
| JNK/Nrf2/NQO1 | 86 |
| Kynurenine pathway | 78 |
| miR‐122/Bax/Bcl | 42 |
| Mitochondrial/caspase‐3 | 37, 38, 39, 40 |
| NF‐κB | 51, 53 |
| NF‐κB/MAPK | 55, 65 |
| NF‐κB/MAPK/ PI3K/AKT | 49 |
| NLRP3 inflammasome | 52, 53 |
| NO‐cGMP | 63 |
| PI3K/AKT/MMPs | 1, 25 |
| RANKL/RANK | 4 |
| RAS/ERK | 41 |
| RhoA/ROCK | 67, 84 |
| STAT3 | 43 |
| TLR‐4 | 45 |
| TIMP‐1/MMPs | 18 |
8. STRUCTURE‐ACTIVITY RELATIONSHIP
Coptisine is a typical quaternary proberberine alkaloid (QPA) with a parent nucleus of isoquinoline (Figure 5), which plays a vital role in the secondary metabolism of herbals like CC.87 By far, efforts to promote natural QPAs’ bioactivities have been conducted by applying structural modifications. In general, C‐8 and C‐13 positions, as well as the aromatic sites, attract the most attention on QPAs’ structural modifications. Among them, nucleophilic reagents (cyano, alkyl and phenyl, etc) are usually introduced to C‐8 position.88 Structure‐activity relationship studies demonstrated that QPAs’ activity was affected by (1) quaternary nitrogen atom, (2) character and size of substituent at C‐13, (3) aromatic substituent on ring C and (4) substituents type on ring A and D. To begin with, tetrahydroprotoberberine’ anti‐acetylcholinesterase activity was elevated after nitro substituents were introduced at ring A.89 Secondly, the presence of hydrophilic radical at C‐13 position could improve QPAs’ anti‐malarial activity against plasmodium falciparum while QPAs with a high lipophilicity exerted more potent anti‐bacterial activity.87 It is reported that an aromatic ring C contributes to a higher activity of G‐quadruplex induction and stabilization ability.90 Moreover, much evidence indicated the substituents on ring A and ring D displayed close relationship with activities of QPAs. For example, Jung et al showed the hydroxyl radical scavenging effect of coptisine and other isoquinoline alkaloids in CC is correlated with the ferrous ion chelating activity which can be promoted by the existence of hydroxyl group at C‐9 or methylenedioxy group at C‐9 and C‐10 in ring D.91 A study conducted by Jung et al verified aldose reductase inhibitory effect of proberberine alkaloids can be enhanced through introducing dioxymethylene group in ring D and oxidized form of dioxymethylene group in ring A.92 Last but not least, di‐methoxy at C‐9 and C‐10 or at C‐10 and C‐11 on ring D led to elevated activity of low‐density lipoprotein receptor (LDLR) gene expression and AMPK activation, showing great potential in clinical use against inflammatory diseases.93 Apart from studies on structure‐activity relationship of QPAs summarized in Table 4, there are also a few research investigating structure‐toxicity relationship of QPAs, such as quaternary 13‐substituted palmatines, once the aliphatic chain of the n‐alkanoyls is elongated more than 5 carbon atoms, the corresponding compounds are to display an apparent increase in cytotoxicity on normal IEC‐6 cell.88 More details are yet unclear and investigations are still needed.
Figure 5.
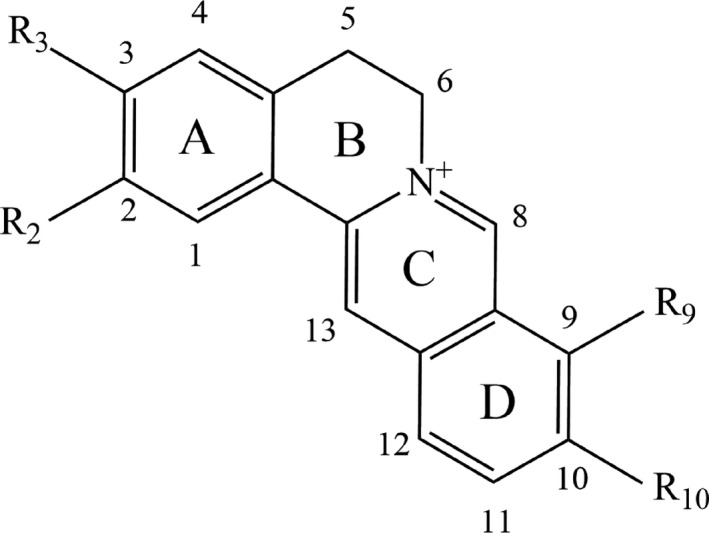
Isoquinoline parent nucleus of quaternary proberberine alkaloid
Table 4.
Structure‐activity relationship of quaternary proberberine alkaloid
| Substituents | Position | Effect | References |
|---|---|---|---|
| Nitro | Ring A | Anti‐acetylcholinesterase activity was elevated | 89 |
| Hydrophilic radical | C‐13, ring C | Anti‐malarial activity was improved | 87 |
| High lipophilicity | C‐13, ring C | More potent anti‐bacterial activity | 87 |
| Aromatic | Ring C | A higher activity of G‐quadruplex induction and stabilization ability | 90 |
| Hydroxyl | C‐9, ring D | Hydroxyl radical scavenging effect was promoted | 91 |
| Methylenedioxy | C‐9 and C‐10, ring D | ||
| Dioxymethylene | Ring D | The inhibitory effect of aldose reductase was enhanced | 92 |
| Oxidized dioxymethylene | Ring A | ||
| Di‐methoxy | Ring D | Increased LDLR expression and AMPK activation | 93 |
9. CLINICAL USE, DOSAGE AND ADVERSE REACTIONS
Documented in ancient pharmaceutical books, CC has been used since 2000 years ago in the treatment for ocular inflammation, diarrhoea and damp‐heat‐caused abdomen disorders, which are also the common indications of CC’s clinical use by now. Since CC exerts effect of clearing away damp‐induced heat, we can also be informed from some Chinese plant medicine books of its anti‐tumour action.94 However, there is still a long journey to transfer the knowledge to clinical application and CC clinical trial still remains at an early stage.95 By far, there is yet no record of single‐dosed coptisine in clinical, while berberine, the most abundant isoquinoline alkaloid in CC, has a history of treating bacteria‐correlative diarrhoeas in the late 1900s.96
On the other hand, dose‐effect relationship determines how well the drug works. In clinical, owing to the affecting factors such as age, disease location, administration route and processing method, CC exhibits a very wide daily single dose range of 1.5‐40 g.97 As for coptisine, shown in Table 5, the dose ranges of in vitro and in vivo experiment are 0.06‐468 μmol/L and 0.0025‐150 mg/kg, respectively. The wide dosage range may be due to the sensitivity variation of different types of cell lines and animal species. To acquire more uncovered detail about the dose‐effect relationship of coptisine, more studies are still warranted.
Table 5.
Coptisine concentration ranges used in reviewed article
| Study Type | Dose | References | Study Type | Dose | References |
|---|---|---|---|---|---|
| In vitro | 0.06‐0.25 μmol/L, 3 h | 86 | In vivo | 0.0025‐0.01 mg/mL, 12 hpf | 86 |
| 0.3‐10 μmol/L, 6 h | 62 | 0.97‐3.87 mg/kg, i.v, single dose, 0‐4 h | 49 | ||
| 0‐25 μmol/L, 24 h | 25 | 2.91‐11.61 mg/kg, i.v, single dose, 0‐24 h | 53 | ||
| 0‐28.11 μmol/L, 24 h | 31 | 10 mg/kg, i.v, single dose, 0.083‐24 h | 20 | ||
| 0‐40 μmol/L, 24 h | 79 | 10‐30 mg/kg, oral, two times | 68 | ||
| 0‐64 μmol/L, 24 h | 35 | 10‐40 mg/kg, once daily for 7d | 84 | ||
| 0‐75 μmol/L, 48 h | 32 | 10‐40 mg/kg, oral, once daily for 7d | 55 | ||
| 2.5‐40 μmol/L, 4 h | 38 | 15‐50 mg/kg, i.v, 5 days a week for 6w | 34 | ||
| 1‐10 μmol/L, 24 h | 82 | 15‐50 mg/kg, oral, once daily for 20d | 82 | ||
| 1‐30 μmol/L, 3.75‐24 h | 53 | 25‐100 mg/kg, oral, once daily for 21d | 67 | ||
| 1‐30 μmol/L, 15 min‐24 h | 49 | 30 mg/kg, oral, twice a day, 4d | 75 | ||
| 0‐3.125 μmol/L, 0‐48 h | 41 | 30‐90 mg/kg, i.p, once daily for 14d | 25 | ||
| 7.8 μmol/L, 48 h | 42 | 37.5‐150 mg/kg, oral, once daily for 7d | 42 | ||
| 10 μmol/L, 5 h | 78 | 50 mg/kg, oral, once daily for 24 h | 43 | ||
| 10‐30 μmol/L, 12 h | 50 | 50 mg/kg, oral, once daily for one month | 78 | ||
| 10‐40 μmol/L, 24‐48 h | 43 | 50 mg/kg, oral, single dose, 0.083‐24 h | 20 | ||
| 10‐100 μmol/L, 20 min | 63 | 50‐150 mg/kg, oral, once daily for 25d | 41 | ||
| 12.5‐50 μmol/L, 48 h | 37 | 50‐200 mg/kg, oral, once daily for 10d | 50 | ||
| 12.5‐50 μmol/L, 48 h | 40 | 70.05 mg/kg, oral, once daily for 4w | 66 | ||
| 12.5‐100 μmol/L, 24 h | 34 | 150 mg/kg, oral, once daily for 12w | 65 | ||
| 15.6‐93.8 μmol/L, 30 min | 76 | ||||
| 25‐50 μmol/L, 24 h | 39 | ||||
| 25‐150 μmol/L, 48 h | 36 | ||||
| 25.6 μmol/L (IC50) | 80 | ||||
| 30 μmol/L, 72 h | 64 | ||||
| 50 μmol/L, 0‐120s | 72 | ||||
| 50‐200 μmol/L, 30 min | 73 | ||||
| 62‐468 μmol/L, 0‐13.3 h | 77 |
When evaluating drug efficacy, toxicity and safety should be firstly taken into consideration. It has been reported that the oral LD50 value of CC in mice was 4.89 g/kg,98 and the common adverse reactions include haemolytic jaundice, nausea, vomiting, shortness of breath and convulsion.97, 99 Concerning the basis of CC’s toxic substance, Ma et al concluded berberine and coptisine were the main constituents responsible for the toxicity of CC.100 The LD50 value of berberine was, respectively, 329 (oral), 9.0386 (i.v) and 57.6103(i.p) mg/kg, and coptisine's LD50 value was 880.10 mg/kg,66, 101 which indicates berberine and coptisine have relatively wide range of safety.We also notice that, even ignoring the distribution loss from blood to tissues, the plasma level of coptisine with the highest dose in animal experiment is in fact, not able to reach the minimum concentration level used in cell experiment, so the main death cause of mice in LD50 study is probably due to the gastrointestinal toxicity, rather than systemic toxicity.
10. FUTURE PROSPECTS
Chemically modified drug plays a dominant role in clinical because it exerts potent therapeutical effect by affecting accurate and specific target.56, 102 Unfortunately, it meanwhile shows many adverse effects such as unselectively killing normal cells in chemotherapeutics.103, 104 During the past decades, natural products are attracting more and more attention for their preferable treating property with low toxicity.105 For example, apigenin from Epimedium koreanum Nakai, emodin from Rheum palmatum L, quercetin from Hypericun ascyron L and curcumin from Curcuma louga L are all reported to modulate various chronic diseases within safe dose range.106, 107, 108, 109 Here in this article, we systematically review the existing literature concerning coptisine's diverse beneficial properties, and this continues to support the recommendation that coptisine can be applied to the treatment for cancer, inflammation, CVDs and metabolic disorders.
The main anti‐cancer approaches include (1) invasion and metastasis prevention, (2) apoptosis or (3) autophagy induction, among which (1) and (2) are reported to be successfully achieved by coptisine treatment in many cancer cell lines to date. Apart from mechanisms mentioned above, cellular senescence is another promising approach in anti‐cancer therapy.110 It has been reported that berberine, a natural product which shares similar structure with coptisine, induced apoptosis and premature senescence, respectively, while used in high concentration of shorter treatment and lower concentration over longer period of treatment.111, 112 Therefore, it will be meaningful to evaluate coptisine's effect on cellular senescence of cancer cells. Another essential issue which needs to be further detected in future investigation is the role of coptisine to act as small‐molecule inhibitor of key markers involved in inflammation, CVDs, etc Berberine is revealed to diminish mRNA overexpression of caspase‐1 and NLRP3 in MSU‐challenged Raw 264.7 cells, and it also regulates pyroptosis in human hepatocellular carcinoma.113, 114 Since caspase‐1 is a key marker participating in inflammasome assembling and pyroptosis occurrence, and coptisine displays considerable suppressing effect on NLRP3 inflammasome priming and assembly, another relevant subject for further study can be the role of coptisine as small‐molecular inhibitor of caspase‐1 and the downstream pyroptosis activation.
Cardiovascular diseases such as hyperlipidaemia and hypertension are global health issue and closely related to metabolic disorder which is accompanied by low‐grade chronic inflammation.115 Similar to coptisine, berberine exerts cardiovascular protection, lipid‐lowering, vascular‐relaxing and anti‐atherosclerosis properties. Besides, berberine also prevents multiple pathophysiologic processes such as myocardial injury, neurohormonal activation and oxidative stress.116, 117, 118 Accordingly, further efforts could focus on coptisine's effect on myocardial injury biomarkers (eg cardiac troponins), neurohormonal activation biomarkers (eg norepinephrine, ET‐1) and oxidative/nitroxidative stress biomarkers (eg nitrotyrosine, MPO).
Autophagy is involved in the progression of multiple disorders including cancer and CADs.119, 120, 121 To date, apart from regulating transduction of pathways such as NF‐κB, MAPK and PI3K/AKT, berberine has been verified to mediate mitochondrial‐induced apoptosis and protective autophagy in human breast cancer cells,122 and it also induces autophagy and the downstream NLRP3 inflammasome activation in macrophages.123 Moreover, berberine ameliorates CADs through triggering autophagy in both Beclin‐1‐dependent and Beclin‐1‐independent ways.124 Since coptisine and berberine exert property of structural homology, the limitation of the existed studies includes the lack of investigation concerning the effect of coptisine on autophagy occurrence, which is closely related to the pathogenesis of multiple cancers, inflammation and CADs (Table 6 and Figure 6).
Table 6.
Future prospects of coptisine
| Type | Future prospect |
|---|---|
| Cancer | 1) Cellular senescence of cancer cells |
| 2) Autophagy occurrence | |
| Inflammation | 1) Caspase‐1‐involved inflammasome activation |
| 2) Autophagy occurrence | |
| CADs | 1) Myocardial injury biomarkers |
| 2) Neurohormonal activation biomarkers | |
| 3) Oxidative/nitroxidative stress biomarkers | |
| 4) Autophagy occurrence |
Figure 6.
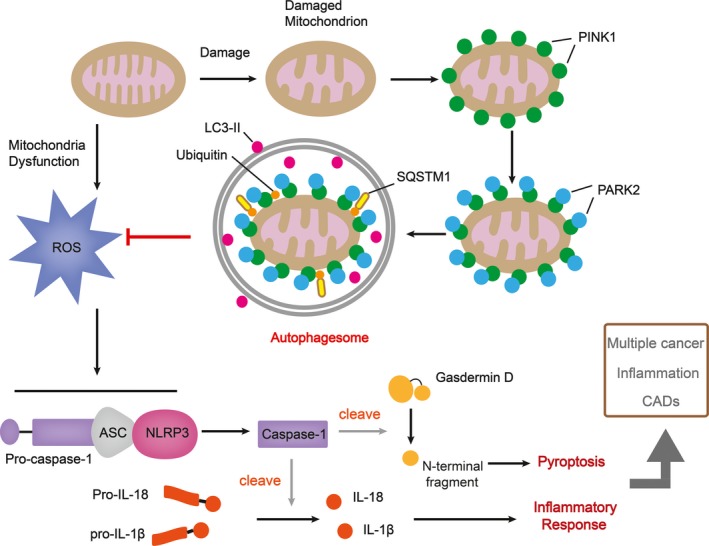
Recommendations for future investigations on coptisine. Once mitochondrion is damaged, PARK2 binds to PINK1 on the surface of mitochondrial and ubiquitinates mitochondrial outer membrane proteins, which then bind to SQSTM1, a receptor which can interact with LC3. The formation of autophagosome inhibits ROS, the overproduction of which causes NLRP3 inflammasome assembling and the downstream pyroptosis and inflammatory response
As mentioned in previous chapters, the plasma concentration of coptisine and other CC isoquinoline alkaloids demonstrates obvious non‐liner relationship with dosage, and the low dissolution in intestinal fluid dominantly limits the absorption amount. In recent years, pharmaceutics methods like nano strategies and microrods have been employed to promote the intestinal dissolution of berberine. Given the similar parent structure shared by berberine and coptisine, further study is advised to focus on new pharmaceutics strategy which is able to improve coptisine's dissolve rate.
To sum up, coptisine is a promising drug with multiple targets, while there is still a knowledge gap before coptisine meets the requirements to be introduced to clinic and further applied to the prevention and therapy of diseases such as cancer, inflammation, anti‐bacteria and CVDs. Besides, more investigation is needed to promote coptisine's bioavailability and meanwhile reach the balance between toxicological safety and therapeutic efficacy.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
JS.W and Y.L wrote the draft; JS. W, S.L and SY.S analysed the data; DH.D, L.X and YF.H searched the database and extracted literatures; JS.W and DH.D prepared all the figures; and P.W and XL.M supervised the work.
ACKNOWLEDGEMENTS
This work is supported by grants from the National Natural Science Foundation of China (No. 81773974 and No. 81473419) and the National Key Research and Development Program of China (No. 2017YFC1703900).
Wu J, Luo Y, Deng D, et al. Coptisine from Coptis chinensis exerts diverse beneficial properties: A concise review. J Cell Mol Med. 2019;23:7946–7960. 10.1111/jcmm.14725
Contributor Information
Ping Wang, Email: viviansector@aliyun.com.
Xianli Meng, Email: xlm9999@hotmail.com.
DATA AVAILABILITY STATEMENT
No data, models or code was generated or used during the study.
REFERENCES
- 1. Han L, Wang R, Zhang X, et al. Advances in processing and quality control of traditional chinese medicine Coptidis rhizoma (Huanglian): A Review. J AOAC Int. 2018; 102(3): 699‐707. [DOI] [PubMed] [Google Scholar]
- 2. Wei X, Tao J, Shen Y, et al. Sanhuang xiexin tang ameliorates type 2 diabetic rats via modulation of the metabolic profiles and NF‐κB/PI‐3K/Akt signaling pathways. Front Pharmacol. 2018;9:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu CP, Shia CS, Tsai SY, Hou YC. Pharmacokinetics and relative bioavailability of flavonoids between two dosage forms of gegen‐qinlian‐tang in rats. Evid Based Complement Alternat Med. 2012;2012:1‐8. 10.1155/2012/308018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee Y‐H, Kim D, Lee MJ, et al. Subchronic toxicity study of Coptidis Rhizoma in rats. J Ethnopharmacol. 2014;152(3):457‐463. 10.1186/s13020-018-0171-3 [DOI] [PubMed] [Google Scholar]
- 5. Meng F‐C, Wu Z‐F, Yin Z‐Q, Lin L‐G, Wang R, Zhang Q‐W. Coptidis rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin Med. 2018;13(1):13 10.1186/s13020-018-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun Y, Xia M, Yan H, et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br J Pharmacol. 2018;175(2):374‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan J, Zhang K, Jin Y, et al. Pharmacological effects of berberine on mood disorders. J Cell Mol Med. 2019;23(1):21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang N, Tan H‐Y, Li L, Yuen M‐F, Feng Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J Ethnopharmacol. 2015;176:35‐48. [DOI] [PubMed] [Google Scholar]
- 9. Hesari AR, Ghasemi F, Cicero A, et al. Berberine: a potential adjunct for the treatment of gastrointestinal cancers? J Cell Biochem. 2018;119(12):9655‐9663. [DOI] [PubMed] [Google Scholar]
- 10. Liang Y, Xu X, Yin M, et al. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta‐analysis. Endocr J. 2019;66(1):51‐63. [DOI] [PubMed] [Google Scholar]
- 11. Lin X, Zhang N. Berberine: pathways to protect neurons. Phytother Res. 2018;32(4):1‐10. [DOI] [PubMed] [Google Scholar]
- 12. Neag MA, Mocan A, Echeverría J, et al. Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front Pharmacol. 2018;9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedemann T, Ying Y, Wang W, et al. Neuroprotective effect of coptis chinensis in mpp [formula: see text] and mptp‐induced parkinson's disease models. Am J Chin Med. 2016;44(5):1. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Deng A, Yu J, et al. Current research status on pharmacological activities of coptisine. China J Chin Mater Med 2013; 38: 2750‐2754. [Chinese Article]. [PubMed] [Google Scholar]
- 15. Zan B, Shi R, Wang T, Wu J, Ma Y, Cheng N. Simultaneous quantification of multiple active components from Xiexin decoction in rat plasma by LC‐ESI‐MS/MS: application in pharmacokinetics. Biomed Chromatogr. 2011;25(7):816‐826. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Zhang Y, Xiao J, Xu R, Wang Q, Wang X. Simultaneous determination of baicalin, baicalein, wogonoside, wogonin, scutellarin, berberine, coptisine, ginsenoside rb1 and ginsenoside re of banxia xiexin decoction in rat plasma by lc–ms/ms and its application to a pharmacokinetic study. Biomed Chromatogr. 2017;32(2): e4083. [DOI] [PubMed] [Google Scholar]
- 17. Fan H, Chen Y, Bei W, et al. In vitro screening for antihepatic steatosis active components within coptidis rhizoma alkaloids extract using liver cell extraction with HPLC analysis and a free fatty acid‐induced hepatic steatosis HepG2 cell assay. Evid Based Complement Alternat Med. 2013;459390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Wang H, Si N, et al. Metabolic profiling analysis of berberine, palmatine, jatrorrhizine, coptisine and epiberberine in zebrafish by ultra‐high performance liquid chromatography coupled with LTQ Orbitrap mass spectrometer. Xenobiotica. 2015;45(4):302‐311. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Qiu F, Jiang J, Gao C, Tan Y. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P‐glycoprotein. Xenobiotica. 2011;41(4):290‐296. [DOI] [PubMed] [Google Scholar]
- 20. Su J, Miao Q, Miao P, et al. Pharmacokinetics and brain distribution and metabolite identification of coptisine, a protoberberine alkaloid with therapeutic potential for cns disorders, in rats. Biol Pharm Bull. 2015;38(10):1518‐1528. [DOI] [PubMed] [Google Scholar]
- 21. Yan YU, Zhang H, Zhang Z, et al. Pharmacokinetics and tissue distribution of coptisine in rats after oral administration by liquid chromatography‐mass spectrometry. Biomed Chromatogr. 2017;31(7):e3918 [DOI] [PubMed] [Google Scholar]
- 22. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2018;17(6):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang LI. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013; 2013:928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baek SH, Ko J‐H, Lee JH, et al. Ginkgolic acid inhibits invasion and migration and tgf‐β‐induced emt of lung cancer cells through pi3k/akt/mtor inactivation. J Cell Physiol. 2016;232(2):346‐354. [DOI] [PubMed] [Google Scholar]
- 25. Cao Q, Hong S, Li Y, et al. Coptisine suppresses tumor growth and progression by down‐regulating MFG‐E8 in colorectal cancer. RSC Adv. 2018;8(54):30937‐30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arun A, Ansari MI, Popli P, et al. New piperidine derivative DTPEP acts as dual‐acting anti‐breast cancer agent by targeting ERα and downregulating PI3K/Akt‐PKCα leading to caspase‐dependent apoptosis. Cell Prolif. 2018;51(6):e12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karatepe K, Zhu H, Zhang X, et al. Proteinase 3 limits the number of hematopoietic stem and progenitor cells in murine bone marrow. Stem Cell Rep. 2018;11(5):1092‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eustace AJ, Conlon NT, McDermott M, et al. Development of acquired resistance to lapatinib may sensitise HER2‐positive breast cancer cells to apoptosis induction by obatoclax and TRAIL. BMC Cancer. 2018;18(1):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang W, Cheng P, Hu W, et al. Inhibition of microrna‐384‐5p alleviates osteoarthritis through its effects on inhibiting apoptosis of cartilage cells via the nf‐κb signaling pathway by targeting sox9. Cancer Gene Ther. 2018;25:326‐338. [DOI] [PubMed] [Google Scholar]
- 30. Zhang H, Wang L, Guo C, et al. Response of mouse thymic cells to radiation after transfusion of mesenchymal stem cells. Medicine. 2016;95(51):e5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han B, Jiang PU, Li Z, et al. Coptisine‐induced apoptosis in human colon cancer cells (HCT‐116) is mediated by PI3K/Akt and mitochondrial‐associated apoptotic pathway. Phytomedicine. 2017;152‐160. [DOI] [PubMed] [Google Scholar]
- 32. Wang XH, Jiang SM, Sun QW. Effects of berberine on human rheumatoid arthritis fibroblast‐like synoviocytes. Exp Biol Med. 2011;236:859‐866. [DOI] [PubMed] [Google Scholar]
- 33. Huang Z, Dong F, Li S, et al. Berberine‐induced inhibition of adipocyte enhancer‐binding protein 1 attenuates oxidized low‐density lipoprotein accumulation and foam cell formation in phorbol 12‐myristate 13‐acetate‐induced macrophages. Eur J Pharmacol. 2012;690:164‐169. [DOI] [PubMed] [Google Scholar]
- 34. Zhou LI, Yang F, Li G, et al. Coptisine induces apoptosis in human hepatoma cells through activating 67‐KD laminin receptor/cGMP signaling. Front Pharmacol. 2018;9:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Qiu D‐M, Chen S‐H, Cao S‐P, Xia X‐L. Suppression of Human Breast Cancer Cell Metastasis by Coptisine in Vitro. Asian Pac J Cancer Prev. 2014;15(14):5747‐5751. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y‐L, Zhang X, Miao X‐Z, et al. Coptisine suppresses proliferation and inhibits metastasis in human pancreatic cancer PANC‐1 cells. J Asian Nat Prod Res. 2019;23:1‐12. 10.1080/10286020.2019.1585820 [DOI] [PubMed] [Google Scholar]
- 37. Han B, Pu J, Xu HS, et al. 8‐Cetylcoptisine, a new coptisine derivative, induces mitochondria dependent apoptosis and G0/G1 cell cycle arrest in human A549 cells. Chem Biol Interact. 2019;299:27‐36. [DOI] [PubMed] [Google Scholar]
- 38. Wei S, Liu JC, Ge WH. The protective effect of coptisine on vascular endothelial cells damage induced by hydrogen peroxide. Chi Med Herald 2019;16:4‐7. [Chinese Article]. [Google Scholar]
- 39. Yang F, Li X, Zhang TT, et al. Apoptosis of NCI⁃H1650 lung cancer cells was induced by active oxygen mediators‐mitochondrial pathway. J Prac Med 2017;33:4033‐4037. [Chinese Article]. [Google Scholar]
- 40. Rao PC, Begum S, Sahai M, Sriram DS. Coptisine‐induced cell cycle arrest at g2/m phase and reactive oxygen species‐dependent mitochondria‐mediated apoptosis in non‐small‐cell lung cancer a549 cells. Tumour Biol. 2017;39(3):101042831769456. [DOI] [PubMed] [Google Scholar]
- 41. Huang T, Xiao Y, Yi L, et al. Coptisine from rhizoma coptidis suppresses HCT‐116 cells‐related tumor growth in vitro and in vivo. Sci Rep. 2017;7(1):38524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chai F‐N, Zhang J, Xiang H‐M, et al. Protective effect of coptisine from, rhizoma coptidis, on lps/d‐galn‐induced acute liver failure in mice through up‐regulating expression of mir‐122. Biomedi Pharmacother. 2018;98:180‐190. [DOI] [PubMed] [Google Scholar]
- 43. Yu DI, Fu S, Cao Z, et al. Unraveling the novel anti‐osteosarcoma function of coptisine and its mechanisms. Toxicol Lett. 2014;226(3):328‐336. [DOI] [PubMed] [Google Scholar]
- 44. Wu J, Hu Y, Xiang LI, et al. San‐huang‐xie‐xin‐tang constituents exert drug‐drug interaction of mutual reinforcement at both pharmacodynamics and pharmacokinetic level: a review. Front Pharmacol. 2016;7:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zou Z‐Y, Hu Y‐R, Ma H, et al. Coptisine attenuates obesity‐related inflammation through LPS/TLR‐4‐mediated signaling pathway in Syrian golden hamsters. Fitoterapia. 2015;105:139‐146. [DOI] [PubMed] [Google Scholar]
- 46. Yu T, Lee YJ, Jang H‐J, et al. Anti‐inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J Ethnopharmacol. 2010;134:493‐500. [DOI] [PubMed] [Google Scholar]
- 47. Winkler S, Rosenwolff A. Caspase‐1: an integral regulator of innate immunity. Semin Immunopathol. 2015;37(4):419‐427. [DOI] [PubMed] [Google Scholar]
- 48. Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer H‐D. Caspase‐1: The inflammasome and beyond. Innate Immunity. 2014;20(2):115‐125. [DOI] [PubMed] [Google Scholar]
- 49. Wu J, Zhang H, Hu B, et al. Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide‐stimulated RAW 264.7 murine macrophage cells. Eur J Pharmacol. 2016;780:106‐114. [DOI] [PubMed] [Google Scholar]
- 50. Fu S, Ni S, Wang D, Hong T. Coptisine suppresses mast cell degranulation and ovalbumin‐induced allergic rhinitis. Molecules. 2018;23(11):3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou K, Hu LI, Liao W, Yin D, Rui F. Coptisine prevented il‐β‐induced expression of inflammatory mediators in chondrocytes. Inflammation. 2016;39(4):1558‐1565. [DOI] [PubMed] [Google Scholar]
- 52. Ren Y, Wang D, Lu F, et al. Coptidis Rhizoma inhibits NLRP3 inflammasome activation and alleviates renal damage in early obesity‐related glomerulopathy. Phytomedicine. 2018;49:52‐65. 10.1016/j.phymed.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 53. Wu J, Luo Y, Jiang Q, et al. Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase‐1. Pharmacol Res. 2019;147:104348 [DOI] [PubMed] [Google Scholar]
- 54. Huang H, Ke C, Chen Q. Study on in‐vivo anti‐inflammatory effect of tetrahydro coptidine. Practl Pharm Clin Remed 2016;190:830‐834. [Chinese Article]. [Google Scholar]
- 55. Chen H‐B, Luo C‐D, Liang J‐L, et al. Anti‐inflammatory activity of coptisine free base in mice through inhibition of NF‐κB and MAPK signaling pathways. Eur J Pharmacol. 2017;811:222‐231. 10.1016/j.ejphar.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 56. Hu Y, Wang LI, Xiang LI, et al. Pharmacokinetic‐pharmacodynamic modeling for coptisine challenge of inflammation in LPS‐stimulated rats. Sci Rep. 2019;9:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kahl KG, Stapel B, Link FH. between depression and cardiovascular diseases due to epigenomics and proteomics: Focus on energy metabolism. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:146‐157. [DOI] [PubMed] [Google Scholar]
- 58. Luis Â, Domingues FC, Pereira L. Association between berries intake and cardiovascular diseases risk factors: a systematic review with meta‐analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018;9(2):740‐757. [DOI] [PubMed] [Google Scholar]
- 59. Satoh K, Fukumoto Y, Shimokawa H. Rho‐kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301(2):H287‐H296. [DOI] [PubMed] [Google Scholar]
- 60. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144‐2153. [DOI] [PubMed] [Google Scholar]
- 61. Wang Z, Hu W, Lu C, et al. Targeting NLRP3 (Nucleotide‐Binding Domain, Leucine‐Rich–Containing Family, Pyrin Domain–Containing‐3) inflammasome in cardiovascular disorders. Arterioscler Thromb Vasc Biol. 2018;38(12):2765‐2779. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Wang Q, Zhang LU, et al. Coptisine protects cardiomyocyte against hypoxia/reoxygenation‐induced damage via inhibition of autophagy. Biochem. Biophys. Res Commun. 2017;490(2):231‐238. [DOI] [PubMed] [Google Scholar]
- 63. Gong L‐L, Fang L‐H, Qin H‐L, Lv Y, Du G‐H. Analysis of the mechanisms underlying the vasorelaxant action of coptisine in rat aortic rings. Am J Chin Med. 2012;40(2):309‐320. [DOI] [PubMed] [Google Scholar]
- 64. Suzuki H, Tanabe H, Mizukami H, Inoue M. Differential gene expression in rat vascular smooth muscle cells following treatment with coptisine exerts a selective antiproliferative effect. J Nat Prod. 2011;74(4):634‐638. [DOI] [PubMed] [Google Scholar]
- 65. Feng M, Kong S‐Z, Wang Z‐X, et al. The protective effect of coptisine on experimental atherosclerosis ApoE−/− mice is mediated by MAPK/NF‐κB‐dependent pathway. Biomed Pharmacother. 2017;721‐729. [DOI] [PubMed] [Google Scholar]
- 66. He K, Ye X, Wu H, et al. The Safety and Anti‐Hypercholesterolemic Effect of coptisine in syrian golden hamsters. Lipids. 2015;50(2):185‐194. [DOI] [PubMed] [Google Scholar]
- 67. Gong L‐L, Fang L‐H, Wang S‐B, et al. Coptisine exert cardioprotective effect through anti‐oxidative and inhibition of RhoA/Rho kinase pathway on isoproterenol‐induced myocardial infarction in rats. Atherosclerosis. 2012;222(1):50‐58. [DOI] [PubMed] [Google Scholar]
- 68. Guo J, Wang S‐B, Yuan T‐Y, et al. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis. 2013;231(2):384‐391. [DOI] [PubMed] [Google Scholar]
- 69. Han Y, Yan D, Zhao Y, Peng C, Xiao X. Toxic effects of protoberberine alkaloids from Rhizoma Coptidis on Tetrahymena thermophila BF5 growth based on microcalorimetry. J Therm Anal Calorim. 2012;108(1):341‐346. [Google Scholar]
- 70. Malik TA, Kamili AN, Chishti MZ, Tanveer S, Ahad S, Johri RK. In vivo anticoccidial activity of berberine [18, 5,6‐dihydro‐9,10‐dimethoxybenzo(g)‐1,3‐benzodioxolo(5,6‐a) quinolizinium]‐an isoquinoline alkaloid present in the root bark of berberis lycium. Phytomedicine. 2014;21(5):663‐669. [DOI] [PubMed] [Google Scholar]
- 71. Zhang G‐B, Maddili SK, Tangadanchu V, et al. Discovery of natural berberine‐derived nitroimidazoles as potentially multi‐targeting agents against drug‐resistant Escherichia coli. Sci China Chem. 2017;61(5):557‐568. [Google Scholar]
- 72. Lang L, Hu Q, Wang J, et al. Coptisine, a natural alkaloid from Coptidis Rhizoma, inhibits plasmodium falciparum dihydroorotate dehydrogenase. Chem Biol Drug Des. 2018;92(1):1324‐1332. [DOI] [PubMed] [Google Scholar]
- 73. Li C, Huang P, Wong K, et al. Coptisine‐induced inhibition of Helicobacter pylori: elucidation of specific mechanisms by probing urease active site and its maturation process. J Enzyme Inhib Med Chem. 2018;33(1):1362‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tan L, Li C, Chen H, et al. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. Eur J Pharm Sci. 2017;77‐86. [DOI] [PubMed] [Google Scholar]
- 75. Zhang XF, Qiao CX, Chen XF, et al. Experimental study on the modulation of coptisine on chemotherapy‐related diarrhea through regulating IκBα/NF‐κB pathway. Chin J Integr Trad West Med. 2018;38:820‐824. [Chinese Article]. [Google Scholar]
- 76. Yan D, Jin C, Xiao X, Dong X. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. J Biochem Biophys Methods. 2008;70(6):845‐849. [DOI] [PubMed] [Google Scholar]
- 77. Yan D, Wei LI, Xiao XH, Zhou DL, Han YM. Microcalorimetric investigation of effect of berberine alkaloids from Coptis chinensis Franch on intestinal diagnostic flora growth. Chin Sci Bull. 2009;54(3):369‐373. [Google Scholar]
- 78. Yu D, Tao B‐B, Yang Y‐Y, et al. The ido inhibitor coptisine ameliorates cognitive impairment in a mouse model of alzheimer's disease. J Alzheimers Dis. 2014;43(1):291. [DOI] [PubMed] [Google Scholar]
- 79. Friedemann T, Schumacher U, Tao Y, et al. Neuroprotective activity of coptisine from coptis chinensis (Franch). Evid Based Complement Alternat Med. 2015;2015;827308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ge AH, Bai Y, Li J, et al. An activity‐integrated strategy involving ultra‐high‐performance liquid chromatography/quadrupole‐time‐of‐flight mass spectrometry and fraction collector for rapid screening and characterization of the ‐glucosidase inhibitors in Coptis chinensis Franch. (Huanglian). J Pharm Biomed Anal. 2014;100:79‐87. [DOI] [PubMed] [Google Scholar]
- 81. Chen L, Wang X, Liu Y, Di X. Dual‐target screening of bioactive components from traditional Chinese medicines by hollow fiber‐based ligand fishing combined with liquid chromatography–mass spectrometry. J. Pharm Biomed Anal. 2017;6:269‐276. [DOI] [PubMed] [Google Scholar]
- 82. Shi L‐L, Jia W‐H, Zhang LI, et al. Glucose consumption assay discovers coptisine with beneficial effect on diabetic mice. Eur J Pharmacol. 2019;859:172523. [DOI] [PubMed] [Google Scholar]
- 83. Kang JA, Rho JK, Park SH, et al. Evaluation of inhibitory effect of coptisine on protein kinase C activity using a RI detection‐assisted biochip. J Radioanal Nucl Chem. 2019;319(3):1103‐1110. [Google Scholar]
- 84. Luo C, Chen H, Wang Y, et al. Protective effect of coptisine free base on indomethacin‐induced gastric ulcers in rats: Characterization of potential molecular mechanisms. Life Sci. 2018;12:47‐56. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Z‐H, Wu L‐Q, Deng A‐J, et al. New synthetic method of 8‐oxocoptisine starting from natural quaternary coptisine as anti‐ulcerative colitis agent. J Asian Nat Prod Res. 2014;16(8):841‐846. [DOI] [PubMed] [Google Scholar]
- 86. Hu Y‐R, Ma H, Zou Z‐Y, et al. Activation of Akt and JNK/Nrf2/NQO1 pathway contributes to the protective effect of coptisine against AAPH‐induced oxidative stress. Biomed Pharmacother. 2016;11:313‐322. [DOI] [PubMed] [Google Scholar]
- 87. Dembitsky VM, Gloriozova TA, Poroikov VV, et al. Naturally occurring plant isoquinoline N‐oxide alkaloids: Their pharmacological and SAR activities. Phytomedicine. 2015;22:183‐202. [DOI] [PubMed] [Google Scholar]
- 88. Song LI, Zhang H‐J, Deng A‐J, et al. Syntheses and structure‐activity relationships on antibacterial and anti‐ulcerative colitis properties of quaternary 13‐substituted palmatines and 8‐oxo‐13‐substituted dihydropalmatines. Bioorg Med Chem. 2018;26:2584‐2598. [DOI] [PubMed] [Google Scholar]
- 89. Huang QQ, Bi JL, Sun QY, et al. Bioactive isoquinoline alkaloids from Corydalis saxicola . Planta Med. 2012;78:65‐70. [DOI] [PubMed] [Google Scholar]
- 90. Ji X, Sun H, Zhou H, Xiang J, Tang Y, Zhao C. The interaction of telomeric DNA and c‐myc22 G‐quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012;22:127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jang MH, Kim HY, Kang KS, Yokozawa T, Park JH. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch Pharm Res. 2009;32:341‐345. [DOI] [PubMed] [Google Scholar]
- 92. Jung HA, Yoon NY, Bae HJ, Min B‐S, Choi JS. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch Pharm Res. 2008;31:1405‐1412. [DOI] [PubMed] [Google Scholar]
- 93. Wang Y‐X, Kong W‐J, Li Y‐H, et al. Synthesis and structure–activity relationship of berberine analogues in LDLR up‐regulation and AMPK activation. Bioorg Med Chem. 2012;20:6552‐6558. [DOI] [PubMed] [Google Scholar]
- 94. Xu JT, Wang LQ, Xu B. Research development of coptis chinensis. Acta Academiae Medicinae Sinicae 2004;26:704‐707 (Chinese article). [PubMed] [Google Scholar]
- 95. Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126(1):5‐17. [DOI] [PubMed] [Google Scholar]
- 96. Rabbani GH, Butler T, Knight J, et al. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic E. coli and Vibrio cholerae. J Infec Dis. 1986;155:979‐984. [DOI] [PubMed] [Google Scholar]
- 97. Wu SP, Xu LP, Liu HX, Tong XL. The explore of clinical application and dosage of Coptidis Rhizoma. Chin J Clin Med. 2015;43(2):92‐94. (Chinese article). [Google Scholar]
- 98. Qiu SH, Tang HB, Li FY et al. Experimental study of the acute toxicity on common‐used bitter and cold medicines. Central South Pharmacy 2004;2:37‐38. (Chinese article). [Google Scholar]
- 99. Yang SY, Gao XS. The research survey of the newborn jaundice induced and enhanced by Huanglian. China J Chin Materia Med 1996;21:187‐188. (Chinese article). [Google Scholar]
- 100. Zhao XY, Tong XL, Zhao LH, et al. Advances in modern research on adverse reactions of Coptidis Rhizoma unilateral and its main components. China J Chin Materia Med 2013;38(2):292‐295. (Chinese article). [Google Scholar]
- 101. Kheir MM, Wang Y, Hua L, et al. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48(4):1105‐1110. [DOI] [PubMed] [Google Scholar]
- 102. Dang Z, Liu X, Wang X, et al. Comparative effectiveness and safety of traditional Chinese medicine supporting Qi and enriching blood for cancer related anemia in patients not receiving chemoradiotherapy: a meta‐analysis and systematic review. Drug Des Dev Ther. 2018;13:221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Alexander MS, Wilkes JG, Schroeder SR, et al. Pharmacological ascorbate reduces radiation‐induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 2018;1:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jalalian SH, Ramezani M, Abnous K, Taghdisi SM. Targeted co‐delivery of epirubicin and NAS‐24 aptamer to cancer cells using selenium nanoparticles for enhancing tumor response in vitro and in vivo. Cancer Lett. 2018;12:87‐93. [DOI] [PubMed] [Google Scholar]
- 105. Nitulescu G, Van De Venter M, Nitulescu G, et al. The Akt pathway in oncology therapy and beyond (Review). Int J Oncol. 2018;53(6):2319‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ozbey U, Attar R, Romero MA, et al. Apigenin as an effective anticancer natural product: Spotlight on TRAIL, WNT/β‐catenin, JAK‐STAT pathways, and microRNAs. J Cell Biochem. 2018;120(2):1060‐1067. [DOI] [PubMed] [Google Scholar]
- 107. Monisha BA, Kumar N, Tiku AB. Emodin and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;47‐73. [DOI] [PubMed] [Google Scholar]
- 108. Kim JK, Park SU. Quercetin and its role in biological functions: an updated review. EXCLI J. 2018;17:856‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit Rev Food Sci Nutr. 2019;11:1‐53. https://doi.org/10(1080/10408398) [DOI] [PubMed] [Google Scholar]
- 110. Madunić J, Madunić IV, Gajski G, Popić J, Garaj‐Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11‐22. 10.1016/j.canlet.2017.10.041 [DOI] [PubMed] [Google Scholar]
- 111. Pan Y, Zhang F, Zhao Y, et al. Berberine enhances chemosensitivity and induces apoptosis through dose‐orchestrated AMPK signaling in breast cancer. J Cancer. 2017;8(9):1679‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Agnarelli A, Natali M, Garcia‐Gil M, et al. Cell‐specific pattern of berberine pleiotropic effects on different human cell lines. Sci Rep. 2018;8:10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dinesh P, Rasool MK. Berberine, an isoquinoline alkaloid suppresses txnip mediated nlrp3 inflammasome activation in msu crystal stimulated raw 264.7 macrophages through the upregulation of nrf2 transcription factor and alleviates msu crystal induced inflammation in rats. Int Immunopharmacol. 2017;44:26‐37. [DOI] [PubMed] [Google Scholar]
- 114. Chu Q, Jiang Y, Zhang W, et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget. 2016;7(51):84658‐84665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension. 2017;70(1):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Qing Y, Dong X, Hongli L, Yanhui L. Berberine promoted myocardial protection of postoperative patients through regulating myocardial autophagy. Biomed Pharmacother. 2018;105:1050‐1053. [DOI] [PubMed] [Google Scholar]
- 117. Chi L, Peng L, Hu X, Pan NA, Zhang Y. Berberine combined with atorvastatin downregulates LOX‐1 expression through the ET‐1 receptor in monocyte/macrophages. Int J Mol Med. 2014;34(1):283‐290. [DOI] [PubMed] [Google Scholar]
- 118. Choy KW, Murugan DD, Mustafa MR. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol Res. 2018;6:119‐129. [DOI] [PubMed] [Google Scholar]
- 119. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2010;469(7329):221‐225. [DOI] [PubMed] [Google Scholar]
- 120. Drake LE, Springer MZ, Poole LP, Kim CJ, Macleod KF. Expanding perspectives on the significance of mitophagy in cancer. Semin. Cancer Biol. 2017;12:110‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bravo‐San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812‐1824. [DOI] [PubMed] [Google Scholar]
- 122. Yao Z, Wan Y, Li B, et al. Berberine induces mitochondrial‐mediated apoptosis and protective autophagy in human malignant pleural mesothelioma NCI‐H2452 cells. Oncol Rep. 2018;40(6):3603‐3610. [DOI] [PubMed] [Google Scholar]
- 123. Zhou H, Feng L, Xu F, et al. Berberine inhibits palmitate‐induced nlrp3 inflammasome activation by triggering autophagy in macrophages: a new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother. 2017;89:864‐874. [DOI] [PubMed] [Google Scholar]
- 124. Hashemzaei M, Entezari Heravi R, Rezaee R, Roohbakhsh A, Karimi G. Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. Eur J Pharmacol. 2017;2:44‐51. [DOI] [PubMed] [Google Scholar]
- 125. Sun L, Ding F, You G, et al. Development and validation of an uplc‐ms/ms method for pharmacokinetic comparison of five alkaloids from jinqi jiangtang tablets and its monarch drug coptidis rhizoma. Pharmaceutics. 2017;10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Liu G, He W, Cai H, et al. The simultaneous determination of berberine, palmatine, coptisine, epiberberine and jatrorrhizine in rat plasma by LC‐MS/MS and a pharmacokinetic comparison after the oral administration of Rhizoma coptidis and Jiao‐Tai‐Wan extract. Anal Methods. 2014;6(9):2998‐3008. [Google Scholar]
- 127. Zhao X, Wang Y, Zheng LU, et al. Comparative pharmacokinetics study of five alkaloids in rat plasma and related compound–herb interactions mechanism after oral administration of shuanghua baihe tablets. Nat Prod Res. 2018;32(17):2031‐2036. [DOI] [PubMed] [Google Scholar]
- 128. Qian P, Zhang Y‐B, Yang Y‐F, Xu W, Yang X‐W. Pharmacokinetics studies of 12 alkaloids in rat plasma after oral administration of zuojin and fan‐zuojin formulas. Molecules. 2017;22(2):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Qian X‐C, Zhang L, Tao YI, et al. Simultaneous determination of ten alkaloids of crude and wine‐processed Rhizoma Coptidis aqueous extracts in rat plasma by UHPLC–ESI–MS/MS and its application to a comparative pharmacokinetic study. J Pharm Biomed Anal. 2015;49:64‐73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data, models or code was generated or used during the study.


