Abstract
Understanding the effects of parasites on host behaviour, of host behaviour on parasite infection, and the reciprocal interactions between these processes is vital to improving our understanding of animal behaviour and disease dynamics. However, behaviour and parasite infection are both highly variable within and between individual hosts, and how this variation affects behaviour–parasite feedbacks is poorly understood. For example, it is unclear how an individual's behaviour before infection might change once it becomes infected, or as the infection progresses, and how these changes depend on the host's parasite susceptibility. Here, using the guppy, Poecilia reticulata, and a directly transmitted ectoparasite, Gyrodactylus turnbulli, I show that parasite-induced behavioural plasticity depends on host sex and susceptibility. Among females, time spent shoaling (sociality), a behaviour that increases parasite transmission, did not depend on infection status (infected/not) or susceptibility. By contrast, male sociality in the absence of infection was negatively correlated with susceptibility, suggesting that the most susceptible males use behaviour to avoid infection. However, in late infection, when parasite transmission is most likely, male sociality and susceptibility became positively correlated, suggesting that susceptible males modify their behaviour upon infection potentially to increase transmission and mating opportunities. I discuss the implications of these patterns for disease dynamics.
Keywords: behavioural disease ecology, behaviour–parasite feedback, parasite-induced behavioural plasticity, Poecilia reticulata, social behaviour, sex-biased parasitism
1. Background
An animal's behaviour is fundamentally linked to the ecology and evolution of its parasites: to fully understand the proximate and ultimate forces driving behavioural evolution, we need to better characterize the role of parasites [1–3]. Similarly, in this time of unprecedented infectious disease emergence, it is imperative we improve our understanding of how variation in individual behaviour affects the spread of diseases at the population level [1,4–6].
Parasite infection affects behaviour: infected hosts often behave quite differently from uninfected hosts. For example, infected hosts may reduce activity levels and social interactions [7,8]. The strength of such infection-induced behavioural plasticity often depends on infection intensity, or parasite susceptibility [2,9–11].
As well as parasite infection affecting behaviour, behaviour affects the risk of parasite infection and transmission. Across taxa, relatively bold individuals harbour different numbers and communities of parasites [12,13], and may be more likely to transmit the infection to conspecifics [14,15]. The majority of such behaviour–parasite risk studies has been conducted on wild-caught, naturally infected hosts (but see [16]). Experimental infection studies complement this approach by testing causality: does behaviour affect parasite risk, does infection affect behaviour, or both [1,2,16,17]?
One approach to investigating the correlation between behaviour and infection risk is to examine how animals balance investment in behavioural and physiological immunity. Even in the absence of infection, mice [18] and house finches [19] with less effective physiological defences avoid behaviours entailing high infection risk. Caveats of this important work include that it is unclear how the measured immune function reflects susceptibility to the most prevalent parasites in nature, nor how its correlation with behaviour changes during the course of parasite infection (the full behaviour–parasite feedback loop [1,2,20]).
Understanding the full feedback loop between behaviour and parasite infection will enable better theoretical predictions of infectious disease transmission. In epidemiological models the effective contact rate, β, is the product of the contact rate between infected and uninfected individuals (behavioural component: βc) and the transmission rate per contact, or ‘infectiousness’, which is often determined by the infected hosts' parasite susceptibility (physiological component: βp) [5,21,22]. Our recent empirical work demonstrates that βc and βp may covary [23], which has important implications for disease dynamics: Hawley et al. [5] showed theoretically that behaviourally mediated negative covariation in βc and βp, such as through the stronger avoidance of more infectious individuals [23], can lead to the parasite fading out of the host population. Positive covariation in βc and βp, however, can lead to rapid epidemic spread. Further empirical tests will help elucidate the conditions under which βc and βp covary, and the factors affecting the sign of this relationship [4,5,22].
In an experimental test of the full feedback loop between host behaviour and parasite infection, I used the guppy, Poecilia reticulata, and Gyrodactylus turnbulli to explore how parasite susceptibility (βp proxy) covaries with host sociality (βc proxy) before and during infection. G. turnbulli is an ectoparasitic monogenean that reproduces on the host's skin with a generation time of 24 h. Infection loads initially increase, and hosts vary in their ability to limit this growth [24]. Gyrodactylus spp. are the most prevalent multicellular parasites in wild populations [25], transmit directly through contact between hosts [26], and reduce host fitness [9,27,28] in proportion to infection load [10,28–30]. My results highlight the dynamic nature of both behaviour and parasite infection, and the importance of accounting for within- and between-individual variation in predicting disease spread [4,5,22].
2. Methods
(a). Host and parasite origin and maintenance
I used laboratory-bred descendants of guppies collected from the Caura River, Trinidad. Guppies were housed at densities of 1–2 fish per litre in 4.5 l tanks in a recirculating system at 24 ± 1°C, on a 12 L : 12 D lighting schedule (overhead fluorescent lighting) and fed daily on flake food and Artemia. I initiated an isogenic line of G. turnbulli using a single parasite individual (collected June 2017 from a local pet shop) and maintained on ornamental guppies (culture fish). Differences in susceptibility between experimental fish cannot, therefore, be ascribed to profound genetic differences between their parasites.
(b). Infection and screening
A total of 68 fish, of which 42 were experimentally infected and 26 were sham-infected controls, were used in five experimental blocks between August and November 2017. All fish were housed individually throughout the experiment to enable individual identification and eliminate the possibility of parasite transmission. To initiate experimental infections, a heavily infected culture fish was killed with an overdose of MS222 (0.02% tricaine methanesulfonate; PHARMAQ Ltd., Fordingbridge, UK) and pithing. This culture fish was placed near an anaesthetized experimental fish until two parasites transmitted, observed under a dissecting microscope. The experimental fish were revived and housed individually in 1 l tanks, and the number of parasites infecting each was counted under anaesthetic every other day. Sham-infected control fish were also held individually in 1 l tanks and anaesthetized and manipulated every other day. All tanks were maintained under standard conditions and received 100% water exchanges every other day. I recorded fish standard length and weight pre- and post-infection, and used these to calculate the change in the scaled mass index, ‘scaled mass change’, through the course of the experiment [31].
(c). Behavioural experiment
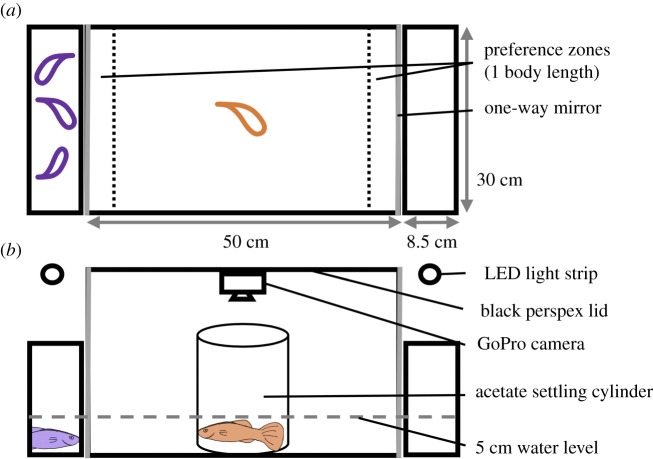
I quantified the sociality of all fish thrice: pre-infection (before I experimentally/sham-infected them); early infection (7–8 days-post-infection); and late infection (15–17 days-post-infection) using an established protocol [32]. A central glass aquarium (test aquarium) was flanked on both short sides by smaller aquaria (‘stimulus aquaria’; figure 1), all with 5 cm water depth. The short sides of the test aquarium were one-way mirrors, such that test fish could see stimulus fish, but not vice versa. The stimulus aquaria were lit from above by 32 cm LED strip lights (350 lm, 5 W, 4000 K, MeRox® Technics). The test aquarium had a black Perspex lid, to which was fastened a GoPro camera (Hero4 Black Edition; GoPro Inc. San Mateo, CA). To start a trial, I placed a shoal of three females in one of the stimulus tanks (n = 23 shoals; used between 3 and 22 times). I used female guppies as stimuli because males have highly polymorphic coloration that affects how conspecifics respond to them [33]. I placed a test fish (of either sex: n = 33 females; 35 males) in an acetate cylinder in the centre of the test tank for a 5 min settling period. I then remotely set the GoPro to record, lifted the settling cylinder using a pulley, and recorded a 10 min test period. The experimental set-up was enclosed in a blackout curtain to ensure even lighting and prevent disturbance. Between trials, the test aquarium was scrubbed with 70% ethanol, rinsed and refilled. Whether the shoal was in the left or right stimulus aquarium was switched between trials haphazardly. Behavioural videos were subsequently analysed by four observers, blind to the treatment, using JWatcher™ 1.0 (www.jwatcher.ucla.edu; [34]).
Figure 1.
(a) Overhead and (b) side views of the set-up for quantifying sociality. (Online version in colour.)
(d). Data analysis
All analyses of the data from this experiment were conducted in R v.3.5.1 [35] (see supplement for code and output; data available online [36]). I used the proportion of trial time the test fish spent within one body length of the stimulus tank (arcsine square-root transformed; ‘sociality’) as the response variable in two linear mixed models using lme4 [37]. In the first analysis (binary model), I tested for sociality differences between infected and uninfected fish. In the second analysis (infected only model), I tested for covariation between parasite susceptibility and sociality among infected fish. Both models had as random effects the identity of the test fish, the identity of the shoal and the experimental block. Fixed effects in both models were: test fish length and mean shoal fish length (size is important in shoaling decisions [38]), test fish sex, the proportion of time spent swimming, scaled mass change, infection stage (pre, early or late), and two- and three-way interactions between sex, scaled mass change and infection stage. I included whether or not the fish was infected (binary model), or the ‘infection integral’ of the fish (the area under the curve when infection load is plotted over time—‘susceptibility’; infected only model), and their two- and three-way interactions with sex and infection stage. I used visreg [39] and ggplot2 [40] for plotting.
3. Results
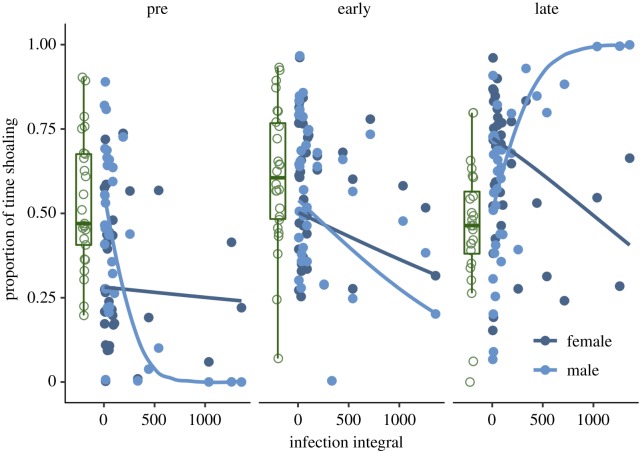
None of the variables included in the binary model explained a significant amount of the variation in test fish sociality. Among infected fish, there was a significant three-way interaction between test fish sex, infection stage and susceptibility (figure 2; χ2 = 4.44, d.f. = 1, p = 0.035). Females consistently showed a weakly negative correlation between sociality and susceptibility across all infection stages. Among males, however, there was a strong negative correlation between sociality and susceptibility in the absence of infection: those males that shoaled less pre-infection went on to develop the highest infection integrals during the subsequent experimental infection. This negative correlation between sociality and susceptibility became weaker in early infection, and reversed, becoming strongly positive, in late infection. No other variable explained a significant amount of variation in sociality.
Figure 2.
The proportion of time guppies spent within one body length of the stimulus shoal tank (sociality) depended on a three-way interaction between infection integral (susceptibility), sex and infection stage. Points are partial residuals from the ‘infected only’ model, back-transformed to the response scale and lines give model fits. Box plots give the median (dark line), interquartile range (box) and values within 1.5× the interquartile range (whiskers) of the sham-infected controls; associated open points give the raw data. (Online version in colour.)
As a post hoc test of the difference between males and females, I ran the ‘infected only’ model (minus sex and its interactions) twice more, using data from each sex separately. No variable explained variation in female sociality, but among males, the interaction between susceptibility and infection stage was almost significant (χ2 = 3.42, d.f. = 1, p = 0.064), and was significant when comparing male sociality during infection (early and late combined) to that pre-infection (χ2 = 4.52, d.f. = 1, p = 0.034). The trend of a negative correlation between susceptibility and sociality among females apparent in figure 2 is, therefore, not significant, and parasite-induced behavioural plasticity among males is driving the main result.
4. Discussion
My results show that correlations between host behaviour and parasite susceptibility are not consistent across contexts and, crucially, can vary with host infection status, duration and sex. In the absence of infection, more susceptible males were less social than resistant males, indicating a negative correlation between parasite susceptibility (βp proxy) and transmission-relevant social behaviour (βc proxy). This pattern is consistent with the most susceptible males reducing social interactions to avoid becoming infected, suggesting male guppies balance investment in behavioural and physiological immunity, as recently documented in other animals [18,19]. The mechanisms underlying this phenomenon remain unclear [20], but recent research suggests the involvement of steroid hormones such as cortisol [17]. While our understanding of the balance between behavioural and physiological immunity is limited, there are clear adaptive advantages to using relatively cheap behavioural defences and reducing investment in expensive immune responses [41], and clear costs of avoiding social interactions. The balance between these two may depend on individual resources, and could vary within as well as between individuals [3].
In late infection, the most susceptible males spent the most time shoaling, suggesting that the correlation between βp and βc became positive. There are at least three non-mutually exclusive explanations for this result. First, as stimulus shoals were all-female, the result may indicate that infection stimulated terminal investment in reproduction among the most susceptible males [42]. Second, susceptible males would benefit most from transmitting parasites to conspecifics, both in terms of the reduced infection load and the decrease in fitness deficit compared to uninfected conspecifics. Indeed, infected guppies have been observed to initiate physical contact with uninfected shoalmates [43], putatively to transmit the parasite, and my results suggest this behaviour correlates positively with susceptibility. Finally, susceptible males may be the most vulnerable to predation [28], and thus perhaps the most likely to seek out protection from the shoal's proximity.
In contrast to males, I found no effect of infection or parasite susceptibility on female guppy sociality. Sex-specific infection-induced behavioural plasticity also occurs in badgers [6]. Behaviours that protect against parasites should only evolve when the cost posed by parasites outweighs the costs of the behaviour [2,44]. Conforming to Bateman's principle [45], female guppies live 1.65 times longer [46] and are less likely to suffer costs owing to Gyrodactylus infection than males [27,28] in the wild. Additionally, females form stable social groups [43] and spend the majority of their time shoaling, while males focus on mating and have low shoal fidelity [47]. Perhaps, then, the cost parasites pose is lower, and the cost of changing their social behaviour higher for female than male guppies.
There was no overall difference in the behaviour of infected and uninfected fish. These data are consistent with infected fish maintaining normal behaviour to conceal their infection: infection-induced behavioural plasticity may be constrained to within the range of the behaviour of uninfected conspecifics because abnormal behaviour is likely to lead to social exclusion [23,48]. Concealing infection would benefit both host and parasite in this system, and is likely to be under strong positive selection: the parasite would gain transmission opportunities and the host would gain the benefits of shoaling. Whether the risk-sensitive infection avoidance behaviour observed previously [23] would successfully mitigate the effects of the most infectious males becoming the most sociable remains untested.
The observed reversal from a negative to a positive correlation between host sociality, βc, and parasite susceptibility, βp, through the course of infection has implications for our ability to predict infectious disease dynamics [4,5]. In addition to the powerful repercussions of the sign of this correlation, White et al. [4] found that heterogeneity between hosts in their susceptibility, sociality and infection-induced behavioural change, such as that shown here, dramatically altered theoretical disease dynamics. My findings suggest that the feedbacks between animal behaviour and parasite infection are dynamic, depend on both host sex and susceptibility and are likely to shape the pace and pattern of disease transmission.
Supplementary Material
Acknowledgements
I thank Annamari Alitalo, John Hansen, Maya Jog, Stephen Kisty, Kirsten Klappert, Pascal Reichlin and Maura Sackett for assistance. Jukka Jokela and Kyle Young made helpful comments.
Ethics
This work was performed with ethical approval from the Veterinäramt of Zürich (licence ZH177/15).
Data accessibility
The analysis code is provided in the supplement. The data are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.q1223rs.
Competing interests
I declare I have no competing interests.
Funding
I was supported by the Center for Adaptation to a Changing Environment (ACE) at ETH Zürich, and the University of Pittsburgh.
References
- 1.Ezenwa VO, Archie EA, Craft ME, Hawley DM, Martin LB, Moore J, White L. 2016. Host behaviour–parasite feedback: an essential link between animal behaviour and disease ecology. Proc. R. Soc. B 283, 20153078 ( 10.1098/rspb.2015.3078). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kortet R, Hedrick AV, Vainikka A. 2010. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 13, 1449–1458. ( 10.1111/j.1461-0248.2010.01536.x) [DOI] [PubMed] [Google Scholar]
- 4.White LA, Forester JD, Craft ME. 2018. Covariation between the physiological and behavioral components of pathogen transmission: host heterogeneity determines epidemic outcomes. Oikos 127, 538–552. ( 10.1111/oik.04527) [DOI] [Google Scholar]
- 5.Hawley DM, Etienne RS, Ezenwa VO, Jolles AE. 2011. Does animal behavior underlie covariation between hosts' exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integr. Comp. Biol. 51, 528–539. ( 10.1093/icb/icr062) [DOI] [PubMed] [Google Scholar]
- 6.Silk MJ, Weber NL, Steward LC, Hodgson DJ, Boots M, Croft DP, Delahay RJ, McDonald RA. 2018. Contact networks structured by sex underpin sex-specific epidemiology of infection. Ecol. Lett. 21, 309–318. ( 10.1111/ele.12898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes PC, Adelman J, Wingfield JC, Bentley GE. 2012. Social context modulates sickness behaviour. Behav. Ecol. Sociobiol. 66, 1421–1428. ( 10.1007/s00265-012-1397-1) [DOI] [Google Scholar]
- 8.Lopes PC, Block P, König B. 2016. Infection-induced behavioural changes reduce connectivity and the potential for disease spread in wild mice contact networks. Sci. Rep. 6, 31790 ( 10.1038/srep31790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houde A, Torio AJ. 1992. Effect of parasitic infection on male color pattern and female choice in guppies. Behav. Ecol. 3, 346–351. ( 10.1093/beheco/3.4.346) [DOI] [Google Scholar]
- 10.López S. 1999. Parasitized female guppies do not prefer showy males. Anim. Behav. 57, 1129–1134. ( 10.1006/anbe.1998.1064) [DOI] [PubMed] [Google Scholar]
- 11.Moore J. 2002. Parasites and the behavior of animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Boyer N, Réale D, Marmet J, Pisnau B, Chapuis JL. 2010. Personality, space use and tick load in an introduced population of Siberian chipmunks, Tamias sibiricus. J. Anim. Ecol. 79, 538–547. ( 10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- 13.Wilson DS, Coleman K, Clark AB, Biederman L. 1993. Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260. ( 10.1037/07357036.107.3.250) [DOI] [Google Scholar]
- 14.Dizney L, Dearing MD. 2013. The role of behavioural heterogeneity on infection patterns: implications for pathogen transmission. Anim. Behav. 86, 911–916. ( 10.1016/j.anbehav.2013.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiser N, Pinter-Wollman N, Augustine DA, Ziemba MJ, Hao L, Lawrence JG, Pruitt JN. 2016. Individual differences in boldness influence patterns of social interactions and the transmission of cuticular bacteria among group-mates. Proc. R. Soc. B 283, 20160457 ( 10.1098/rspb.2016.0457). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koprivnikar J, Gibson CH, Redfern JC. 2012. Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc. R. Soc. B 279, 1544–1550. ( 10.1098/rspb.2011.2156). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber I, Mora AB, Payne EM, Weinersmith KL, Sih A. 2017. Parasitism, personality and cognition in fish. Behav. Processes 141, 205–219. ( 10.1016/j.beproc.2016.11.012). [DOI] [PubMed] [Google Scholar]
- 18.Filiano AJ, et al. 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429. ( 10.1038/nature18626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zylberberg M, Klasing KC, Hahn TP. 2013. House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol. Lett. 9, 20120856 ( 10.1098/rsbl.2012.0856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes PC. 2017. Why are behavioral and immune traits linked? Horm. Behav. 88, 52–59. ( 10.1016/j.yhbeh.2016.09.008) [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWaal KL, Ezenwa V. 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. 30, 1606–1622. ( 10.1111/1365-2435.12645) [DOI] [Google Scholar]
- 23.Stephenson JF, Perkins SE, Cable J. 2018. Transmission risk predicts avoidance of infectious conspecifics in Trinidadian guppies. J. Anim. Ecol. 87, 1525–1533. ( 10.1111/1365-2656.12885) [DOI] [PubMed] [Google Scholar]
- 24.Stephenson JF, Young KA, Fox J, Jokela J, Cable J, Perkins SE. 2017. Host heterogeneity affects both parasite transmission to and fitness on subsequent hosts. Phil. Trans. R. Soc. B 372, 20160093 ( 10.1098/rstb.2016.0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephenson JF, van Oosterhout C, Mohammed RS, Cable J. 2015. Parasites of Trinidadian guppies: evidence for sex- and age-specific trait-mediated indirect effects of predators. Ecology 96, 489–498. ( 10.1890/14-0495.1) [DOI] [PubMed] [Google Scholar]
- 26.Richards EL, van Oosterhout C, Cable J. 2010. Sex-specific differences in shoaling affect parasite transmission in guppies. PLoS ONE 5, e13285 ( 10.1371/journal.pone.0013285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson JF, van Oosterhout C, Cable J. 2015. Pace of life, predators and parasites: predator-induced life history evolution in Trinidadian guppies predicts decrease in parasite tolerance. Biol. Lett. 11, 20150806 ( 10.1098/rsbl.2015.0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson JF, Kinsella C, Cable J, van Oosterhout C. 2016. A further cost for the sicker sex? Evidence for male-biased parasite-induced vulnerability to predation. Ecol. Evol. 6, 2506–2515. ( 10.1002/ece3.2049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hockley FA, Wilson CAME, Brew A, Cable J. 2013. Fish responses to flow velocity and turbulence in relation to size, sex and parasite load. J. R. Soc. Interface 11, 20130814 ( 10.1098/rsif.2013.0814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López S. 1998. Acquired resistance affects male sexual display and female choice in guppies. Proc. R. Soc. B 265, 717–723. ( 10.1098/rspb.1998.0352). [DOI] [Google Scholar]
- 31.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 32.Wright D, Krause J. 2006. Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nat. Protoc. 1, 1828–1831. ( 10.1038/nprot.2006.287) [DOI] [PubMed] [Google Scholar]
- 33.Houde A. 1997. Sex, color and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 34.Blumstein DT, Daniel JC. 2007. Quantifying behaviour the JWatcher way. Sunderland, MA: Sinauer. [Google Scholar]
- 35.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 36.Stephenson JF. 2019. Data from: Parasite-induced plasticity in host social behaviour depends on sex and susceptibility Dryad Digital Repository ( 10.5061/dryad.q1223rs). [DOI] [PMC free article] [PubMed]
- 37.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 38.Hoare DJ, Krause J, Peuhkuri N, Godin JGJ. 2000. Body size and shoaling in fish. J. Fish Biol. 57, 1351–1366. ( 10.1111/j.1095-8649.2000.tb02217.x) [DOI] [Google Scholar]
- 39.Breheny P, Burchett W. 2017. Visualisation of regression models using visreg. R J. 9, 56–71. ( 10.32614/RJ-2017-046) [DOI] [Google Scholar]
- 40.Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer New York. [Google Scholar]
- 41.Loehle C. 1995. Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335. ( 10.2307/1941192) [DOI] [Google Scholar]
- 42.Duffield KR, Bowers EK, Sakaluk SK, Sadd BM. 2017. A dynamic threshold model for terminal investment. Behav. Ecol. Sociobiol. 71, 185 ( 10.1007/s00265-017-2416-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croft DP, Edenbrow M, Darden SK, Ramnarine IW, van Oosterhout C, Cable J. 2011. Effect of gyrodactylid ectoparasites on host behaviour and social network structure in guppies, Poecilia reticulata. Behav. Ecol. Sociobiol. 65, 2219–2227. ( 10.1007/s00265-011-1230-2) [DOI] [Google Scholar]
- 44.Lafferty KD. 1992. Foraging on prey that are modified by parasites. Am. Nat. 140, 854–867. ( 10.1086/285444) [DOI] [Google Scholar]
- 45.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. B 269, 867–872. ( 10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arendt JD, Reznick DN, López-Sepulcre A. 2014. Replicated origin of female-biased adult sex ratio in introduced populations of the Trinidadian guppy (Poecilia reticulata). Evolution 68, 2343–2356. ( 10.1111/evo.12445) [DOI] [PubMed] [Google Scholar]
- 47.Griffiths SW, Magurran AE. 1998. Sex and schooling behaviour in the Trinidadian guppy. Anim. Behav. 56, 689–693. ( 10.1006/anbe.1998.0767) [DOI] [PubMed] [Google Scholar]
- 48.Stephenson JF, Reynolds M. 2016. Imprinting can cause a maladaptive preference for infectious conspecifics. Biol. Lett. 12, 20160020 ( 10.1016/j.cub.2010.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stephenson JF. 2019. Data from: Parasite-induced plasticity in host social behaviour depends on sex and susceptibility Dryad Digital Repository ( 10.5061/dryad.q1223rs). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The analysis code is provided in the supplement. The data are archived in the Dryad Digital Repository: https://doi.org/10.5061/dryad.q1223rs.