To the editor,
Sepsis deeply perturbs immune homeostasis by inducing a complex immune response that varies over time and associates a tremendous systemic inflammatory response to anti-inflammatory mechanisms. As a delayed consequence, some septic patients enter a state of profound immunosuppression [1]. As the latter may persist for weeks, leaving the patient at increased risk of secondary infections, immunostimulation recently appeared as a reasonable therapeutic option in patients with signs of persistent and severe immunosuppression [2].
Septic patients develop marked T lymphocyte dysfunctions such as profound lymphopenia, increased expression of inhibitory co-receptor molecules, decreased repertoire diversity, and reduced functionality (proliferation and cytokine production). These alterations have been repeatedly associated with deleterious outcomes [1, 2]. However, mechanisms leading to these alterations are only partially understood. For instance, while the role of deactivated mTORC1 is established [3], the intrinsic capacity of T cell receptor (TCR) to be activated and to transduce intracellular signalling remains unexplored. Among determinants of T cell response, immediate calcium signaling following TCR ligation is of paramount importance and serves crucial effector functions. Thus, we developed a flow cytometry protocol to follow calcium flux after TCR stimulation in patients’ CD4+ T lymphocytes. In addition, phosphorylation of molecules from the proximal and downstream TCR signaling cascade was analyzed (Additional file 1). We included patients with septic shock (according to SEPSIS-3 definition) presenting with features of immunosuppression, i.e., decreased monocyte HLA-DR and lymphocyte count (clinical and immunological characteristics in Additional file 1: Table S1).
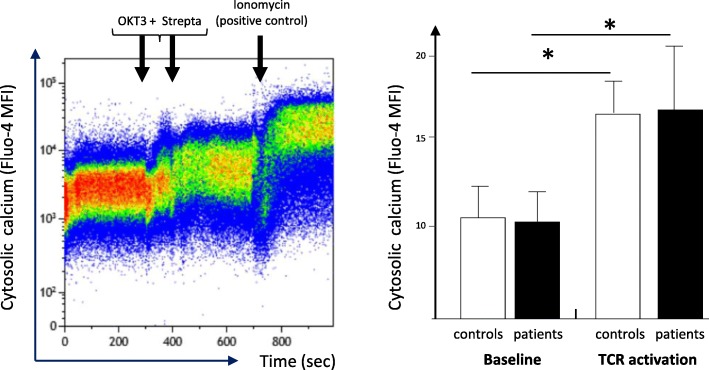
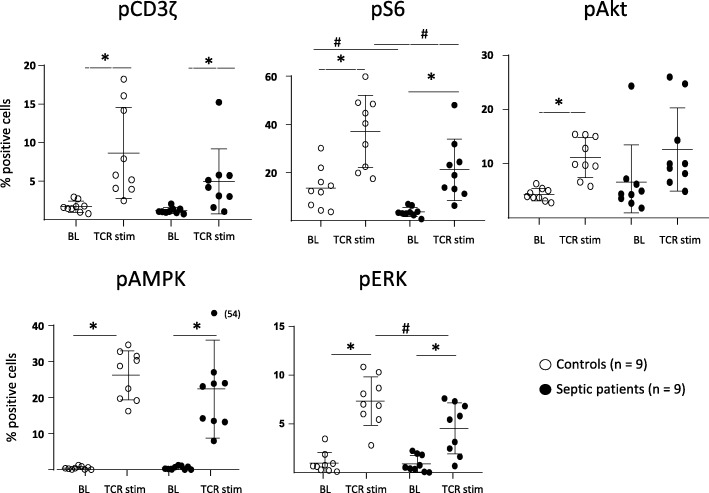
We show that immediate signaling downstream TCR stimulation was not altered in circulating CD4 lymphocytes from septic shock patients. Indeed, cells exhibited no deregulation of intracytoplasmic calcium influx after TCR ligation compared with healthy controls (OKT3 response, Fig. 1). In agreement, we observed a significant CD3ζ phosphorylation (one of the first molecules to be phosphorylated after TCR engagement) after T cell stimulation in both cells from septic patients and controls (Fig. 2). This showed that immediate response after TCR activation was unaffected after sepsis.
Fig. 1.
Intracellular calcium signaling in CD4+ T cells from septic patients and controls upon TCR ligation. Peripheral blood mononuclear cells (PBMC) were isolated from patients and controls and loaded with Fluo-4AM for 30 min at 37 °C. Cells were analyzed by flow cytometry for 5 min at baseline then stimulated by biotinylated anti-CD3 (OKT3) antibody during 90 s before addition of streptavidin to induced TCR stimulation and analyzed for another 5 min. As a positive control, ionomycin was added at the end of experiment. Left, representative example of overtime intracellular calcium staining in one healthy volunteer. Right, intracellular calcium staining following TCR ligation in septic patients (n = 7) and healthy volunteers (controls, age-matched, n = 7) before (baseline), after TCR stimulation (biotinylated anti-CD3 antibodies + streptavidin) and after ionomycin addition. MFI of Fluo-4AM was measured in CD4+ T lymphocytes on 3 periods of 100 s at baseline and after complete TCR stimulation. For each stimulation condition, the maximum MFI was considered. Data are represented as means +/− SD. *p < 0.05 compared to baseline, Wilcoxon paired test
Fig. 2.
PI3/Akt/mTOR pathway activation in CD4+ T cells from septic patients and controls upon TCR ligation. PBMCs were isolated from septic patients and controls, stimulated for 7 min with anti-CD2-CD3-CD28 Ab-coated beads (bead/cell ratio = 3/1) and stained with anti-CD4 and anti-CD3z, anti-pS6, anti-pAkt, anti-pERk, and anti-pAMPK antibodies protein phosphorylation was assessed by flow cytometry. Data represent the percentage of positive cells based on FMO staining and as individual values and means +/− SD in 9 septic patients and 9 healthy volunteers (controls) before (baseline, BL) and after TCR stimulation (TCR stim). *p < 0.05 (Wilcoxon paired test, baseline vs TCR stim), #p < 0.05 (Mann-Whitney, healthy volunteers vs patients after stimulation)
In contrast, activation of more distal molecules in the TCR signaling cascade was impacted. For example, stimulation-induced rise of pAkt and pERK was affected leading to a limited mTORC1 activation capacity as measured by S6 phosphorylation after stimulation. In contrast, activation of AMPK, an inhibitor of mTORC1 was mostly unaltered in patients compared to controls (Fig. 2).
In conclusion, the present results obtained in septic shock patients show that proximal TCR signaling remains functional in circulating CD4+ T cells from septic shock patients while downstream activation of mTORC1 pathway is markedly diminished. PI3K-Akt pathway integrates signals from both co-activating/inhibitory receptors and an increased expression of such co-inhibitory receptors has been described on circulating T cells from septic patients [4]. In that respect, this suggests that inhibitory receptors known to block downstream signaling are likely of utmost importance in sepsis-induced T lymphocyte dysfunctions. As TCR from septic lymphocytes remains actionable [5], the present results reinforce the rational for blocking co-inhibitors (e.g., with anti-PD-1) or stimulating mTORC1 (for example with rhIL-7) as reasonable immunoadjuvant approaches to tackle sepsis-induced immunosuppression [6–8].
Supplementary information
Additional file 1: Table S1. Clinical and biological data from septic shock patients.
Acknowledgements
Not applicable
Abbreviations
- anti-PD-1
Anti-Program-Death-1
- HLA-DR
Human leukocyte antigen–DR isotype
- mTORC1
Mammalian target of rapamycin complex 1
- OKT3
Monoclonal antibody anti-CD3 receptor (muromomab)
- pAkt
Phosphor-Akt (aka phospho-protein kinase B)
- pAMPK
Phosphor-mitogen-activated protein kinases
- pERK
Phosphor-extracellular signal-regulated kinases
- rhIL-7
Recombinant human interleukin-7
- TCR
T cell receptor
Authors’ contributions
CDR, KK, MG, EP, FV, and GM designed the experiments. CDR and KK performed the experiments and the statistical analyses. TR, CM, MC, and LA included patients. All authors discussed the data, drafted or revised critically the manuscript for important intellectual content, and approved the final manuscript.
Funding
This work was supported by Université Lyon 1, Hospices Civils de Lyon, and bioMerieux. bioMérieux had no role in the study design; in the collection, analysis, and interpretation of data; and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Septic shock patients: this project was approved by our Institutional Review Board for ethics (“Comité de Protection des Personnes Sud-Est II”, number 11236).This study is registered at the French Ministry of Research and Teaching (#DC-2008-509), at the Commission Nationale de l’Informatique et des Libertés, and on ClinicalTrials.gov (NCT02803346). Oral information and non-opposition to inclusion in the study were mandatory and recorded in patients’ clinical files.
Healthy volunteers: peripheral blood from healthy volunteers was provided by the “Etablissement Français du Sang” from Lyon. According to EFS standardized procedures for blood donation and to provisions of the articles R.1243–49 and following ones of the French public health codes, a written non-opposition to the use of donated blood for research purposes was obtained from HV. The blood donors’ personal data were anonymized before being transferred to our research laboratory.
Consent for publication
Not applicable.
Competing interests
EP is an employee of bioMérieux. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Charles de Roquetaillade and Khalil Kandara contributed equally to the work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40635-019-0287-5.
References
- 1.Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14:121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venet F, Demaret J, Blaise BJ, Rouget C, Girardot T, Idealisoa E, Rimmele T, Mallet F, Lepape A, Textoris J, Monneret G. IL-7 restores T lymphocyte immunometabolic failure in septic shock patients through mTOR activation. J Immunol. 2017;199:1606–1615. doi: 10.4049/jimmunol.1700127. [DOI] [PubMed] [Google Scholar]
- 4.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaret J, Dupont G, Venet F, Friggeri A, Lepape A, Rimmele T, Morel J, Monneret G. STAT5 phosphorylation in T cell subsets from septic patients in response to recombinant human interleukin-7: a pilot study. J Leukoc Biol. 2015;97:791–796. doi: 10.1189/jlb.5AB1114-545R. [DOI] [PubMed] [Google Scholar]
- 6.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3:e98960. doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL. Nivolumab plus interferon-gamma in the treatment of intractable mucormycosis. Lancet Infect Dis. 2017;17:18. doi: 10.1016/S1473-3099(16)30541-2. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB, Park PK, Ye J, Catlett IM, Girgis IG, Grasela DM. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559) Crit Care Med. 2019;47:632–642. doi: 10.1097/CCM.0000000000003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Clinical and biological data from septic shock patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.