Abstract
The present study aimed to investigate the therapeutic potential of the methanolic extract of Lepidium sativum seeds in mice experimentally infected with Trypanosoma evansi. A total of thirty-two male Swiss albino mice were randomly divided into four groups: the first group was the normal control, while the second, third and fourth groups were infected intraperitoneally with 1 × 104 trypanosomes. The third and fourth groups were treated with 100 μl of Lepidium sativum seed extract (LSSE) at a dose of 200 mg/kg body weight intraperitoneally (infected + LSSEI) and orally (infected + LSSEO) respectively, once a day, for a period of four days.
Parasitaemia was found to be significantly raised in the untreated infected group, reaching 2 × 107 at day 4 post-infection, but was significantly reduced by 65.5% and 88% in the mice treated orally and intraperitoneally with LSSE, respectively. The erythrocyte count, HCT, haemoglobin content, leucocyte count and the percentage of lymphocytes was significantly reduced in the untreated infected group, while the treatment with LSSE returned these parameters to their pre-infection values. In addition, our study proved that LSSE provided protection against liver tissue damage and decreased the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The present study also established that intraperitoneal injection of LSSE is more effective than oral administration in the treatment of trypanosome infection in mice. In conclusion, the infection caused haematological, biochemical and histological changes that were ameliorated following treatment with LSSE.
Keywords: Lepidium sativum, Antitrypanosomal activity, Trypanosoma evansi, Mice
1. Introduction
Animal trypanosomiasis is catastrophic parasitic protozoan disease that is caused by Trypanosoma evansi, the most common and widespread trypanosome in domestic and wild animals (Desquesnes et al., 2013, Molefe et al., 2017). Trypanosoma evansi was the first mammalian trypanosome in the world to be described (by Evans in 1880); originally in the blood of camels and equines and later in the blood of a wide range of other hosts (Desquesnes et al., 2013). Although this trypanosome is able to infect most mammals, camels and horses are the principal hosts and represent the major economic loss (Baldissera et al., 2017). Trypanosoma evansi is not usually considered to be of zoonotic concern, but human infection with this trypanosome has recently been confirmed (Powar et al., 2006, Habila et al., 2011). In Saudi Arabia, camels of the Eastern, Jazan, Northern Frontiers, Riyadh and Tabouk regions were found to be infected with T. evansi with a considerably high prevalence of infection (40%) in Jazan (Al-Khalifa et al., 2009). Trypanosoma evansi disease can occur in either acute form or chronic forms. The chronic form is the more common one and is characterized by relapsing parasitaemia, anaemia, emaciation, lachrymation, lymphadenitis, oedema of the abdomen and legs, abortion, and death in some animals (Haroun et al., 2000, Gutierrez et al., 2005). The acute form shows high fever with progressive anaemia, anorexia, marked depression, weakness and in some cases, death (Haroun et al., 2000, Omer et al., 2007). The treatment of trypanosomiasis is based on just a few chemotherapeutic compounds that have been in use for decades. Most of these, however, do not provide total control of the infection and are associated with high rates of disease recurrence, severe toxicity, severe side effects and sometimes mortality (Da Silva et al., 2008, Baldissera et al., 2017). In addition, the parasite has probably developed resistance towards these longstanding treatments (Steverding, 2010, Molefe et al., 2017). There is therefore an urgent need for new compounds as alternative and less toxic treatments.
In this context, Lepidium sativum is a popular herb, commonly known as “Hab el Rashaad” or “Thufa”, which is grown in many regions in Saudi Arabia such as Hijaz, Al-Qaseem and the Eastern province (Gilani et al., 2013). It is frequently used in Saudi traditional medicine, as well as in many African countries, for the treatment of gut disorders like diarrhoea, among other medicinal uses, while in the Western world its leaves are used in salads (Usmanghani et al., 1997, Rahman et al., 2004, Azaizeh et al., 2006, Gilani et al., 2013). Phytochemical studies of Lepidium sativum seeds have revealed the presence of tannins, benzyl isothiocyanate, flavonoids, alkaloids, triterpenes and sterols, which are known to have antioxidant, anti-inflammatory, analgesic and anti-parasitic activities (Adamu and Boonkaewwan, 2014, Bahrami et al., 2016, Raish et al., 2016). The present work was therefore suggested to evaluate the potential activity of the extract of Lepidium sativum seeds against trypanosomiasis in a mice model.
2. Materials and methods
2.1. Extract of Lepidium sativum seeds
Lepidium sativum seeds were purchased from the local markets in Riyadh and identified by a taxonomist in the Botany Department at the College of Science, King Saud University, Riyadh, Saudi Arabia. Before extraction, the seeds were ground with a mortar and then extraction was done in methanol in a closed glass vessel with continuous shaking at room temperature for two days. The mixture was then filtered through Whatman filter paper and the filtrate was evaporated in a vacuum evaporator to dryness. Finally, the dried Lepidium sativum seeds extract (LSSE) was dissolved in Dimethyl sulfoxide (DMSO) for use in this experiment.
2.2. Mice
In the present study, thirty-two males of Swiss albino mice of age ranged from eight to nine weeks were obtained from the animal house at King Saud University, Riyadh, Saudi Arabia. The animals were bred under pathogen-free conditions and fed on a standard pellet diet with water ad libitum. Experiments were performed in accordance with the rules of the Ethical Committee of Research on Animals at King Saud University.
2.3. Trypanosoma evansi isolate
The cryopreserved isolate of T. evansi that was used in the present study was kindly provided by Professor Mehlhorn, Department of Parasitology, Heinrich Heine University, Düsseldorf, Germany. The cryopreserved isolate of T. evansi was passaged twice in mice in order to obtain a large amount of viable parasites before use in the experiment.
2.4. Experimental design
The adult male mice were divided into four groups with eight animals in each group. The first group (non-infected) was only treated with 100 μl of DMSO and served as a negative control. The second, third and fourth groups were infected intraperitoneally with 1 × 104 trypanosomes. Then, the third, and fourth groups were treated with 100 μl of LSSE extract at a dose of 200 mg/kg body weight Intraperitonealy (infected + LSSEI) and orally (infected + LSSEO) respectively, once a day, for a period of four days. The dose has been chosen based on the fact that LSEE showed no toxicity with dose between 150 and 300 mg/kg b.w in animal model (Raish et al., 2016). Parasitaemia was individually checked every day by direct microscopic counting of trypanosomes in 5 µl of blood according to the Pizzi-Brener method (Brener, 1962).
2.5. Haematology
On day 4 p.i., blood samples were collected from the heart into tubes containing heparin in order to estimate the erythrocyte count, HCT, haemoglobin content, leucocyte count and percent lymphocytes using a VetScan haematology analyser (VetScan HM5; Abaxis UK Limited, Yorkshire, UK).
2.6. Liver enzymes
Liver enzymes (aspartate aminotransferase (AST), alanine aminotransferase (ALT) were estimated by commercial kits (Biodiagnostic Co., Dokki, Giza, Egypt) as described in the manufacturer’s instructions.
2.7. Preparation of liver tissue
Livers of both non-infected and infected mice were aseptically removed and small pieces were fixed in 10% neutral buffered formalin. These were then processed as per the standard procedure for histological preparations. 5 µm thick paraffin sections were cut and double-stained haematoxylin and eosin (H & E).
3. Results
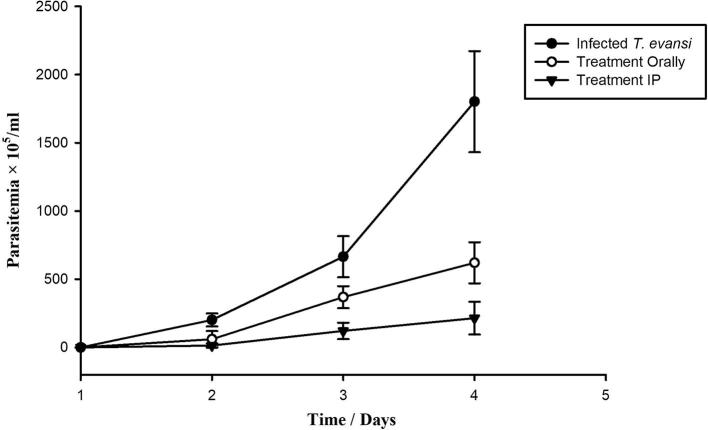
The present study evaluates the trypanocidal activity of LSSE against T. evansi. After infection of mice with T. evansi, trypanosomes appeared in the blood of all three infected groups during day 2 post infection (p.i.). Parasitaemia increased quickly in the untreated group, reaching 2 × 107 trypanosomes/ml by day 4 p.i. At this level of parasitaemia, mice succumbed to death within a few hours (Fig. 1). With oral and intraperitoneal LSSE treatment, however, parasitaemia was gradually but significantly suppressed to the extent that it was 65.5% and 88% less than in the untreated infected mice by day 4 p.i.
Fig. 1.
Parasitemia profiles of mice infected with T. evansi and treated with 100 μl of L. sativum seed extract (LSSE) at a dose of 200 mg/kg body weight intraperitoneally and orally (All values presented as Mean ± SD).
Trypanosome infection induced a maximum decrease in the WBCs count to 2.8 × 109 WBCs/mm3 in the untreated infected group on day 4 p.i., but this was significantly improved (P < 0.050) to 4.3 × 109 WBCs/mm3 and 6.7 × 109 WBCs/mm3 in the orally and intraperitoneally LSSE treated groups respectively (Table 1). Similarly, the percentage of lymphocytes was significantly reduced in the infected group compared to the non-infected one which was significantly (P < 0.050) improved in the treated groups (Table 1). Both the RBCs count and haemoglobin content in the untreated infected group were significantly decreased (P < 0.050) when compared with those of the non-infected one, while LSSE treatment revealed a significant improvement in both these parameters (Table 1). The haematocrit (HCT), meanwhile, was significantly decreased in the untreated infected group when compared to the control group (P < 0.050) but, with treatment, HCT% was greatly improved to close to the normal level (Table 1). The levels of the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were significantly increased in the untreated infected group (P < 0.050) comparing to the control uninfected one (Table 2). Upon treatment with LSSE, however, the levels of AST and ALT were significantly ameliorated, nearly returning to their levels in the control uninfected group. All the previously measured parameters showed more effect evident for intraperitoneal injection than for oral administration (Fig. 1, Table 1, Table 2).
Table 1.
Some haematological indices of mice infected with T. evansi and treated with LSSE on day 4 p.i. (Data expressed as Mean ± SD).
| Groups | RBCs (×1012/mm3) | HB (g/dl) | WBCs (×109/mm3) | HCT (%) | Lymphocytes (%) |
|---|---|---|---|---|---|
| Control | 8.7 ± 0.5 | 13.5 ± 0.5 | 7.0 ± 1.2 | 43.6 ± 1.5 | 5.5 ± 1.1 |
| Infected | 7.1 ± 0.5* | 11.3 ± 0.8* | 2.8 ± 0.7* | 36.3 ± 1.6* | 0.9 ± 0.4* |
| Infected + LSSEI | 8.9 ± 0.7# | 13.2 ± 0.9# | 6.7 ± 1.1 | 43.1 ± 2.0 | 3.5 ± 1.0 |
| Infected + LSSEO | 8.8 ± 0.6# | 14.2 ± 0.6# | 4.3 ± 0.8# | 41.9 ± 1.9# | 3.3 ± 1.3# |
P < 0.05 infected vs. control.
P < 0.05 treatment groups vs. infected group.
Table 2.
Plasma levels of liver enzymes; ALT and AST; of mice infected with T. evansi and treated with LSSE on day 4 p.i. (Data expressed as Mean ± SD).
| Groups | ALT U/L | AST U/L |
|---|---|---|
| Control | 32.3 ± 3.6 | 71.1 ± 3.8 |
| Infected | 73.2 ± 7.1* | 145.9 ± 5.3* |
| Infected + LSSEI | 35.2 ± 2.7# | 99.2 ± 4.7# |
| Infected + LSSEO | 51.1 ± 1.3# | 119.0 ± 5.5# |
P < 0.05 infected vs. control.
P < 0.05 treatment groups vs. infected group.
3.1. Liver histology
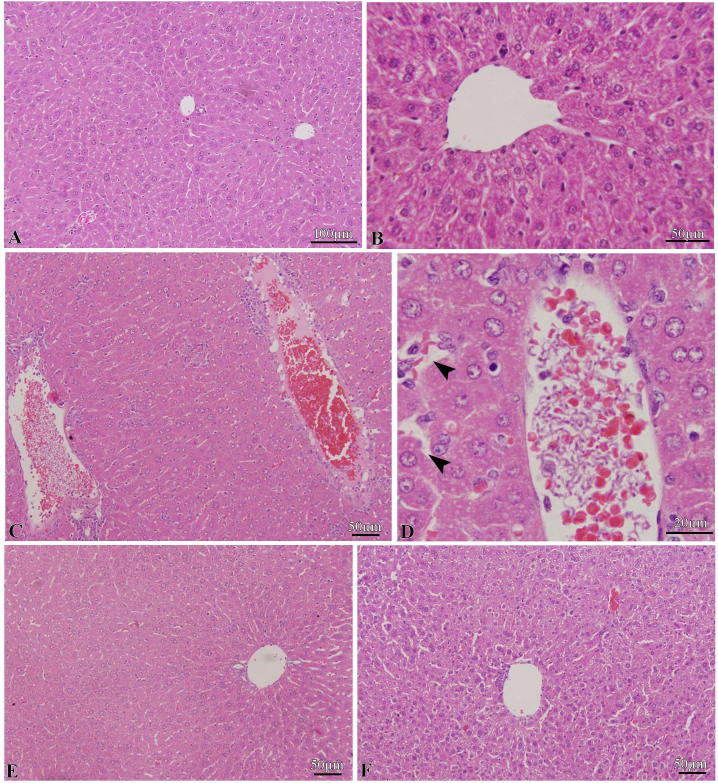
The control mice had livers with normal a histological organization, as shown in Fig. 2A. The sections of normal liver revealed the presence of hepatic lobules composed of distinct hepatocytes, with well-preserved cytoplasm and prominent nucleus, radially arranged around the central vein (Fig. 2B).
Fig. 2.
A and B. Liver histology of normal mice showing the normal liver. C and D. Liver histology of T. evansi infected mice at day 4 p.i. showing congested blood vessel of liver clogged with clumps of trypanosomes with some vacuolar degeneration (arrowheads). E and F. Liver histology LSSE treated groups showing improvement in the liver histopathological changes induced by T. evansi (E. IP treatment and F. Oral treatment).
In the infected group, the liver sections showed some degenerative changes, including great disruption in the normal arrangement and integrity of liver cells due to congestion of blood vessels and clogging with clumps of trypanosomes (Fig. 2C,D). Some hepatocytes were damaged in certain areas and vacuolar degeneration was evident. Upon treatment with LSSE, however, the liver retained nearly the original architecture with no trypanosomes seen in the blood vessel (Fig. 2D,E). In other words, LSSE extract significantly ameliorated the histopathological impacts of trypanosome infection and improved the liver histology to close to normal levels, especially with IP treatment (Fig. 2D).
4. Discussion
The greatest challenge facing the treatment of T. evansi infection today is the drug resistance and toxicity associated with the available chemotherapy (Baldissera et al., 2017). Natural products and their compounds are considered to be promising sources of new drugs and alternative treatment strategies to medicate trypanosomes (Habila et al., 2011, Dyary et al., 2015, Baldissera et al., 2017). In this context, the antitrypanosomal activity of LSSE was screened against T. evansi. The present study showed a significant reduction in parasitaemia following the treatment with LSSE. Similarly, Habila et al. (2011) reported a significant reduction in T. evansi parasitaemia upon treatment rats with 400 mg/kg methanolic extract of Azadirachta indica seeds. Also, Tesfaye et al. (2015) demonstrated that daily administration of 400 mg/kg methanolic extract of Albizia schimperiana leaves significantly reduced the levels of parasitaemia of Trypanosoma congolense in experimentally infected mice.
Lepidium sativum is an antiparasitic with documented efficacy against Eimeria tenella (Adamu and Boonkaewwan, 2014) and Echinococcous granulosus (Bahrami et al., 2016). The antiparasitic activity of L. sativum might be related to the seed coating, which may contain various amounts of different active components that are able to prohibit the division and development of parasites or provoke immunity in the host (Delaquis et al., 2002, Adamu and Boonkaewwan, 2014).
This study also showed that the infection with trypanosome induced leukopenia associated with lymphocytopenia, but that this was significantly improved following the treatment with LSSE. Mahassni and Khudauardi (2017) have similarly noticed that daily oral administration of an aqueous extract of L. sativum seeds was associated with a gradual but significant increase in WBC count in mice. Mahassni and Khudauardi (2017) also reported that an aqueous L. sativum seed extract is useful for enhancing and strengthening the immune system by increasing the weight of the spleen, which may result from the increased production and storage of immune system cells in the spleen evident in the significant increase in the mean WBC count. In the present study, the RBCs count, HCT and Hb values were significantly reduced in the infected group, which might be due to increased susceptibility of the membrane of red blood cells to oxidative damage, as suggested by Taiwo et al., 2003, Sivajothi et al., 2015. The treatment with LSSE greatly improved the haematological parameters, possibly linked to LSSE inducing a significant decrease in oxidative stress markers, as proposed by Raish et al. (2016).
The plasma level of liver enzymes (AST and ALT) were significantly increased in the infected group while LSEE treatment markedly ameliorated these liver enzymes, nearly restoring them to their normal levels. The increased levels of ALT and AST during trypanosome infection is compatible with the findings of earlier studies (e.g. Ezeokonkwo et al., 2012, Nwoha et al., 2013, Sivajothi et al., 2015) and may be due to the tissue breakdown, particularly liver tissue damage that accompanies trypanosomiasis (Ekanem and Yusuf, 2005, Nwoha et al., 2013). In agreement with our results, Sakran et al., 2014, Raish et al., 2016 proved that LSSE has the ability to reduce hepatotoxicity by limiting damage to the liver cells, with a significant improvement and normalizing of the liver enzyme levels. They attributed the improvement of the liver functions to the presence of isoflavonoids and glycosilated phenols (Aranda et al., 2007, Sakran et al., 2014). The histological complications of Trypanosoma infection for the liver range from mild to moderate degenerative changes, including granular to vacuolar degeneration, degeneration in the original polyhedral shape of hepatocytes and congestion of the central vein and sinusoids with blood cells and clumps of trypanosomes, all of which are noticeable in our histological findings (Biswas et al., 2001, Bal et al., 2012, Ghaffar etal., 2017). These pathological changes are likely to be due to the multiplication of trypanosomes, which is accompanied by increased oxygen consumption, provoking a hypoxic state and consequent degenerative changes (Bal et al., 2012, Ghaffar etal., 2017). Additionally, some authors have attributed these pathological changes to toxins liberated from T. evansi (Darganetes et al., 2005, Ghaffar etal., 2017). Upon treatment with LSSE, however, a significant improvement in the condition of the liver tissues was observed, giving further support for the efficacy of LSSE in respect to T. evansi infection. Similar ameliorative effects of LSSE on paracetamol and d-galactosamine/lipopolysaccharide induced hepatotoxicity that have been previously reported by Sakran et al., 2014, Raish et al., 2016 respectively. They established that LSEE mitigates liver histopathological conditions; an effect they attributed to its biologically active ingredients such as isoflavonoids and glucosinolates which have the ability to reducing oxidative stress, inflammation, and apoptosis in the liver (Sakran et al., 2014, Raish et al., 2016).
In conclusion, our results demonstrate that LSSE have trypanocidal activities with more effect evident for intraperitoneal injection than for oral administration. Therefore, further screening of the toxicology, and isolation and identification of the bioactive compounds of LSSE may reveal that the seeds of this plant could be exploited for the development of a new generation of trypanocidal agents.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgements
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adamu M., Boonkaewwan C. Effect of Lepidium sativum L. (Garden Cress) seed and its extract on experimental Eimeria tenella infection in broiler chickens. Kasetsart J. (Nat. Sci.) 2014;48:28–37. [Google Scholar]
- Al-Khalifa M.S., Hussein H.S., Diab F.M., Khalil G.M. Blood parasites of livestock in certain regions in Saudi Arabia. Saudi J. Biol. Sci. 2009;16:63–67. doi: 10.1016/j.sjbs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda E., García-Romera I., Ocampo J.A., Carbone V., Mari A., Malorni A., Sannino F., De Martino A., Capasso R. Chemical characterization and effects on Lepidium sativum of the native and bioremediated components of dry olive mill residue. Chemosphere. 2007;69:229–239. doi: 10.1016/j.chemosphere.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Azaizeh H., Saad B., Khalil K., Said O. The state of the art of traditional Arab herbal medicine in the eastern region of the Mediterranean: a review. Evid. Based Complem. Alternat. Med. 2006;3:229–235. doi: 10.1093/ecam/nel034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami S., Razi Jalali M.H., Ramezani Z., Pourmehdi Boroujeni M., Toeimepour F. In vitro scolicidal effect of Lepidium sativum essential oil. J. Ardabil Univ. Med. Sci. 2016;15:395–403. [Google Scholar]
- Bal M.S., Singla L.D., Kumar H., Vasudev A., Gupta K., Juy P.D. Pathological studies on experimental Trypanosoma evansi infection in Swiss albino mice. J. Parasit. Dis. 2012;36:260–264. doi: 10.1007/s12639-012-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera M.D., Souza C.F., Boligon A.A., Grando T.H., De Sá M.F., Da Silva A.S., Stefani L.M., Baldisserotto B., Monteiro S.G. Solving the challenge of the blood-brain barrier to treat infections caused by Trypanosoma evansi: evaluation of nerolidol-loaded nanospheres in mice. Parasitology. 2017;144:1543–1550. doi: 10.1017/S003118201700110X. [DOI] [PubMed] [Google Scholar]
- Biswas D., Choudhury A., Misra K.K. Histopathology of Trypanosoma (Trypanozoon) evansi infection in bandicoot rat. I. Visceral organs. Exp. Parasitol. 2001;99:148–159. doi: 10.1006/expr.2001.4664. [DOI] [PubMed] [Google Scholar]
- Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- Da Silva C.F., Batista M.M., Batista Dd.a.G., de Souza E.M., da Silva P.B., de Oliveira G.M., Meuser A.S., Shareef A.R., Boykin D.W., Soeiro Mde N. In vitro and in vivo studies of the trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2008;52:3307–3314. doi: 10.1128/AAC.00038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darganetes A.P., Compbell R.S.F., Copeman D.B., Reid S.A. Experimental Trypanosoma evansi infection in the goat. J. Comp. Pathol. 2005;133:267–276. doi: 10.1016/j.jcpa.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Delaquis P.J., Stanich K., Girard B., Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002;74:101–109. doi: 10.1016/s0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]
- Desquesnes M., Dargantes A., Lai D.H., Lun Z.R., Holzmuller P., Jittapalapong S. Trypanosoma evansi and surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. Biomed. Res. Int. 2013;2013:321237. doi: 10.1155/2013/321237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyary H.O., Arifah A.K., Sharma R.S., Rasedee A., Aspollah M.S., Zakaria Z.A., Zuraini A., Somchit M.N. In vivo antitrypanosomal activity of Garcinia hombroniana aqueous extract. Res. Vet. Sci. 2015;100:226–231. doi: 10.1016/j.rvsc.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Ekanem J.T., Yusuf O.K. Activities of alkaline phosphatase, glutamate oxaloacetate transaminase and glutamate pyruvate transaminase in liver and serum of Trypanosoma brucei-infected rats treated with honey. Biokemistri. 2005;17:185–191. [Google Scholar]
- Ezeokonkwo R.C., Ezeh I.O., Onunkwo J.I., Onyenwe I.W., Iheagwam C.N., Agu W.E. Comparative serum biochemical changes in mongrel dogs following single and mixed infections of Trypanosoma congolense and Trypanosoma brucei brucei. Vet. Parasitol. 2012;190:56–61. doi: 10.1016/j.vetpar.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Ghaffar M.A., El-Melegy M., Afifi A.F., El-Aswad B.W., El-Kady N., Atia A.F. The histopathological effects of Trypanosoma evansi on experimentally infected mice. Menoufa Med. J. 2017;29:868–873. [Google Scholar]
- Gilani A.H., Rehman N.U., Mehmood M.H., Alkharfy K.M. Species differences in the antidiarrheal and antispasmodic activities of Lepidium sativum and insight into underlying mechanisms. Phytother. Res. 2013;27:1086–1094. doi: 10.1002/ptr.4819. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Corbera J.A., Juste M.C., Doreste F., Morales I. An outbreak of abortions and high neonatal mortality associated with Trypanosoma evansi infection in dromedary camels in the Canary Islands. Vet. Parasitol. 2005;130:163–168. doi: 10.1016/j.vetpar.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Habila N., Humphrey N.C., Abel A.S. Trypanocidal potentials of Azadirachta indica seeds against Trypanosoma evansi. Vet. Parasitol. 2011;180:173–178. doi: 10.1016/j.vetpar.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Haroun E.M., Magzoub M., Mahmoud O.M., Qarawi A.A., Hawas A., Omer O.H. Some clinicopathological aspects of experimental Trypanosoma evansi infection in Najdi camels (Camelus dromedarius) J. Camel Pract. Res. 2000;7:107–108. [Google Scholar]
- Mahassni S.H., Khudauardi E.R. A pilot study: The effects of an aqueous extract of Lepidium sativum seeds on levels of immune cells and body and organs weights in Mice. J. Ayurvedic. Herb. Med. 2017;3:27–32. [Google Scholar]
- Molefe N.I., Yamasaki S., Macalanda A.M.C., Suganuma K., Watanabe K., Xuan X., Inoue N. Oral administration of azithromycin ameliorates trypanosomosis in Trypanosoma congolense-infected mice. Parasitol. Res. 2017;16:2407–2415. doi: 10.1007/s00436-017-5542-7. [DOI] [PubMed] [Google Scholar]
- Nwoha R.I.O., Eze I.O., Anene B.M. Serum biochemical and liver enzymes changes in dogs with single and conjunct experimental infections of Trypanosoma brucei and Ancylostoma caninum. Afr. J. Biotechnol. 2013;12:618–624. [Google Scholar]
- Omer O.H., Mousa H.M., Al-Wabel N. Study on the antioxidant status of rats experimentally infected with Trypanosoma evansi. Vet. Parasitol. 2007;145:142–145. doi: 10.1016/j.vetpar.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Powar R.M., Shegokar V.R., Joshi P.P., Dani V.S., Tankhiwale N.S., Truc P., Jannin J., Bhargava A. A rare case of human trypanosomiasis caused by Trypanosoma evansi. Indian J. Med. Microbiol. 2006;24:72–74. doi: 10.4103/0255-0857.19904. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Mossa J.S., Al-Said M.S., Al-Yahya M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia. 2004;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Raish M., Ahmad A., Alkharfy K.M., Ahamad S.R., Mohsin K., Al-Jenoobi F.I., Al-Mohizea A.M., Ansari M.A. Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/lipopolysaccharide induced hepatotoxicity in animal model. BMC Complem. Altern. Med. 2016;16:501. doi: 10.1186/s12906-016-1483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakran M., Selim Y., Zidan N. A new isoflavonoid from seeds of Lepidium sativum L. and its protective effect on hepatotoxicity induced by paracetamol in male rats. Molecules. 2014;19:15440–15451. doi: 10.3390/molecules191015440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivajothi S., Rayulu V.C., Sudhakara B.R. Haematological and biochemical changes in experimental Trypanosoma evansi infection in rabbits. J. Parasit. Dis. 2015;39:216–220. doi: 10.1007/s12639-013-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasite Vec. 2010;3:15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo V.O., Olaniyi M.O., Ogunsanmi A.O. Comparative plasma biochemical changes and susceptibility of erythrocytes to in vitro peroxidation during experimental Trypanosoma congolense and Trypanosoma brucei infection in sheep. J. Vet. Med. 2003;58:30–33. [Google Scholar]
- Tesfaye A., Terefe G., Giday M., Shibeshi W. In vivo anti-trypanosomal activity of the leaf extracts of Albizia Schimperiana (Fabaceae) against Trypanosoma congolense infection in mice. Clin. Exp. Pharmacol. 2015;5:2161–11459. [Google Scholar]
- Usmanghani K., Saeed A., Alam M.T. University of Karachi Press; Karachi: 1997. Indusyunic Medicine; pp. 276–277. [Google Scholar]