Abstract
The voltage-gated potassium channel Kv1.5, which mediates the cardiac ultra-rapid delayed-rectifier (IKur) current in human cells, has a crucial role in atrial fibrillation. Therefore, the design of selective Kv1.5 modulators is essential for the treatment of pathophysiological conditions involving Kv1.5 activity. This review summarizes the progress of molecular structures and the functionality of different types of Kv1.5 modulators, with a focus on clinical cardiovascular drugs and a number of active natural products, through a summarization of 96 compounds currently widely used. Furthermore, we also discuss the contributions of Kv1.5 and the regulation of the structure-activity relationship (SAR) of synthetic Kv1.5 inhibitors in human pathophysiology. SAR analysis is regarded as a useful strategy in structural elucidation, as it relates to the characteristics that improve compounds targeting Kv1.5. Herein, we present previous studies regarding the structural, pharmacological, and SAR information of the Kv1.5 modulator, through which we can assist in identifying and designing potent and specific Kv1.5 inhibitors in the treatment of diseases involving Kv1.5 activity.
Keywords: potassium channel, Kv1.5, KCNA5, modulators, SAR
1. Introduction
The voltage-gated potassium channel Kv1.5, which mediates the cardiac ultra-rapid delayed-rectifier (IKur) current in cells [1], is an attractive familial atrial fibrillation (AF) type 7 drug target, because it is selectively expressed in the atria but not in the ventricles of human cells [2]. AF is the most common cardiac arrhythmia facing physicians, afflicting 13% of men and 11% of women over 85 years of age. In atrial tissue from AF donors, the inhibition of IKur extends the repolarization phase of the atrial cardiac action potential, thereby providing desirable antiarrhythmic effects without the risk of drug-induced torsade de pointes. It is noteworthy that loss-of-function Kv1.5 mutations are associated with AF, and many companies are currently exploring IKur modulators for the treatment of AF [3].
The Kv1.5 protein is encoded by the KCNA5 gene with a length of 602 amino acids in mice (Unitprot Entry: Q61762) and rat (Unitprot Entry: P19024) sequences and 613 amino acids in the human sequence (Unitprot Entry: P22460). According to the Basic Local Alignment Search Tool (BLAST) result, the sequence of Kv1.5 is similar to homology targets Kv1.1, Kv1.2, and Kv1.3 in most regions, whereas differences mainly occur toward the start and end terminals of the sequence (see Figure 1C,D). The Kv1.5 channel belongs to the shaker-type voltage-gated K+ channel family, and it comprises four pore-forming α-subunits, each containing six transmembrane segments, named S1–S6 [4,5]. A pore region is formed between the pore helix and S6 domain of each subunit, which contains the selectivity filter through which K+ ions flow across the plasma membrane [6,7]. Currently, the structure of the Kv1.5 protein is still awaiting identification; however, alanine-scanning mutagenesis and homologous modeling studies provide us with some amino acids, including Thr479, Ile502, Val505, Ile508, and Val512, which reside within the deep pore (Thr479-Val481) and lower S6 (Cys500-Val512) regions as putative binding sites for open-channel blockers [8,9,10,11,12,13] (Figure 1B). This not only helps us to understand the drug targets more comprehensively, but also saves time with regard to the development of potential clinical candidates in the future. From this perspective, we highlight recent advances in the discovery of small molecules as modulators of Kv1.5, and we discuss the structure-activity relationship (SAR) studies of currently used synthetic Kv1.5 inhibitors.
Figure 1.
(A) Schematic representation of the hKv1.5 α-subunit with the sequence of the S6 region listed. (B) Homologous model of Kv1.5 (Q61672) with 67.2% similarity for the Kv1.5 sequence, obtained from the SWISS-MODEL database; some of the residues are slightly different from those published in previous research. (C) Basic Local Alignment Search Tool (BLAST) result of KCNA5_HUMAN (P22460), obtained from the NCBI BLAST+ database. (D) Sequence alignment ofKCNA1_HUMAN (Q09470), KCNA3_HUMAN (P22001), KCNA2_HUMAN (P16389), and KCNA5_HUMAN (P22460), acquired from the ESPript database.
2. Summarization of Models and Mechanisms of Kv1.5 Modulators
To date, various kinds of Kv1.5 modulators have been disclosed, herein, we summarize the molecular structures and functionality of different types of Kv1.5 modulators with their chemical structure as follows (Table 1, Figure 2). As shown in Table 1, the existing Kv1.5 modulators can be divided into four categories: clinical cardiovascular drugs (1–14), other clinical drugs (15–28), drugs in development (29–37), and natural products (38–56). With the development of pharmacology, more and more experiment models including rats, HEK cells, CHO cells, Xenopus laevis oocytes, and Ltk- cells have been used to evaluate the effect of Kv1.5 channel modulators; the parameters containing mRNA expression, IKur, effective refractory period (ERP), and action potential duration (APD) were utilized to reveal the improvement degree of AF. In principle, the Kv1.5 modulators can lengthen the time course of ERP and APD to protect heart from the harm of AF.
Table 1.
Active Kv1.5 modulators.
| No. | Name | CAS | Status | Model | Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Clinical Cardiovascular Drugs | ||||||
| 1 |

|
54-96-6 | Approved | Smooth muscle cells | Blocking hKv1.5 current with a threshold fur activation near –45 mV. | [30] |
| 2 |

|
504-24-5 | Approved | HEK cells | Inhibiting hKv1.5 current after long-term treatment, abbreviating the prolongation of action potential duration in chronic atrial fibrillation (AF). | [31] |
| 3 |

|
794466-70-9 | Approved, investigational | HEK cells | Selective blocking of the Kv1.5 channel by interacting with important residues including Thr 479, Thr 480, Ile 502, Val 505, and Val 508. | [32] |
| 4 |

|
1951-25-3 | Approved, investigational | Papillary muscles or single ventricular cells | Decreasing the amount of mRNA for Kv1.5. | [33] |
| 5 |

|
54143-55-4 | Approved, withdrawn | Xenopus laevis oocytes | Producing open-channel block of Kv1.5 by sensitively interacting with key residues including Asp 469, Val 481, and Ile 502 in the S6 region of Kv1.5. | [34] |
| 6 |

|
21829-25-4 | Approved | HEK cells | Blocking hKv1.5 channels with 6.3 μM of Kd was affected by mutations like Arg 487 similar to those known to affect outer pore C-type inactivation. | [35] |
| 7 |

|
54063-53-5 | Approved | Ltk- cells | Inhibiting hKv1.5 current with Kdvalue of 9.2 μM, showing time-dependent and dose-dependent manners simultaneously. | [36] |
| 8 |

|
86384-10-3 | - | Ltk- cells | Inhibiting hKv1.5 current with Kdvalue of 4.4 μM, showing time-dependent and dose-dependent manners simultaneously. | [36] |
| 9 |

|
56-54-2 | Approved, investigational | HEK cells | Producing a voltage-dependent block between +30 and +120 mV (Kd at +60 mV = 7.2 μM) with an equivalent electrical distance in the steady state. | [37] |
| 10 |

|
42399-41-7 | Approved, investigational | CHO cells | Blocking hKv1.5 channels, in a frequency-dependent manner exhibiting a biphasic dose-response curve (IC50: 4.8 nM and 42.3 μM) by binding to the open and inactivated state of the channels. | [38] |
| 11 |

|
66-40-0 | Experimental, investigational | BT-474 breast cancer cell | Blocking hKv1.5 channels in a delayed rectifier manner. | [39] |
| 12 |

|
68379-03-3 | - | CHO cells | Inhibiting hKv1.5 current with concentration-dependent acceleration of the apparent channel inactivation in both outside-out and inside-out patches. | [40] |
| 13 |

|
163163-23-3 | - | CHO cells | Blocking hKv1.5 current stereoselectivity, the results showed that (-)-[3R, 4S] was more potent than the (-)-enantiomer. | [41] |
| 14 |

|
64706-54-3 | Approved, withdrawn | HEK cells | Inhibiting the hKv1.5 channel current with IC50 value of 6.6 μM. | [42] |
| Other Clinical Drugs | ||||||
| 15 |

|
120014-06-4 | Approved | HEK cells | Resulting in a rapid and reversible block of Kv1.5 currents (IC50: 72.5 μM) with a significant delay in the duration of activation and deactivation, and the outer mouth region proved to be the target site. | [15] |
| 16 |

|
61869-08-7 | Approved, investigational | CHO cells | Slowing the deactivation time course, resulting in a tail crossover phenomenon when the tail currents, recorded in the presence and absence of paroxetine, were superimposed. | [43] |
| 17 |

|
54910-89-3 | Approved, vet approved | Human Pulmonary Artery Smooth Muscle Cells | Protecting against big endothelin-1 induced anti-apoptosis and rescued Kv1.5 channels in human pulmonary arterial smooth muscle cells. | [44] |
| 18 |

|
79617-96-2 | Approved | CHO cells | Reducing Kv1.5 whole-cell currents in a reversible dose-dependent manner and accelerating the decay rate of inactivation of Kv1.5 currents without modifying the kinetics of current activation. | [45] |
| 19 |

|
53-06-5 | Approved | Xenopus oocytes | Suppressing the amplitude of Kv1.5 channel current with IC50 value of 50.2 μM. | [46] |
| 20 |

|
50-23-7 | Approved, vet approved | Xenopus oocytes | Suppressing the amplitude of Kv1.5 channel current with IC50 value of 33.4 μM. | [46] |
| 21 |

|
52-01-7 | Approved | Male Wistar rats | Shorting the APD90(action potential duration) and increasing the expression of Kv1.5. | [47] |
| 22 |

|
169590-42-5 | Approved, investigational | Ltk- cells | Blocking hKv1.5 channels with an IC50 of 26.2 μM for the peak current and 5.5 μM for the current at the end of a 250 ms pulse to +60 mV. | [48] |
| 23 |

|
38396-39-3 | Approved, investigational | Ltk- cells | Blocking the opening of hKv1.5 channels stereoselectivity; the results showed the Kd value for R(+)-enantiomer (4.1 μM) was six-fold more potent than the S(-)-enantiomer (27.3 μM). | [49,50] |
| 24 |

|
2078-54-8 | Approved, investigational, vet approved | CHO cells | Inducing a time-dependent decline of the hKv1.5 current (IC50: 62.9 μM) during depolarizing steps and slowing the time course of tail current decay upon repolarization. | [4] |
| 25 |

|
59467-70-8 | Approved | HEK cells | Inhibited Kv1.5 current (IC50: 17 μM) without influence on the half-maximal activation voltage of Kv1.5 channels. | [51] |
| 26 |

|
64-77-7 | Approved, investigational | Insulin-secreting (INS-1) cells | Activating Kv1.5 channel and the activation of secretion can be counteracted by an excessive stimulation of Kv channels in INS-1 cells which shorten the Ca2+ signal and confine the insulin secretion. | [52] |
| 27 |

|
94-09-7 | Approved | Ltk- cells | Blocking hKv1.5 channels in a voltage-dependent manner and modifying the voltage-dependence of channel activation. | [53] |
| Drugs in Development | ||||||
| 28 |

|
1163-36-6 | Phase 2 Clinical | HEK cells | Decreasing IKs and human Kv1.5 channel current at doses of 3 and 10 μM at voltages ranging from –14.3 to +34.7 mV. | [54] |
| 29 |

|
767334-89-4 | Phase 1 discontinued | CHO cells | Inhibiting hKv1.5 current with IC50 value of 3.6 μM, blocking early atrial K+ channels, and prolonging atrial refractoriness with no effects on electrocardiography intervals and ventricular repolarization. | [55] |
| 30 |

|
864368-79-6 | Phase 2 discontinued | CHO cells | Blocking hKv1.5 current with IC50 value of 27 μM with a slight decrease at higher frequency. | [56] |
| 31 |

|
343246-73-1 | Phase 1 discontinued | Mouse fibroblast L929 cells | Showing excellent activity in blocking Kv1.5 (IC50: 0.05 μM) and very good selectivity over hERG, sodium, and L-type calcium ion channels. | [57] |
| 32 |

|
1272353-82-8 | Phase 1 discontinued | Mammalian L-929 cells | Blocking hKv1.5 current with IC50 value of 0.05 μM with an acceptable in vitroselectivity and liability profile and a good pharmacokinetic profile across species. | [58] |
| 33 |

|
875562-81-5 | Phase 1 discontinued | HK2BN9 cells | Blocking Kv1.5 current in an expression system and concentration-dependently elevated the plateau phase of atrial action potentials (APs). | [59] |
| 34 | XEN-D0103 (Undisclosed structure) |
1410180-16-3 | Phase 2 discontinued | CHO cells | Prolongating action potential duration (APD) and suppressed APs at high stimulation rates in sinus rhythm (SR) and paroxysmal AF (pAF) tissue. | [60] |
| 35 |

|
154447-36-6 | Experimental | CHO cells | Acting directly on hKv1.5 currents as an open channel blocker with key interacting residues located in the pore region (Thr 480, Arg 487) and the S6 segment (Ile 502, Ile 508, Leu 510, Val 516). | [9] |
| 36 |

|
752253-75-1 | - | CHO cells | Inhibiting several potassium currents including IKr, IKs, IK(ACh), and IKv1.5 at doses of 0.01–30 μM. | [61] |
| 37 |

|
1034194-07-4 | - | HEK cells | Inhibiting hKv1.5 current slightly when specially blocked by the Kv11.1 channel. | [62] |
| Natural Products | Type | |||||
| 38 |

|
2334247-91-3 | Terpenoid | CHO cells | Blocking Kv1.5 with an IC50 value of 6.94 μM. | [63] |
| 39 |

|
2334247-94-6 | Terpenoid | CHO cells | Blocking Kv1.5 with an IC50 value of 0.30 μM. | [63] |
| 40 |

|
57444-62-9 | Terpenoid | C6 glioma cells | Inhibiting the hKv1.5 current in time and dose-dependent manners. | [64] |
| 41 |

|
13018-10-5 | Terpenoid | Ltk- cells | Inhibiting the hKv1.5 current in time- and voltage-dependent manners, with an IC50 value of 2.51 μM at +60 mV accelerated the inactivation kinetics of the hKv1.5 channel and slowed the deactivation kinetics of the hKv1.5 current, resulting in a tail crossover phenomenon. | [65] |
| 42 |

|
1394-48-5 | Alkaloid | Guinea pigs | Blocking I-Kv1.5 slightly with a ratio of 20.6% at a dosage of 200 μM. | [66] |
| 43 |

|
90-69-7 | Alkaloid | HEK cells | Accelerating the decay rate of Kv1.5 inactivation, decreased the current amplitude at the end of the pulse in a concentration-dependent manner with an IC50 value of 15.1 μM. | [67] |
| 44 |

|
4360-12-7 | Alkaloid | Xenopus oocytes | Inhibiting Kv1.5 with an IC50 of 1.70 μM in Xenopus expression system, resulting in a mild leftward shift of Kv1.5 activation curve. | [68] |
| 45 |

|
58-74-2 | Alkaloid | Ltk- cells | Blocking hKv1.5 channels and native hKv1.5 channels in a concentration-, voltage-, state-, and time-dependent manner. | [69] |
| 46 |

|
2934-97-6 | Alkaloid | HEK cells | Blocking Kv1.5 currents dose-dependently with an IC50 value of 53.2 μM inhibited the delayed rectifier effect of Kv1.5 resulting in a potential left shift of the inactivation curve. | [70] |
| 47 |

|
302-27-2 | Alkaloid | Xenopus laevis oocytes | Producing a voltage-, time-, and frequency-dependent inhibition of Kv1.5 (IC50: 0.796 μM). | [71] |
| 48 |

|
529-44-2 | Flavonoid | HEK cells | Inhibiting Ikur and the expression of hKv1.5 in a dose-, time-, and frequency-dependent manner. | [72] |
| 49 |

|
5631-70-9 | Flavonoid | HEK cells | Suppressing hKv1.5 current in HEK 293 cell line (IC50: 6.4 μM) and the ultra-rapid delayed rectify K+ current IKur in human atrial myocytes (IC50: 8.0 μM) by binding to open channels in a use- and frequency-dependent manner. | [73] |
| 50 |

|
117-39-5 | Flavonoid | Xenopus laevisoocytes | Activating hKv1.5 channels (EC50: 37.8 μM) by interacting with key residue Ile 502 in S6 region. | [74] |
| 51 |

|
480-44-4 | Flavonoid | HEK cells | Blocking open hKv1.5 channels by binding to their S6 domain influenced by the interaction of V505A, I508A, and V512A. | [75] |
| 52 |

|
501-36-0 | Phenol | Human PASMCs | Reducing the expression of Kv1.5 mRNA to reverse monocrotaline-induced pulmonary vascular and cardiac dysfunction. | [76] |
| 53 |

|
5928-25-6 | Coumarin | Ltk− cells | Inhibiting hKv1.5 current in a concentration- and use-dependent manner, with an IC50 value of 2.7 μM at +60 mV accelerated the inactivation kinetics of the hKv1.5 channel, resulting in a tail crossover phenomenon. | [77] |
| 54 | Kaliotoxin | 145199-73-1 | Polypeptide | T cell | Inhibiting hKv1.5 current in a dose-dependent manner. | [64] |
| 55 |

|
190017-00-6 | Nor-triterpenoid | CHO cells | Inhibiting Kv1.5 with an IC50 of 1.77 μM and influenced by the mutations T480A, V505A, I508A, as well as V516A. | [78] |
| 56 |

|
107-35-7 | Amino acid | Male Wistar rats | Down-regulating the mRNA expression level of Kv1.5. | [79] |
Figure 2.
(A) Pharmacophore model of vernakalant (cyan ball: hydrophobic center; yellow ball: aromatic center; green ball: hydrogen bond receptor; pink ball: hydrogen bond donor; red ball: ionizable positive center); (B) potential binding domain of vernakalant in Kv1.5 (H-bond is expressed as green dashed).
Although the structure of Kv1.5 protein has not been characterized yet, current researches provide information for the development of Kv1.5 inhibitors according to fragment-based drug design and structure-based drug design. In regard to the design of Kv1.5 inhibitor, for the instance of the typical candidate vernakalant, in the pharmacophore model, hydrogen bond receptor, hydrogen bond donor, and hydrophobic groups should be present in the structure (Figure 2A) to play a role in the transmembrane effect to interact with the Kv1.5 channel. From the potential binding domain of vernakalant in Kv1.5 [8,14] (Figure 2B), we can see that the positively charged moiety bound in the cationophilic inner pore (mainly formed by electron-donating residues including alanine, leucine, and valine) formed a cationic “blocking particle” causing a block of the potassium channel; additionally, the uncharged dimethoxyphenyl moiety of a vernakalant has a tendency to bind in hydrophobic subunit interfaces including residues Ile 502 and Val 505. Functionally important residue isoleucine I502 in the inner helix S6 is exposed into the subunit interface of the pore module rather than into the inner pore. It is worth noting that mutations of Ile 502 decrease the potency of vernakalant, flecainide, and AVE0118, which are the ligands with a long hydrophobic tail in the side chain of the structure.
It seems that the introduction of heterocyclic rings including pyrrole (vernakalant, bepridil, clemizole, and BMS-394136) and piperdine (lobeline, CD-160130, bupivacaine, paroxetine, and donepezil) is important because these moieties usually influence the acidification conditions of the molecules, in which a potentially protonated and thus positively charged drug may enter deeply into the channel pore in a voltage-dependent way [15].
As a result of the definite curative effects and pharmacokinetic parameters proved by clinical trials, conventional drugs in new use trends seem to be a feasible way to develop new therapy. Multiple cardiovascular drugs not designed for targeting Kv1.5 have shown Kv1.5 inhibitory effect including quinidine (9) and diltiazem (10), however, the selectivity of these compounds on Kv1.5 still needs to be investigated.
As for other clinical drugs, CNS agents include: donepezil (15), which is generally used as an anti-Alzheimer’s agent; paroxetine (16), fluoxetine (17), and sertraline (18), which are usually used as antidepressant agents; and bupivacaine (23), propofol (24), midazolam (25), tolbutamide (26), and benzocaine (27), which are utilized as anesthetic agents. hERGs (human ether-à-go-go-related genes) are widely associated with CNS diseases [16,17,18], thus it is not strange that active CNS agents can effectively modulate Kv1.5 according to the homology of the protein. Especially the neurotransmitter acetylcholine, which is an important substance that modulates the acetylcholine-activated K+ current [19], however, only the piperidine type acetylcholine inhibitor donepezil showed significant inhibitory effect on Kv1.5, the same phenomenon was not present in another inhibitor tacrine [15], suggesting the selectivity of the binding site of Kv1.5.
Generally, Kv1.5 drugs in development are not going smoothly. The projects listed in Table 1 have been discontinued till now. Effectiveness, toxicity, and druggability should be taken into account at this stage. Persistence of investigation in this field is necessary because the listed compound like AZD-7009 (30) can not only alleviate the suffering of patients from intermittent AF but also plays a role in relieving durative AF which continues to attack for more than 48 h [20]. The major voltage-gated K+ channels expressed in the vasculature are Kv1.2, Kv1.5, Kv2.1, and Kv7.4/7.5 [21]. Kv1.3, another Shaker-related family voltage-gated K+ channel, is closely related to the hERG channels regulated by Kv1.1 [22], which are the important targets influencing the prolongation of Q band to the end of T band (QT) syndrome and torsade pointes attributed to the gain-of-function mutations of clinical candidates whose details are being requested by drug regulatory authorities. Limitations in the ability of high-throughput screening methods to monitor the complex behavior of hERG have restricted the discovery of activators. It is noteworthy that some inhibitors of Kv1.5 channels listed in Table 1 are not specific voltage-gated K+ channels for Kv1.5, and some of which also block Kv1.3 channels (e.g., 4-aminopiridine (2), nifedipine (6), diltiazem (10), tetraethylammonium (11), propofol (24) [23], resveratrol (52) [24], and correolide (55)). Application of these drugs may result in side effects related to the inhibition of Kv1.3 channels like immune suppression, thus more attention should be paid to the toxicity to hERG-related targets of Kv1.5 developing candidates. Additionally, in the field of immunization [25], nuclear factor erythroid 2-related factor (Nrf2)-induced oxidative stress-inducible protein 1/p62 enhances the inhibition of pulmonary arterial Kv1.5 channels under acute hypoxia, and the 1/p62-Kv1.3-integrin axis provides novel insight into the molecular mechanisms underlying redox-regulated cell signaling in stress-induced biological responses, which broaden future potential directions.
A variety of natural products have been proven to modulate Kv1.5, but the exploration of novel skeleton could be helpful for the current dilemma. Among the isolated compounds, the main types are terpenoids (38–41), alakaloids (42–47), and flavonoids (48–50). Terpenoids are widely reported to inhibit potassium channels [26,27,28], however, the stability and difficulty in preparation because of the lack of a fluorescence group and the abundance in chiral carbon are worth worrying about in the development. Alkaloids, as well as polypeptides like kaliotoxin (54) and toxins from marine animals like tetrodotoxin, have been disclosed to inhibit ion channel activity, but the toxicity of these types of compounds is also concerning; after all, hERG toxicity has attracted the attention of the FDA and drugs like bepridil have been withdrawn because of their toxicity [29]. Bioactive flavonoids are also proven to modulate the Kv1.5 channel; among them is quercetin (50), a minor compound and activator of Kv1.5, with the tendency of developing flavonoids and phenols as health care products or food additives.This class of compounds may play a role in the daily prevention against Kv1.5 disease.
3. Synthetic Kv1.5 Inhibitors and SAR Investigations
In this section we collated information about chemical synthesis, pharmacological properties, and SAR investigations in the published literature from 2003 to 2019 and summarized them in a timeline. The previous work was briefly introduced in the description ofthe potential synthetic derivatives and chemical structure of compounds, and the SAR studies are listed in the corresponding figures in the perspective of medicinal chemistry. As we can see, multiple scaffolds include 5-methoxypsoralen (60,68), tetrahydroindolone (62–65), benzopyran sulfonamides (70–72), dihydropyrazolopyrimidine (73,81), and phenylquinazoline (90–92). Compounds (86–88) have been reported to be effective in inhibiting Kv1.5, suggesting potential future directions for investigations about Kv1.5 inhibitors. It is noteworthy that research from Bristol-Myers Squibb has contributed greatly with data about pharmacology and pharmacokinetics of active compounds in blocking Kv1.5, increasing the possibility that we can conquer the diseases targeting Kv1.5.
In 2003, Peukert and co-workers [80] synthesized a series of ortho-disubstituted bisaryl compounds as blockers of the Kv1.5 channel. Among the derivatives, the most potent compounds 57 (IC50: 0.7 μM) and 58 (IC50: 0.16 μM) inhibited the Kv1.5 channel with sub-micromolar half-blocking concentrations and displayed three fold selectivity over Kv1.3 and no significant effect on the hERG channel and sodium currents (Figure 3).
Figure 3.
Biphenyl derivatives.
In 2004, Peukert et al. [81] synthesized several anthranilic amides as novel blockers of the Kv1.5 channel. The most hopeful analogue 59 showed moderate Kv1.5 inhibition (IC50: 0.7 μM) with good oral bioavailability, however, no significant effect on the IKr current of 59 was detected (Figure 4).
Figure 4.
Anthranilic amides.
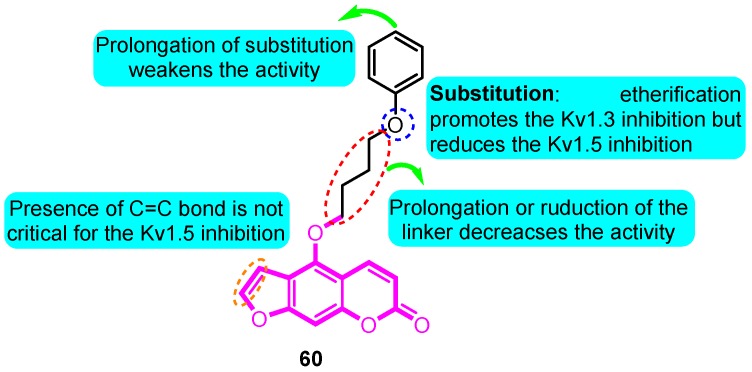
Inspired from the precursor 5-methoxypsoralen isolated from Rutagraveolens, Schmitz and colleagues [82] prepared a series of phenoxyalkoxypsoralen analogues and evaluated their voltage-gated ion channel blocker potency. The most potent and “druglike” compound of this series, 5-(4-phenoxybutoxy) psoralen (PAP-1, 60), blocks Kv1.3 in a use-dependent manner, with a Hill coefficient of 2 and an EC50 of 2 nM, by preferentially binding to the C-type inactivated state of the channel. PAP-1 is 23 fold selective over Kv1.5, 33–125 fold selective over other Kv1 family channels, and 500–7500 fold selective over Kv2.1, Kv3.1, Kv3.2, Kv4.2, hERG, calcium-activated K channels, Na, Ca, and Cl channels. PAP-1 does not exhibit cytotoxic or phototoxic effects, is negative in the Ames test, and affects cytochrome P450-dependent enzymes only at micromolar concentrations (Figure 5).
Figure 5.
Phenoxyalkoxypsoralen analogues.
In 2006, Blass et al. [83] synthesized a cluster of (2-phenethyl-2H-1,2,3-triazol-4-yl) (phenyl) methanone and examined for utility as Kv1.5 channel blockers for the treatment of atrial fibrillation. The results showed that O substitution in the 4-position of the acetophenone-derived portion of the scaffold is highly favored, and the most active compound 61 blockaded Kv1.5 for 99% at a concentration of 1 μM (Figure 6).
Figure 6.
(2-phenethyl-2H-1,2,3-triazol-4-yl)(phenyl) methanones.
Fluxe and co-workers [84] synthesized multiple tetrahydroindolone-derived carbamates as potent Kv1.5 blockers. The most promising analogues 62 and 63 exhibited the strongest Kv1.5 inhibitory effect with IC50 values of 67 and 21 nM, respectively. They were also very selective over hERG (> 450 fold) and L-type calcium channels (> 450 fold) (Figure 7).
Figure 7.
Tetrahydroindolone-derived carbamates.
Subsequently, Wu et al. [85] designed and synthesized tetrahydroindolone derived semicarbazones as selective Kv1.5 blockers. Compounds 64 and 65 showed good selectivity for the blockade of Kv1.5 (IC50: 0.13 μM for two compounds), moreover, in an anesthetized pig model, compounds 64 and 65 increased atrial ERP by about 28% and 18%, respectively, in the right atrium without affecting ventricular ERP (Figure 8).
Figure 8.
Tetrahydroindolone-derived semicarbazones.
Based on a diisopropyl amide scaffold, a series of potent Kv1.5 ion channel antagonists were synthesized by Nanda and colleagues [86]. The most active derivative 66, which was a single active enantiomer of the diastereomerically pure racemic analog, exhibited significant atrial-selective effects in an in vivo model (IC50: 150 nM) (Figure 9).
Figure 9.
Diisopropyl amide derivatives.
Trotter and co-workers [87] designed and synthesized a group of isoquinoline-3-nitriles as orally Kv1.5 antagonists for the treatment of AF. The ethanolamide derivative 67 exhibited improved potency (Kv1.5 HT-Clamp IC50: 60 nM), excellent selectivity versus hERG, and good pharmacokinetic properties. Rat EP experiments confirmed that the compound potently increased ARP without significant effects on AVRP− (Figure 10).
Figure 10.
Isoquinoline-3-nitriles.
In 2007, Eun et al. [88] synthesized multiple psoralen derivatives as hKvl.5 channel blockers. Among them, compound 68 was the most potent in blocking hKv1.5 (IC50: 27.4 nM), much stronger than the lead compound psoralen. Compound 68 accelerated the inactivation kinetics of the hKvl.5 channel and slowed the deactivation kinetics of the hKv1.5 current resulting in a tail crossover phenomenon. Compound 68 inhibited the hKvl.5 current in a use-dependent manner (Figure 11).
Figure 11.
Psoralen derivatives.
Jackson and co-workers [89] prepared several classes of thiazolidine-based Kv1.5 blockers. The most promising analogue 69 derived from 3,4-dimethylacetophenone exhibited the strongest inhibitory effect with an IC50 value of 69 nM (Figure 12).
Figure 12.
Thiazolidine derivatives.
Lloyd et al. [90] synthesized a series of benzopyran sulfonamides and determined Kv1.5 potassium channel blocking effects. Among the productions, derivative 70 exhibited the most significant activity (IC50: 57 nM), and a moderate inhibition (35%) of hERG at a concentration of 10 μM (Figure 13).
Figure 13.
Benzopyran sulfonamides.
In 2008, benzopyran sulfonamides derivatives were further investigated [91]. Compound 71 and 72 were considered as the most active derivatives in the two series of compounds with IC50 values of 46 and 378 nM in the inhibition of current in a L-929 cell model, respectively. Additionally, at the concentration of 1.0 μM, compound 72 displayed the most significant inbitory effect in the current of L-929 cells with an inhibitory ratio of 89% (Figure 14).
Figure 14.
Thiazolidine derivatives.
Vaccaro and co-workers [90] synthesized a series of dihydropyrazolopyrimidine analogues as Kv1.5 inhibitors. The most promising compound 73 showed the best potential in suppressing Kv1.5, with inhibitory effects on hERG (69%) and INa10 (42%) at a concentration of 10 μM (Figure 15).
Figure 15.
Dihydropyrazolopyrimidine derivatives.
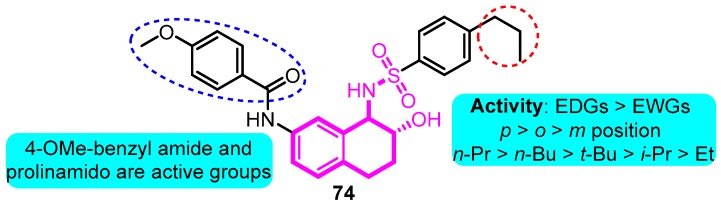
In 2008, Gross and co-workers [92] synthesized aryl sulfonamido tetralin as a Kv1.5 inhibitor according to the basis of previous work. Among the productions, compound 74 exhibited remarkable Kv1.5 inhibitions with an IC50 value of 90 nM; in addition, moderate hERG inhibition was detected at the dose of 10 μM (39%), indicating the potential for further development of clinical candidates (Figure 16).
Figure 16.
Aryl sulfonamido tetralin derivatives.
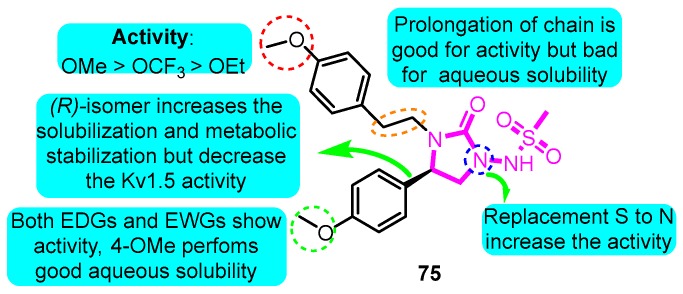
According to the structure of marketed drugs amiodarone and vernakalant, Blass et al. [93] synthesized a series of imidazolidinone derivatives as a potential treatment for atrial arrhythmia. KVI-020/WYE-160020 (75) exhibited the efficacy in clinically relevant models of AF and mechanistic models of the cardiac action potential with acceptable pharmacokinetic and pharmaceutical properties. The pharmacology IC50 values for compound 75 in Kv1.5, hERG, Nav1.5, Cav1.3, Cav1.2, Kv1.1, Kv1.3, and Kv4.3 were 0.48, 15.1, >30, 23.4, >30, 2.66, 1.41, and 3.87 μM in vitro, respectively (Figure 17).
Figure 17.
Structure-activity relationship (SAR) of imidazolidinone derivatives.
In 2010, Lloyd and co-workers [58] developed a series of pyrazolodihydropyrimidines as potent and selective Kv1.5 blockers based on previous studies. The most promising analogue BMS-394136 (76) displayed excellent activity in blocking Kv1.5 (IC50: 50 nM) and very good selectivity over hERG, sodium, and L-type calcium ion channels with good pharmacokinetic parameters (Figure 18).
Figure 18.
SAR of pyrazolodihydropyrimidines.
In 2012, Blass [94] prepared several heteroarylsulfonamides as Kv1.5 inhibitors. The active analogues 77, 78 and 79 exhibited 100% inhibition of Kv1.5 using stably transfected HEK293 cells and the FLIPR potassium ion channel assay, suggesting good potential for further investigation (Figure 19).
Figure 19.
SAR of heteroarylsulfonamides.
Finlay and colleagues [95] prepared several dihydropyrazolo[1,5-a]pyrimidine derivatives. Among the synthetic compounds, compound 80 showed potential to be a selective IKur inhibitor with Kv1.5 IC50 of 0.15 μM and hERG with an IC50 value >10 μM. Furthermore, favorable pharmacokinetic properties in rats and dogs of 80 were determined; compound 80 was identified with less than 1% GSH adducts formation with an improved PK profile and equivalent PD efficacy to the lead compound (Figure 20).
Figure 20.
SAR of dihydropyrazolo[1,5-a]pyrimidine derivatives.
In 2013, triazolo and imidazo were introduced into the active scaffold dihydropyrazolopyrimidine [96]. Trifluoromethylcyclohexyl triazole analogue 81 was identified as a potent and selective Kv1.5 inhibitor (IC50: 133 nM) with an acceptable PK and liability profile. Compound 81 demonstrated an improved rat PK profile and was advanced to the rat PD model (Figure 21).
Figure 21.
SAR of trifluoromethylcyclohexyl triazole analogues.
With the help of a pharmacophore model, Guo et al. [97] designed and synthesized a series of indole derivatives as potent Kv1.5 inhibitors. The most promising compound 82 displayed significant INa, HEK 293 hKv1.5, and CHO hERG inhibitory activities with IC50 values of 52.6, 0.51, and 418.35 μM, respectively, which displayed remarkable selectivity and ameliorating effects on atrial effective refractory period (AERP) and VERP (Figure 22).
Figure 22.
SAR of indole derivatives.
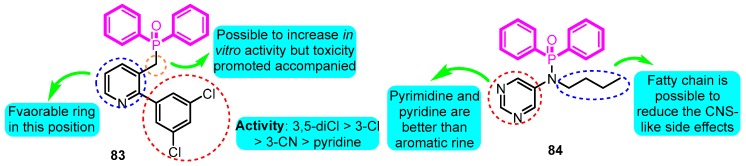
Olsson and co-workers [98] possessed design and pharmacological evaluation of multiple potential hits targeting on Kv1.5. The compound 83 performed the best in vitro activity with Kv1.5 IC50 of 0.08 μM in diphenylphosphinic amide and diphenylphosphine oxide analogues (Figure 23). However, both hERG and IKs active and remarkable safety in rats of compound 83 was detected and judged unsuitable for in vivo testing; conversely, the derivative 84 was regarded as a hopeful compound for further development with Kv1.5 IC50, IKs, Ceu20, and QTmax change values for 1.0 μM, >33%, 0.6 μM, and <10%, respectively.
Figure 23.
SAR of diphenylphosphinic amides and diphenylphosphine oxides.
In 2014, the subsequent study was updated [99], and a series of lactam sulfonamide derivatives was prepared and the Kv1.5 inhibitory potency was evaluated. The most promising candidate 85 inhibited Kv1.5 with an IC50 value of 0.21 μM and caused a marked increase in the atrium ERP with a Ceu20 of 0.35 μM, which was at the same order of magnitude as the IC50 value from the human cellular assay. The human hERG channel was blocked by compound 85 with an IC50 value of 30 μM, indicating a 140 fold margin of the hERG and Kv1.5 in vitro values. No measurable change was noted in the QT-interval in the rabbit experiments, which also indicated a good margin to block of the hERG channel. The compound 85 was well tolerated in rabbits with no signs of the CNS-like side effects observed for other Kv1.5 blockers (Figure 24).
Figure 24.
SAR of lactam sulfonamides.
Johnson et al. [100] synthesized phenethylaminoheterocycles and assayed for inhibition of the Kv1.5 potassium ion channel as a potential approach to the treatment of atrial fibrillation. Combination of the indazole with a cyclohexane-based template gave the most promising derivative 86 (Kv1.5 IC50: 138 nM) which demonstrated significant prolongation of AERP in the rabbit pharmacodynamic model (Figure 25).
Figure 25.
SAR of phenethylaminoheterocycles.
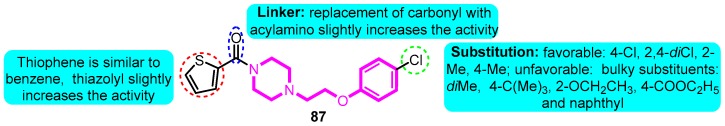
Guo and colleagues [101] prepared a series of 1-aryloxyethyl piperazine derivatives as Kv1.5 potassium channel inhibitors. The most potent compound 87 exerted significant activity on hKv1.5 (IC50: 0.72 μM), balanced Log D, and permeability. In addition, comparable in vivo potency with sotalol and dronedarone and remarkable safety in rats of compound 87 were detected as well (Figure 26).
Figure 26.
SAR of 1-aryloxyethyl piperazine derivatives.
In 2016, Kajanus et al. [102] synthesized multiple isoindolinone compounds as Kv1.5 blockers. The most potent compounds 88 and 89 exhibited an inhibitory effect with the IC50 values of 0.4 and 0.7 µM on Kv1.5, respectively. The above-mentioned two compounds were found to have desirable in vivo PK properties in a mouse model (Figure 27).
Figure 27.
SAR of isoindolinones.
Finlay and co-workers [103] explored phenylquinazoline derivatives as Kv1.5 inhibitors. 5-Phenyl-N-(pyridin-2-ylmethyl)-2-(pyrimidin-5-yl)quinazolin-4-amine (90) was identified as a potent and ion channel selective inhibitor (Kv1.5 IC50: 90 nM, hERG inhibition: 43% at 10 μM) with robust efficacy in the pre-clinical rat ventricular effective refractory period (VERP) model and the rabbit atrial effective refractory period (AERP) model (Figure 28).
Figure 28.
SAR of phenylquinazoline derivatives.
Subsequently in 2017, Gunaga et al. [58] modified the structure of 91 with a series of analogues and evaluated the IKur inhibitory effect. 5-[5-Phenyl-4-(pyridin-2-ylmethylamino)-quinazolin-2-yl] pyridine-3-sulfonamide (92) was identified as the lead compound in this series with good selectivity over hERG (Kv1.5 IC50: 50 nM, hERG IC50: 1.9 μM). Compound 91 exhibited robust effects in rabbit and canine pharmacodynamic models and an acceptable cross-species pharmacokinetic profile which was then advanced as a clinical candidate. Further optimization of 91 to mitigate pH-dependent absorption resulted in identification of the corresponding phosphoramide prodrug (92) with an improved solubility and pharmacokinetic profile (Figure 29).
Figure 29.
SAR of phenylquinazoline sulfonamide derivatives.
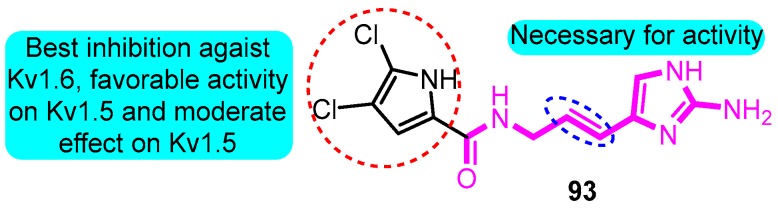
According to the skeleton of Agelas alkaloids clathrodin, oroidin, and hymenidin, Zidar and colleagues [104] synthesized multiple derivatives as inhibitors of the voltage-gated potassium channels. The most potent inhibitor was (E)-N-(3-(2-amino-1H-imidazol-4-yl)allyl)-4,5-dichloro-1H-pyrrole-2-carboxamide (93) with IC50 values between 1.4 and 6.1 mM against Kv1.3, Kv1.4, Kv1.5, and Kv1.6 channels (Kv1.5 IC50: 6.1 μM) (Figure 30).
Figure 30.
SAR of oroidin derivatives.
Wolkenberg et al. [105] told the story of the development of prospective candidate MK-1832 (94) (Figure 31). Based on the structure of MK-0448, a cluster of derivatives were synthesized and tested the Kv1.5 inhibitory effect and in vivo and in vitro toxicity. MK-1832 (94) was considered to be the best derivative with pharmacological parameters including Kv1.5, Ikur, and Ikr(hERG) IC50 values for 29, 11 and 1.28 × 10 5 nM, respectively, and pharmacokinetic parameters including dog in vivo atrial refractory period EC10 for 14 nM and threshold change in ventricular refractory period >25 μM.
Figure 31.
SAR of oroidin MK-1832.
In 2019, Kajanus and colleagues [106] prepared potassium channel blocking 1,2-bis(aryl)ethane-1,2-diamines active as antiarrhythmic agents. The most promising analogue 95 displayed significant nanomolar potency in blocking Kv1.5 in human atrial myocytes (IC50: 1.7 μM, IKur IC50: 60 nM) and based on the PD data, the estimated dose for men was 700 mg/day (Figure 32).
Figure 32.
SAR of 1,2-bis(aryl)ethane-1,2-diamines.
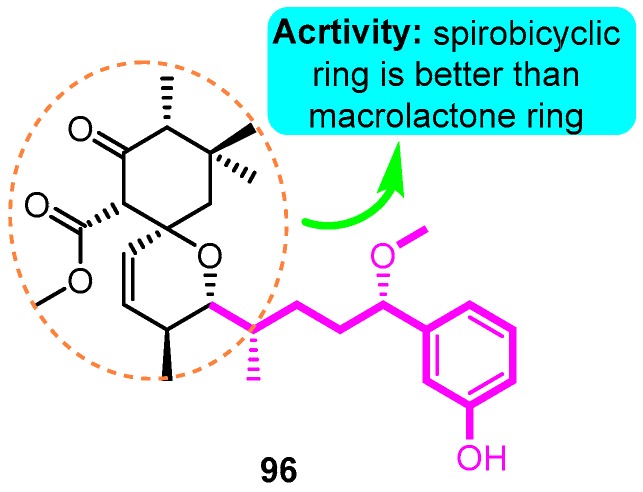
Recently, natural products with novel structural motif as a Kv1.5 inhibitor also gained progress in this field. In the sequence of the isolation of compound debromoaplysiatoxin A (38) and debromoaplysiatoxin B (39) [63], Tang and co-workers [14] identified other novel aplysiatoxin derivatives from the marine cyanobacterium Lyngbya sp. Among them, compound oscillatoxin E (96) with the hexane-tetrahydropyran of a spirobicyclic system skeleton exhibited the strongest Kv1.5 inhibition (IC50: 0.79 μM) in the CHO cells at an HP of -80 mV (Figure 33).
Figure 33.
SAR of aplysiatoxin derivatives.
4. Conclusions
Herein the target and the pharmacological properties with structural, pharmacological, and SAR information of Kv1.5 modulators were discussed. Detailed descriptions of pharmacology parameters and SAR studies provide an actionable path forward for medicinal chemists to optimize the structure of Kv1.5 modulators. Further experiments should improve the PK and safety after the effectiveness is proven. Design and development of potential and selective Kv1.5 modulators are important and challenging tasks. Based on the existing pharmacophoric requirements and potential protein structure parsed in the future, some novel effective Kv1.5 modulators may be designed and prepared [107,108]. However, gaps exist in the scientific studies on Kv1.5 modulators. Firstly, the selectivity of existing Kv1.5 modulators remains to be investigated, and more specific modulators aiming at the Kv1.5 channel are needed in the future. Secondly, from the point of application, the market of AF is relatively small, and the sales condition of marked anti-AF agents is not satisfactory as a whole, thus more in-depth pharmacological investigation of roles of Kv1.5 are required in the future. Moreover, the definite structure of Kv1.5 protein is still vacant, difficulties and potential fallacy are still consistent in the design of modulators only estimating by the pocket of homologous models.
SAR investigation is crucial for the development of novel promising clinical candidates. It is anticipated that the information compiled in this review article not only updates researchers with the recently reported pharmacology and SAR of Kv1.5 modulators, but also motivates them to design and synthesize promising Kv1.5 modulators with improved medicinal properties.
Abbreviations
| AF | Atrial fibrillation |
| BLAST | Basic Local Alignment Search Tool |
| Ceu20 | Unbound steady-state plasma concentration |
| CHO cells | Chinese hamster ovary cells |
| CNS | Central nervous system |
| EDGs | Electron donating groups |
| EWGs | Electron withdrawing groups |
| HEK cells | Human embryonic kidney 293 cells |
| hERG | Human ether-à-go-go-related gene |
| hKv1.5 channels | Human Kv1.5 channels |
| Human PASMCs | Human pulmonary arterial smooth muscle cells |
| I Kur | Cardiac ultra-rapid delayed-rectifier |
| IC50 | 50% inhibitory concentration |
| Ile | Isoleucine |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| SAR | Structure–activity relationship |
| Thr | Threonine |
| Val | Valine |
| VERP | Ventricular effective refractory period |
Funding
This work was supported by the Changjiang Scholars and Innovative Research Team in Universities, Ministry of Education of China (IRT_15R55), the 9th Group of Hundred Talent Program of Shaanxi Province (2017), Biomedicine Key Laboratory of Shaanxi Province (No. 2018SZS41), and the International Science and Technology Cooperation Program of Shaanxi Province (No. 2019KWZ-001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Milnes J.T., Madge D.J., Ford J.W. New pharmacological approaches to atrial fibrillation. Drug Discov. Today. 2012;17:654–659. doi: 10.1016/j.drudis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Amos G.J., Wettwer E., Metzger F., Li Q., Himmel H.M., Ravens U. Differences between outward currents of human atrial, and subepicardial ventricular myocytes. J. Physiol. 1996;491:31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphries E.S.A., Dart C. Neuronal and cardiovascular potassium channels as therapeutic drug targets: Promise and pitfalls. J. Biomol. Screen. 2015;20:1055–1073. doi: 10.1177/1087057115601677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima A., Ito Y., Ding W.-G., Kitagawa H., Matsuura H. Interaction of propofol with voltage-gated human Kv1.5 channel through specific amino acids within the pore region. Eur. J. Pharmacol. 2015;764:622–632. doi: 10.1016/j.ejphar.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Nerbonne J.M., Kass R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 6.Tamargo J., Caballero R., Gomez R., Delpon E. I-Kur/Kv1.5 channel blockers for the treatment of atrial fibrillation. Expert Opin. Inv. Drug. 2009;18:399–416. doi: 10.1517/13543780902762850. [DOI] [PubMed] [Google Scholar]
- 7.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 8.Tikhonov D.B., Zhorov B.S. Homology modeling of Kv1.5 channel block by cationic and electroneutral ligands. Biochim. Biophys. Acta. 2014;1838:978–987. doi: 10.1016/j.bbamem.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Ding W.G., Matsuura H., Tsuji K., Zang W.J., Horie M. Inhibitory actions of the phosphatidylinositol 3-kinase inhibitor LY294002 on the human Kv1.5 channel. Brit. J. Pharmacol. 2009;156:377–387. doi: 10.1111/j.1476-5381.2008.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guex N., Peitsch M.C., Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 11.Chen R., Chung S.-H. Inhibition of Voltage-Gated K+ Channel Kv1.5 by Antiarrhythmic Drugs. Biochemistry. 2018;57:2704–2710. doi: 10.1021/acs.biochem.8b00268. [DOI] [PubMed] [Google Scholar]
- 12.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert X., Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y.H., Wu J., Fan T.T., Zhang H.H., Gong X.X., Cao Z.Y., Zhang J., Lin H.W., Han B.N. Chemical and biological study of aplysiatoxin derivatives showing inhibition of potassium channel Kv1.5. RSC Adv. 2019;9:7594–7600. doi: 10.1039/C9RA00965E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K., Cheng N., Li X.T. Inhibitory effects of cholinesterase inhibitor donepezil on the Kv1.5 potassium channel. Sci. Rep. 2017;7:41509–41518. doi: 10.1038/srep41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Xue B., Wang J., Liu H., Shi L., Xie J. Potassium channels: A potential therapeutic target for Parkinson’s disease. Neurosci. Bull. 2018;34:341–348. doi: 10.1007/s12264-017-0177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P., Chen Z., Xu H., Sun H., Li H., Liu H., Yang H., Gao Z., Jiang H., Li M. The gating charge pathway of an epilepsy-associated potassium channel accommodates chemical ligands. Cell Res. 2013;23:1106–1118. doi: 10.1038/cr.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert G., Henneberger C., Steinhaeuser C. Diversity of astrocyte potassium channels: An update. Brain Res. Bull. 2018;136:26–36. doi: 10.1016/j.brainresbull.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt N., Grunnet M., Olesen S.P. Cardiac potassium channel subtypes: New roles in repolarization and arrhythmia. Physiol. Rev. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 20.Geller J.C., Egstrup K., Kulakowski P., Rosenqvist M., Jansson M.A., Berggren A., Edvardsson N., Sager P., Crijns H.J. Rapid conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. J. Clin. Pharmacol. 2009;49:312–322. doi: 10.1177/0091270008329549. [DOI] [PubMed] [Google Scholar]
- 21.Ng F.L., Davis A.J., Jepps T.A., Harhun M.I., Yeung S.Y., Wan A., Reddy M., Melville D., Nardi A., Khong T.K., et al. Expression and function of the K plus channel KCNQ genes in human arteries. Br. J. Pharmacol. 2011;162:42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros F., Pardo L.A., Dominguez P., Maria Sierra L., De la Pena P. New Structures and Gating of Voltage-Dependent Potassium (Kv) Channels and Their Relatives: A Multi-Domain and Dynamic Question. Int. J. Mol. Sci. 2019;20:248. doi: 10.3390/ijms20020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozrzymas J.W., Teisseyre A., Vittur F. Propofol blocks voltage-gated potassium channels in human T lymphocytes. Biochem. Pharmacol. 1996;52:843–849. doi: 10.1016/0006-2952(96)00350-4. [DOI] [PubMed] [Google Scholar]
- 24.Teisseyre A., Michalak K. Inhibition of the activity of human lymphocyte Kv1.3 potassium channels by resveratrol. J. Membr. Biol. 2006;214:123–129. doi: 10.1007/s00232-007-0043-8. [DOI] [PubMed] [Google Scholar]
- 25.Ishii T., Warabi E., Siow R.C.M., Mann G.E. Sequestosome1/p62: A regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth. Free Radic. Biol. Med. 2013;65:102–116. doi: 10.1016/j.freeradbiomed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Dos Santos-Nascimento T., Veras K.M., Cruz J.S., Leal-Cardoso J.H. Inhibitory Effect of Terpinen-4-ol on Voltage-Dependent Potassium Currents in Rat Small Sensory Neurons. J. Nat. Prod. 2015;78:173–180. doi: 10.1021/np4009249. [DOI] [PubMed] [Google Scholar]
- 27.Kulcitki V., Harghel P., Ungur N. Unusual cyclic terpenoids with terminal pendant prenyl moieties: From occurrence to synthesis. Nat. Prod. Rep. 2014;31:1686–1720. doi: 10.1039/C4NP00081A. [DOI] [PubMed] [Google Scholar]
- 28.Menezes P.M.N., Brito M.C., de Paiva G.O., dos Santos C.O., de Oliveira L.M., Ribeiro L.A.D., De Lima J.T., Lucchese A.M., Silva F.S. Relaxant effect of Lippia origanoides essential oil in guinea-pig trachea smooth muscle involves potassium channels and soluble guanylyl cyclase. J. Ethnopharmacol. 2018;220:16–25. doi: 10.1016/j.jep.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Kalyaanamoorthy S., Barakat K.H. Development of safe crugs: The hERG challenge. Med. Res. Rev. 2018;38:525–555. doi: 10.1002/med.21445. [DOI] [PubMed] [Google Scholar]
- 30.Cheong A., Dedman A.M., Beech D.J. Expression and function of native potassium channel (K-v alpha 1) subunits in terminal arterioles of rabbit. J. Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y., Ding W., Liu Y., Yu M., Sun X., Matsuura H. Long-term 4-AP treatment facilitates functional expression of human Kv1.5 channel. Eur. J. Pharmacol. 2019;844:195–203. doi: 10.1016/j.ejphar.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Eldstrom J., Wang Z., Xu H., Pourrier M., Ezrin A., Gibson K., Fedida D. The molecular basis of high-affinity binding of the antiarrhythmic compound vernakalant (RSD1235) to Kv1.5 channels. Mol. Pharmacol. 2007;72:1522–1534. doi: 10.1124/mol.107.039388. [DOI] [PubMed] [Google Scholar]
- 33.Kodama I., Kamiya K., Honjo H., Toyama J. Acute and chronic effects of amiodarone on mammalian ventricular cells. Jpn. Heart J. 1996;37:719–730. doi: 10.1536/ihj.37.719. [DOI] [PubMed] [Google Scholar]
- 34.Herrera D., Mamarbachi A., Simoes M., Parent L., Sauve R., Wang Z.G., Nattel S. A single residue in the S6 transmembrane domain governs the differential flecainide sensitivity of voltage-gated potassium channels. Mol. Pharmacol. 2005;68:305–316. doi: 10.1124/mol.104.009506. [DOI] [PubMed] [Google Scholar]
- 35.Lin S., Wang Z., Fedida D. Influence of permeating ions on Kv1.5 channel block by nifedipine. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1160–H1172. doi: 10.1152/ajpheart.2001.280.3.H1160. [DOI] [PubMed] [Google Scholar]
- 36.Franqueza L., Valenzuela C., Delpon E., Longobardo M., Caballero R., Tamargo J. Effects of propafenone and 5-hydroxy-propafenone on hKv1.5 channels. Brit. J. Pharmacol. 1998;125:969–978. doi: 10.1038/sj.bjp.0702129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedida D. Gating charge and ionic currents associated with quinidine block of human Kv1.5 delayed rectifier channels. J. Physiol. 1997;499:661–675. doi: 10.1113/jphysiol.1997.sp021959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballero R., Gomez R., Nunez L., Moreno I., Tamargo J., Delpon E. Diltiazem inhibits hKv1.5 and Kv4.3 currents at therapeutic concentrations. Cardiovasc. Res. 2004;64:457–466. doi: 10.1016/j.cardiores.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Chow L.W.C., Cheng K.-S., Wong K.-L., Leung Y.-M. Voltage-gated K+ channels promote BT-474 breast cancer cell migration. Chin. J. Cancer Res. 2018;30:613–622. doi: 10.21147/j.issn.1000-9604.2018.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malayev A.A., Nelson D.J., Philipson L.H. Mechanism of clofilium block of the human Kv1.5 delayed rectifier potassium channel. Mol. Pharmacol. 1995;47:198–205. [PubMed] [Google Scholar]
- 41.Yang I.C.H., Scherz M.W., Bahinski A., Bennett P.B., Murray K.T. Stereoselective interactions of the enantiomers of chromanol 293B with human voltage-gated potassium channels. J. Pharmacol. Exp. 2000;294:955–962. [PubMed] [Google Scholar]
- 42.Kobayashi S., Reien Y., Ogura T., Saito T., Masuda Y., Nakaya H. Inhibitory effect of bepridil on hKv1.5 channel current: Comparison with amiodarone and E-4031. Eur. J. Pharmacol. 2001;430:149–157. doi: 10.1016/S0014-2999(01)01381-4. [DOI] [PubMed] [Google Scholar]
- 43.Lee H.M., Hahn S.J., Choi B.H. Blockade of Kv1.5 by paroxetine, an antidepressant drug. Korean J. Physiol. Pharmacol. 2016;20:75–82. doi: 10.4196/kjpp.2016.20.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai F.F., Mao Z.F., Xia J., Zhu S.P., Wu Z.Y. Fluoxetine protects against big endothelin-1 induced anti-apoptosis by rescuing Kv1.5 channels in human pulmonary arterial smooth muscle cells. Yonsei Med. J. 2012;53:842–848. doi: 10.3349/ymj.2012.53.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H.M., Hahn S.J., Choi B.H. Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J. Physiol. Pharmacol. 2016;20:193–200. doi: 10.4196/kjpp.2016.20.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J., Park M.-H., Jo S.-H. Inhibitory effects of cortisone and hydrocortisone on human Kv1.5 channel currents. Eur. J. Pharmacol. 2015;746:158–166. doi: 10.1016/j.ejphar.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Lammers C., Dartsch T., Brandt M.C., Rottlander D., Halbach M., Peinkofer G., Ockenpoehler S., Weiergraeber M., Schneider T., Reuter H., et al. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol. Biochem. 2012;29:833–840. doi: 10.1159/000178483. [DOI] [PubMed] [Google Scholar]
- 48.Frolov R.V., Singh S. Celecoxib and ion channels: A story of unexpected discoveries. Eur. J. Pharmacol. 2014;730:61–71. doi: 10.1016/j.ejphar.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Luzhkov V.B., Nilsson J., Arhem P., Aqvist J. Computational modelling of the open-state K(v)1.5 ion channel block by bupivacaine. Biochim. Biophys. Acta. 2003;1652:35–51. doi: 10.1016/j.bbapap.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Valenzuela C., Delpon E., Tamkun M.M., Tamargo J., Snyders D.J. Stereoselective block of a human cardiac potassium channel (Kv1.5) by bupivacaine enantiomers. Biophys. J. 1995;69:418–427. doi: 10.1016/S0006-3495(95)79914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vonderlin N., Fischer F., Zitron E., Seyler C., Scherer D., Thomas D., Katus H.A., Scholz E.P. Inhibition of cardiac Kv1.5 potassium current by the anesthetic midazolam: Mode of action. Drug Des. Dev. 2014;8:2263–2271. doi: 10.2147/DDDT.S70461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su J.P., Huang Y., Lenka N., Hescheler J., Ullrich S. The expression and regulation of depolarization-activated K+ channels in the insulin-secreting cell line INS-1. Pflugers Arch. 2001;442:49–56. doi: 10.1007/s004240000508. [DOI] [PubMed] [Google Scholar]
- 53.Caballero R., Moreno I., Gonzalez T., Valenzuela C., Tamargo J., Delpon E. Putative binding sites for benzocaine on a human cardiac cloned channel (Kv1.5) Cardiovasc. Res. 2002;56:104–117. doi: 10.1016/S0008-6363(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 54.Jie L., Wu W., Li G., Xiao G., Zhang S., Li G., Wang Y. Clemizole hydrochloride blocks cardiac potassium currents stably expressed in HEK 293 cells. Brit. J. Pharmacol. 2017;174:254–266. doi: 10.1111/bph.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wirth K.J., Brendel J., Steinmeyer K., Linz D.K., Ruetten H., Goegelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J. Cardiovasc. Pharmacol. 2007;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- 56.Persson F., Carlsson L., Duke G., Jacobson I. Blocking characteristics of hKv1.5 and hKv4.3/hKChIP2.2 after administration of the novel antiarrhythmic compound AZD7009. J. Cardiovasc. Pharmacol. 2005;46:7–17. doi: 10.1097/01.fjc.0000161405.37198.c1. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd J., Finlay H.J., Vacarro W., Hyunh T., Kover A., Bhandaru R., Yan L., Atwal K., Conder M.L., Jenkins-West T., et al. Pyrrolidine amides of pyrazolodihydropyrimidines as potent and selective KV1.5 blockers. Bioorg. Med. Chem. Lett. 2010;20:1436–1439. doi: 10.1016/j.bmcl.2009.12.085. [DOI] [PubMed] [Google Scholar]
- 58.Gunaga P., Lloyd J., Mummadi S., Banerjee A., Dhondi N.K., Hennan J., Subray V., Jayaram R., Rajugowda N., Reddy K.U., et al. Selective I-Kur inhibitors for the potential treatment of atrial fibrillation: Optimization of the phenyl quinazoline series leading to clinical candidate 5- 5-phenyl-4-(pyridin-2-ylmethylamino)quinazolin-2-yl pyridine-3-sulfon amide. J. Med. Chem. 2017;60:3795–3803. doi: 10.1021/acs.jmedchem.6b01889. [DOI] [PubMed] [Google Scholar]
- 59.Loose S., Mueller J., Wettwer E., Knaut M., Ford J., Milnes J., Ravens U. Effects of IKur blocker MK-0448 on human right atrial action potentials from patients in sinus rhythm and in permanent atrial fibrillation. Front. Pharmacol. 2014;5:26–32. doi: 10.3389/fphar.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ford J., Milnes J., El Haou S., Wettwer E., Loose S., Matschke K., Tyl B., Round P., Ravens U. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm. 2016;13:555–564. doi: 10.1016/j.hrthm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gautier P., Guillemare E., Djandjighian L., Marion A., Planchenault J., Bernhart C., Herbert J.M., Nisato D. In vivo and in vitro characterization of the novel antiarrhythmic agent SSR149744C-Electrophysiological, anti-adrenergic, and anti-angiotensin II effects. J. Cardiovasc. Pharmacol. 2004;44:244–257. doi: 10.1097/00005344-200408000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Gasparoli L., D’Amico M., Masselli M., Pillozzi S., Caves R., Khuwaileh R., Tiedke W., Mugridge K., Pratesi A., Mitcheson J.S., et al. New pyrimido-indole compound CD-160130 preferentially inhibits the K(V)11.1B isoform and produces antileukemic effects without cardiotoxicity. Mol. Pharmacol. 2015;87:183–196. doi: 10.1124/mol.114.094920. [DOI] [PubMed] [Google Scholar]
- 63.Han B., Liang T., Keen L.J., Fan T., Zhang X., Xu L., Zhao Q., Wang S., Lin H. Two marine cyanobacterial aplysiatoxin polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Org. Lett. 2018;20:578–581. doi: 10.1021/acs.orglett.7b03672. [DOI] [PubMed] [Google Scholar]
- 64.Grissmer S., Nguyen A.N., Aiyar J., Hanson D.C., Mather R.J., Gutman G.A., Karmilowicz M.J., Auperin D.D., Chandy K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 65.Kwak Y.G., Kim D.K., Ma T., Park S.-A., Park H., Jung Y.H., Yoo D.-J., Eun J.S. Torilin from Torilis japonica (Houtt.) DC. blocks hKv1.5 channel current. Arch. Pharmacol. Res. 2006;29:834–839. doi: 10.1007/BF02973902. [DOI] [PubMed] [Google Scholar]
- 66.Jin S., Guo Q., Xu J., Yu P., Liu J., Tang Y. Antiarrhythmic ionic mechanism of Guanfu base A -Selective inhibition of late sodium current in isolated ventricular myocytes from guinea pigs. Chin. J. Nat. Med. 2015;13:361–367. doi: 10.1016/S1875-5364(15)30027-3. [DOI] [PubMed] [Google Scholar]
- 67.Jeong I., Choi B.H., Hahn S.J. Effects of lobeline, a nicotinic receptor ligand, on the cloned Kv1.5. Pflugers Arch. 2010;460:851–862. doi: 10.1007/s00424-010-0868-3. [DOI] [PubMed] [Google Scholar]
- 68.Fischer F., Vonderlin N., Zitron E., Seyler C., Scherer D., Becker R., Katus H.A., Scholz E.P. Inhibition of cardiac Kv1.5 and Kv4.3 potassium channels by the class Ia anti-arrhythmic ajmaline: Mode of action. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:991–999. doi: 10.1007/s00210-013-0901-0. [DOI] [PubMed] [Google Scholar]
- 69.Choe H., Lee Y.K., Lee Y.T., Choe H., Ko S.H., Joo C.U., Kim M.H., Kim G.S., Eun J.S., Kim J.H., et al. Papaverine blocks hKv1.5 channel current and human atrial ultrarapid delayed rectifier K+ currents. Can. J. Cardiol. 2003;304:706–712. doi: 10.1124/jpet.102.042770. [DOI] [PubMed] [Google Scholar]
- 70.Li K., Pi M., Li X. The inhibitory effects of levo-tetrahydropalmatine on rat Kv1.5 channels expressed in HEK293 cells. Eur. J. Pharmacol. 2017;809:105–110. doi: 10.1016/j.ejphar.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 71.Li Y.F., Tu D.N., Xiao H., Du Y.M., Zou A.R., Liao Y.H., Dong S.H. Aconitine blocks HERG and Kv1.5 potassium channels. J. Ethnopharmacol. 2010;131:187–195. doi: 10.1016/j.jep.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 72.Ou X., Bin X., Wang L., Li M., Yang Y., Fan X., Zeng X. Myricetin inhibits K (v) 1.5 channels in HEK293 cells. Mol. Med. Rep. 2016;13:1725–1731. doi: 10.3892/mmr.2015.4704. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y., Xu X., Liu Z., Du X., Chen K., Xin X., Jin Z., Shen J., Hu Y., Li G., et al. Effects of the natural flavone trimethylapigenin on cardiac potassium currents. Biochem. Pharmacol. 2012;84:498–506. doi: 10.1016/j.bcp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Yang L., Ma J., Zhang P., Zou A., Tu D. Quercetin activates human Kv1.5 channels by a residue I502 in the S6 segment. Clin. Exp. Pharmacol. Physiol. 2009;36:154–161. doi: 10.1111/j.1440-1681.2008.05061.x. [DOI] [PubMed] [Google Scholar]
- 75.Wu H.-J., Wu W., Sun H.-Y., Qin G.-W., Wang H.-B., Wang P., Yalamanchili H.K., Wang J., Tse H.-F., Lau C.-P., et al. Acacetin causes a frequency- and use-dependent blockade of hKv1.5 channels by binding to the S6 domain. J. Mol. Cell. Cardiol. 2011;51:966–973. doi: 10.1016/j.yjmcc.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 76.Paffett M.L., Lucas S.N., Campen M.J. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: A potential role for atrogin-1 in smooth muscle. Vasc. Pharmacol. 2012;56:64–73. doi: 10.1016/j.vph.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwak Y.G., Choi B.-H., Kim D.K., Eun J.S. Decursin from Angelica gigas Nakai blocks hKv1.5 channel. Biomol. Ther. 2011;19:33–37. doi: 10.4062/biomolther.2011.19.1.033. [DOI] [Google Scholar]
- 78.Karczewski J., Kiss L., Kane S.A., Koblan K.S., Lynch R.J., Spencer R.H. High-throughput analysis of drug binding interactions for the human cardiac channel, Kv1.5. Biochem. Pharmacol. 2009;77:177–185. doi: 10.1016/j.bcp.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 79.Yang Q., Lv Q., Feng M., Liu M., Feng Y., Lin S., Yang J., Hu J. Taurine prevents the electrical remodeling in ach-CaCl2 induced atrial fibrillation in rats. In: Lee D.H., Schaffer S.W., Park W., Kim H.W., editors. Taurine 10. Volume 975. Springer; Dordrecht, The Netherlands: 2017. pp. 821–830. [DOI] [PubMed] [Google Scholar]
- 80.Peukert S., Brendel J., Pirard B., Bruggemann A., Below P., Kleemann H.W., Hemmerle H., Schmidt W. Identification, synthesis, and activity of novel blockers of the voltage-gated potassium channel Kv1.5. J. Med. Chem. 2003;46:486–498. doi: 10.1021/jm0210461. [DOI] [PubMed] [Google Scholar]
- 81.Peukert S., Brendel J., Pirard B., Strubing C., Kleemann H.W., Bohme T., Hemmerle H. Pharmacophore-based search, synthesis, and biological evaluation of anthranilic amides as novel blockers of the Kv1.5 channel. Bioorg. Med. Chem. Lett. 2004;14:2823–2827. doi: 10.1016/j.bmcl.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 82.Schmitz A., Sankaranarayanan A., Azam P., Schmidt-Lassen K., Homerick D., Hansel W., Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol. Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 83.Blass B.E., Coburn K., Lee W., Fairweather N., Fluxe A., Wu S., Janusz J.M., Murawsky M., Fadayel G.M., Fang B., et al. Synthesis and evaluation of (2-phenethyl-2H-1,2,3-triazol-4-yl)(phenyl) methanones as Kv1.5 channel blockers for the treatment of atrial fibrillation. Bioorg. Med. Chem. Lett. 2006;16:4629–4632. doi: 10.1016/j.bmcl.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Fluxe A., Wu S.D., Sheffer J.B., Janusz J.M., Murawsky M., Fadayel G.M., Fang B., Hare M., Djandjighian L. Discovery and synthesis of tetrahydroindolone-derived carbamates as Kv1.5 blockers. Bioorg. Med. Chem. Lett. 2006;16:5855–5858. doi: 10.1016/j.bmcl.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 85.Wu S., Fluxe A., Janusz J.M., Sheffer J.B., Browning G., Blass B., Cobum K., Hedges R., Murawsky M., Fang B., et al. Discovery and synthesis of tetrahydroindolone derived semicarbazones as selective Kv1.5 blockers. Bioorg. Med. Chem. Lett. 2006;16:5859–5863. doi: 10.1016/j.bmcl.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 86.Nanda K.K., Nolt M.B., Cato M.J., Kane S.A., Kiss L., Spencer R.H., Wang J., Lynch J.J., Regan C.P., Stump G.L., et al. Potent antagonists of the Kv1.5 potassium channel: Synthesis and evaluation of analogous N,N-diisopropyl-2-(pyridine-3-yl)acetamides. Bioorg. Med. Chem. Lett. 2006;16:5897–5901. doi: 10.1016/j.bmcl.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 87.Trotter B.W., Nanda K.K., Kett N.R., Regan C.P., Lynch J.J., Stump G.L., Kiss L., Wang J., Spencer R.H., Kane S.A., et al. Design and synthesis of novel isoquinoline-3-nitriles as orally bioavailable Kv1.5 antagonists for the treatment of atrial fibrillation. J. Med. Chem. 2006;49:6954–6957. doi: 10.1021/jm060927v. [DOI] [PubMed] [Google Scholar]
- 88.Eun J.S., Kim K.S., Kim H.N., Park S.A., Ma T.-Z., Lee K.A., Kim D.K., Kim H.K., Kim I.S., Jung Y.H., et al. Synthesis of psoralen derivatives and their blocking effect of hKv1.5 channel. Arch. Pharmacol. Res. 2007;30:155–160. doi: 10.1007/BF02977688. [DOI] [PubMed] [Google Scholar]
- 89.Jackson C.M., Blass B., Coburn K., Djandjighian L., Fadayel G., Fluxe A.J., Hodson S.J., Janusz J.M., Murawsky M., Ridgeway J.M., et al. Evolution of thiazolidine-based blockers of human Kv1.5 for the treatment of atrial arrhythmias. Bioorg. Med. Chem. Lett. 2007;17:282–284. doi: 10.1016/j.bmcl.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 90.Lloyd J., Atwal K.S., Finlay H.J., Nyman M., Huynh T., Bhandaru R., Kover A., Schmidt J., Vaccaro W., Conder M.L., et al. Benzopyran sulfonamides as K(v)1.5 potassium channel blockers. Bioorg. Med. Chem. Lett. 2007;17:3271–3275. doi: 10.1016/j.bmcl.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Finlay H.J., Lloyd J., Nyman M., Conder M.L., West T., Levesque P., Atwal K. Pyrano- [2,3b] -pyridines as potassium channel antagonists. Bioorg. Med. Chem. Lett. 2008;18:2714–2718. doi: 10.1016/j.bmcl.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 92.Gross M.F., Castle N.A., Zou A., Wickenden A.D., Yu W., Spear K.L. Aryl sulfonamido tetralin inhibitors of the Kv1.5 ion channel. Bioorg. Med. Chem. Lett. 2009;19:3063–3066. doi: 10.1016/j.bmcl.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Blass B.E., Fensome A., Trybulski E., Magolda R., Gardell S.J., Liu K., Samuel M., Feingold I., Huselton C., Jackson C.M., et al. Selective Kv1.5 blockers: Development of (R)-1-(methylsulfonylamino)-3-2-(4-methoxyphenyl)ethyl -4-(4-methoxyphe nyl)-2-imidazolidinone (KVI-020/WYE-160020) as a potential treatment for atrial arrhythmia. J. Med. Chem. 2009;52:6531–6534. doi: 10.1021/jm901042m. [DOI] [PubMed] [Google Scholar]
- 94.Blass B. Derivatives of heteroarylsulfonamides, their peparation, and their application in human therapy patent highlight. Acs Med. Chem. Lett. 2012;3:618–619. doi: 10.1021/ml3001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finlay H.J., Lloyd J., Vaccaro W., Kover A., Yan L., Bhave G., Prol J., Tram H., Bhandaru R., Caringal Y., et al. Discovery of ((S)-5-(methoxymethyl)-7-(1-methyl-1H-indol-2-yl)-2-(trifluoromethyl)-4, 7-dihydropyrazolo 1,5-a pyrimidin-6-yl)((S)-2-(3-methylisoxazol-5-yl)pyr rolidin-1-yl)methanone as a potent and selective I-Kur inhibitor. J. Med. Chem. 2012;55:3036–3048. doi: 10.1021/jm201386u. [DOI] [PubMed] [Google Scholar]
- 96.Finlay H.J., Jiang J., Caringal Y., Kover A., Conder M.L., Xing D., Levesque P., Harper T., Hsueh M.M., Atwal K., et al. Triazolo and imidazo dihydropyrazolopyrimidine potassium channel antagonists. Bioorg. Med. Chem. Lett. 2013;23:1743–1747. doi: 10.1016/j.bmcl.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 97.Guo X., Yang Q., Xu J., Zhang L., Chu H., Yu P., Zhu Y., Wei J., Chen W., Zhang Y., et al. Design and bio-evaluation of indole derivatives as potent Kv1.5 inhibitors. Bioorg. Med. Chem. 2013;21:6466–6476. doi: 10.1016/j.bmc.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 98.Olsson R.I., Jacobson I., Bostrom J., Fex T., Bjore A., Olsson C., Sundell J., Gran U., Ohrn A., Nordin A., et al. Synthesis and evaluation of diphenylphosphinic amides and diphenylphosphine oxides as inhibitors of Kv1.5. Bioorg. Med. Chem. Lett. 2013;23:706–710. doi: 10.1016/j.bmcl.2012.11.098. [DOI] [PubMed] [Google Scholar]
- 99.Olsson R.I., Jacobson I., Iliefski T., Bostrom J., Davidsson O., Fjellstrom O., Bjore A., Olsson C., Sundell J., Gran U., et al. Lactam sulfonamides as potent inhibitors of the Kv1.5 potassium ion channel. Bioorg. Med. Chem. Lett. 2014;24:1269–1273. doi: 10.1016/j.bmcl.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 100.Johnson J.A., Xu N., Jeon Y., Finlay H.J., Kover A., Conder M.L., Sun H., Li D., Levesque P., Hsueh M.-M., et al. Design, synthesis and evaluation of phenethylaminoheterocycles as K(v)1.5 inhibitors. Bioorg. Med. Chem. Lett. 2014;24:3018–3022. doi: 10.1016/j.bmcl.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 101.Guo X., Ma X., Yang Q., Xu J., Huang L., Jia J., Shan J., Liu L., Chen W., Chu H., et al. Discovery of 1-aryloxyethyl piperazine derivatives as Kv1.5 potassium channel inhibitors (part I) Eur. J. Med. Chem. 2014;81:89–94. doi: 10.1016/j.ejmech.2014.03.075. [DOI] [PubMed] [Google Scholar]
- 102.Kajanus J., Jacobson I., Astrand A., Olsson R.I., Gran U., Bjore A., Fjellstrom O., Davidsson O., Emtenas H., Dahlen A., et al. Isoindolinone compounds active as Kv1.5 blockers identified using a multicomponent reaction approach. Bioorg. Med. Chem. Lett. 2016;26:2023–2029. doi: 10.1016/j.bmcl.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 103.Finlay H.J., Johnson J.A., Lloyd J.L., Jiang J., Neels J., Gunaga P., Baneriee A., Dhondi N., Chimalakonda A., Mandlekar S., et al. Discovery of 5-Phenyl-N-(pyridin-2-ylmethyl)-2-(pyrimidin-5-yl)quinazolin-4-amine as a Potent I-Kur Inhibitor. Acs Med. Chem. Lett. 2016;7:831–834. doi: 10.1021/acsmedchemlett.6b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zidar N., Zula A., Tomasic T., Rogers M., Kirby R.W., Tytgat J., Peigneur S., Kikelj D., Ilas J., Masic L.P. Clathrodin, hymenidin and oroidin, and their synthetic analogues as inhibitors of the voltage-gated potassium channels. Eur. J. Med. Chem. 2017;139:232–241. doi: 10.1016/j.ejmech.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 105.Wolkenberg S.E., Nolt M.B., Bilodeau M.T., Trotter B.W., Manley P.J., Kett N.R., Nanda K.K., Wu Z.C., Cato M.J., Kane S.A., et al. Discovery of MK-1832, a Kv1.5 inhibitor with improved selectivity and pharmacokinetics. Bioorg. Med. Chem. Lett. 2017;27:1062–1069. doi: 10.1016/j.bmcl.2016.12.054. [DOI] [PubMed] [Google Scholar]
- 106.Kajanus J., Antonsson T., Carlsson L., Jurva U., Pettersen A., Sundell J., Inghardt T. Potassium channel blocking 1,2-bis(aryl)ethane-1,2-diamines active as antiarrhythmic agents. Bioorg. Med. Chem. Lett. 2019;29:1241–1245. doi: 10.1016/j.bmcl.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Banerjee S., Adhikari N., Amin S.A., Jha T. Histone deacetylase 8 (HDAC8) and its inhibitors with selectivity to other isoforms: An overview. Eur. J. Med. Chem. 2019;164:214–240. doi: 10.1016/j.ejmech.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 108.Zhao Z., Song H., Xie J., Liu T., Zhao X., Chen X., He X., Wu S., Zhang Y., Zheng X. Research progress in the biological activities of 3,4,5-trimethoxycinnamic acid (TMCA) derivatives. Eur. J. Med. Chem. 2019;173:213–227. doi: 10.1016/j.ejmech.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]