Abstract
Background and Purpose:
Patients with active malignancy are at risk for intracerebral hemorrhage (ICH). We aimed to characterize perihematomal edema (PHE) and hematoma volumes after spontaneous non-traumatic ICH in patients with cancer without central nervous system involvement.
Methods:
Patients with active malignancy who developed ICH were retrospectively identified through automated searches of institutional databases. Control patients were identified with ICH and without active cancer. Demographic and cancer-specific data were obtained by chart review. Hematoma and PHE volumes were determined using semi-automated methodology. Univariate and multivariate linear regression models were created to assess which variables were associated with hematoma and PHE expansion.
Results:
Patients with cancer (N=80) and controls (N=136) had similar demographics (all P>0.20), although hypertension was more prevalent among controls (P=0.004). Most cancer patients had received recent chemotherapy (N=45, 56%) and had recurrence of malignancy (N=43, 54%). Cancer patients were thrombocytopenic (median platelet count 90,000 [interquartile range, 17,500–211,500]), and most had undergone blood product transfusion (N=41, 51%), predominantly platelets (N=38, 48%). 30-day mortality was 36% (N=29). Cancer patients had significantly increased PHE volumes (23.67 mL versus 8.61 mL; P=1.88×10−9) and PHE-to-ICH volume ratios (2.26 versus 0.99; P=2.20×10−16). In multivariate analyses, variables associated with PHE growth among cancer patients were ICH volume (β=1.29; 95% CI, 1.58–1.30; P=1.30×10−5) and platelet transfusion (β=15.67; 95% CI, 3.61–27.74; P=0.014). Variables associated with 30-day mortality were ICH volume (odds ratio [OR], 1.06; 95% CI, 1.03–1.10; P=6.76×10−5), PHE volume (OR, 1.07; 95% CI, 1.04–1.09; P=7.40×10−6), PHE growth (OR, 1.05; 95% CI, 1.01–1.10; P=0.01), and platelet transfusion (OR, 1.48; 95% CI, 1.22–1.79; P=0.0001).
Conclusions:
Patients with active cancer who develop ICH have increased PHE volumes. PHE growth was independent of thrombocytopenia but associated with blood product transfusion. 30-d mortality was associated with PHE and ICH volumes and blood product transfusion.
Keywords: intracerebral hemorrhage, perihematomal edema, cancer, neoplasms, blood component transfusion, brain injuries, intracranial hemorrhage, Computerized Tomography (CT)
INTRODUCTION
Intracerebral hemorrhage (ICH) is a devastating form of stroke and remains without effective treatment. Cerebrovascular events are common with cancer, half of which are ICH.1,2 ICH may occur due to different mechanisms in the context of cancer including coagulopathy, aberrant neovascularization, or overexpression of factors such as metalloproteinases.3,4 However, optimal treatment of ICH in patients with active cancer remains unclear, and outcomes in these patients are worse.5 Secondary injury occurs after ICH due to inflammation caused by hematoma components such as iron as well as hemostatic mechanisms causing clot retraction, namely thrombin.6 Perihematomal edema (PHE) develops after ICH and is considered to be a radiographical surrogate of secondary injury.6,7 PHE has been associated with mortality and functional outcomes after ICH,8–10 prompting the development of strategies to minimize PHE.11,12
The characteristics of hematoma and PHE volumes in patients with active cancer have not been well studied. Cancer fundamentally alters the immune system; therefore the inflammatory response and secondary injury occurring in response to ICH in the setting of active malignancy may differ. This may be particularly true with hematologic malignancies, with ICH exposing the brain parenchyma directly to malignant cells and often in the context of significant coagulopathy. Chemotherapy and radiation therapy also alter the immune system as well as vascular permeability and may affect hematoma and PHE volumes. Coagulopathy and thrombocytopenia occurring due to myelosuppression from hematologic malignancies or chemotherapy also present a particular treatment challenge.2,13
We sought to characterize both baseline hematoma and PHE volumes as well as expansion of both neuroimaging markers in patients with active cancer who developed spontaneous ICH in the absence of any known central nervous system (CNS) presence of malignancy. We further characterized factors specific to patients with active cancer affecting PHE and hematoma volumes.
METHODS
All data supporting the findings reported in this study are available upon request made to the corresponding author.
Patient Selection
Patients were retrospectively identified from institutional review board (IRB) approved cohorts of patients through searches of institutional databases. Patients with active malignancy who developed spontaneous ICH were identified at both Memorial Sloan Kettering Hospital and the Johns Hopkins Hospital between 2007 and 2016. Given the retrospective nature of this study, the IRBs at both institutions waived the need for written informed consent. Patients with presence of CNS malignancy were excluded. Patients with CNS disease were excluded given the inability to deconvolute edema due to ICH from edema due to a CNS metastatic lesion. All patients had follow-up magnetic resonance imaging after ICH to ensure absence of underlying tumor or metastatic disease. Control patients with spontaneous ICH were identified from a cohort of patients at Yale New Haven Hospital. All control patients had no evidence of active malignancy and had at least two CT scans available after onset of ICH.
All patients were 18 years of age or older and had a known time of ICH onset. All patients had at least two computed tomography (CT) scans of the head available for review. In addition to CNS malignancy, exclusion criteria included patients undergoing surgery prior to the second scan and presence of intraventricular hemorrhage.
Clinical and Laboratory Data
Clinical and laboratory data as well as cancer-specific treatment information were obtained from chart review. Laboratory data were collected at the timepoint closest to ICH ictus. Data regarding chemotherapy and radiation therapy were included up to 30 days prior to ICH. Data regarding blood transfusions were collected up to 48 hours after ICH. Only transfusions occurring after ICH were included.
Neuroimaging analysis
Images from each CT scan were exported as DICOMs, and the date and time of each image was documented. Volumetric analyses of ICH and PHE were performed using OsiriX v9.5 (Geneva, Switzerland). Regions of interest were initially drawn around areas of PHE and ICH. A semi-automated approach, highly reliable and reproducible method, was implemented using a Hounsfield unit (HU) range of 5–33 HU to identify areas of PHE.8,14 The upper limit was adjusted as necessary in order to obtain the best detection of edema. Volumes were generated by OsiriX after HU limits were set. Blood was calculated in a similar fashion, setting HU greater than 45. All volumes were calculated by A.M.G. and R.M., with a subset of volumes confirmed by a second reader.
Statistical Analysis
All statistical analyses were performed with R (R Foundation for Statistical Computing). Discrete variables are summarized as count (%), and continuous variables are summarized using medians and interquartile ranges. Mann-Whitney U tests were used to compare variables that were not normally distributed. P values less than 0.05 were considered to be statistically significant. Univariate regression analysis was performed for each variable using either linear or logistic regression models as appropriate. Multivariate models were created to assess the impact of variables contributing to PHE growth and 30-day mortality. Given the a priori hypothesis that chemotherapy, radiation therapy, and bleed location would affect PHE, models were created to include these variables, as well as those variables found to be significant in univariate analysis.
RESULTS
Demographics and clinical information
Clinical characteristics are shown in Table 1, including patients with active malignancy (cancer, N=80) and those who developed spontaneous ICH without active malignancy (controls, N=136). Details regarding each type of cancer in those patients with active malignancy are shown in Supplemental Table I. Approximately half (N=38) of the patients had a hematologic malignancy (leukemia or lymphoma), with the rest of the tumor types comprising a variety of solid tumors. Control and cancer patients had similar age at bleed onset, were sex matched, and had similar frequencies of diabetes, atrial fibrillation, cigarette smoking, antiplatelet use, and anticoagulation use. There was a higher prevalence of hypertension among control patients. Control patients had similar rates of cortical and subcortical bleeds, while cancer patients tended to have more cortical bleeds.
Table 1.
Patient Demographics and ICH Characteristics
| Cancer | Controls | P Value | |
|---|---|---|---|
| N | 80 | 136 | |
| Age | 66 (55.5–73.5)* | 66.5 (56.8–77.0) | 0.181 |
| Female sex | 41 (51%)† | 71 (52%) | 0.539 |
| Hypertension | 43 (53.8%) | 103 (75.7%) | 0.004 |
| Diabetes | 10 (12.5%) | 18 (13.2%) | 0.933 |
| Atrial fibrillation | 10 (12.5%) | 15 (11.0%) | 0.848 |
| Smoking history | 21 (26.3%) | 35 (25.7%) | 0.851 |
| Anticoagulation | 13 (16%) | 19 (14.0%) | 0.624 |
| Antiplatelets | 14 (18%) | 21 (15.4%) | 0.635 |
| Location | |||
| Cortical | 51 (63.8%) | 59 (43.4%) | 0.0039 |
| Subcortical | 19 (23.8%) | 61 (44.9%) | 0.0019 |
| ;Posterior Fossa | 10 (12.5%) | 16 (11.8%) | 0.875 |
Continuous variables are presented as median (interquartile range).
Categorical variables are presented as N (%).
Additional clinical details regarding the cancer patients is shown in Table 2. The majority (N=45, 56%) of patients had been treated with chemotherapy within 30 days. Only 13 patients (16%) had received radiation therapy within 30 days, with no patients having received CNS radiation. The majority of patients (54%) were being treated during a relapse of their cancer, while 40% were on first line treatment, and 6% had yet to initiate treatment. Thrombocytopenia was common (median platelet count 90,000/m3), while median international normalized ratio (INR), prothrombin time (PT), and partial thromboplastin time (PTT) were within normal limits. Blood products were transfused after ICH in 41 patients (51%). Given the predominant thrombocytopenia, 38 patients (48%) received platelet transfusion after ICH. By 30 days after ICH, 29 patients (36%) had died.
Table 2.
Cancer-Specific Treatment and Laboratory Values
| Treatment | Value |
|---|---|
| Chemotherapy | 45 (56%)* |
| Radiation therapy | 13 (16%) |
| Average Gy | 3600 (2225–6450)† |
| Treatment stage | |
| First line | 32 (40%) |
| Recurrence | 43 (54%) |
| Pre-treatment | 5 (6%) |
| Coagulopathy | |
| Platelets | 90,000 (17,500–211,500) |
| INR | 1.16 (1.08–1.40) |
| PT | 12.9 (11.6–15.2) |
| PTT | 30.0 (25.6–32.1) |
| Transfusions | 41 (51%) |
| PRBC | 3 (3.8%) |
| Platelets | 38 (48%) |
| FFP | 6 (7.5%) |
| Cryoprecipitate | 1 (1.25%) |
| 30-day mortality | 29 (36%) |
Gy indicates Gray; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; PRBC, packed red blood cells; and FFP, fresh frozen plasma.
Categorical variables are presented as N (%).
Continuous variables are presented as median (interquartile range).
ICH and PHE Characteristics
Initial and follow-up ICH and PHE volumetric analysis comparing cancer patients and controls are shown in Table 3. Time from symptom onset to bleed was similar between both groups, as was the time from first to second scan. There was no significant difference in baseline ICH volume between the two groups. Initial PHE volume was significantly higher in patients with active cancer (P=1.88×10−9). The ratio of initial PHE to ICH volume was also significantly higher among patients with active cancer (P=2.20×10−16). There was a trend toward increased PHE growth in patients with active malignancy, which did not reach significance (P=0.078). There was no significant difference in ICH growth between the two groups.
Table 3.
Hematoma and PHE Volumes Comparing Patients with Cancer and Controls
| Cancer | Controls | P value | |
|---|---|---|---|
| ICH volume [mL (IQR)] | 9.1 (4.68–15.54) | 10.4 (5.71–19.73) | 0.704 |
| PHE volume [mL (IQR)] | 23.67 (13.16–40.96) | 8.61 (5.93–15.73) | 1.88×10−9 |
| PHE to ICH volume ratio (IQR) | 2.26 (1.69–3.68) | 0.99 (0.79–1.16) | 2.20×10−16 |
| PHE growth [mL (IQR)] | 2.78 (−1.71 to 8.05) | 0.37 (−0.87 to 2.84) | 0.078 |
| ICH growth [mL (IQR)] | 0.955 (−0.25 to 2.58) | 0.54 (−0.52 to 1.10) | 0.105 |
| Time from symptom onset to first scan (h) | 5.3 (2.1–9.9) | 6.1 (2.6–10.5) | 0.922 |
| Time from first to second scan (h) | 11.0 (6.7–17.3) | 12.1 (8.3–18.5) | 0.739 |
ICH indicates intracerebral hemorrhage; mL, milliliters; IQR, interquartile range; PHE, perihematomal edema; and h, hours.
ICH and PHE characteristics were assessed in patients with hematologic malignancies (Supplementary Table I) and all solid tumors (Supplementary Table II). Compared with control patients, those with hematological malignancies had no difference in ICH volume (P=0.514) but had higher PHE volumes (P=4.00×10−7) and ratio of PHE to ICH volume (P=1.11×10−14). There was a trend toward increased PHE growth in patients with hematologic malignancies compared with controls, which did not reach significance (P=0.07), and there was no change in ICH growth (P=0.231). Similar results were found in those patients with solid tumors, with comparable ICH volumes (P=0.955), increased PHE volumes (2.35×10−6), increased PHE to ICH ratio (2.16×10−11), and no difference in PHE (P=0.221) or ICH (P=0.116) growth.
There was no significant difference in ICH volume, PHE volume, and PHE to ICH volume ratio in cancer patients with cortical or subcortical bleeds (Supplemental Table IV), although there was a trend toward larger ICH (median 10.3 mL [IQR, 6.10–15.5] versus median 6.26 mL [IQR, 3.32–12.1]) and PHE volumes (median 26.2 mL [IQR, 15.9–41.6] versus median 15.4 mL [IQR, 7.89–44.9]) in those with cortical bleeds. There was no significant difference in ICH growth; however, there was more PHE growth among those with cortical bleeds (median 5.43 mL [IQR, 1.10–12.8] versus 0.70 mL [IQR, −1.17 to 1.65]).
Longitudinal analysis of ICH and PHE in patients with cancer
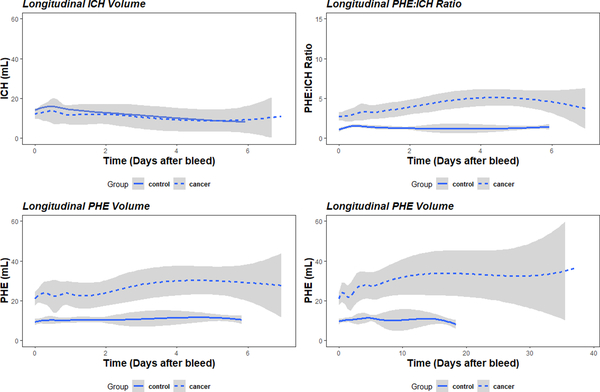
Trends in ICH and PHE volumes were determined after the initial bleed. ICH volume remained stable and trended down gradually in both control and cancer patients (Figure 1A). PHE increased after ICH (Figure 1B), with control and cancer patients demonstrating an increase in PHE to hematoma ratio early after ICH, with cancer patients demonstrating a more sustained increase (Figure 1C). PHE volumes in cancer patients continued to gradually increase, reaching a maximum at 15 days after ICH (Figure 1D).
Figure 1.
Temporal analysis of intracerebral hemorrhage (ICH) and perihematomal edema (PHE) volumes. Hematoma and PHE volumes are shown over time after ICH in control (solid line) and cancer (dashed line) patients. Hematoma volume (A), PHE volume (B), and ratio of PHE to ICH volume (C) are shown over the first 6 days after ICH. PHE volume over 30 days after ICH is also shown (D).
Univariate analysis of cancer specific variables
Univariate linear or logistic regression analysis was performed on patients with active cancer, as shown in Supplemental Table V. As expected, initial ICH volume had a significant association with initial PHE volume (β=1.98, P=2.0×10−16) as well as PHE expansion (β=1.15, P=9.3×10−6). No significant associations were found with any bleed characteristic and age, gender, hypertension, or treatment with anticoagulation. There were no associations between recent (within 30 days) treatment with chemotherapy or radiation therapy and any bleed characteristic. While significant thrombocytopenia was present, there were no associations between platelet count, ICH volume or expansion, PHE volume or expansion, and PHE to ICH ratio. There were no associations between other markers of coagulopathy (INR, PT, PTT). Patients who had received blood product transfusions after ICH demonstrated significantly increased PHE growth (β=16.64, P=0.03). The majority of blood product transfusions were platelets (Table 2). Similarly, patients who had received platelet transfusion after ICH had increased PHE growth (β=15.41, P=0.04). ICH and PHE volumes, PHE expansion, any transfusion, and platelet transfusion were associated with increased 30-day mortality (β=24.74 [P=0.001], β=29.94 [P=0.0003], β=22.27 [P=0.01], β=3.12 [P=0.0057], and β=3.43 [P=0.0061], respectively). Subcortical bleed location was associated with less PHE expansion (β=−18.90 [P=0.04]), while cortical bleed location was associated with great PHE expansion (β=17.74 [P=0.02]). ICH volume, PHE volume, PHE growth, blood transfusions, and platelet transfusions were associated with increased 30-day mortality (P=0.001, 0.0003, 0.01, 0.0057, and 0.0061, respectively).
Multivariate analysis
Multivariate models were created to control for individual variables associated with PHE growth and 30-day mortality. Three models were created to control for factors associated with PHE growth, as shown in Table 4. ICH volume and receipt of platelet transfusion after ICH remained significantly associated with PHE growth after controlling for chemotherapy and radiation therapy (Model 2), or chemotherapy, radiation therapy, and bleed location (Model 3).
Table 4.
Associations with PHE Growth
| Covariates | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| ICH volume | 1.15 [0.70–1.60 (7.66×10−6)]* | 1.23 [0.77–1.68 (2.90×10−6)] | 1.29 [0.67–1.58 (1.30×10−5)] |
| Platelet transfusion | 14.16 [1.85–26.47 (0.028)] | 16.73 [4.23–29.14 (0.011)] | 15.67 [3.61–27.74 (0.014)] |
| Chemotherapy | −1.19 [−24.59 to 0.61 (0.07)] | −1.74 [−30.41 to −4.36 (0.18)] | |
| Radiation therapy | 6.19 [−9.06 to 21.43 (0.43)] | 6.46 [−8.65 to 21.57 (0.41)] | |
| Subcortical | −11.32 [−33.28 to 10.63 (0.32)] | ||
| Cortical | 8.09 [−10.73 to 26.92 (0.40)] |
PHE indicates perihematomal edema and ICH, intracerebral hemorrhage.
Values are represented as β coefficient [95% CI (P value)].
Models were created to control for the associations between 30-day mortality and ICH volume, PHE volume, and PHE growth (Table 5). Each volumetric parameter as well as receipt of platelet transfusion after ICH maintained a significant association with 30-day mortality after controlling for chemotherapy, radiation therapy, and bleed location.
Table 5.
Associations with 30-Day Mortality
| ICH Volume | PHE Volume | PHE Growth | |
|---|---|---|---|
| Unadjusted | 1.05 [1.02–1.09 (0.002)]* | 1.06 [1.03–1.09 (0.0003)] | 1.05 [1.01–1.09 (0.01)] |
| Adjusted | 1.06 [1.03–1.1 (6.76×10−5)] | 1.07 [1.04–1.09 (7.40×10−6)] | 1.05 [1.01–1.1 (0.01)] |
| Covariates | |||
| Platelet transfusion | 1.48 [1.22–1.79 (0.0002)] | 1.47 [1.23–1.78 (0.0001)] | 1.29 [1.03–1.61 (0.03)] |
| Chemotherapy | 1.04 [0.85–1.27 (0.70)] | 1.04 [0.86–1.26 (0.69)] | 1.01 [0.79–1.28 (0.95)] |
| Radiation therapy | 1.22 [0.87–1.73 (0.15)] | 1.31 [1.01–1.70 (0.08)] | 1.27 [0.78–1.65 (0.08)] |
| Subcortical | 1.23 [0.87–1.73 (0.24)] | 1.18 [0.85–1.65 (0.33)] | 1.30 [0.88–1.91 (0.19)] |
| Cortical | 0.92 [0.68–1.24 (0.58)] | 0.89 [0.67–1.20 (0.45)] | 0.98 [0.71–1.37 (0.93)] |
ICH indicates intracerebral hemorrhage and PHE, perihematomal edema.
Values are represented as odds ratio [95% CI (P value)].
DISCUSSION
This manuscript provides a detailed analysis of ICH characteristics and PHE development in patients with active cancer and without primary or metastatic CNS disease. In addition to demonstrating increased PHE in patients with cancer, we show that platelet transfusion after ICH may be associated with increased PHE growth and 30-day mortality.
Our results are consistent with prior reports that have suggested that PHE volume is dependent on hematoma volume and that PHE volume and growth are associated with worse outcomes after ICH.8,15,16 The natural history of PHE has been well described, with the most rapid PHE growth occurring within the first 24 to 48 hours9,17 after ICH but not peaking until around 12 days.17 The temporal profile of PHE in patients with active malignancy reported herein was similar, with peak PHE volume occurring at 15 days (Figure 1). However, patients with active cancer had larger PHE volumes and higher ratios of PHE to hematoma (Table 3 and Figure 1). ICH location does not predict PHE volume;17,18 similarly, we did not find significant differences in PHE volume by bleed location (Supplemental Table IV). While cortical bleeds were associated with more PHE growth (Supplemental Tables IV and V), this effect was lost in multivariate models (Table 4). It remains possible that PHE may affect functional outcomes according to bleed location;8 however, this study was not adequately powered to make this determination. It has recently been shown that relative PHE is greater in patients with ICH caused by an underlying tumor.19 While our study also demonstrates an increased relative PHE in patient with cancer, our patients had no underlying tumor, suggesting that systemic alterations in immune function or the inflammatory system may be contributing to PHE rather than a direct effect from underlying tumor cells.
Inflammation plays an important role in secondary brain injury after ICH, with the activation of microglia and infiltration of neutrophils, monocytes, and T cells as well as development of PHE.20,21 The interactions between cancer and the immune system were recognized as early as 1863 when Virchow suggested that cancer develops as a result of unresolved inflammation.22 Both the innate and adaptive immune system contribute to cancer-associated inflammation.23,24 CD4+ T cells are critical for immunosurveillance of cancer cells, and produce proinflammatory cytokines such as IL-1α/β and IL-6.25 Furthermore, tumor cells themselves secrete various cytokines and chemokines that attract leukocytes, which are capable of producing cytokines such as TNF-α, interleukins, and interferons (IFNs). In addition to its effects on the immune system, other systemic effects of cancer may also modulate PHE. Alternations in microRNAs (miRNA) have been linked to cancinogensis.26 Recent evidence has implicated miRNAs in PHE formation,27 suggesting that miRNA modulation by cancer may also affect PHE. Additionally, cancer modulates a plethora of signaling pathways, affecting the expression of matrix metalloproteinases and the process of angiogenesis,28,29 both of which may affect PHE.6
We hypothesize that the already primed immune system in patients with active cancer results in a more robust inflammatory response to ICH, leading to relatively more PHE. Given the direct exposure of brain parenchyma to malignant cells that occurs after ICH in patients with hematologic malignancies, more PHE and secondary brain injury may be expected compared with solid tumors. However, we found similar PHE volumes and PHE to hematoma ratios in hematologic and solid tumors (Supplemental Tables II and III). This may not be entirely unexpected, since although hematologic malignancies are robustly recognized by the immune system, they employ unique immune evasions strategies.30 Both solid tumor and hematologic malignancies utilize similar immune evasion mechanisms such as induction of programmed death-ligand 1 (PD-L1), downregulation of antigen presenting complexes, and recruitment of immunosuppressive cells.30 However, hematologic malignancies do not activate the innate immune system as robustly as solid tumors.31,32 Danger-associated molecular patterns (DAMPs) released by lysed cancer cells are required to activate the innate immune system, but they may not reach significant concentrations given the body-wide dissemination of many hematologic malignancies.33 In solid tumors, IFN production by dendritic cells results in cross-priming of tumor-specific CD8+ T cells; however, a diminished IFN response seems to be generated in response to leukemia.31 Leukemias also tends to have lower mutations burdens than solid tumors and therefore may harbor fewer neoantigens resulting in less T cell activation.30,33 Therefore, the mechanisms of PHE formation in patients with active cancer may vary by cancer type and stage and may also be modulated by immunotherapy. Discerning the exact mechanisms driving PHE formation in patients with cancer will require further prospective evaluation and studies to evaluate the underlying pathophysiological processes.
Coagulopathy has been suggested as a major factor contributing to the development of ICH in patients with cancer.2,34 This has likely affected the practice of providing blood product transfusions for patients who develop ICH. However, our results suggest that the degree of coagulopathy did not affect ICH or PHE growth (Supplemental Table V). Of note, the most prevalent coagulopathy present in the cohort of patients with cancer was thrombocytopenia. Chemotherapy, radiation therapy, and myelosuppression by hematologic cancers can all contribute to thrombocytopenia.13 However, patients with thrombocytopenia above 10,000/μL tend not to bleed,35 which may be related to contributions made by platelets to vascular integrity.36,37 Given the small, retrospective nature of this study, the results should be considered hypothesis generating; however, the lack of association between platelet count and ICH and PHE volumes raises questions about the role of thrombocytopenia in the pathogenesis of ICH in this cohort as well as the optimal platelet count to target after bleed.
We found that having received platelet transfusion after ICH results in significantly increased PHE growth (Table 4). In addition to their role in promoting hemostasis, platelets have much more complicated effects on cell signaling and can modulate the inflammatory response. Each leukocyte subtype has been shown to be able to adhere to platelets,38 largely dependent on interactions with P-selectin.39 Platelets contain CD40L, which allows for stimulation of neutrophils and T-cell, as well as endothelial cells.40–42 Activated platelets can also synthesize IL-1β, derived from pre-mRNA containing in the cytosol.43 Additionally, high-mobility group protein 1 (HMGB1) is expressed on the surface of activated platelets, which engages toll-like receptors leading to NF-κB activation further promoting inflammation.44,45 We suspect that the proinflammatory role of platelets may account for the increase in PHE observed in those patients who received transfusions after ICH. This may be consistent with a prior report demonstrating an association between increased platelet count and PHE.46 While we found an association with platelet transfusion and PHE, we may not have seen a similar association between platelet count and PHE given the prevalence of thrombocytopenia in this population.
The potentially adverse effect of platelet transfusions after spontaneous ICH was demonstrated in the PATCH trial.47 Given the increase risk of death or dependence shown after platelet transfusion for ICH, routine transfusion of platelets in those patients being treated with anti-platelet agents is no longer recommended. However, it is unknown whether platelet transfusions should be used in patients who are thrombocytopenic, and it remains common practice to target a platelet goal of 50–100 after intracranial hemorrhage. Thrombocytopenia was common in the patients with active malignancy resulting in a high percentage of patients receiving platelet transfusions. We are unable to conclude whether transfusions had an effect on functional outcome; however, blood product was consistently associated with worse 30-day mortality (Table 5). It will be important to perform future prospective studies to determine the effects of platelet transfusions in thrombocytopenic patients who develop intracranial hemorrhage.
This study has several limitations. Importantly, the study was based on retrospective data, and the sample size was also relatively small. Though we were able to capture 30-day mortality, one of the limitations of this study is that we do not have 30-day morbidity outcomes. This is in part due to the lack of standardized reporting of morbidity, such as modified Rankin Scale scores, in this patient population. Further, outcomes were also impacted by changes in level of care post-ICH. In our cancer population, the event of ICH led to withdrawal of care in a subset of patients, thus potentially affecting outcome data. The patients with active malignancy also comprised a relatively heterogenous group of patients with a variety of tumor types and treatment regimens. While we did not find any associations between recent chemotherapy or radiation therapy and bleed characteristics, it remains possible that certain types of chemotherapy may affect hematoma of PHE volumes. It is also of note that none of the patients in this study with active malignancy had any CNS metastatic disease. Therefore, these results cannot be extrapolated to the common scenario of patients with primary brain tumors or CNS metastases who develop tumor-related hemorrhage. This population of patients also warrants further study.
SUMMARY:
We have shown that patients with active cancer and no CNS disease who develop spontaneous ICH have large PHE volumes and more PHE relative to ICH volume. We further show that receipt of platelet transfusion after ICH may contribute to increased PHE growth. This is of particular significance, as it raises questions about the management of ICH in cancer patients. Future prospective studies will be needed to identify the optimal platelet targets to limit hematoma expansion while also minimizing PHE growth.
Supplementary Material
ACKNOWLEDGMENTS:
This research was supported by an NIH/NCI Cancer Center Support Grant (P30-CA008748) to Memorial Sloan Kettering Cancer Center.
SOURCES OF FUNDING:
DFH is supported by the NIH (U01NS080824 and U24TR001609). KNS receives support from the NIH (R01NS110721, R03NS112859, U01NS106513, R01NR018335, U24NS107136, and U24NS107215) and American Heart Association (19CSLOI34770004). GJF is supported by the NIH (K76AG059992 and R03NS112859), American Heart Association (18IDDG34280056), Yale Pepper Scholar Award (P30AG021342), and Neurocritical Care Society Research Fellowship. LHS is supported by the NIH (R21NS088972 and R01NS097728).
DISCLOSURES: LHS has received consultant fees from Genentech not relevant to this work. DFH receives personal fees from BrainScope, Neurotrope, Op2Lysis, Portola Pharmaceuticals, and Medicolegal not relevant to this work. KNS has received grant funding from Bard, Novartis, Biogen, Hyperfine, Zoll, Alva, and Astrocyte not relevant to this work.
REFERENCES
- 1.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine. 1985;64:16–35. [DOI] [PubMed] [Google Scholar]
- 2.Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012;14:373–381. [DOI] [PubMed] [Google Scholar]
- 3.Oka K, Tsuda H, Sakamoto S, Go Y, Tomonaga M. Plasminogen activator and hemorrhage in brain tumors. J Neurooncol. 1994;22:183–187. [DOI] [PubMed] [Google Scholar]
- 4.Jung S, Moon KS, Jung TY, Kim IY, Lee YH, Rhu HH, et al. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006;76:257–263. [DOI] [PubMed] [Google Scholar]
- 5.Murthy SB, Shastri A, Merkler AE, Hanley DF, Ziai WC, Fink ME, et al. Intracerebral hemorrhage outcomes in patients with systemic cancer. J Stroke Cerebrovasc Dis. 2016;25:2918–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urday S, Beslow LA, Goldstein DW, Vashkevich A, Ayres AM, Battey TW, et al. Measurement of perihematomal edema in intracerebral hemorrhage. Stroke. 2015;46:1116–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC. Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke. 2015;46:3088–3092. [DOI] [PubMed] [Google Scholar]
- 9.Wu TY, Sharma G, Strbian D, Putaala J, Desmond PM, Tatlisumak T, et al. Natural history of perihematomal edema and impact on outcome after intracerebral hemorrhage. Stroke. 2017;48:873–879. [DOI] [PubMed] [Google Scholar]
- 10.Urday S, Beslow LA, Dai F, Zhang F, Battey TW, Vashkevich A, et al. Rate of perihematomal edema expansion predicts outcome after intracerebral hemorrhage. Crit Care Med. 2016;44:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leasure A, Kimberly WT, Sansing LH, Kahle KT, Kronenberg G, Kunte H, et al. Treatment of edema associated with intracerebral hemorrhage. Curr Treat Options Neurol. 2016;18:9. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Edwards NJ, Choi HA, Chang TR, Jo KW, Lee K. Treatment strategies to attenuate perihematomal edema in patients with intracerebral hemorrhage. World Neurosurg. 2016;94:32–41. [DOI] [PubMed] [Google Scholar]
- 13.Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133:S63–S69. [DOI] [PubMed] [Google Scholar]
- 14.Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. Minimally invasive surgery plus recombinant tissuetype plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, et al. Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial studies. Stroke. 2015;46:1009–1013. [DOI] [PubMed] [Google Scholar]
- 16.Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, et al. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol. 2015;11:111–122. [DOI] [PubMed] [Google Scholar]
- 17.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke. 2011;42:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631–2635. [DOI] [PubMed] [Google Scholar]
- 19.Nawabi J, Hanning U, Broocks G, Schön G, Schneider T, Fiehler J, et al. Neoplastic and non-neoplastic causes of acute intracerebral hemorrhage on CT: the diagnostic value of perihematomal edema. [published online March 21, 2019]. Clin Neuroradiol. 2019. 10.1007/s00062-019-00774-4. Accessed May 30, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Askenase MH, Sansing LH. Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol. 2016;36:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusdon AM, Gialdini G, Kone G, Baradaran H, Merkler AE, Mangat HS, et al. Neutrophil–lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48:2589–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus A, Gowen BG, Thompson TW, Iannello A, Deng W, Wang L, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haabeth OAW, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lujambio A, Lowe SW. The microsomes of cancer. Nature. 2012;482:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Wang H, Huang Q, Wang G, Zhang H. MicroRNA-23a-3p promotes the perihematomal edema formation after intracerebral hemorrhage via ZO-1. Eur Rev Med Pharmacol Sci. 2018;22:2809–2816. [DOI] [PubMed] [Google Scholar]
- 28.Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. [DOI] [PubMed] [Google Scholar]
- 30.Curran EK, Godfrey J, Kline J. Mechanisms of immune tolerance in leukemia and lymphoma. Trends Immunol. 2017;38:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curran E, Chen X, Corrales L, Kline DE, Dubensky TW, Duttagupta P, et al. STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep. 2016;15:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Chen X, Liu X, Kline DE, Teague RM, Gajewski TF, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest. 2013;123:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran E, Corrales L, Kline J. Targeting the innate immune system as immunotherapy for acute myeloid leukemia. Front Oncol. 2015;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navi B, Reichman J, Berlin D, Reiner A, Panageas K, Segal A, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stolla M, Refaai MA, Heal JM, Spinelli SL, Garraud O, Phipps RP, et al. Platelet transfusion: the new immunology of an old therapy. Front Immunol. 2015;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho-Tin-Noé B, Goerge T, Wagner DD. Platelets: Guardians of tumor vasculature. Cancer Res. 2009;69:5623–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulaftali Y, Hess PR, Kahn ML, Bergmeier W. Platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling and vascular integrity. Circ Res. 2014;114:1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. [DOI] [PubMed] [Google Scholar]
- 39.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. [DOI] [PubMed] [Google Scholar]
- 40.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogné M, Richard Y, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35:1376–1387. [DOI] [PubMed] [Google Scholar]
- 41.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. [DOI] [PubMed] [Google Scholar]
- 42.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. [DOI] [PubMed] [Google Scholar]
- 45.Rouhiainen A, Imai S, Rauvala H, Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost. 2000;84:1087–1094. [PubMed] [Google Scholar]
- 46.Sansing LH, Kaznatcheeva EA, Perkins CJ, Komaroff E, Gutman FB, Newman GC. Edema after intracerebral hemorrhage: correlations with coagulation parameters and treatment. J Neurosurg. 2003;98:985–992. [DOI] [PubMed] [Google Scholar]
- 47.Baharoglu MI, Cordonnier C, Salman RA, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–2613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.