Abstract
Head and neck cancer is one of the most common cancers in the world, accounting for up to 30–40% malignancies in India. Research is always on the lookout for parameters that help in early diagnosis of such disease and to explore the possibility of discovering such parameters that would assist in management of the disease by its potential to predict and prognosticate the disease. To estimate serum ADA levels and to correlate with response to therapy and also to correlate between different clinical stages and serum ADA levels and to correlate HPV status to response to therapy. A prospective cohort study. 30 patients who were diagnosed with squamous cell carcinoma of the oropharynx, hypopharynx and larynx were considered in this study. The pre and post treatment values of serum ADA was estimated in these patients and the tumour was assessed for HPV status. The difference in the in the serum ADA levels before and after treatment was 9.982 which was statistically significant with a p value of < 0.001. HPV positive status and response to therapy in the form of recurrence shows a p value of 0.485 which is not statistically significant. Serum ADA level can be used as a parameter to assess the severity of the disease and the response to treatment in cases of carcinoma of the oropharynx, hypopharynx and larynx. HPV status of the disease has its limitation in prediction and prognosis of the disease.
Keywords: Squamous cell carcinoma, Oropharynx, Hypopharynx, Larynx, Serum ADA, HPV
Introduction
Head and neck cancer is one of the most common cancers in the world, accounting for up to 30–40% malignancies in India. Failure or delay in the early diagnosis of squamous cell carcinoma is one of the primary causes of high mortality and morbidity in cancer patients [1]. High incidence is featured to several predisposing factors such as male sex, age greater than 55 years, low socioeconomic status, smoking and alcohol [2]. Early finding of cancer offers the best opportunity for cure with the goal being to diagnose cancer at an early stage so as to reduce the morbidity and mortality. A large number of serological, molecular or genetic markers have been examined in carcinomas of the head and neck all of which are controversial regarding their prognostic significance and also clinical effectiveness.
Further insights into the mechanisms leading to malignancy are prerequisite for identifying new biomarkers for squamous cell carcinoma from the serum. The idea of screening and following individuals with malignancy by serum analysis is appealing from several points of view including its ease, economical advantage, non-invasiveness and possibility of repeated sampling.
Serum SCCA (squamous cell carcinoma antigen), CYFRA 21-1, and CEA (carcino-embryonic antigen) are the most frequent tumor markers for head and neck squamous cell carcinoma however their diagnostic sensitivity still requires improvement, especially at early stages [3]. There is a need for simple biochemical examinations that can be easily assayed, are less expensive and can detect metastasis. ADA (adenosine deaminase) is an enzyme of the purine salvage pathway which catalyses the hydrolytic deamination of adenosine to form inosine and 2′-deoxyadenosine to 2′-deoxyinosine respectively [4, 5]. ADA in individuals is involved in the development and maintenance of the immune system particularly differentiation and proliferation of T-cells [6]. It is associated with epithelial cell differentiation, neurotransmission, and gestation maintenance as well [7]. Inflammatory and neoplastic state are likely to cause release of cytoplasmic enzymes. The concentration of the enzymes released reflects the degree of membrane damage. Increase in the level of serum ADA has been observed in various cancers such as breast cancer [8], liver cancer [9], colorectal cancer [10] etc. Reactive oxygen species have also been implicated to play a vital role in carcinogenesis and lead to oxidative cellular damage.
Objectives
To estimate serum ADA levels and to correlate with response to therapy and also to correlate between different clinical stages and serum ADA levels and to correlate HPV status to response to therapy.
Materials and Methods
A minimum of 30 patients attending the outpatient and inpatient department from M. S. Ramaiah hospital satisfying the inclusion and exclusion criteria were included in the study. Based on a previous study by Lal et al. it was found that the serum ADA level was 27.77 ± 2.94 units/l before the start of therapy and it was decreased to 18.28 ± 1.77 units/l post therapy. In the present study expecting similar difference with the power of 99% and with α error of 5% the minimum sample size was evaluated to be 30. A prospective cohort study being conducted in patients who satisfy the inclusion and exclusion criteria, after obtaining an informed consent. Histopathologically proved cases of laryngeal carcinoma. Histopathologically proved cases of oropharyngeal or hypopharyngeal carcinoma were included in the study. Patients with acute infections, cancers of other parts of the body, patients who have received any previous treatment for malignancy, diagnosis of tuberculosis and nasopharyngeal carcinoma were excluded in the study.
Demographic variables were recorded such as age, gender, history of smoking/chewing tobacco, history of consumption of alcohol. All the quantitative variables in the present study such as ADA levels, age, number of pack years of smoking history etc. will be summarized in terms of mean and standard deviation. All qualitative variables like HPV status, gender, family history etc. will be expressed as proportion. The mean ADA levels before and after therapy will be compared for statistical significance using paired T test/Wilcoxon’s signed rank test. The difference in the mean ADA levels with respect to clinical stages will be compared using ANOVA/Krushkal Wallis test.
The thirty patients included in this study received treatment as per the NCCN guidelines version 1. 2015 Head and Neck cancers. Serum ADA level were estimated prior to commencement of treatment and on follow up 3 months after the completion of treatment. Estimation of adenosine deaminase (ADA) in serum was done by non-Giusti method. The ADA assay is based on a technique developed by Delacour et al. [11] in which one unit of ADA is defined as the amount of ADA that generates one micromole of inosine from adenosine per minute at 37 °C.
The cases then underwent Direct laryngoscopy + biopsy or Microlaryngoscopy + biopsy under general anaesthesia. Tissues were processed by standard formalin fixation, paraffin embedding and sectioning. Following microtome, retrieval sections will be incubated with primary antibody. The sections were then washed and biotinylated secondary antibodies were added followed by addition of streptavidin/biotin Horse Radish Protein (HRP) conjugate. After haematoxylin staining the sections were mounted using Distrene Polystyrene Xylene (DPX) and visualized under bright field microscope. For quantitation, five randomly selected bright field microscope images (20×) per sample were obtained. The total cell number in each image was counted by counting haematoxylin positive cells. 3,3′-diaminobenzidene (DAB) positive cells were also counted the same way by performing colour deconvolution command and expressed as positive percentage cells (Fig. 1).
Fig. 1.
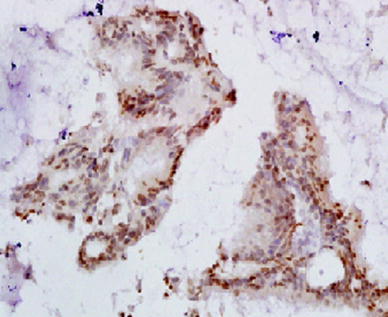
Immunohistochemistry staining of HPV P16 in laryngeal tissue
Patients in this study received maximum of 66 Gy and 37 fractions of Intensity Modulated RadioTherapy (IMRT) with 6 mV Linac dosed as 5 h per week and maximum of 5 cycles of Cisplatin. These patients were followed up for a period of 3 months following combined chemoradiotherapy and clinically assessed for recurrence with the aid of Videolaryngoscopy and CT [12].
Statistical Analysis
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on mean ± SD (min–max) and results on categorical measurements are presented in number (%) [13]. Significance is assessed at 5% level of significance. The following assumptions on data is made, Assumptions: (1) Dependent variables should be normally distributed, (2) Samples drawn from the population should be random, Cases of the samples should be independent. Student t test (two tailed, independent) has been used to find the significance of study parameters on continuous scale between two groups (Inter group analysis) on metric parameters and Student t test (two tailed, dependent) has been used to find the significance of study parameters on continuous scale with in each group. Chi square/Fisher Exact test has been used to find the significance of study parameters on categorical scale between two or more groups.
Statistical Software
The Statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc.
Results
Study Design
A prospective cohort study was carried out in 30 included 30 patients. These patients were diagnosed with oropharyngeal/hypopharyngeal/laryngeal Squamous cell carcinomas. Pre-treatment and post-treatment levels of serum Adenosine deaminase were measured. The biopsy samples were also evaluated for HPV status. The most common age group of patients affected were 51–60 years. In the present study 63% of patients were males and 37% of patients were females. The maximum numbers of patients in this study were from Bengaluru and Kolar districts. In this study oropharynx and hypopharynx were the most commonly involved sites. The Pyriform fossa is the most commonly involved subsite. Highest number of patients presented at stage III of the disease. 90% of the patients had histopathological diagnosis of moderately differentiated carcinoma. There does not seem to be a strong correlation between gender and HPV positive status in this study. In this study there is no relation between alcohol intake, tobacco smoking and HPV positivity. With p < 0.001, the oropharynx is the site with the maximum predilection for HPV positive malignancies in this study (Fig. 2).
Fig. 2.
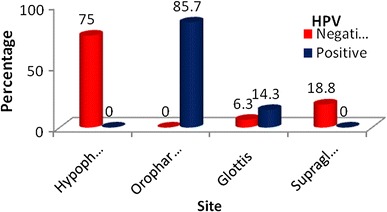
Site distribution of patients studied in relation to HPV positivity
The HPV status does not affect the change in serum ADA levels before and after treatment (Table 1; Fig. 3).
Table 1.
Serum ADA distribution: assessment before and after treatment
| Serum ADA | Before treatment | After treatment | % change |
|---|---|---|---|
| HPV negative (n=16) | |||
| < 35 | 4 (25%) | 13 (81.3%) | 56.3% |
| 35–40 | 7 (43.8%) | 2 (12.5%) | − 31.3% |
| > 40 | 5 (31.3%) | 1 (6.3%) | − 25% |
| HPV positive (n=14) | |||
| < 35 | 4 (28.6%) | 14 (100%) | 71.4% |
| 35–40 | 5 (35.7%) | 0 (0%) | − 35.7% |
| > 40 | 5 (35.7%) | 0 (0%) | − 35.7% |
| p value | 1.000 | 0.485 | – |
Chi square test/Fisher exact test
Fig. 3.
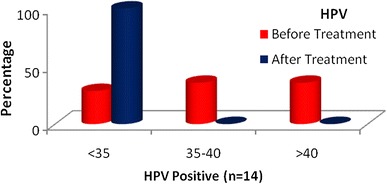
Serum ADA distribution: assessment before and after treatment
In this study, irrespective of the HPV status of the tumour, significant lowering in the value of serum ADA was seen post treatment. The HPV status does not affect the change in serum ADA levels before and after treatment (Table 2).
Table 2.
Serum ADA: comparative assessment of serum ADA before and after treatment
| Serum ADA | Before treatment | After treatment | Difference | t value | p value |
|---|---|---|---|---|---|
| HPV | |||||
| Negative | 37.50 ± 5.00 | 28.99 ± 5.50 | 8.505 | 21.366 | < 0.001** |
| Positive | 37.97 ± 5.32 | 26.30 ± 4.85 | 11.669 | 55.540 | < 0.001** |
| p value | 0.803 | 0.170 | – | – | – |
Between Group: Student T Test (Unpaired); Within Group: Student T Test (Paired)
In this study p = 0.485 which denotes the HPV status does not aid in determining the risk of recurrence following treatment. The difference in the serum ADA levels before and after treatment was 9.982 which was statistically significant with p value of < 0.001. HPV positive status and response to therapy in the form of recurrence shows a P value of 0.485 which is not statistically significant (Table 3; Fig. 4).
Table 3.
Recurrence in relation to HPV
| Recurrence | HPV | Total | |
|---|---|---|---|
| Negative | Positive | ||
| No | 14 (87.5%) | 14 (100%) | 28 (93.3%) |
| Yes | 2 (12.5%) | 0 (0%) | 2 (6.7%) |
| Total | 16 (100%) | 14 (100%) | 30 (100%) |
Fig. 4.
HPE findings of patients
Conclusion
We would like to conclude that serum ADA level is significantly high in cases of oropharyngeal, hypopharyngeal and laryngeal malignancies and the study also shows significant fall in serum ADA levels post treatment.
Our study also shows that a more advanced stage of oropharyngeal/hypopharyngeal/laryngeal malignancy is associated with higher levels of serum ADA at the time of presentation which continued to be relatively higher following treatment when compared to an early stage disease. However, our study was not successful in correlating the HPV positive status with better prognosis in the form of non-recurrence of the disease.
Discussion
The incidence of squamous cell carcinoma (SCC) in the oropharynx has increased greatly over this period of time, more than other sites of the upper aerodigestive tract (UADT). This has mainly been due to the rise in non-smokers with human papilloma virus (HPV)-induced tumours, something that has been described as an ‘epidemic’. Carcinoma of the hypopharynx is uncommon approximately 3600 new cases are diagnosed in the United States each year [14]. The incidence of these cancers is relatively high in India (11% against 1% world-wide, among males) [15].
Carcinoma larynx accounts for approximately 1% of newly diagnosed cancers in the world [16]. Laryngeal carcinoma is the most common head and neck cancer worldwide, contributing 25% of all head and neck malignancies and it is the 14th most common cancer in the world. In India, it is 1.3–8.8 per 1 lakh population, (ICMR,1992) [17, 18]. The purpose of this study is to estimate serum ADA levels and to correlate with response to therapy and also to correlate between different clinical stages and serum ADA levels and to correlate HPV status to response to therapy.
All the patients in this study underwent estimation of serum ADA level before commencement of treatment and 3 months after completion of treatment along with assessment of the HPV status of the tumour. A study by Ashok et al. showed elevated levels of ADA was seen in the study group (34.89 ± 6.8 IU/l) when compared to control group (20.47 ± 3.33 IU/l). The conclusion of the study was that serum ADA levels can be used to assess prognosis of head and neck malignancies [19].
A study by Sharma et al. [20] showed that serum ADA level was significantly high in histologically proven cases of laryngeal cancers and the ADA level changed with respect to the severity of the disease.
A study by Mishra et al. came to the conclusion that the rise in serum ADA level was directly proportional to the stage of the disease and did not depend on the duration of the disease. The study also concluded that the activity of ADA reduced with radiotherapy and after surgery [21].
A study by Metgudmath et al. [22] proved that serum ADA can be used as a diagnostic parameter in cases of head and neck malignancies. A study by Lal et al. [23] proved that ADA activity increased with advanced stages of the disease and significantly reduced following treatment.
Our study included patients who were diagnosed with oropharyngeal/hypopharyngeal/laryngeal malignancies. The serum ADA levels of the patients were quantified immediately after diagnosis and 3 months after completion of treatment. Our study showed a significant fall in the level of serum ADA following treatment. Our study also showed a significant variation in the levels of the serum ADA based on the stage of the disease.
A study by Fakhry et al. showed that patients with HPV positive status had a higher response rate after induction chemotherapy when compared to HPV negative cases. They concluded that better survival and response to treatment was seen in HPV positive cases of HNSCC of the oropharynx [24].
A study by Maura et al. showed that disease specific survival was significantly improved in HPV positive cases. However they concluded that disease specific survival was the same in both the groups [25], but our study could not come to a similar conclusion of better prognosis in the presence of HPV positive status similar to a study by Benjamin et al. also came to a conclusion that the 3-year survival rates between the HPV positive and HPV negative head and neck malignancies was not statistically significant [26]. A study by Maura et al. came to a conclusion that subjects who neither smoked tobacco nor drank alcohol had an increased risk of HPV-16 negative HNSCC [27] but our study could not come to such a conclusion.
A study by Elaine et al. concluded that infection of oral exfoliated cells with HPV-HR types is a risk factor for head and neck cancer. It is independent of alcohol and tobacco use and acts synergistically with alcohol use [28].
Acknowledgements
We sincerely thank all the individuals who have supported us for the study. We thank Mr. Pradeep B K (Biocon) for scientific assistance.
References
- 1.Kelgandre DC, et al. Adenosine deaminase—a novel diagnostic and prognostic biomarker for oral SCC. Asian Pac J Cancer Prev. 2016;17(4):1865–1868. doi: 10.7314/APJCP.2016.17.4.1865. [DOI] [PubMed] [Google Scholar]
- 2.Kapil U, Singh P, Bahadur S, Dwivedi SN, Singh R, Shukla N. Assement of risk factors in laryngeal cancer in India: a case control study. Asia Pac J Cancer Prev. 2005;6(2):202–207. [PubMed] [Google Scholar]
- 3.Ayude D, Gacio G, Páez de la Cadena M, Pallas E, Martínez-Zorzano VS, de Carlos A, et al. Combined use of established and novel tumour markers in the diagnosis of head and neck squamous cell carcinoma. Oncol Rep. 2003;10(5):1345–1350. [PubMed] [Google Scholar]
- 4.Bansal SK, Singh RP, Narang RK, Joshi LD, Bansal AB, Agrawal AK. Serum ADA activity in pulmonary tuberculosis, malignancy and non tubercular respiratory diseases. Indian J Chest Dis Allied Sci. 1991;33(4):189–193. [PubMed] [Google Scholar]
- 5.Satyanarayan U. Biochemistry. Calcutta: Calcutta Books and Allied (P) Ltd.; 2001. [Google Scholar]
- 6.Hovi T, Smyth JF, Allison AC, Williams SC. Role of ADA in lymphocyte proliferation. Clin Exp Immunol. 1976;1276:395–403. [PMC free article] [PubMed] [Google Scholar]
- 7.Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism—a review of histochemical localization and functional implications. Histol Histopathol. 1999;14(4):1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- 8.Orban C, ILker D, Cetin R, et al. Activities of adenosine deaminase, 5′-nucleotidase, guanase and cytidine deaminase enzymes in cancerous and noncancerous human breast tissues. Breast Cancer Res Treat. 1996;37:189–193. doi: 10.1007/BF01806500. [DOI] [PubMed] [Google Scholar]
- 9.Raczynska J, Jonas S, Krawczinski J. Diagnostic value of adenosine in some liver diseases. Clin Chem Act. 1996;13:151–154. doi: 10.1016/0009-8981(66)90288-9. [DOI] [PubMed] [Google Scholar]
- 10.Ten Kate J, Van den Ingh HF, Khan PM, Bosman FT. Adenosine deaminase complexing protein (ADCP) immunreactivity in colorectal adenocarcinoma. Int J Cancer. 1986;37:479–485. doi: 10.1002/ijc.2910370402. [DOI] [PubMed] [Google Scholar]
- 11.Delacour H, Sauvanot C, et al. Analytical performances of the diazyme ADA assay on the Cobas 6000 system. Clin Biochem. 2010;43(18):1468–1471. doi: 10.1016/j.clinbiochem.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 12.David GP, Sharon S, David M (eds) et al (2015) National comprehensive cancer network (NCCN) Guidelines version 1.2015 head and neck cancers
- 13.Rosner B (2000) Fundamentals of biostatistics, 5th edn. Duxbury, pp 80–240
- 14.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36(7):945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best SR, Niparko KJ, Pai SI. Biology of human papillomavirus infection and immune therapy for HPV-related head and neck cancers. Otolaryngol Clin N Am. 2012;45(4):807–822. doi: 10.1016/j.otc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schache AG, Liloglou T, Risk JM, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17(19):6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.http://www.icmr.nic.in/library_bull/lib_bulletin.htm
- 18.Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashok KJ, Pinto GJO, Kavitha AK, Palathra MJ. The diagnostic and prognostic value of serum adenosine deaminase levels in head and neck cancer. J Clin Diagn Res. 2008;3:833–837. [Google Scholar]
- 20.Sharma S, Desai PB, Metgudmath RB. Evaluation of serum adenosine deaminase and retinol in patients with laryngeal cancer. Indian J Pharm Biol Res. 2013;1(4):30–34. doi: 10.30750/ijpbr.1.4.6. [DOI] [Google Scholar]
- 21.Mishra R, Agarwal MK, Chansuria JPN. Serum adenosine deaminase levels as an index of tumor growth in head and neck malignancy. Indian J Otolaryngol Head Neck Surg. 2000;52:360–363. doi: 10.1007/BF02991478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metgudmath RB, Sharma S, Desai PB. Role of purine metabolic enzymes and vitamin A in patients with oral cancer. Int J Pharm Biol Chem Sci. 2013;2:31–37. [Google Scholar]
- 23.Lal H, Munjal SK, Wig U, Saini AS. Serum enzymes in head and neck cancer III. J Laryngol Otol. 1987;101(10):1062–1065. doi: 10.1017/S0022215100103226. [DOI] [PubMed] [Google Scholar]
- 24.Carole F, William H, Sigui L, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 25.Maura L, Wayne M, Randolph B, et al. Evidence for a casual association between human papillomavirus and a subset of head and neck cancer. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin P, Nathan C, Tamara O. Human papilloma virus in head and neck cancer—an association of HPV 16 with squamous cell carcinoma of Waldeyer’s Tonsillar Ring. Cancer. 1997;79:595–604. doi: 10.1002/(SICI)1097-0142(19970201)79:3<595::AID-CNCR24>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Maura L, Gypsyamber D, William W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 28.Elaine M, Justine M, Kurt F, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]


