Abstract
STUDY QUESTION
Is there a difference in functional outcomes and recurrence rate between conservative versus radical rectal surgery in patients with large deep endometriosis infiltrating the rectum 5 years postoperatively?
SUMMARY ANSWER
No evidence was found that long-term outcomes differed when nodule excision was compared to rectal resection for deeply invasive endometriosis involving the bowel.
WHAT IS KNOWN ALREADY
Functional outcomes of nodule excision and rectal resection for deeply invasive endometriosis involving the bowel are comparable 2 years after surgery. Despite numerous previously reported case series enrolling patients managed for colorectal endometriosis, long-term data remain scarce in the literature.
STUDY DESIGN, SIZE, DURATION
From March 2011 to August 2013, we performed a two-arm randomized trial, enrolling 60 patients with deep endometriosis infiltrating the rectum up to 15 cm from the anus, measuring >20 mm in length, involving at least the muscular layer in depth, and up to 50% of rectal circumference. Among them, 55 women were enrolled at one tertial referral centre in endometriosis, using a randomization list drawn up separately for this centre. Institute review board approval was obtained to continue follow-up to 10 years postoperatively. One patient requested to stop the follow-up 2 years after surgery.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Patients underwent either nodule excision by shaving or disc excision, or segmental resection. Randomization was performed preoperatively using sequentially numbered, opaque, sealed envelopes, and patients were informed of randomization results. The primary endpoint was the proportion of patients experiencing one of the following symptoms: constipation (1 stool/>5 consecutive days), frequent bowel movements (≥3 stools/day), anal incontinence, dysuria or bladder atony requiring self-catheterization 24 months postoperatively. Secondary endpoints were values taken from the Knowles–Eccersley–Scott-symptom questionnaire (KESS), the gastrointestinal quality of life index (GIQLI), the Wexner scale, the urinary symptom profile (USP) and the Short Form 36 Health Survey (SF36).
MAIN RESULTS AND THE ROLE OF CHANCE
Fifty-five patients were enrolled. Among the 27 patients in the excision arm, two were converted to segmental resection (7.4%). One patient managed by segmental resection withdrew from the study 2 years postoperatively, presuming that associated pain of other aetiologies may have jeopardized the outcomes. The 5 year-recurrence rate for excision and resection was 3.7% versus 0% (P = 1), respectively. For excision and resection, the primary endpoint was present in 44.4% versus 60.7% of patients (P = 0.29), respectively, while 55.6% versus 53.6% of patients subjectively reported normal bowel movements (P = 1). An intention-to-treat comparison of overall KESS, GIQLI, Wexner, USP and SF36 scores did not reveal significant differences between the two arms 5 years postoperatively. Statistically significant improvement was observed shortly after surgery with no further improvement or impairment recorded 1–5 years postoperatively. During the 5-year follow-up, additional surgical procedures were performed in 25.9% versus 28.6% of patients who had undergone excision or resection (P = 0.80), respectively.
LIMITATIONS, REASONS FOR CAUTION
The presumption of a 40% difference concerning postoperative functional outcomes in favour of nodule excision resulted in a lack of power for demonstration of the primary endpoint difference.
WIDER IMPLICATIONS OF THE FINDINGS
Five-year follow-up data do not show statistically significant differences between conservative and radical rectal surgery for long-term functional digestive and urinary outcomes in this specific population of women with large involvement of the rectum.
STUDY FUNDING/COMPETING INTEREST(S)
No specific funding was received. Patient enrolment and follow-up until 2 years postoperatively was supported by a grant from the clinical research programme for hospitals in France. The authors declare no competing interests related to this study.
TRIAL REGISTRATION NUMBER
This randomized study is registered with ClinicalTrials.gov, number NCT 01291576.
TRIAL REGISTRATION DATE
31 January 2011.
DATE OF FIRST PATIENT’S ENROLMENT
7 March 2011.
Keywords: colorectal resection, shaving, disc excision, digestive symptoms, bladder dysfunction
Introduction
Surgical management of deep infiltrating endometriosis of the rectum (DIER) has become a topic of increasing interest in gynaecological surgery. Surgical procedures employed to remove nodules of DIER may be classified as radical (employing segmental resection, i.e. the nodule is removed along with a segment of the rectum, followed by colorectal anastomosis) or conservative (the nodule is removed by excision, with or without opening the rectum, employing disc excision or shaving, respectively) (Donnez and Squifflet, 2010; Fanfani et al., 2010; Abrao et al., 2015; Roman et al., 2018a). It has been presumed that outcomes related to these procedures differ in terms of postoperative complications (Abo et al., 2018; Donnez and Roman, 2017), functional outcomes (Roman et al., 2013) and delayed DIER recurrences (Meuleman et al., 2011; Afors et al., 2016). However, owing to the rarity of studies with long-term follow-up (Roman et al., 2016; Soto et al., 2016) and their retrospective design, little is known about long-term outcomes related to this surgery.
We recently reported the results of the first randomized trial comparing conservative and radical surgery in DIER (Roman et al., 2018a), with the primary endpoint assessed 2 years after surgery. Sixty patients were enrolled in this study in three different centres, using a randomization procedure independent of each centre. For patients enrolled at the Rouen University Centre, an institute review board (IRB)-approved extension was obtained for follow-up until 10 years after surgery, to assess long-term functional outcomes, pelvic pain and recurrences.
The aim of our study was to compare digestive and urinary outcomes, pelvic pain and recurrence risk in patients managed for DIER by either conservative or radical surgery 5 years postoperatively.
Materials and Methods
We conducted an unblinded, 1:1 parallel-arms, randomized controlled trial to assess the hypothetical superiority of conservative rectal surgery over segmental resection in the management of deep endometriosis infiltrating the rectum (ENDORE; NCT 01291576) (Roman et al., 2018a). Eligible patients were >18 and <45 years and managed for deep endometriosis infiltrating the rectum up to 15 cm from the anus, measuring >20 mm in length, involving at least the muscular layer in depth, and up to 50% of rectal circumference. Between March 2011 and August 2013, patients were enrolled in three French referral centres: Rouen University Hospital, Tenon University Hospital and Lille University Hospital. Assignment of a patient to nodule excision or colorectal resection was based on randomization lists drawn up separately for the centre of Rouen by a statistician with no clinical involvement in the trial (M.B.). In the present study, in accordance with IRB approval, only patients enrolled in Rouen were followed up from 2 to 5 years after surgery. The methodology of the randomized trial was extensively presented in our previous article (Roman et al., 2018a).
According to the trial design, patients were followed-up at 6, 12, 18, 24, 36, 48 and 60 month visits after surgery. Digestive and urinary outcomes were assessed using the same questionnaires employed before surgery. Complete data were not recorded in women whose stoma was not yet closed at the time of the visit. The primary endpoint at 24 months postsurgery related to the proportion of patients experiencing one of the following symptoms: constipation (1 stool/>5 consecutive days), frequent bowel movements (≥3 stools/day), defecation pain, anal incontinence (involuntary loss of gas or stools), dysuria (urinary symptom profile (USP) score for dysuria ≥1) or bladder atony requiring bladder voiding by self-catheterization. Secondary endpoints taken were the visual analog scale (VAS), the Knowles–Eccersley–Scott-symptom questionnaire (KESS) (Knowles et al., 2000), the gastrointestinal quality of life index (GIQLI) (Nieveen van Dijkum et al., 2000), the Wexner scale (Jorge and Wexner, 1993), the USP (Haab et al., 2008) and the Short Form 36 Health Survey (SF36) (Brazier et al., 1992).
Statistical analysis
Statistical analyses were carried out using SAS 9.3 software (SAS Institute, Cary, NC, USA) and IBM SPSS 23.0 statistic software (IBM Corporation, Armonk, NY, USA). The population at the time of randomization and at different time points of the follow-up, i.e. just before the intervention started, was described using median, first and third quartile (Q1–Q3) if characteristics reached at least ordinal level and were not categorized. To compare treatment arms with respect to the presence of functional symptoms at a given time, Fisher’s exact test or its generalization by Freeman and Halton was used for categorical characteristics and Wilcoxon’s test for independent samples. Each time the P-value was <0.05 the corresponding differences were considered to be significant.
In order to study the trend of repeated measures during the follow-up period, a mixt-effect model was used to analyse the effect of intervention type on digestive outcomes (KESS; Wexner) and on dimensions of quality of life (SF36; GIQLI). The chi-square for linear trend (extended Mantel–Haenszel) was used to assess the evolution of digestive outcomes. For the purpose of comparing the baseline and follow-up after surgery, the trends of quality of life and digestive outcomes were assessed for nodule excision and colorectal resection, separately. The evolution of quality of life and digestive symptoms were tested globally, based on the records for all time points.
The trial was funded by the clinical research programme for hospitals in France and locally registered as 2009/069/HP by the sponsor. Funders took no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The follow-up from 2 to 5 years after surgery did not benefit from specific funding. This study is registered with ClinicalTrials.gov, number NCT 01291576.
Results
Fifty-five patients were enrolled in the Rouen centre. Twenty-seven patients, recruited from March 2011 to August 2013, were randomly assigned to arm A and 28 to arm B and received surgery from March 2011 to October 2013. They attended clinical visits at the time of randomization (baseline) and at 6-month intervals for 2 years, then yearly for 5 years, with the final follow-up in October 2018. One patient managed by radical surgery asked to stop follow-up after 2 years (1.8%), believing her answers could be jeopardized by concomitant painful diseases (lumbar degenerative disc disease). As she was considered to have unfavourable functional outcomes, all 55 patients were analysed for the primary endpoint. The trial flow chart is presented in Figure 1.
Figure 1. Flow diagram (CONSORT 2010) for study of patients who underwent excision or colorectal resection for deep endometriosis infiltrating the rectum.
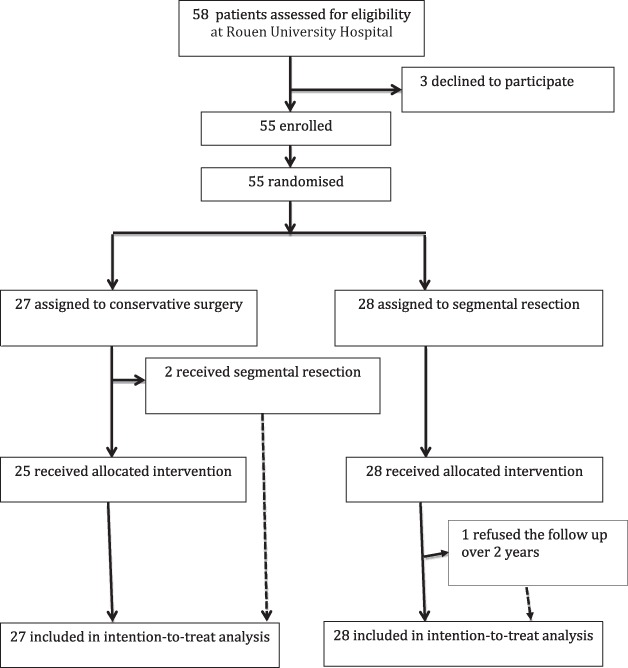
Table I presents baseline characteristics for each group. Multifocal colorectal endometriosis was recorded in half of the patients. The median distance of rectal nodules from the anus corresponded to an infiltration of the mid rectum. Adenomyosis was present in more than two-thirds of patients.
Table I. Baseline characteristics of patients who underwent excision or colorectal resection for deep endometriosis infiltrating the rectum.
| Parameter | Excision (n=27) | Colorectal resection (n=28) | P |
|---|---|---|---|
| Age (years) | 30 (27-36) | 28 (27-33) | 0.18 |
| Number of previous surgeries for endometriosis | 0.50 | ||
| 0 | 19 (70.4%) | 19 (67.9%) | |
| 1 | 6 (22.2%) | 5 (17.9%) | |
| 2 | 2 (7.4%) | 1 (3.6%) | |
| 3 | 0 | 3 (10.7%) | |
| Nullipara | 20 (74.1%) | 20 (71.4%) | 1 |
| Infertility care | 8 (29.6%) | 10 (35.7%) | 0.78 |
| Dysmenorrhoea: | 27 (100%) | 28 (100%) | 1 |
| VAS of dysmenorrhoea | 8 (8-10) | 9 (8-10) | 0.57 |
| Sexual intercourse during past year | 24 (88.9%) | 27 (96.4%) | 0.35 |
| Dyspareunia: | 20 (74.1%) | 24 (85.7%) | 0.33 |
| VAS of dyspareunia | 5 (4-7) | 5 (3 – 6.5) | 0.73 |
| Chronic intermenstrual pelvic pain: | 19 (70.4%) | 23 (82.1%) | 0.36 |
| VAS of intermenstrual chronic pelvic pain | 6 (5-7) | 5 (3-8) | 0.30 |
| Biberoglou & Behrman score | 4 (3-6) | 5 (3.5-6) | 0.46 |
| Digestive symptoms: | |||
| <= than 1 stool/5 days | 8 (29.6%) | 12 (42.9%) | 0.40 |
| Defecation pain | 21 (77.8%) | 22 (78.6%) | 1 |
| >=3 stools/day | 12 (44.4%) | 10 (35.7%) | 0.59 |
| Involuntary gas or stool loss | 6 (22.2%) | 9 (32.1%) | 0.55 |
| GIQLI score | 89 (82-105) | 93.5 (86-107) | 0.40 |
| KESS score | 13 (9-18) | 10 (6.5 – 17.5) | 0.26 |
| Wexner score | 0 (0-3) | 0 (0 – 3.5) | 0.46 |
| How long are you able to defer defecation? | 0.07 | ||
| <5 min | 6 (22.2%) | 1 (3.6%) | |
| 5 to 10 min | 6 (22.2%) | 8 (28.6%) | |
| 10 to 15 min | 4 (14.8%) | 1 (3.6%) | |
| > 15 min | 11 (40.7%) | 18 (64.2%) | |
| Urinary symptoms: | |||
| USP score | 0 (0-3) | 0 (0-1.5) | 0.25 |
| Catamenial urinary pain or haematuria | 8 (29.6%) | 7 (25%) | 0.77 |
| Localisation of deep nodules on the digestive tract: | |||
| - Rectal nodules | 1 | ||
| 1 nodule | 26 (96.3%) | 27 (96.4%) | |
| 2 nodules | 1 (3.7%) | 1 (3.6%) | |
| - Sigmoid colon nodules | 0.89 | ||
| 1 nodule | 10 (37%) | 12 (42.9%) | |
| 2 nodules | 1 (3.7%) | 1 (3.6%) | |
| 3 nodules | 1 (3.7%) | 0 | |
| - Left, transverse, right colon and caecum nodules | 2 (7.4%) | 2 (7.1%) | 1 |
| - Small bowel nodules | 2 (7.4%) | 1 (3.6%) | 1 |
| Diameter of largest rectal nodule (mm) | 30 (26-40) | 30 (25-40) | 0.45 |
| Deepest infiltration of the rectum: | 0.89 | ||
| - muscular layer | 17 (63%) | 19 (67.9%) | |
| - submucosa | 9 (33.3%) | 8 (28.6%) | |
| - mucosa | 1 (3.7%) | 1 (3.6%) | |
| Height of the lowest nodule (mm from anal verge) | 80 (60-100) | 90 (70-105) | 0.45 |
| Stenosis of the rectum | 22 (81.5%) | 17 (61.7%) | 0.14 |
| Diameter of the lumen at the level of the nodule (mm) | 12.5 (6-18) | 12 (9-15) | 0.95 |
| Adenomyosis | 21 (77.8%) | 17 (60.7%) | 0.25 |
Data are n (%) or median, first and third quartile (Q1-Q3). VAS: visual analog scale; GIQLI: gastrointestinal quality of life index; KESS: Knowles-Eccersley-Scott-symptom; USP: urinary symptom profile. Fisher’s exact test or its generalisation by Freeman and Halton was used for categorical characteristics and Wilcoxon’s test for independent samples.
Table II presents intraoperative findings and additional surgical procedures. In arm A, of the 15 patients managed by disc excision, 8 were treated by the Rouen technique, 6 by disc excision using transanal end-to-end circular stapler and one by direct transvaginal disc excision. In two women allocated to the excision arm, infiltration was both too large and deep to be accurately treated by shaving and too high to be managed by the Rouen technique (Roman et al., 2017). Colorectal resection was thus considered by the surgeon to be the most appropriate technique, and in accordance with the intention-to-treat principle both women were analysed in the patient group managed by excision.
Table II. Intraoperative findings and surgical procedures.
| Parameter | Excision (n=27) | Colorectal resection (n=28) | P |
|---|---|---|---|
| Operative route: | 0.35 | ||
| Laparoscopic | 24 (88.9%) | 27 (96.4%) | |
| Laparoscopic converted to open surgery | 3 (11.1%) | 1 (3.6%) | |
| Operative time (min) | 280 (190-360) | 270 (240-300) | 0.59 |
| Procedure performed on the rectum: | <0.001 | ||
| Shaving | 10 (37%) | 0 | |
| Full thickness disc excision | 15 (55.6%) | 0 | |
| Colorectal segmental resection | 2 (7.4%) | 28 (100%) | |
| Full thickness disc excision: | 15 (55.6%) | 0 | |
| Diameter of the specimen (mm) | 40 (40-50) | - | |
| Height of rectal suture (mm) | 70 (50-90) | - | |
| Colorectal segmental resection: | 2 | 33 | |
| Length of colorectal specimen (mm) | 125 (100-150) | 80 (50-130) | |
| Length of rectal segment removed (mm) | 60 (60-60) | 54 (40-70) | |
| Length of sigmoid colon segment removed (mm) | 65 (40-90) | 25 (0-60) | |
| Height of colorectal anastomosis (mm) | 90 (90-90) | 80 (60-100) | |
| Diameter of the end-to-end transanal circular stapler used to perform colorectal anastomosis (mm) | |||
| 31 | 1 (3.7%) | 21 (75%) | |
| 28 | 1 (3.7%) | 7 (25%) | |
| Temporary stoma | 0.009 | ||
| None | 11 (40.7%) | 8 (28.6%) | |
| Ileostoma | 0 | 8 (28.6%) | |
| Colostoma | 16 (59.3%) | 12 (42.9%) | |
| rAFS score | 55 (19-98) | 53 (12-91) | 0.72 |
| Management of ovarian endometrioma: | 8 (29.6%) | 13 (39.4%) | 0.06 |
| None | 15 (55.6%) | 15 (53.6) | |
| Vaporization of the right ovary | 3 (11.1%) | 1 (3.6%) | |
| Vaporization of the left ovary | 1 (3.7%) | 6 (21.4%) | |
| Bilateral ovarian vaporization | 3 (11.1%) | 6 (21.4%) | |
| Unilateral oophorectomy | 2 (7.4%) | 0 | |
| Bilateral oophorectomy | 3 (11.1%) | 0 | |
| Resection of bladder nodule | 3 (11.1%) | 1 (3.6%) | 0.35 |
| Management of ureteral endometriosis: | 0.83 | ||
| Advanced ureterolysis for stenosis of the ureter | 3 (11.1%) | 3 (10.7%) | |
| Resection of the ureter and reimplantation into the bladder | 1 (3.7%) | 0 | |
| Segmental resection of sigmoid colon (separated from rectal procedure) | 3 (11.1%) | 0 | 0.11 |
| Selective resection of left, transverse, right colon or caecum | 3 (11.1%) | 2 (7.1%) | 0.67 |
| Appendectomy | 4 (14.8%) | 2 (7.1%) | 0.42 |
| Resection of posterior vagina | 24 (88.9%) | 20 (71.4%) | 0.18 |
| Hysterectomy | 7 (25.9%) | 1 (3.6%) | 0.025 |
| Omentoplasty | 18 (66.7%) | 21 (75%) | 0.56 |
| Intraoperative blood loss (mL) | 200 (200-300) | 200 (200-275) | 0.31 |
| Blood transfusion | 0 | 0 | |
| Immediate postoperative outcomes (Day 1-Day 30) | |||
| Abscess/infected hematoma of the Douglas cul de sac | 0 | 2 (7.1%) | 0.49 |
| Second laparoscopy | 0 | 2 (7.1%) | 0.49 |
| Digestive tract fistula | 1 (3.7%) | 1 (3.6%) | 1 |
| Bladder delayed healing | 1 (3.7%) | 0 | 0.49 |
| Bladder self-catheterisation after recovery, due to bladder atony | 6 (22.2%) | 1 (3.6%) | 0.05 |
Data are n (%) or median (Q1-Q3). rAFS: revised American Fertility Society Score, Fisher’s exact test or its generalisation by Freeman and Halton was used for categorical characteristics and Wilcoxon’s test for independent samples.
Additional surgical procedures, excluding those dedicated to stoma closure, were performed during 5 postoperative years in 27.3% of patients (25.9% and 28.6% in the excision and resection arm, respectively; P = 0.8) and were justified by complications or pelvic pain. In the excision group, two patients had surgeries for rectovaginal fistula, and one for peritonitis and hemoperitoneum following stoma closure, hysterectomy for adenomyosis and salpingectomy for hydrosalpinx. In the radical surgery group, two patients underwent secondary segmental resection for stenosis of the colorectal anastomosis, and one patient had surgery for Douglas abscess, adhesions with negative impact on IVF feasibility, abdominal adhesions presumed to be painful, vaginal scar responsible for deep dyspareunia, hernia following stoma closure and gastric sleeve before IVF. In each group, one patient benefited from sacral roots neuromodulation for bladder atony. In one patient managed by radical surgery, chronic pelvic pain and pelvic hypersensitivity postoperatively occurred, and secondary pelvic laparoscopy and cholecystectomy were performed before definitively affirming the diagnosis.
All 55 patients were included in the intention-to-treat analysis, in the arm assigned by randomization. Analysis of primary outcomes 5 years postoperatively revealed 12 patients in the excision arm and 17 in the resection arm presenting one or more functional symptoms after surgery (44.4% versus 60.7%, P = 0.29) (Table III). As regards secondary outcomes, the values of KESS, GIQLI, Wexner, USP, SF36 and VAS scores were comparable between the two arms (Table III).
Table III. Clinical assessment 5 years after surgery.
| Parameter | Excision (n=27) | Colorecta resection (n=28) | P |
|---|---|---|---|
| Rectal nodule recurrence | 1 (3.7%) | 0 | 1 |
| Assessment of digestive and urinary function | |||
| Patients presenting primary outcome | 12 (44.4%) | 17 (60.7%) | .29 |
| Digestive symptoms: | |||
| <= than 1 stool/5 days | 4 (14.8%) | 3 (11.1%) | 1 |
| Defecation pain | 6 (22.2%) | 8 (29.6%) | 0.76 |
| >=3 stools/day | 5 (18.5%) | 8 (29.6%) | 0.53 |
| Involuntary gas or stool loss | 2 (7.4%) | 5 (18.5%) | 0.42 |
| GIQLI score | 119 (99-130) | 116 (97-126) | .67 |
| KESS score | 10 (6-15) | 7.5 (4-15) | .65 |
| Wexner score | 0 (0-1) | 0 (0-2) | .98 |
| How long are you able to defer defecation? | .86 | ||
| <5 min | 6 (23.1%) | 5 (19.2 %) | |
| 5 to 10 min | 6 (23.1 %) | 6 (23.1 %) | |
| 10 to 15 min | 1 (3.9 %) | 3 (11.5 %) | |
| > 15 min | 13 (50%) | 13 (50%) | |
| USP of Dysuria | 0 (0-1) | 0 (0-0) | .39 |
| Bladder self-catheterization | 0 | 0 | 1 |
| Short Form 36 Health Survey score: | |||
| Physical functioning | 95 (85-100) | 95 (85-100) | .99 |
| Physical role functioning | 100 (50-100) | 100 (50-100) | .82 |
| Bodily pain | 84 (58-100) | 85 (45-90) | .44 |
| General health perceptions | 63 (46-83) | 63 (38-75) | .18 |
| Vitality | 63 (30-75) | 55 (30-60) | .18 |
| Social functioning | 75 (50-100) | 88 (75-100) | .48 |
| Emotional role functioning | 100 (67-100) | 100 (67-100) | .90 |
| Mental health | 74 (56-80) | 68 (56-76) | .63 |
| Physical Score | 85 (61-95) | 82 (63-91) | .32 |
| Mental Score | 72 (61-90) | 76 (58-83) | .66 |
| “Do you consider your bowel movements as being normal?” | 1 | ||
| No | 12 (44.4%) | 13 (46.4%) | |
| Yes | 15 (55.6%) | 15 (53.6%) | |
| Assessment of postoperative pelvic pain | |||
| Patients with menstruation during preceding 6 months | 9 (33%) | 15 (45%) | .77 |
| Among whom, patients with dysmenorrhoea | 4/9 (44%) | 8/15 (53%) | 1.00 |
| VAS of dysmenorrhoea | 3 (2-4) | 4 (3-6) | .86 |
| Months until first recurrence of dysmenorrhoea | 12 (5-18) | 10 (4-18) | 1.00 |
| Patients having sexual intercourse after surgery during preceding 6 months | 24 (89%) | 32 (97%) | .32 |
| Among whom, patients with dyspareunia | 8/24 (33%) | 9/32 (28%) | .77 |
| VAS of dyspareunia | 4 (3-6) | 4 (3-7) | 1.00 |
| Patients with intermenstrual pelvic pain during preceding 6 months | 6 (22%) | 10 (3%) | .57 |
| VAS of intermenstrual pelvic pain | 4 (3-5) | 4 (3-6) | .83 |
Data are n (%) or median (Q1-Q3). Fisher’s exact test or its generalisation by Freeman and Halton was used for categorical characteristics and Wilcoxon’s test for independent samples.
One recurrence was observed in a patient managed for a large endometriosis nodule infiltrating the whole posterior vagina, the mid rectum and both uterosacral ligaments. Computed tomography-based virtual colonoscopy revealed a second 2 cm nodule on the sigmoid colon. We performed the Rouen technique and removed a 60 mm-diameter rectal patch, with a transversal rectal stapled line at 6 cm above the anal verge, along with a 3 cm-diameter vaginal patch. The nodule on the sigmoid colon was removed by separate disc excision, and a diverting colostoma was required for 3 months. The patient presented with a rectovaginal fistula requiring secondary surgery with rectal suture and omentoplasty and bladder atony requiring 6-month self-catheterization. Having unsuccessfully attempted to conceive for 6 months, she then abandoned pregnancy intention without taking the contraceptive pill. Eighteen months later, MRI revealed a 2 cm-diameter fibrous nodule on the stapled line and was concluded to be a local recurrence. To date, the patient has resumed taking the contraceptive pill and has received no further surgery for recurrent nodules.
Yearly assessment of gastrointestinal function and quality of life using standardized scores was compared between the two arms. The results revealed a rapid and significant improvement of GIQLI and SF36 score in both arms, as for the KESS score in the conservative surgery arm. The improvement was observed at 1 year after surgery and remained constant for 5 years. No statistically significant differences were observed between the two arms in terms of GIQLI and SF36 values assessed at 5 years postoperatively, or for the improvement trends in each arm.
Discussion
Our study demonstrates that long-term postoperative functional outcomes, pain improvement and recurrence rates are comparable between the radical and conservative techniques employed to treat DIER. The benefits of the surgery in symptomatic patients with DIER, became significant 6 months after surgery (Roman et al., 2019), then remained constant for 5 years. Our data recommend both conservative and radical rectal surgery as an efficient and lasting treatment of patients suffering from pain and digestive troubles caused by DIER. On the basis of previous retrospective studies, we overvalued excision when compared to colorectal resection in terms of functional outcomes. This is the essence of randomization and the reason for which it is the pinnacle of scientific methodology.
Our study presents some limitations. The design of the randomized trial was based on the presumption of a 40% difference favourable to nodule excision in terms of postoperative functional outcomes. This hypothesis was not realistic, and at 5 years after surgery we observed only a 16% difference in favour of nodule excision, which did not reach statistical significance. In addition, the differences between the frequencies of primary endpoint functional symptoms were routinely <11%, and our sample was insufficient to reveal statistically significant differences. GIQLI, KESS and SF36 scores in the two arms were found to be comparable, indicating that functional outcomes of the two surgical approaches differ less than initially presumed. With the exception of rectal stenosis after segmental resection (Roman et al., 2018a), unfavourable outcomes and additional surgical procedures were comparable between the two arms. However, increasing the trial’s sample size and consequently the statistical power of the analysis could in theory reveal a difference favourable to rectal excision, particularly in terms of postoperative frequency of stools and involuntary loss of gas or stools.
Our trial also has several strengths. The allocation was randomized, which allowed comparison between two arms with similar characteristics. Patients were managed, followed up and assessed by experienced surgeons, which allowed for accurate results. Only two conversions were observed in the excision arm, with 96.7% of patients enrolled in the trial receiving the allocated procedure. The trial focused on a question of major interest relating to the management of deep endometriosis. Only one patient was not followed up until 5 years postoperatively, and all patients were analysed for the primary endpoint. Lastly, the extensive follow-up, among the longest previously reported in the literature relating to deep endometriosis surgery, allows our study to provide consistent data about long-term outcomes of rectal surgery in deep endometriosis.
Overall, a 5-year recurrence rate of 1.8% demonstrates that surgical removal of DIER is a valid procedure with excellent long-term remission. Furthermore, recurrence rates were comparable between the conservative and radical surgery arms. It should be noted that randomization to the conservative arm allocated patients with very large rectal infiltration for which disc excision could be challenging, as well as patients with multiple rectosigmoid nodules, managed by separate excisions (Millochau et al., 2018) instead of classically recommended en bloc segmental resection (Abrao et al., 2015). Despite these circumstances, the rate of recurrences in the conservative arm reached only 3.7%. In the literature, the main argument in support of the radical approach in DIER is a presumed excess of recurrences after conservative procedures (Meuleman et al., 2011). Our study demonstrates the invalidity of this argument during the first 5-year period following the procedure.
Our results are in agreement with previous reports suggesting that complete removal of large deep endometriosis infiltrating the rectum does not guarantee relief from digestive complaints (Erdem et al., 2018; Kupelian and Cutner, 2016; Riiskjaer et al., 2016; Soto et al., 2016). Our patients received multiple opportunities to answer follow-up questions concerning the quality of their digestive function. Answers showed that patients would sometimes consider their bowel movements as normal, despite concomitantly affirming the primary endpoint (constipation, frequent stools, defecation pain or anal incontinence). This paradox may be explained by the general improvement in health and gastrointestinal quality of life after surgery, rendering some symptoms simply less embarrassing when compared with more severe complaints at baseline (Roman et al., 2019).
Similar to other reports in the literature (Riiskjaer et al., 2018), quality of life was assessed using the SF36, which revealed significant and lasting improvement in quality of life during the 5 years of follow-up. Overall improvement was not only statistically significant but also clinically relevant, as the number of patients presenting symptoms related to the main outcome decreased by >50%, while the median values of Biberoglou and Behrman, KESS and GIQLI scores improved by 75%, 50 and 30%, respectively. All score variations were concordant with general health improvement, from as early as 6 months after surgery. Our study shows no observed further significant improvements or impairments during the period from 1 to 5 years postoperatively. This information should be given to patients prior to surgery for rectal endometriosis.
There is an overall improvement in pelvic pain and quality of life after surgery, which is comparable between the two arms and remains constant during the 5 years of follow-up. It should be emphasized that these favourable outcomes cannot be attributed solely to the procedure on the rectum but also to the complete excision of endometriosis lesions spread into the pelvis and the abdomen. In our opinion, excision of bowel nodules must be accompanied by that of associated localizations of the disease.
Pelvic pain and quality of life improvement may also be the consequence of postoperative medical treatment. It is our philosophy to routinely recommend postoperative amenorrhea to those women who do not intend to get pregnant. As stated in a recent article, 36 patients (65.5%) attempted to conceive after surgery, of whom 81% conceived, in most cases naturally (Roman et al., 2018b). During the 6 months preceding the 5-year-follow up visit, only 33% of patients managed by excision and 45% of those treated by resection had periods (Table III), which demonstrates overall patient compliance with our recommendations.
In contrast to rectal cancer, rectal endometriosis nodules are rarely if ever limited to the digestive tract, but they concomitantly infiltrate the vagina, uterosacral ligaments and the parametriums. When splanchnic nerves are involved, there is risk of nerve injury during surgery with unavoidable impairment of rectal function, despite its conservation, in addition to voiding troubles, vaginal dryness and sensitivity loss. Bladder voiding was included in the primary outcome, though not directly related to digestive tract function. Postoperative occurrence of bladder voiding troubles, due to splanchnic nerve injury resulting from surgery or the endometriosis itself, may provide an explanation as to why anatomical conservation of the rectum is not necessarily associated with conservation of rectal function (Darwish and Roman, 2017).
DIER surgery can be challenging, and Clavien–Dindo 3 postoperative complications (Dindo et al., 2004) may involve up to 27% of patients (Roman et al., 2018a). However, it should be emphasized that postoperative complications do not have a negative impact on either 1-year outcomes (Riiskjaer et al., 2018) or postoperative pregnancy rate (Ferrier et al., 2018; Roman et al., 2018b). A fear of postoperative complications should not therefore outweigh the expected benefits in pelvic pain, bowel movements, quality of life and ability to conceive. The intention-to-treat analysis revealed a higher rate of immediate postoperative bladder atony in the excision arm (one patient in the excision arm underwent resection), most probably a chance occurrence. However, 5 years after surgery, bladder function was restored for all patients, in two cases due to sacral root neurostimulation.
We report a high rate of temporary stoma, which is directly related to the proximity of rectal and vaginal sutures and related increases in risk of rectovaginal fistula. Vaginal excision was performed in 88.9% and 71.4% of patients undergoing, nodules excision or colorectal resection, respectively, and a temporary stoma was considered in those patients who were managed by disc excision or rectal resection. Conversely, women managed by shaving did not receive stoma. Although we recently showed that a diverting stoma is responsible for specific morbidity (Bonin et al., 2019), no arguments suggest that this procedure could have an impact on 5-year functional outcomes or quality of life.
The choice of performing separate excision for multiple rectosigmoid nodules may be disputable, as performing two bowel sutures logically increases two-fold the risk of leakage. The risk of leakage is, however, low and probably inferior to that of low anterior resection syndrome when low en bloc rectosigmoid resection is performed (Millochau et al., 2018); although, these presumptions are as yet unproven.
The rate of secondary procedures carried out during the 5 years after surgery may appear unusual. However, of these procedures, only five were directly due to the surgery of the rectum (9.1%), i.e. repair of rectovaginal fistulae, pelvic abscess drainage and resection of stenotic colorectal anastomosis, while three other surgeries were directly linked to a stoma (5.5%). Other additional surgeries were not directly related to rectal surgery and could have occurred following laparoscopic management of any other localization of deep endometriosis.
We recently reported that pregnancy rates in our 55 patient cohorts reached as high as 81% when assessed after a postoperative period varying from 4 to 6 years, with a majority of natural conceptions (Roman et al., 2018b). These results show that surgical management of DIER leads to both clinical improvement and conception and accordingly can be safely recommended to young women with a pregnancy wish who are experiencing pelvic or digestive complaints.
In conclusion, our study demonstrates that DIER surgery by either conservative procedures or segmental resection allows an overall significant long-term improvement in pelvic pain, gastrointestinal complaints and quality of life, as well as a negligible recurrence rate. This improvement occurs shortly after surgery and remains stable >5 years postoperatively. Our data demonstrate that symptomatic patients may benefit from laparoscopic surgery for DIER with favourable long-term outcomes. A surgeon’s goal should be complete excision of digestive tract endometriosis using the least invasive procedure possible for each patient, along with excision of all associated localizations of the disease.
Acknowledgements
The authors are grateful to Amélie Bréant and Karim Lallouche for the management of data and Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Authors’ roles
H.R. and J.J.T. developed the original design. H.R. enrolled patients. H.R., J.J.T., V.B., E.H. and H.K. performed surgical procedures. L.A.B. and M.B. performed statistical analysis. H.R. and C.H. wrote the first draft of the report. All authors contributed to the writing of the final manuscript.
Funding
This study did not benefit from specific funding. Patient enrolment and follow-up until 2 years postoperatively was supported by a grant from the clinical research programme for hospitals in France. Open access was supported by the SELARL du Docteur Horace Roman.
Conflict of interest
The authors declare no competing interests related to this study.
References
- Abo C, Moatassim S, Marty N, Saint Ghislain M, Huet E, Bridoux V, Tuech JJ, Roman H. Postoperative complications after bowel endometriosis surgery by shaving, disc excision, or segmental resection: a three-arm comparative analysis of 364 consecutive cases. Fertil Steril 2018;109:172–178. [DOI] [PubMed] [Google Scholar]
- Abrao MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update 2015;21:329–339. [DOI] [PubMed] [Google Scholar]
- Afors K, Centini G, Fernandes R, Murtada R, Zupi E, Akladios C, Wattiez A. Segmental and discoid resection are preferential to bowel shaving for medium-term symptomatic relief in patients with bowel endometriosis. J Minim Invasive Gynecol 2016;23:1123–1129. [DOI] [PubMed] [Google Scholar]
- Bonin E, Bridoux V, Chati R, Kermiche S, Coget J, Tuech JJ, Roman H. Diverting stoma-related complications following colorectal endometriosis surgery: a 163-patient cohort. Eur J Obstet Gynecol Reprod Biol 2019;232:46–53. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Harper R, Jones NMB, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Bruni F, D’Urso E, Gagliardi ML, Roviglione G, Minelli L, Ruffo G. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc 2012;26:2029–2045. [DOI] [PubMed] [Google Scholar]
- Darwish B, Roman H. Nerve sparing and surgery for deep infiltrating endometriosis: pessimism of the intellect or optimism of the will. Semin Reprod Med 2017;35:72–80. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod 2010;25:1949–1958. [DOI] [PubMed] [Google Scholar]
- Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril 2017;108:931–942. [DOI] [PubMed] [Google Scholar]
- Erdem S, Imboden S, Papadia A, Lanz S, Mueller MD, Gloor B, Worni M. Functional outcomes after rectal resection for deep infiltrating pelvic endometriosis: long-term results. Dis Colon Rectum 2018;61:733–742. [DOI] [PubMed] [Google Scholar]
- Fanfani F, Fagotti A, Gagliardi ML, Ruffo G, Ceccaroni M, Scambia G, Minelli L. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril 2010;94:444–449. [DOI] [PubMed] [Google Scholar]
- Ferrier C, Roman H, Alzahrani Y, d’Argent EM, Bendifallah S, Marty N, Perez M, Rubod C, Collinet P, Daraï E et al. Fertility outcomes in women experiencing severe complications after surgery for colorectal endometriosis. Hum Reprod 2018;33:411–415. [DOI] [PubMed] [Google Scholar]
- Haab F, Richard F, Amarenco G, Coloby P, Arnould B, Benmedjahed K, Guillemin I, Grise P. Comprehensive evaluation of bladder and urethral dysfunction symptoms: development and psychometric validation of the urinary symptom profile (USP) questionnaire. Urology 2008;71:646–656. [DOI] [PubMed] [Google Scholar]
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77–97. [DOI] [PubMed] [Google Scholar]
- Knowles CH, Eccersley AJ, Scott SM, Walker SM, Reeves B, Lunniss PJ. Linear discriminant analysis of symptoms in patients with chronic constipation. Validation of a new scoring system (KESS). Dis Colon Rectum 2000;43:1419–1426. [DOI] [PubMed] [Google Scholar]
- Kupelian AS, Cutner A. Segmental bowel resection for deep infiltrating endometriosis. BJOG 2016;123:1368. [DOI] [PubMed] [Google Scholar]
- Meuleman C, Tomassetti C, D’Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I, Hooghe T. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update 2011;17:311–326. [DOI] [PubMed] [Google Scholar]
- Millochau JC, Stochino-Loi E, Darwish B, Abo C, Coget J, Chati R, Tuech JJ, Roman H. Multiple nodule removal by disc excision and segmental resection in multifocal colorectal endometriosis. J Minim Invasive Gynecol 2018;25:139–146. [DOI] [PubMed] [Google Scholar]
- Nieveen van Dijkum EJM, Terwee CB, Oosterveld P, van der Meulen JHP, Gouma DJ, de Haes JCJM. Validation of the gastrointestinal quality of life index for patients with potentially operable periampullary carcinoma. Br J Surg 2000;87:110–115. [DOI] [PubMed] [Google Scholar]
- Riiskjær M, Forman A, Kesmodel US, Andersen LM, Ljungmann K, Seyer-Hansen M. Pelvic pain and quality of life before and after laparoscopic bowel resection for rectosigmoid endometriosis: a prospective, observational study. Dis Colon Rectum 2018;61:221–229. [DOI] [PubMed] [Google Scholar]
- Riiskjaer M, Greisen S, Glavind-Kristensen M, Kesmodel US, Forman A, Seyer-Hansen M. Pelvic organ function before and after laparoscopic bowel resection for rectosigmoid endometriosis: a prospective, observational study. BJOG 2016;123:1360–1367. [DOI] [PubMed] [Google Scholar]
- Roman H. Rectal shaving using PlasmaJet in deep endometriosis of the rectum. Fertil Steril 2013;100:e33. [DOI] [PubMed] [Google Scholar]
- Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Collinet P, Daraï E, Tuech JJ. Baseline severe constipation negatively impacts functional outcomes of surgery for deep endometriosis infiltrating the rectum: results of the ENDORE randomized trial. J Gynecol Obstet Hum Reprod 2019;48:625–629. doi: 10.1016/j.jogoh.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Daraï E, Collinet P, Tuech JJ. Conservative surgery versus colorectal resection in deep endometriosis infiltrating the rectum: a randomized trial. Hum Reprod 2018a;33:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Chanavaz-Lacheray I, Ballester M, Bendifallah S, Touleimat S, Tuech JJ, Farella M, Merlot B. High postoperative fertility rate following surgical management of colorectal endometriosis. Hum Reprod 2018b;33:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Darwish B, Bridoux V, Chati R, Kermiche S, Coget J, Huet E, Tuech JJ. Functional outcomes after disc excision in deep endometriosis of the rectum using transanal staplers: a series of 111 consecutive patients. Fertil Steril 2017;107:977–986. [DOI] [PubMed] [Google Scholar]
- Roman H, Milles M, Vassilieff M, Resch B, Tuech JJ, Huet E, Darwish B, Abo C. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol 2016;215:762.e1–762.e9. [DOI] [PubMed] [Google Scholar]
- Roman H, Vassilieff M, Tuech JJ, Huet E, Savoye G, Marpeau L, Puscasiu L. Postoperative digestive function after radical versus conservative surgical philosophy for deep endometriosis infiltrating the rectum. Fertil Steril 2013;99:1695–1704. [DOI] [PubMed] [Google Scholar]
- Soto E, Catenacci M, Bedient C, Jelovsek JE, Falcone T. Assessment of long-term bowel symptoms after segmental resection of deeply infiltrating endometriosis: a matched cohort study. J Minim Invasive Gynecol 2016;23:753–759. [DOI] [PubMed] [Google Scholar]


