Abstract
Purpose of Review
Asthma is a chronic respiratory condition with increasing domestic and worldwide prevalence that burdens individuals and the healthcare system with high costs associated with long-term treatments and acute emergency room (ER) visits. It can be triggered by ambient microbes, including bacteria, viruses, and fungi. In this review, we examine the outcomes of asthma patients in relation to environmental exposures to ambient microbe products, focusing on whether exposure leads to asthma development from birth to childhood and if particular microbes are associated with worsened asthma exacerbations.
Recent Findings
Bacterial endotoxin is more prominent in homes with pets and may cause cytokine cascades that lead to asthma exacerbation. However, some studies have demonstrated a protective effect with early exposure. Patients with positive Aspergillus skin testing are more prone to moderate-severe or severe-uncontrolled asthma. Fungal sensitization is also associated with earlier onset of asthma and demonstrates a dose-dependent relationship of symptom severity and duration. Among viruses, rhinovirus has the greatest association with decreased lung function, severe asthma, and asthma-related hospital admissions. Distribution of microbial products and associated asthma symptoms depends on the geographical climate. Genetic variations among individuals also mitigate the effects of microbial products on asthma development and symptom severity.
Summary
Microbial products of bacteria, fungi, and viruses are associated with the development of asthma, more severe asthma symptoms, and worse outcomes. However, some early exposure studies have also demonstrated a protective effect. Bacterial and fungal products are related to decreased lung function and earlier onset of asthma. Viral products are related to asthma-associated hospital admissions; and the climate and patient genetics can also temper or intensify the relationships between microbial products, asthma development, and asthma symptom severity. Further research should focus on the effects of early microbe exposure and its interaction with human immune systems and asthma-related outcomes.
Keywords: Early microbial exposure, Asthma outcomes, Allergen sensitivity, Bacteria, Fungi, Virus
Introduction
Every day, 10 people in the USA die from asthma [1]. In 2017 alone, there were 3,564 asthma-related deaths [1, 2]. Within the USA, the prevalence of asthma is 1 in 13 people, producing annual economic costs of over $81.9 billion, related to missed school and work, medical costs, and asthma-related mortality [1–3]. Ambient microbes have been shown to trigger asthma attacks [4–6]. The most common microbes are bacteria, viruses, and fungi [7]. Though the literature has clearly demonstrated that microbes and allergens can trigger asthma exacerbations, controversy still remains whether or not microbes promote the development of asthma with early-life exposure or if they have a preventative effect against asthma symptoms in an individual with established disease [4–6]. Many longitudinal studies have demonstrated that early exposure to ambient microbial products increases the risk of the development of asthma [8•, 9, 10, 11•]. Whereas, other studies, particularly related to human contact with animal microbes, have shown protective effects against the development of asthma [8•, 12, 13]. With over 1.8 million asthma-related ER visits each year, which may be potentiated by exposure to various ambient microbial products in the environment, early-life exposure to microbial products and its effect on the respiratory system later in childhood warrants further investigation [2, 6, 14, 15].
Purpose
The purpose of this literature review was to specifically examine asthma outcomes related to environmental exposures to microbial products, pertaining to endotoxin from bacteria—(1,3)-β-d-glucan and ergosterol from fungus, and common viruses associated with worsening asthma morbidity (rhinovirus, respiratory syncytial virus (RSV), enterovirus, and the influenza virus) during infancy, and to assess the risk of asthma development later in childhood [15–18] (see Table 1).
Table 1.
Microbes and associated microbial products related to selected studies
| Microbe | Microbial product/ type |
|---|---|
| Bacteria | Endotoxin |
| Fungi | (1,3)-β-d-glucan |
| Ergosterol | |
| Virus | Human rhinovirus (HRV) |
| Respiratory syncytial virus (RSV) | |
| Enterovirus | |
| Influenza virus |
While other reviews have focused on common routes of exposure and the concentration of microbial products (endotoxin, fungal spores, virus, etc.) in urban vs. suburban regions [19, 20], this review investigates if the exposure to microbial products during infancy leads to the development of asthma during childhood, and whether particular microbes have a higher severity for triggering asthma exacerbations over others. Primary questions for this review consisted of the following: What microbes are associated with asthma severity and outcomes? What microbial products are known for triggering asthma symptoms and/or exacerbations? Does the exposure of microbial products in the environment lead to the development of asthma?
For this review, we selected original manuscripts in English, published in peer-review journals on the basis of bacterial, fungal, and viral products within the environmental matrix with children exposed to these microbial products. Thus, we selected articles examining health outcomes for the development of asthma related to microbial product exposure during the time of infancy until childhood. However, we also highlighted factors in the literature that contribute to microbial exposure and their effect on asthma for people with an established diagnosis of asthma. Only journal articles within the last 5 years were chosen; however, there were no restrictions pertaining to specific populations or geographical locations.
Exclusion criteria consisted of animal studies, investigative procedures using only in vitro methods, studies not translated into English, articles focusing on the measurement of microbial products without their effect on asthma outcomes, and articles not assessing microbial exposure from the time of infancy. With this thought process in mind, the following word criteria was applied using PubMed as the primary search engine:
(“Asthma”[Mesh] OR asthma[tiab] OR asthmatic*[tiab]) AND (“Microbiota”[Mesh] OR “Bacteria”[Mesh] OR “Antigens, Bacterial”[Mesh] OR “Bacterial Toxins”[Mesh] OR “Enterotoxins”[Mesh] OR “Fungi”[Mesh] OR “Mycotoxins”[Mesh] OR “Viruses”[Mesh] OR bacteria[tiab] OR bacterial[tiab] OR endotoxin*[tiab] OR enterotoxin*[tiab] OR fungal[tiab] OR fungi[tiab] OR fungus[tiab] OR lipopolysaccharides[tiab] OR microbial [tiab] OR microbiome*[tiab] OR microbiota*[tiab] OR mold[tiab] OR molds[tiab] OR mycotoxin*[tiab] OR peptidoglycan[tiab] OR staph[tiab] OR Staphylococcus[tiab] OR teichoic acid*[tiab] OR virus*[tiab] OR viral[tiab]) NOT (“Animals”[Mesh] NOT “Humans”[Mesh]) AND (“2014/01/01”[PDat] : “2019/12/31”[PDat]) AND English[lang].
Results
The search returned a total of 2999 articles. After the removal of 2924 studies, consisting of reviews, duplicates, mouse studies, and irrelevant articles to asthma health outcomes, this then left 76 studies. 65 Studies were pertaining to adults, which left a total of N = 11 studies (see Fig. 1).
Fig. 1.
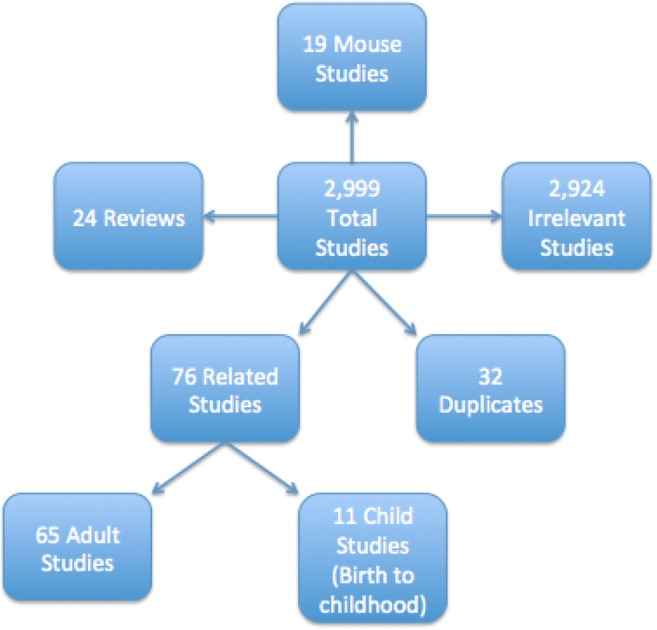
Selection criteria for related studies
Characteristics of Included Studies
The majority of the studies had a prospective cohort design (9/11), while the other two studies were retrospective cohort designs. Environmental sampling consisted of different locations in participants’ homes (living room, kitchen, bedroom, mattress), outside air, and settled dust in classrooms. All of the studies included examined asthma health outcomes related to ambient exposure to bacterial, fungal, and viral microbial products. Considering bacterial products and asthma outcomes, the literature has discussed endotoxin, β-glucan, and lipopolysaccharide (LPS). Of these microbial products, endotoxin has been well established with asthma outcomes [5, 14, 21, 22].
Endotoxin
When bacteria are destroyed or lysed, LPS is dispersed into the environment or blood as endotoxin, which can lead to a cascade of inflammatory cytokines (IL-6, IL-12, IL1β, and TNF-α) and exacerbation of asthma symptoms [22, 23]. The main route of contact to endotoxin is via inhalation, which has been demonstrated in multiple studies [5, 14, 21, 22]. Of the 11 studies selected for review, there were two that specifically discussed endotoxin exposure from infancy [8•, 24••] (see Table 2). Tischer et al. conducted a prospective longitudinal study examining whether early exposure to microbial products in dust was associated with allergy and asthma later in childhood for children in suburban areas using the following three birth cohort studies for children born between 1996 and 1999: Prevention and Incidence of Asthma and Mite Allergy (PIAMA) from the Netherlands, Infancia y Medio Ambiente- Childhood and Environment (INMA) from Spain, and Influence of Life-Style factors (LISAplus) on the development of the Immune System and Allergies in East and West Germany [8•]. The researchers collected dust samples from living room floors shortly after children were born and found that high endotoxin levels were positively associated with asthma at 6 years of age in the PIAMA cohort (adjusted OR = 1.96; 95% CI (1.07–3.58)), but there was no association with the LISAplus cohort [8•]. However, there was an inverse relationship between endotoxin and asthma in the INMA cohort from Spain at age 10 (OR = 0.39; 95% CI (0.16–0.94)). The INMA cohort from Spain had the highest number of pets in terms of dogs and cats with exposure from 0 to 1 year of age, compared to the other groups [8•]. Whereas, in a study conducted by O’Connor et al. [24••], dust samples were collected from children’s mattresses, bedroom floors, and living room floors; and showed no association between endotoxin nor the fungal membrane lipid ergosterol in the development of asthma with exposure from birth to 7 years of age. Previous research has shown that endotoxin levels tend to be higher in homes with a pet such as a cat or dog in addition to homes of families that are low-income and/or may be in community projects that contain cockroaches [5, 31]. Similar results were seen in children by a study conducted by Mendy et al. [32] who found that overall for people who had a cat or dog, endotoxin levels were higher in the household with a pet and was associated with wheeze (OR = 1.30; 95% CI (1.04–1.62)) but not with asthma [32]. Co-exposure with dog and cat allergens modified the association with asthma, increasing the risk when exposed to endotoxin (OR = 2.00; 95% CI (1.04–3.83)) [32]. However, the O’Connor et al. [24••] study found that exposure of a pet cat or dog from the time of infancy decreased the chances of developing asthma later in childhood (OR = 0.71, 95% CI (0.51–1.00) and OR = 0.78, 95% CI (0.56–1.09), respectively). For people with an established asthma diagnosis, regardless of allergen sensitization, Thorne et al. [5] demonstrated that endotoxin exposure is associated with wheeze with exercise, office or ER visits, and use of prescription medication for wheezing [5]. Studies have indicated that lung function and capacity can be decreased due to continuous exposure to endotoxin in patients with atopic asthma [22, 33].
Table 2.
Select studies on relationship between microbial products and asthma outcomes
| Reference | Population | Design | Focus | Investigation and methods | Outcomes | Comments/limitations |
|---|---|---|---|---|---|---|
| Tischer et al. (2015) [8•] | 3 European birth cohorts: PIAMA (Dutch, n = 553); INMA (Spanish, n = 481); and LISAplus (German, n = 395) | Prospective cohort study | Endotoxin/beta-glucan and development of asthma | Endotoxin, beta-d-glucan, and LPS were measured in dust from living room floors shortly after birth of patients. Current asthma at age 6 and 10, and ever having asthma in children up to age 10 were assessed by parental questionnaires. IgE levels at age 8 (PIAMA) and age 10 (LiSAplus) were analyzed with logistic regression |
• Higher endotoxin levels were associated with current asthma at age 6 years of age in PIAMA cohort • Higher endotoxin levels were inversely related with ever having asthma up to age 10 in INMA cohort • No associations were observed with atopic sensitization in all cohorts. |
Unknown whether a single bio-contaminant measurement is able to represent the overall exposure within a home, as the microbial components in the house dust samples may change over time. Unable to determine the effect of exposures on asthma beyond age 6 due to limited data |
| Tischer et al. (2015) [25] |
2441 children from birth to age 10 (Munich and Leipzig, Germany) |
Prospective cohort study | Association of animal fur and endotoxin with asthma | Parental questionnaires surveyed exposure to animal fur, cofactors, and health outcomes of children up to age 10. Information on IgE aeroallergens and cytokine-producing T cells were obtained |
• 55% of children slept with animal fur • Sleeping with animal fur was inversely associated with early wheezes at age 4 and current asthma at age 6 • Sleeping on animal fur during the first 3 months of life was associated with persistently stimulated interferon-γ response until age 3 • Animal fur exposure might act as an immune system stimulant and offer protective mechanisms against asthma and allergy, as observed in farm/rural environments |
Not able to determine the microbial profile of the animal fur nor the intensity of the exposure. Lifestyle factors also play a role in developing asthma |
| Bonnelykke et al. (2015) [10] | 313 children with episodes of respiratory symptoms during first 3 years of life (Copenhagen, Denmark) | Prospective cohort study | Episodes of respiratory symptoms in the first 3 years of life | Documentation of lung symptoms in symptom diaries. Identification of RSV; rhinoviruses; other picornaviruses; coronaviruses 229E and OC43; parainfluenza viruses 1–3; influenza viruses Ah1, Ah3, and B; human metapneumovirus; adenoviruses; bocaviruses; Streptococcus pneumonia; Haemophilus influenza; and Moraxella catarrhalis from nasopharyngeal and hypopharngeal aspirates |
• Viruses were identified in 65% of samples • Bacteria were identified in 87% of samples • Asthma prevalence by age 7 was 15% • After adjusting for total respiratory episodes and number of episodes per pathogen, only the total number of episodes were significantly associated with asthma development • Risk factor for development is asthma is the number of respiratory episodes, not the specific viral trigger |
Assumed that children who did not present to clinic did not have clinically significant respiratory episodes |
| Teo et al. (2015) [26] | 234 children’s nasopharynx microbiomes during first year of life (Perth, Australia) | Prospective cohort study | Microbiomes in relation to acute respiratory illnesses (ARI) and development of asthma | Nasopharyngeal samples collected during episodes of ARI were sequenced to identify different types of bacteria |
• Staphylococcus was the dominant colonizing bacteria in the early healthy NP microbiomes, but its presence declined rapidly with age • Streptococcus was associated with higher severity of ARI vs. other bacteria or RSV • Early asymptomatic colonization with Streptococcus increases risk of asthma • Any febrile lower respiratory tract infection and human rhinovirus C-positive lower respiratory tract infection were risk factors for developing chronic wheeze |
Unable to sample nasomicrobiome while in the womb |
| den Hollander et al. (2016) [27] | 3797 children at 6 years of age, and their mothers during mid-pregnancy (Rotterdam, The Netherlands) | Prospective cohort study | Relationship between H. pylori and asthma | Measured anti-H. pylori and anti-CagA antibodies in serum of children and their mothers during mid-pregnancy. Asthma or related conditions were reported for children at age 6 |
• Colonization of a European child with a CagA-negative-H. pylori at age 6 was associated with increased prevalence of asthma • Positive association for asthma with H. pylori-positive child and H. pylori-negative mother • European child colonized with CagA-negative-H. pylori strain at age 6 had increased prevalence of asthma • Higher prevalence of recent asthma, but not of wheezing, eczema or inhalant allergy, was observed in H. pylori-positive children compared with H. pylori-negative children |
Study population were of higher socioeconomic status, from European ethnic background, fewer adverse lifestyle factors—limits generalizability |
| Lu et al. (2016) [28] | 343 children aged 3–24 months with their 1st or 2nd episode of severe RSV bronchiolitis (multiple global sites) | Prospective longitudinal observation study | Biomarkers associated with asthma or RSV and relationship with asthma/atopic disorders later in childhood | Surveys and blood samples were collected at baseline and at 6 years of age for biomarker measurement |
• Prevalence of asthma in children at 6 years of age was 6.1% • Factors predictive of asthma at 6 years of age: male gender, relative with asthma, RAST positivity for dog dander • Factors predictive for atopic disorders: allergic rhinitis, relative with asthma, IL-5, IL-16, IL-18 • Factors predictive of chronic asthma therapy: asthma diagnosis before enrollment, living in Europe or Africa • Among patients with RSV bronchiolitis, hereditary factors and RSV bronchiolitis severity were predictors of asthma and atopic disorders at 6 years of age |
No gold standard used for diagnosis of asthma and comparing sensitivity and specificity. Diagnosis of asthma was based on parental identification of a wheeze in the child vs. physician diagnosis |
| Lukkarinen et al. (2016) [11•] | 127 steroid-naïve children with first severe wheezing episode requiring hospitalization or emergency room visit (Turku, Finland) | Prospective cohort study | Prevalence of asthma, and risk factors for asthma | Nasopharyngeal aspirates were collected and analyzed for viral diagnostics; serum IgE and eosinophil counts were analyzed |
• Factors predictive of atopic asthma at school age: sensitization, eczema, and rhinovirus etiology at the first severe wheezing episode • Factors predictive of non-atopic asthma: RSV/rhinovirus-negative etiology, age less than 12 months, and parental smoking • Different asthma phenotypes can be predicted using clinical markers at the time of first severe wheezing episode • Virus-testing and atopic status can be assessed in early episodes of severe wheezing to determine patients at high risk for asthma and distinguish the risk between asthma phenotypes |
Sample size was small after excluding steroid-treated patients and rhinovirus typing was not done |
| Rubner et al. (2017) [9] | 217 children (Madison, WI) | Prospective cohort study | Associations between viral wheezing illnesses, presence and pattern of aeroallergen sensitization, and asthma diagnosis at age 13 |
Nasopharyngeal samples were collected at scheduled visits and during times of acute respiratory illnesses. Samples were analyzed for viruses. Allergen-specific IgE levels were measured |
• After adjustment of viral etiologies, wheezing with rhinovirus (not RSV) was associated with asthma at age 13 • 65% of children sensitized by aeroallergen by age 1 had asthma at age 13 (compared to 40% of children sensitized at age 5) • Asthma at age 13 was associated with wheezing illnesses with rhinovirus and aeroallergen sensitization in early life |
Enrollment criterion included only patients at higher risk for the development of asthma based on parental history of atopy and/or asthma. COAST cohort is comprised primarily of nonminority, suburban children, and the role of viral infections and sensitization may differ in other populations |
| Homaira et al. (2017) [29] | 847,516 children ages ≤ 2 born between 2000 and 2010 (New South Wales, Australia) | Retrospective cohort study | Impact of RSV on development of asthma and asthma hospitalizations | Population-based administrative data was used to investigate relationship of RSV hospitalization and asthma hospitalization |
• 4% of subjects had at least 1 occurrence of RSV hospitalization before age 2, of which 7.5% also had an occurrence of asthma hospitalization after age 2 • RSV-related hospitalizations in ages ≤ 2 is associated with subsequent asthma-related hospitalizations |
Study performed using administrative data; did not include information for atopic predisposition and risk of subsequent asthma |
| Kitsantas and Nirmalrag (2018) [30] | 1542 children born at term with no medical issues followed until age 6 (USA) | Retrospective cohort study | Association between RSV in early infancy and asthma or respiratory allergies and age 6 | Descriptive statistics and logistic regression used to analyze associations between RSV infection during infancy with various children’s health conditions |
• Patients with RSV infections as infants had significant increased risk of developing asthma or respiratory allergy by age 6 • Risk was even greater in patients who had family history of asthma or respiratory allergy |
Lacked underrepresented populations. No power analysis. RSV reported by mother only and not validated by clinical record |
| O’Connor et al. (2018) [24••] | 442 inner-city children collectively, followed from birth to age 7 in Boston, Baltimore, St. Louis and New York City | Prospective cohort study | Assessment of the risk of asthma development with early exposure to allergens (cat and dog dander, cockroach, mouse) and microbe products (endotoxin and ergosterol) by age 7 | Dust samples were taken from children’s beds, bedroom floors, and living room floors. Maternal questionnaires about depression, stress and smoking were administered prenatally and annually after the child’s birth with follow-up every 3 months up to age 7. Relationships of environmental exposures, perinatal, demographic, and family factors were assessed by logistic regression |
• Higher allergen exposure at 3 months associated with lower risk of asthma development at age 7. • Maternal history of asthma and depression were associated within higher risk of asthma by age 7. • Bacterial species Kocuria, Bifidobacterium, Alloiococcus, and Acinetobacter were found in homes of children without asthma, but not in homes of children with asthma. • Endotoxin and ergosterol were not found to be associated with the development of asthma |
Gastrointestinal and airway microbiome were not measured during early childhood |
Lipopolysaccharide has also been shown to promote eosinophil airway inflammation in patients with asthma despite maintenance treatment with inhaled corticosteroids (ICS) [34].
Besides having a pet, there are other factors that could have contributed to the varying results between the cohorts within the Tischer et al.’s study. The climate also plays a role in the concentration of endotoxin in the surrounding environment [14, 31]. Researchers have found a positive correlation between log10-endotoxin and current asthma (OR = 1.56, 95% CI (1.11–2.18), p = 0.046) for hot-humid regions and a higher incidence of exercise-induced wheeze (OR = 1.48, 95% CI (1.22–1.80), p = 0.009) for subarctic/very cold/cold regions [31]. High levels of endotoxin, regardless of being inside or outside the home environment, are associated with severe asthma [14, 35]. A study conducted in Japan by Khan and colleagues showed that asthma-related visits to the ER for patients 15-years-old or older was highest during the seasons of autumn and spring, when the environmental endotoxin was highest in the air [14]. Oluwole et al. [35] showed that for 7 to 17-year-olds, high concentrations of endotoxin within households, including mattresses, are associated with an elevated risk of moderate or severe asthma (adjusted OR = 11.40, 95% CI (1.45–89.43)). Tischer et al. [8•] also supported these results for children from infancy to 6 years of age. In addition, these findings are also in accordance with previous investigations in the early 2000s [36–39].
Non-environmental patient characteristics can also impact the development or severity of asthma. Mendy et al.’s [40] study determined that the combination of high levels of endotoxin exposure and low vitamin D levels are associated with an asthma diagnosis or recurrent wheeze (OR = 1.88, 95% CI (1.33–2.66), p = 0.02), current asthma (OR = 1.97, 95% CI (1.09–2.23), p = 0.03), wheeze over the last 12 months (OR = 1.72, 95% CI (1.10–3.7), p = 0.002), and recurrent wheeze (OR = 1.97, 95% CI (1.0–4.0), p < 0.001) [40]. Nevertheless, this study was conducted in adults and the effect from infancy to childhood, regarding vitamin D levels in combination with endotoxin exposure, would need to be assessed. No studies on fungal spore exposure were found for the time period from birth to childhood to assess the development of asthma.
Fungi
Fungal exposure plays a vital role in asthma health outcomes. For the Tischer et al.’s [8•] study, no correlations were found between the fungal product (1,3)-β-d-glucan and the PIAMA and LISAplus studies [8•]. (1,3)-β-d-glucan was not evaluated for in the INMA cohort study [8•]. Sensitization to fungal antigens is a key component to understanding asthma severity. Chopra and colleagues showed that asthma patients with skin reactivity to the Aspergillus skin test (AST) had more cases of moderate and severe asthma versus nonreactive AST patients (98.6% vs. 3.2%, p < 0.001) in a cohort of 282 adult asthma patients over a 1-year period. Sensitization to Aspergillus has been found to have a more significant impact on asthma outcomes compared to other species of fungus [41, 42]. For instance, Vincent et al. [41] demonstrated that adult asthma patients who were sensitized to Penicillium chrysogenum, Cladosporium cladosporiodes, Cladosporium sphaerospermum, or Penicillium brevicompactum were comparable to the control group in having a lower risk of severe asthma compared with the Aspergillus fumigatus group (15.6% vs. 50%, OR = 5.4, 95% CI (1.0–29.06), p = 0.059) [41]. Masaki and colleagues’ study in Japan implicates a different species; their study investigated sensitization to Candida and found that out of 124 adults with asthma, more people had sensitization to Candida than Aspergillus (16 vs. 11%) [6]. The researchers also found that fungal sensitization leads to a higher rate of early-onset asthma (< 16 years of age) compared to those without sensitization (45% vs. 25%) and sensitization increases with age [6, 43]. However, further investigation is needed to examine early exposure of various fungal species during infancy and its effect on the development of asthma during childhood.
The fungal genus Alternaria has also provided more insight into fungal sensitization and asthma outcomes. Recently, Baxi and colleagues conducted a study in 37 inner-city schools in New England with school-aged children with asthma and observed that children sensitized to Alternaria had an association with asthma symptom days (OR = 3.61, 95% CI (1.34–9.76), p = 0.01); however, this did not apply to children not sensitized to Alternaria (OR = 1.04, 95% CI (0.72–1.49), p = 0.85) [44]. Researchers found that asthmatic children who were sensitized to Alternaria and exposed to high levels had 3.2 more symptom days per 2-week period when compared to asthmatic and sensitized children exposed to lower levels of Alternaria. This suggests a dose-dependent relationship between mold exposure and asthma severity [44]. This finding is supported by Oluwole et al. [45], who demonstrated that high mold levels in children’s play areas are associated with current asthma (adjusted OR = 3.0, 95% CI (1.11–8.0)). Furthermore, additional studies have demonstrated that sensitization to Alternaria increases with higher mold concentration exposure and may lead to a risk of hospitalization, especially in the urban setting [46–49].
Fungal exposure also impacts lung function [41]. Asthma patients with sensitization to Aspergillus fumigatus have been found to have higher total serum IgE levels and an increased degree of broncho-obstruction (as measured by FEV1/FVC), compared to asthmatic patients without sensitization [41, 50]. The impact of fungal exposure on lung function has been evaluated in adults; however, this has not been thoroughly evaluated in infants and young children.
The fungal product (1,3)-β-d-glucan has been shown to have a low association with severe asthma (OR = 0.55, 95% CI (0.24–1.26), p = 0.003) in children [51, 52]. Ergosterol has also demonstrated a low affinity for the development of asthma [52]. However, exposure to fungal spores has been shown by multiple studies in adults to be associated with asthma exacerbations; yet, there are very few studies involving children [53–55]. In a study analyzing the fungal microbiome in exhaled breath condensate (EBC) of patients with asthma in Italy, Carpagnano et al. [53] found that in 47 adults with asthma, 94% of the people were colonized in the EBC by Cladodosporium species, 21% by Alternaria, and 24% by Penicillium species [53]. Fungal colonization tended to be higher in asthmatics with severe and uncontrolled asthma with sensitization to fungal species such as Alternaria [53–55]. The differing levels of fungal species, with Cladosporium being the most abundant, and Alternaria and Penicillium in lower quantities, was also found in Manitoba, Canada, by Polyzoi and Polyzois, examining mold within the bedrooms and basements of 3424 school-aged children [56]. Cladosporium was the most common mold found in homes (98.2% of bedrooms and 97.8% of basements), followed by Alternaria (82.4% of bedrooms and 77% of basements), and Penicillium (35.4% of bedrooms and 48.8% of basements). In addition, visible mold in bedrooms and basements in participants’ homes were associated with persistent asthma and colds (16.8%, p < 0.0001). Other fungal studies conducted in Europe, Canada, and Australia have also found similar results [45, 57, 58]. Viral exposure also plays a critical role in the development of asthma and asthma morbidity [9, 11•].
Viruses
Rhinovirus
Two studies were found that were conducted on early-life exposure to rhinovirus and asthma outcomes: One by Rubner et al. [9] and another by Lukkarinen et al. [11•]. Rubner et al. [9] conducted a prospective cohort study with 217 children, examining early-life rhinovirus exposure from birth to 13 years of age. The researchers observed that wheezing with rhinovirus was associated with asthma at age 13 (OR = 3.3, 95% CI (1.5–7.1), p = 0.02) and sensitization to allergens increased with age [9]. FEV1% predicted has also been shown to be lower in asthmatic patients admitted to the hospital with an asthma exacerbation with concurrent rhinovirus infection, compared to children with asthma admitted for an exacerbation but negative for rhinovirus [29, 59].
Lukkarinen et al. [11•] performed a 7-year long prospective cohort study following 127 Finnish children from birth to 7 years of age and found that the risk factors for atopic asthma at study entry were sensitization to common allergens (dust, mold, etc.), and wheezing with rhinovirus infection (aOR 4.8, p < 0.014) [11•].
Zheng et al. [15] conducted one of the numerous studies that demonstrated that human rhinovirus (HRV) was the most common viral microbe to induce an asthma exacerbation in children [60–62]. Zheng and colleagues collected data from 143 inpatient children with asthma exacerbations, 131 outpatients, including 88 patients with asthma exacerbations, and 43 controls with stable asthma [15]. Researchers tested for common viruses including HRV, respiratory syncytial virus (RSV), parainfluenza virus type 3 (PIV3), influenza virus (IFV), human bocavirus (HB0V1), atadenovirus (ADV), human coronavirus (HCOV), and hepatitis E virus. Of all the viruses, HRV was the most prominent among inpatients with severe asthma exacerbations at 50.3% and HCOV was the least at 0% [15]. The researchers found three variants of the HRV: HRV-A, HRV-B, and HRV-C, with HRV-C being the most virulent for severe asthma exacerbations [15]. Other studies conducted in Japan, France, and Australia have also identified rhinovirus as the most common viral culprit for asthma exacerbations [28, 60–62]. White blood cells and neutrophil counts were significantly higher in patients with all HRV variants versus those who were negative for HRV (12.38 ± 5.13 vs. 10.77 ± 4.87 and 64.70 ± 20.27 vs. 56.44 ± 21.95, respectively) [15].
Thymic stromal lymphopoietin (TSLP), a cytokine that impacts T cell maturation, also plays a role in asthma severity in patients with viral illnesses. It has been demonstrated to have a positive outcome on patients during viral infections in patients with asthma [60]. Bjerregaard et al. [60] performed a study in Australia with 44 virus-positive adolescent and adult patients (including rhinovirus, human coronavirus, parainfluenza virus, and human metapneumovirus) and 44 controls. The researchers found that patients with TSLP expression had lower levels of sputum eosinophils, lower fractional exhaled nitric oxide, higher blood neutrophils, lower asthma control questionnaire scores, and higher FEV1 in comparison to patients with low TSLP expression levels [60]. Again, these types of studies are rare for examining variances in lung function and asthma development from birth to childhood.
Respiratory Syncytial Virus
In total, four studies were found on respiratory syncytial virus (RSV) that followed children from birth to childhood for the development of asthma [10, 28–30]. Two of the RSV studies showed that males from infancy to childhood have a high tendency of developing asthma after an infection, compared to females [28, 29] (see Table 2). Two out of the four studies were retrospective cohort studies and the other two were prospective cohort studies [10, 28–30]. One of the retrospective studies showed that peak time for RSV-related hospitalizations was during autumn [29]. The RSV studies showed a high risk for asthma with RSV infection and that the cytokines IL-6, IL-1β, and IL-8 are elevated during infection, which leads to respiratory inflammation [10, 28]. Kitsantas et al. [30] conducted a 6-year long study from birth to 6 years of age, examining the risk of the development of asthma by 6 years with early exposure of RSV infection during infancy. Results showed that exposure to RSV did increase the risk of asthma by age 6 (OR = 1.99, 95% CI (1.06–3.74)). However, children who had a parent with a history of asthma posed a higher risk for childhood asthma by age 6 (OR = 3.86, 95% CI (2.61–5.71)) [30]. A maternal history of asthma has also been demonstrated by O’Connor et al. [24••] to increase the likelihood of asthma in childhood by age 7 (OR = 1.79, 95% CI (1.18–2.74)).
Similar to what was discussed about fungi, asthma patients can also be colonized by viruses. Nguyen-Thi-Dieu et al. [63] conducted a cohort study in Hanoi, Vietnam, on 115 hospitalized children under the age of 15 with a diagnosis of asthma exacerbation and a control group of healthy children under the age of 15. Patients underwent a clinical examination, blood analysis for a cytokine profile, pediatric asthma score (PAS) for severity of asthma exacerbation, and nasopharyngeal aspirates to determine viral infection using real-time polymerase chain reaction (PCR). Of the 115 children diagnosed with an asthma exacerbation, there were 18.2% with a mild PAS, 37.4% with a moderate PAS, and 44% with a severe PAS [63]. Nguyen-Thi-Dieu and colleagues found that 54.8% of the asthmatic children were positive for HRV [63]. The high number of asthma patients with a severe PAS score (44%) was associated with a high number of children diagnosed with HRV [63]. The researchers also found that the percentage of leukocytes in asthmatic children with HRV was significantly higher, compared to children without HRV (76.2% vs. 59.6%, p < 0.05) [63]. No enterovirus longitudinal studies from birth to childhood were available, assessing asthma development; however, studies have been done in children with a diagnosis of asthma [64].
Enterovirus
Smith-Norowitz et al. [64] studied patients in Ireland, examining the levels of IgE and IgM antibodies (Ab) for enterovirus 71 with a mix of 77 children and adolescents, and found that asthmatic children have higher IgE Ab levels, compared to non-asthmatic children, who have higher IgM levels of Ab (p < 0.001). The levels of enterovirus IgE Ab were shown to increase with age especially among the asthmatic group [64].
Another study analyzed data on a 16-year longitudinal study with 36,935 children from infancy to 18 years of age in Taiwan and found that children were diagnosed with asthma within 2.77 years after an enterovirus infection [16]. There was also a higher incidence of asthma associated with enterovirus infection (HR = 1.65, 95% CI (1.60–1.71), p < 0.0001) [16]. Overall, the literature suggests that the rate of hospitalization due to enterovirus infection is low and does not increase asthma severity or length of hospital stay for asthma-related admissions [65, 66].
Influenza Virus
The influenza virus is a major cause of acute exacerbation of bronchial asthma (AEBA); however, rhinovirus has still been shown to be the number one viral cause of asthma exacerbations [67]. Yoshi et al. and other researchers have shown that obtaining the flu vaccine reduces AEBA morbidity significantly when compared to individuals who are unvaccinated for the influenza virus (20.5% vs. 0%), p = 0.047 [67, 68]. Influenza can worsen asthma morbidity; however, it has yet to be determined if there is a relationship pertaining to the development of asthma in association with an influenza infection [69]. Recent studies have found that certain bacteria play a role in preventative effects against the development of asthma.
Bacteria
Helicobacter pylori
Den Hollander et al. [27] performed a population-based prospective cohort study of pregnant women and their children in Rotterdam, Netherlands, and followed the children from pregnancy to 6 years of age for evaluation of asthma. Colonization of a European child with a cytotoxin-associated protein A (CagA)-negative-Helicobacter pylori (H. pylori) strain at age 6 was associated with an increased prevalence of asthma overall (OR = 2.11, 95% CI (1.23–3.60)) but was different for European children (OR = 3.64, 95% CI (1.97–6.73)) and significantly less for non-European children (OR = 0.52, 95% CI (0.14–1.89)) [11•]. There are also common bacteria within the environmental matrix that have been shown to have an effect on the development of asthma in children.
Staphylococcus
Teo et al. [26] found that early childhood nasopharynx (NP) colonization typically involved Staphylococcus or Corynebacterium, which was later replaced by Moraxella or Alloiococcus. Staphylococcus was the dominant colonizing bacteria in the early healthy NP microbiomes, but its presence declined rapidly with age. These researchers found that early asymptomatic colonization with Streptococcus increased the risk of developing asthma [26]. Most infants were initially colonized with Staphylococcus or Corynebacterium before stable colonization with Alloiococcus or Moraxella [26]. Transient incursions of Streptococcus, Moraxella, or Haemophilus marked virus-associated acute respiratory infections (ARIs) [26]. O’Connor et al. [24••] found supporting results in a study examining early-life exposure to environmental factors, including dust, fungal products, bacterial taxa; cockroach, mouse, and cat allergen; from infancy to 7 years of age. Staphylococcus, Haemophilus (Pasturellaceae), several Sphingomonas species members, and Corynebacterium were among the taxa found in children’s homes who had asthma [24••]. However, for children who did not have asthma, there was a prevalence of Alloiococcus (Aerobacteriaceae), Kocuria (Micrococcaceae), Acinetobacter (Moraxellaceae) species, and Bifidobacterium [24••]. More research is needed to investigate the mechanism of transition of bacteria from one species to another in the infant microbiome and how this may possibly contribute to the development of asthma. In addition to the microbiome, children’s genes affect how their bodies will react to their environment.
Genes and Asthma Health Outcomes
Like most illnesses, symptoms and severity can vary at the individual level based on unique genetic differences. Carnes et al. [22] found that there is a gene-by environment interaction for CD14 variant rs2569190 (pinteraction = 0.16) but not for the TLR4 variants rs4986790 and rs4986791. This research is also supported by an older study conducted by Kljaic-Bukvic et al. [70], who found that there are two genetic variants related to CD14 and endotoxin signaling that are highly related with asthma exacerbation hospital admissions. For CD14 single nucleotide polymorphism (SNP) rs5744455, carriers of the T-allele in children 5 to 18 years old had a decreased risk of repeated hospital asthma-related admissions, compared to homozygotes for the C-allele (OR = 0.42, 95% CI (0.25–0.88), p = 0.01) [70]. C-allele carriers were at lower risk of asthma-related hospitalizations in comparison with T-allele homozygotes (OR = 0.59, 95% CI (0.38–0.90), p = 0.01) [70].
A human interleukin 4 receptor alpha chain gene (IL4R) variant that results in a glutamine to arginine substitution at amino acid residue 576 (IL4Rα-Q576R polymorphism) has previously been associated with asthma diagnosis and severity [71]. Lai et al. [72] have demonstrated that there is a gene-by-environment interaction between the IL4Rα-Q576R polymorphism and environmental endotoxin exposure in school-aged children with asthma. Higher classroom endotoxin levels were associated with fewer asthma symptoms for children with the Q/Q genotype (OR = 0.76 [0.55–1.04]), an equivocal effect for the Q/R genotype (OR = 1.10 [0.78–1.55]), and increased asthma symptoms for the R/R genotype (OR = 1.34 [0.85–2.13]) [72]. In addition to genetics, exposure to animals, especially within the farm setting, has shown to play a role in asthma.
Exposure to Microbial Products Growing up on a Farm and Asthma Outcomes
The environment of a farm is a great example of exposure to all of the microbes and microbial products discussed in this review. Carnes et al. [22] found that high concentrations of endotoxin exposure at homes of people not born on a farm have a risk for the development of asthma (OR = 1.67, 95% CI (1.26–2.20), p = 0.05), compared to individuals who were born on a farm (OR = 1.18, 95% CI (1.02–1.37), p = 0.05). In addition, Lampi et al. [12] found that people born on a farm had a higher FEV1, compared to those not raised on a farm. The limitation here is that these participants in the Lampi study could be more physically fit having to do the chores of farm work, compared to people not living on a farm. Prior studies have attributed farm-specific protection from the development of asthma from endotoxin exposed in utero and during early childhood [13, 73, 74].
An older study conducted on Amish and Hutterite communities whom participate in farming activities has revealed two very different results [75, 76]. The Amish farm children of Indiana were found to have a lower prevalence of asthma and allergen sensitization (5.2% vs. 21.3% and 7.2% vs. 33.3%, respectively), compared to the Hutterite farm children of South Dakota [75, 76]. The difference between these two farming communities was that the Amish have constant direct contact with animals to do all of their farming traditionally, and the Hutterite community relies on machinery and maintains the animals in one localized area away from the community homes [75, 76]. This finding brings up a few questions. Why is early farm exposure to endotoxin related to protective effects against the development of asthma and asthma symptoms; whereas, non-farm house endotoxin exposure is generally associated with asthma and asthma exacerbations? Are there different microbes related to farm animals versus household pets, which are associated with increasing asthma symptoms [5, 31]? In a different study, Tischer et al. [25] examined the German LISAplus birth cohort and found that children who slept on animal fur during the first 3 months of life had higher exposure to endotoxin in their mattress, compared to children who did not sleep on fur. More importantly, the researchers found a decrease in the risk of asthma development by age 6 for children who slept on animal fur during the first 3 months of life (aOR = 0.56, 95%, CI (0.31–1.01). In addition, for TNF-α, IL–2, and IL–4 producing T cells, there were no significant changes found in blood levels associated with sleeping on animal fur the first 3 months of life [25].
Conclusion
This review has shed light on several different findings within the literature. Based on the information provided, sensitization within the non-farming community for bacterial, fungal, and viral products increases with IgE Ab expression and over time with age [43, 64, 77]. In terms of the fungal species, Aspergillus fumigatus has shown to induce one of the highest sensitizations for moderate/severe asthma [54, 78]. In addition, human colonization of the genera Cladosporium, Alternaria, and Penicillium have been associated with severe asthma and their ambient environmental concentration is similarly proportional in various parts of the world [41, 45, 53, 56–58]. For viruses, rhinovirus has been shown to be one of the most common triggers of a viral source for an asthma exacerbation [9, 11•, 15, 63, 79, 80]. Early exposure to endotoxin at a young age within the farm setting with direct animal contact may reduce the chances of developing asthma later in life [12, 22, 75, 76]. For children not living on a farm, growing up with a cat or dog may have a protective effect against asthma symptoms [8•, 24••]. The mechanism of this effect is yet to be understood and it appears that genetics plays a role in asthma severity especially among ethnic minorities [72].
Further Research
Further research is needed to investigate the difference in microbes between farm animals and household pets and how early exposure effects the human microbiome and the immune system’s response to inflammatory triggers. In addition, since studies have shown that people with severe asthma may be colonized with various genus of fungi, it would be worthwhile to further investigate the treatment of moderate/severe asthmatics colonized with fungi with other methods not associated with toxic side effects related to current medications for anti-fungals. In addition, more studies are needed examining the mechanism(s) for the transition of bacteria in the microbiome during childhood and how this may effect asthma outcomes. Biomarkers such as dimethylamine, a metabolite produced by bacteria within the gastrointestinal tract, may pose a clue as it has been demonstrated to decrease in concentration from higher to lower levels with the development of asthma in children [81]. Further advances are also being made with dupilumab, a monoclonal antibody that blocks a common receptor for interleukin-4 (IL-4) and interleukin-13 (IL-13), involved in type 2 inflammation [82]. In adult patients with atopic asthma, it has been shown to reduce severe asthma exacerbations [82]. This medication has recently been approved for children 12 years old and up for the treatment of severe asthma [83]. Continued exploration of the human microbiome and its interaction with the environment hopefully may lead to more discoveries.
Funding
Research reported in this publication is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Grant No: K24AI106822)
Compliance with Ethical Standards
All reported studies/experiments with human subjects performed by the authors have been previously published and compiled with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Allergies and the Environment
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.CDC. Most Recent National Asthma Data. 2017. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed 1 Sept 2019.
- 2.CDC. Asthma. 2019. Available from: https://www.cdc.gov/asthma/default.htm. Accessed 1 Sept 2019.
- 3.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Cox M, Liang Z, Brinkmann F, Cardenas PA, Duff R, Bhavsar P, Cookson W, Moffatt M, Chung KF. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One. 2016;11(4):e0152724. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorne PS, et al. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192(11):1287–1297. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masaki K, et al. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol. 2017;119(3):253–257. doi: 10.1016/j.anai.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Longo DL. Harrison’s principles of internal medicine. 18. New York: McGraw-Hill Companies; 2011. [Google Scholar]
- 8.Tischer C, et al. Early exposure to bio-contaminants and asthma up to 10 years of age: results of the HITEA study. Eur Respir J. 2015;45(2):328–337. doi: 10.1183/09031936.00060214. [DOI] [PubMed] [Google Scholar]
- 9.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Gern JE, Lemanske RF Jr Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139(2):501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnelykke K, et al. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136(1):81–86.e4. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukkarinen M, et al. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140(4):988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampi J, Koskela H, Hartikainen AL, Ramasamy A, Couto Alves A, Järvelin MR, Pekkanen J. Farm environment during infancy and lung function at the age of 31: a prospective birth cohort study in Finland. BMJ Open. 2015;5(7):e007350. doi: 10.1136/bmjopen-2014-007350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E, ALEX Study Team Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 14.Khan MS, Coulibaly S, Matsumoto T, Yano Y, Miura M, Nagasaka Y, Shima M, Yamagishi N, Wakabayashi K, Watanabe T. Association of airborne particles, protein, and endotoxin with emergency department visits for asthma in Kyoto, Japan. Environ Health Prev Med. 2018;23(1):41. doi: 10.1186/s12199-018-0731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng SY, Wang LL, Ren L, Luo J, Liao W, Liu EM. Epidemiological analysis and follow-up of human rhinovirus infection in children with asthma exacerbation. J Med Virol. 2018;90(2):219–228. doi: 10.1002/jmv.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YC, Tsai CS, Yang YH, Huang KY, Hsieh WC, Kuo TY, Chin-Hung Chen V, Wong J, Ponton L, Wang TN. Association between enterovirus infection and asthma in children: a 16-year nationwide population-based cohort study. Pediatr Infect Dis J. 2018;37(9):844–849. doi: 10.1097/INF.0000000000001918. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Perez JA, et al. EV-D68 infection in children with asthma exacerbation and pneumonia in Mexico City during 2014 autumn. Influenza Other Respir Viruses. 2016;10(3):154–160. doi: 10.1111/irv.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjerregaard A, et al. High fractional exhaled nitric oxide and sputum eosinophils are associated with an increased risk of future virus-induced exacerbations: a prospective cohort study. Clin Exp Allergy. 2017;47(8):1007–1013. doi: 10.1111/cea.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanchongkittiphon W, Gaffin JM, Phipatanakul W. The indoor environment and inner-city childhood asthma. Asian Pac J Allergy Immunol. 2014;32(2):103–110. [PMC free article] [PubMed] [Google Scholar]
- 20.Shamsollahi HR, Ghoochani M, Jaafari J, Moosavi A, Sillanpää M, Alimohammadi M. Environmental exposure to endotoxin and its health outcomes: a systematic review. Ecotoxicol Environ Saf. 2019;174:236–244. doi: 10.1016/j.ecoenv.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 21.Lai PS, et al. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest. 2015;148(5):1251–1258. doi: 10.1378/chest.15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carnes MU, et al. House dust endotoxin levels are associated with adult asthma in a U.S. farming population. Ann Am Thorac Soc. 2017;14(3):324–331. doi: 10.1513/AnnalsATS.201611-861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor GT, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2018;141(4):1468–1475. doi: 10.1016/j.jaci.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tischer C, Standl M, Lehmann I, Schaaf B, von Berg A, Heinrich J. Sleeping on animal fur is related to asthma outcomes in later childhood. Eur Respir J. 2015;46(1):107–114. doi: 10.1183/09031936.00204914. [DOI] [PubMed] [Google Scholar]
- 26.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, Bochkov YA, Grindle K, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Hollander WJ, et al. Helicobacter pylori in children with asthmatic conditions at school age, and their mothers. Aliment Pharmacol Ther. 2016;43(8):933–943. doi: 10.1111/apt.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu S, Hartert TV, Everard ML, Giezek H, Nelsen L, Mehta A, Patel H, Knorr B, Reiss TF. Predictors of asthma following severe respiratory syncytial virus (RSV) bronchiolitis in early childhood. Pediatr Pulmonol. 2016;51(12):1382–1392. doi: 10.1002/ppul.23461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homaira N, et al. Association between respiratory syncytial viral disease and the subsequent risk of the first episode of severe asthma in different subgroups of high-risk Australian children: a whole-of-population-based cohort study. BMJ Open. 2017;7(11):e017936. doi: 10.1136/bmjopen-2017-017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitsantas P, Nirmalraj L. Effects of respiratory syncytial virus infection in infancy on asthma and respiratory allergy in 6-year-old children. South Med J. 2018;111(11):698–702. doi: 10.14423/SMJ.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 31.Mendy A, Wilkerson J, Salo PM, Cohn RD, Zeldin DC, Thorne PS. Endotoxin predictors and associated respiratory outcomes differ with climate regions in the U.S. Environ Int. 2018;112:218–226. doi: 10.1016/j.envint.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendy A, et al. Exposure and sensitization to pets modify endotoxin association with asthma and wheeze. J Allergy Clin Immunol Pract. 2018;6(6):2006–2013.e4. doi: 10.1016/j.jaip.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delfino RJ, Staimer N, Tjoa T, Gillen DL. Relations of exhaled nitric oxide and FEV1 to personal endotoxin exposure in schoolchildren with asthma. Occup Environ Med. 2015;72(12):830–836. doi: 10.1136/oemed-2014-102651. [DOI] [PubMed] [Google Scholar]
- 34.Berger M, de Boer JD, Bresser P, van der Poll T, Lutter R, Sterk PJ, van der Zee J. Lipopolysaccharide amplifies eosinophilic inflammation after segmental challenge with house dust mite in asthmatics. Allergy. 2015;70(3):257–264. doi: 10.1111/all.12544. [DOI] [PubMed] [Google Scholar]
- 35.Oluwole O, Rennie DC, Senthilselvan A, Dyck R, Afanasieva A, Kirychuk S, Katselis G, Lawson JA. The association between endotoxin and beta-(1-->3)-D-glucan in house dust with asthma severity among schoolchildren. Respir Med. 2018;138:38–46. doi: 10.1016/j.rmed.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Leung TF, Wong YS, Chan IH, Yung E, Wong CK, Lam CW, Wong GW. Indoor determinants of endotoxin and dust mite exposures in Hong Kong homes with asthmatic children. Int Arch Allergy Immunol. 2010;152(3):279–287. doi: 10.1159/000283039. [DOI] [PubMed] [Google Scholar]
- 37.Phipatanakul W, Celedón JC, Raby BA, Litonjua AA, Milton DK, Sredl D, Weiss ST, Gold DR. Endotoxin exposure and eczema in the first year of life. Pediatrics. 2004;114(1):13–18. doi: 10.1542/peds.114.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litonjua AA, et al. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110(5):736–742. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 39.Tavernier GO, et al. Endotoxin exposure in asthmatic children and matched healthy controls: results of IPEADAM study. Indoor Air. 2005;15(Suppl 10):25–32. doi: 10.1111/j.1600-0668.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 40.Mendy A, Cohn RD, Thorne PS. Endotoxin exposure, serum vitamin D, asthma and wheeze outcomes. Respir Med. 2016;114:61–66. doi: 10.1016/j.rmed.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent M, et al. Relationship between mold exposure, specific IgE sensitization, and clinical asthma: a case-control study. Ann Allergy Asthma Immunol. 2018;121(3):333–339. doi: 10.1016/j.anai.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka A, et al. Evaluation of the association between sensitization to common inhalant fungi and poor asthma control. Ann Allergy Asthma Immunol. 2016;117(2):163–168.e1. doi: 10.1016/j.anai.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann S, Sprünken A, Wagner N, Tenbrock K, Ott H. Clinical relevance of IgE-mediated sensitization against the mould Alternaria alternata in children with asthma. Ther Adv Respir Dis. 2017;11(1):30–39. doi: 10.1177/1753465816680786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baxi SN, et al. Association between fungal spore exposure in inner-city schools and asthma morbidity. Ann Allergy Asthma Immunol. 2019;122(6):610–615.e1. doi: 10.1016/j.anai.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oluwole O, Kirychuk SP, Lawson JA, Karunanayake C, Cockcroft DW, Willson PJ, Senthilselvan A, Rennie DC. Indoor mold levels and current asthma among school-aged children in Saskatchewan, Canada. Indoor Air. 2017;27(2):311–319. doi: 10.1111/ina.12304. [DOI] [PubMed] [Google Scholar]
- 46.Kim JL, Henneberger PK, Lohman S, Olin AC, Dahlman-Höglund A, Andersson E, Torén K, Holm M. Impact of occupational exposures on exacerbation of asthma: a population-based asthma cohort study. BMC Pulm Med. 2016;16(1):148. doi: 10.1186/s12890-016-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medrek SK, et al. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract. 2017;5(4):1025–1031.e2. doi: 10.1016/j.jaip.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Cavaleiro Rufo J, Madureira J, Paciência I, Aguiar L, Pereira C, Silva D, Padrão P, Moreira P, Delgado L, Annesi-Maesano I, Oliveira Fernandes E, Teixeira JP, Moreira A. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatr Allergy Immunol. 2017;28(4):332–339. doi: 10.1111/pai.12704. [DOI] [PubMed] [Google Scholar]
- 49.Tham R, Katelaris CH, Vicendese D, Dharmage SC, Lowe AJ, Bowatte G, Taylor P, Burton P, Abramson MJ, Erbas B. The role of outdoor fungi on asthma hospital admissions in children and adolescents: a 5-year time stratified case-crossover analysis. Environ Res. 2017;154:42–49. doi: 10.1016/j.envres.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Woolnough KF, Richardson M, Newby C, Craner M, Bourne M, Monteiro W, Siddiqui S, Bradding P, Pashley CH, Wardlaw AJ. The relationship between biomarkers of fungal allergy and lung damage in asthma. Clin Exp Allergy. 2017;47(1):48–56. doi: 10.1111/cea.12848. [DOI] [PubMed] [Google Scholar]
- 51.Dannemiller KC, et al. Indoor microbial communities: influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol. 2016;138(1):76–83.e1. doi: 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi H, Byrne S, Larsen LS, Sigsgaard T, Thorne PS, Larsson L, Sebastian A, Bornehag CG. Residential culturable fungi, (1-3, 1-6)-beta-d-glucan, and ergosterol concentrations in dust are not associated with asthma, rhinitis, or eczema diagnoses in children. Indoor Air. 2014;24(2):158–170. doi: 10.1111/ina.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpagnano GE, Malerba M, Lacedonia D, Susca A, Logrieco A, Carone M, Cotugno G, Palmiotti GA, Foschino-Barbaro MP. Analysis of the fungal microbiome in exhaled breath condensate of patients with asthma. Allergy Asthma Proc. 2016;37(3):41–46. doi: 10.2500/aap.2016.37.3943. [DOI] [PubMed] [Google Scholar]
- 54.Farrant J, Brice H, Fowler S, Niven R. Fungal sensitisation in severe asthma is associated with the identification of Aspergillus fumigatus in sputum. J Asthma. 2016;53(7):732–735. doi: 10.3109/02770903.2016.1154073. [DOI] [PubMed] [Google Scholar]
- 55.Segura N, Fraj J, Cubero JL, Sobrevía MT, Lezaun A, Ferrer L, Sebastián A, Colás C. Mould and grass pollen allergy as risk factors for childhood asthma in Zaragoza, Spain. Allergol Immunopathol (Madr) 2016;44(5):455–460. doi: 10.1016/j.aller.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Polyzoi E, Polyzois D. Presence of household mold, children’s respiratory health, and school absenteeism: cause for concern. J Environ Health. 2017;79(7):28–35. [PubMed] [Google Scholar]
- 57.Mueller-Rompa S, Janke T, Schwaiger K, Mayer M, Bauer J, Genuneit J, Braun-Fahrlaender C, Horak E, Boznanski A, von Mutius E, Ege MJ, GABRIELA study group Identification of fungal candidates for asthma protection in a large population-based study. Pediatr Allergy Immunol. 2017;28(1):72–78. doi: 10.1111/pai.12665. [DOI] [PubMed] [Google Scholar]
- 58.Tham R, et al. Associations between outdoor fungal spores and childhood and adolescent asthma hospitalizations. J Allergy Clin Immunol. 2017;139(4):1140–1147.e4. doi: 10.1016/j.jaci.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 59.Lewis TC, Metitiri EE, Mentz GB, Ren X, Carpenter AR, Goldsmith AM, Wicklund KE, Eder BN, Comstock AT, Ricci JM, Brennan SR, Washington GL, Owens KB, Mukherjee B, Robins TG, Batterman SA, Hershenson MB, Community Action Against Asthma Steering Committee Influence of viral infection on the relationships between airway cytokines and lung function in asthmatic children. Respir Res. 2018;19(1):228. doi: 10.1186/s12931-018-0922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjerregaard A, Laing IA, Poulsen N, Backer V, Sverrild A, Fally M, Khoo SK, Barrett L, Baltic S, Thompson PJ, Chidlow G, Sikazwe C, Smith DW, Bochkov YA, le Souëf P, Porsbjerg C. Characteristics associated with clinical severity and inflammatory phenotype of naturally occurring virus-induced exacerbations of asthma in adults. Respir Med. 2017;123:34–41. doi: 10.1016/j.rmed.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saraya T, Kimura H, Kurai D, Ishii H, Takizawa H. The molecular epidemiology of respiratory viruses associated with asthma attacks: a single-center observational study in Japan. Medicine (Baltimore) 2017;96(42):e8204. doi: 10.1097/MD.0000000000008204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verduyn M, et al. Serum IgG concentrations in adult patients experiencing virus-induced severe asthma exacerbations. J Allergy Clin Immunol Pract. 2019;7(5):1507–1513.e1. doi: 10.1016/j.jaip.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen-Thi-Dieu T, et al. Study of clinical characteristics and cytokine profiles of asthmatic children with rhinovirus infection during acute asthma exacerbation at National Hospital of Pediatrics. Can Respir J. 2018;2018:9375967. doi: 10.1155/2018/9375967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith-Norowitz TA, et al. Increased seroprevalence of enterovirus 71 IgE antibodies in asthmatic compared with non-asthmatic children. Ir J Med Sci. 2017;186(2):495–503. doi: 10.1007/s11845-016-1480-0. [DOI] [PubMed] [Google Scholar]
- 65.Itagaki T, Aoki Y, Matoba Y, Tanaka S, Ikeda T, Mizuta K, Matsuzaki Y. Clinical characteristics of children infected with enterovirus D68 in an outpatient clinic and the association with bronchial asthma. Infect Dis (Lond) 2018;50(4):303–312. doi: 10.1080/23744235.2017.1400176. [DOI] [PubMed] [Google Scholar]
- 66.Funakoshi Y, Ito K, Morino S, Kinoshita K, Morikawa Y, Kono T, Doan YH, Shimizu H, Hanaoka N, Konagaya M, Fujimoto T, Suzuki A, Chiba T, Akiba T, Tomaru Y, Watanabe K, Shimizu N, Horikoshi Y. Enterovirus D68 respiratory infection in a children’s hospital in Japan in 2015. Pediatr Int. 2019;61(8):768–776. doi: 10.1111/ped.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshii Y, Shimizu K, Morozumi M, Chiba N, Ubukata K, Uruga H, Hanada S, Wakui H, Minagawa S, Hara H, Numata T, Saito K, Araya J, Nakayama K, Kishi K, Kuwano K. Detection of pathogens by real-time PCR in adult patients with acute exacerbation of bronchial asthma. BMC Pulm Med. 2017;17(1):150. doi: 10.1186/s12890-017-0494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaiwong C, Ngamphaiboon J. Effects of inactivated influenza vaccine on respiratory illnesses and asthma-related events in children with mild persistent asthma in Asia. Asian Pac J Allergy Immunol. 2015;33(1):3–7. doi: 10.12932/AP0511.33.2.2015. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein E, Finelli L, O’Halloran A, Liu P, Karaca Z, Steiner CA, Viboud C, Lipsitch M. Hospitalizations associated with respiratory syncytial virus (RSV) and influenza in children, including children diagnosed with asthma. Epidemiology. 2019;30:918–926. doi: 10.1097/EDE.0000000000001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kljaic-Bukvic B, Blekic M, Aberle N, Curtin JA, Hankinson J, Semic-Jusufagic A, Belgrave D, Simpson A, Custovic A. Genetic variants in endotoxin signalling pathway, domestic endotoxin exposure and asthma exacerbations. Pediatr Allergy Immunol. 2014;25(6):552–557. doi: 10.1111/pai.12258. [DOI] [PubMed] [Google Scholar]
- 71.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104(5):1008–1014. doi: 10.1016/s0091-6749(99)70082-5. [DOI] [PubMed] [Google Scholar]
- 72.Lai PS, et al. Gene-environment interaction between an IL4R variant and school endotoxin exposure contributes to asthma symptoms in inner-city children. J Allergy Clin Immunol. 2018;141(2):794–796.e3. doi: 10.1016/j.jaci.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, Cunningham C, le Gros G, von Mutius E, Pearce N. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32(3):603–611. doi: 10.1183/09031936.00033707. [DOI] [PubMed] [Google Scholar]
- 74.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 75.Holbreich M, Genuneit J, Weber J, Braun-Fahrländer C, Waser M, von Mutius E. Amish children living in northern Indiana have a very low prevalence of allergic sensitization. J Allergy Clin Immunol. 2012;129(6):1671–1673. doi: 10.1016/j.jaci.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 76.Motika CA, Papachristou C, Abney M, Lester LA, Ober C. Rising prevalence of asthma is sex-specific in a US farming population. J Allergy Clin Immunol. 2011;128(4):774–779. doi: 10.1016/j.jaci.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, Lee SE, Yang MS, Kim SH, Park HK, Kwon YE, Kim TB, Kim SH, Park HW, Chang YS, Lee BJ, Jee YK, Choi BW, Bachert C, Cho SH. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016;46(3):411–421. doi: 10.1111/cea.12652. [DOI] [PubMed] [Google Scholar]
- 78.Chopra V, et al. Correlation of aspergillus skin hypersensitivity with the duration and severity of asthma. Monaldi Arch Chest Dis. 2017;87(3):826. doi: 10.4081/monaldi.2017.826. [DOI] [PubMed] [Google Scholar]
- 79.Tovey ER, et al. Rhinoviruses significantly affect day-to-day respiratory symptoms of children with asthma. J Allergy Clin Immunol. 2015;135(3):663–9.e12. doi: 10.1016/j.jaci.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stelzer-Braid S, Tovey ER, Willenborg CM, Toelle BG, Ampon R, Garden FL, Oliver BG, Strachan R, Belessis Y, Jaffe A, Reddel HK, Crisafulli D, Marks GB, Rawlinson WD. Absence of back to school peaks in human rhinovirus detections and respiratory symptoms in a cohort of children with asthma. J Med Virol. 2016;88(4):578–587. doi: 10.1002/jmv.24371. [DOI] [PubMed] [Google Scholar]
- 81.Chiu CY, et al. Longitudinal urinary metabolomic profiling reveals metabolites for asthma development in early childhood. Pediatr Allergy Immunol. 2018;29(5):496–503. doi: 10.1111/pai.12909. [DOI] [PubMed] [Google Scholar]
- 82.Corren, J, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2019. 10.1016/j.jaip.2019.08.050. [DOI] [PubMed]
- 83.Licari A, Manti S, Castagnoli R, Parisi GF, Salpietro C, Leonardi S, Marseglia GL. Targeted therapy for severe asthma in children and adolescents: current and future perspectives. Paediatr Drugs. 2019;21(4):215–237. doi: 10.1007/s40272-019-00345-7. [DOI] [PubMed] [Google Scholar]


