Abstract
Published studies indicate that TMEM184A is a heparin receptor that interacts with and transduces stimulation from heparin in vascular cells. Previous studies have indicated that heparin increases endothelial nitric oxide synthase (eNOS) activity in bovine endothelial cells. However, the precise mechanism remains unknown. In this study, we investigated the impact of heparin treatment and TMEM184A on eNOS’s activation and the role of eNOS in heparin signaling in the cloned A7r5 rat vascular smooth muscle cell line and confirmed results in endothelial cells. We employed a combination of TMEM184A knockdown A7r5 cells along with transient eNOS knockdown and enzyme inhibitor strategies. The results indicate that heparin induces phosphorylation of eNOS. eNOS can be immunoprecipitated with TMEM184A and is internalized to the perinuclear region in a TMEM184A-dependent manner in response to heparin. We also examined how heparin treatment leads to phosphorylation of eNOS and confirmed that TMEM184A and Ca2+ were required to mediate heparin-elicited eNOS phosphorylation. Evidence supporting the involvement of transient receptor potential cation channel subfamily V member 4 with TMEM184A in this eNOS activation process is also presented.
Keywords: eNOS, heparin, vascular smooth muscle
INTRODUCTION
Heparin binds vascular cells and regulates their physiology. While heparin is best known for its anticoagulant characteristics, heparin and the related heparan sulfate (HS) chains can interact with and influence the functions of a wide variety of structural proteins, receptors, and other signaling molecules (15). For example, treatment of vascular cells with heparin can suppress proliferation and inflammation (19, 48). Heparin can bind to, and be taken up by, endothelial cells (ECs) (6, 47), inhibiting their inflammatory responses via decreasing NF-κB and stress kinase pathways (19, 38, 59). Heparin can also bind vascular smooth muscle cells (VSMCs) (13) and decreases MAPK-dependent cell proliferation (20, 28, 44, 51). Protein kinase G (PKG) signaling is at least partially responsible for heparin’s antiproliferative effects on VSMCs (28). This evidence suggests a receptor-mediated model for the heparin responses and heparin transport across the membrane. A recently identified heparin receptor, transmembrane protein 184A (TMEM184A), supports this idea (48).
In unstimulated cell lines including rat aortic SMCs (RAOSMCs) and bovine aortic endothelial cells (BAOECs), the heparin receptor TMEM184A was found to colocalize with caveolin-1 (cav-1) (48), which is one of the most important endothelial nitric oxide synthase (eNOS)-associating proteins that regulates eNOS activity (50). Although not necessary for localizing eNOS to caveolae, cav-1 interacts with and inhibits the activity of eNOS (60, 61). Caveolin not only hinders caveolar-targeted receptor signaling but also blocks calmodulin binding sites in eNOS (2, 27, 41). Dissociation from cav-1 is a vital early step in activating eNOS. Intracellular calcium, calmodulin, and shear stress can promote detachment of eNOS from cav-1 (22).
Numerous studies provide evidence supporting the hypothesis that heparin and its derivatives can regulate eNOS, an important producer of NO, which leads to the activation of PKG through activation of soluble guanylyl cyclase to yield cGMP. Heparin treatment of VSMCs results in cGMP-dependent protein kinase activation (28). Both heparin and its nonanticoagulant derivative, N-acetyl heparin, dose dependently increase eNOS activity by promoting citrulline and nitric oxide metabolite formation in BAOECs (35). In vivo experiments suggest that heparin can increase NOS activity and cGMP levels in pulmonary tissue (32).
Primary posttranslational regulation of eNOS activity is through multisite phosphorylation and dephosphorylation at key serine and threonine residues in eNOS. Stimulatory phosphorylation loci include Ser 1177 (human, Ser 1179 in bovine, we will consistently use Ser 1177 in the text), Ser 617, and Ser 635, while inhibitory sites are Thr 495 and Ser 116 (31). Various kinases such as Akt, CaMKII, and AMPK and stimuli including shear stress and hormones can cause eNOS phosphorylation at Ser 1177. Shear stress, which induces similar antiproliferative and anti-inflammatory vascular cell responses to those of heparin (1, 10, 62), resulted in eNOS Ser 1177 phosphorylation through activation of the PI3K-Akt pathway (9, 17, 23). An increase in flow in intact resistance vessels induces NO production by short term Ca2+-dependent eNOS translocation and long-term Ca2+-independent phosphorylation through PI3K-Akt pathways (22). Phosphoprotein phosphatase 2A (PP2A) and PP1 are phosphatases found to dephosphorylate pThr 495 and p-Ser 1177 of eNOS (30, 46, 56). Heparin has been reported to inhibit PP1 (65). Hence, it is also possible that heparin treatment inhibits PP1 or PP2A and consequently upregulates the level of eNOS p-Ser 1177.
While eNOS was identified as an endothelial cell (EC) protein and significant research supports its function there, it is also expressed in VSMCs (11) and other SMCs (66). Laminar shear stress and heparin can both reduce SMC proliferation and ERK1/2 activity. As with heparin, laminar shear stress can suppress proliferation of ECs and VSMCs (1, 62). In addition, heparin and shear stress can modulate the alignment and morphology of ECs in a similar way. Thus heparin with its receptor TMEM184A may play roles in mechanotransduction by interacting with some classic members of the mechanotransduction signaling system. We anticipated that integrin, which is vital in mechanosensing, is one of those members. Integrins are upstream factors of eNOS and ERK1/2 (33, 36) which are also regulated in response to heparin treatment. Interactions between heparin and integrins have been reported to be necessary in various signaling events. Specifically, heparin binds to αvβ3 integrin (4, 21) and α5β1 integrin (21). In VSMCs, the occupancy of the heparin binding domain of αvβ3 ligand is necessary for proliferative effects of IGF-1 including phosphorylation of ERK 1/2 (42).
In this study, we examined heparin-induced effects on ERK and Elk in VSMC and confirmed a requirement for eNOS activity. Interactions between heparin and TMEM184A elicited activation of eNOS by increasing its Ser 1177 phosphorylation in a calcium-dependent manner. We also identified an apparent focal adhesion location for eNOS p-Ser 1177 and an interaction of integrin αV with TMEM184A. Inhibition of CaMKII and transient receptor potential cation channel subfamily V member 4 (TRPV4) indicates that both are involved in the VSMC heparin-induced eNOS activation. We also confirmed a similar heparin induction of eNOS Ser 1177 phosphorylation in primary endothelial cells.
Experimental Procedures
Materials.
Cell culture chemicals, MEM, 2.5% trypsin-EDTA, porcine gelatin, heparin, penicillin/streptomycin, PDGF, and glutamine were obtained from Sigma (https://www.sigmaaldrich.com). Pretested FBS was obtained from Invitrogen (https://www.thermofisher.com/us/en/home/brands/invitrogen.html), Atlanta Biologicals (https://www.atlanta-biologicals.com/), or Biowest (https://www.biowest.net/) and heat inactivated for 1 h at 55°C or purchased as heat inactivated. Anti-active ERK (4370), phospho-Elk-1 (9181), eNOS (32027, 5880), and phospho-eNOS (9571) antibodies were from Cell Signaling Technology (https://www.cellsignal.com). Anti-TMEM184A [cat. no. sc-292006, NH2-terminal domain (NTD), rabbit; cat. no. sc-163460, internal domain (INT), goat], anti-integrin αV antibodies (sc-6618), and anti-NOS3 (sc-8311 and sc-376751) were from Santa Cruz Biotechnology (https://www.scbt.com/scbt/home). Loading control antibodies against actin (A5441) purchased from Sigma and against α-tubulin (ab7291) purchased from Abcam (https://www.abcam.com/) were used in Western blotting. NOS3 siRNA for rat (sc-270518) was also from Santa Cruz. Secondary antibodies with fluorescent tags and others tagged with biotin and processed to exhibit minimal cross-reactivity were obtained from Jackson ImmunoResearch Laboratories, Inc. (https://www.jacksonimmuno.com/). Extra-avidin-alkaline phosphatase, 5-bromo-4-chloro-3-indolyl phosphate, nitro blue tetrazolium, and EZview red protein G affinity gel beads were obtained from Sigma. EGTA and TRPV4 inhibitors GSK2193874 (SML0942) and RN1734 (R0658) were also from Sigma. CaMKII inhibitor KN-93 was obtained from EMD-Millipore (https://www.emdmillipore.com/). VEGF (8065) was obtained from Cell Signaling Technology.
Cell culture.
A7r5 rat smooth muscle cells were obtained from the ATCC (https://www.atcc.org/). Bovine aortic endothelial cells (BAOECs) were obtained from Cell Applications (https://www.cellapplications.com/) and were cultured in MEM as described previously (19).
TMEM184A knockdown.
Stable transfection of A7r5 cells was accomplished using the TMEM184A shRNA construct as described in our earlier study (48). TMEM184A knockdown A7r5s were cultured in MEM, harvested and analyzed as described previously (48).
Coimmunoprecipitation.
Confluent 150-mm dishes of BAOECs or A7r5 cells were washed three times with ice-cold phosphate-buffered saline (PBS). Ice-cold RIPA buffer (1 mL) supplemented with two protease inhibitor cocktails (cat. no. P8340 and P2714; Sigma) was added after PBS was drained. The cells were then agitated on a rocker for 30 min at 4°C. After incubation, the cells were scraped off the dishes and transferred to a cold microcentrifuge tube and centrifuged for 10 min at 10,000 g at 4°C. The supernatant was incubated at 4°C on a rocker overnight with antibody targeting eNOS as noted above. Following that, 75 μL of equilibrated EZview red protein G affinity gel beads were added and the mixture was incubated on a rocker at 4°C overnight. The beads were washed with the RIPA buffer (with protease inhibitors) three times. The beads were suspended in an equal volume of 2× sample buffer and were boiled for 5 min, and the supernatant was collected for Western blotting.
Western blot analysis.
Sample buffer (2×) was added to cells or immunoprecipitated (IP) samples and boiled for 5 min. For Western blotting of eNOS protein or p-Ser 1177, the cell samples were sonicated and cleared by centrifugation as recommended by the antibody supplier. The protein samples were separated by SDS-PAGE. The IP blots were developed with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate and alkaline phosphatase system as described in our earlier study (48). Other Western blots were developed with fluorescent secondary antibodies and imaged using the Bio-Rad ChemiDoc MP system (48).
eNOS knockdown.
A7r5 cells were trypsinized; rinsed with PBS; suspended in HEPES-buffered saline; mixed with 20 µg/mL of eNOS siRNA, control siRNA, or buffer only; and electroporated with the preset HeLa protocol using the Bio-Rad electroporator. The cells were immediately plated onto glass coverslips in MEM culture medium and returned to the incubator. At 48 h after electroporation, the cells were treated and harvested as noted in specific experiments.
Cell treatments.
Cells cultured on individual coverslips were treated with heparin at 200 µg/mL for length of time as stated in the figure legends. EGTA treatment at 5 mmol/L was added to medium for calcium chelation for 30 min, followed by heparin added as above for the final times as noted in the figures. Treatment with the CaMKII inhibitor KN-93 at 0.001 µmol/mL was for 30 min with heparin addition as above. TRPV4 inhibitors GSK2193874 at 15 µM or RN1734 at 10 µM were also incubated with cells for 30 min with heparin addition as above. Endothelial cells were treated with VEGF at 100 ng/mL final concentration for Fig. 8A.
Fig. 8.
Endothelial nitric oxide synthase (eNOS) in bovine aortic endothelial cells (BAOECs) also gains p-Ser 1177 in response to heparin treatment. A: BAOECs were left untreated (C) or treated with either VEGF at 100 ng/mL (V) or heparin at 200 μg/mL (H) for 5 min. Cells were harvested for eNOS Western blotting for p-Ser 1177 eNOS and tubulin as a loading control. B: BAOECs were pretreated with EGTA as in Fig. 5 and then treated with heparin or not. Cells were harvested and analyzed by Western blotting for p-Ser 1177 eNOS, total eNOS (5880 mouse antibody) and tubulin. Blots were developed with fluorescently tagged antibodies. C: BAOECs were treated with RN1734 as in Fig. 7 and analyzed as in B.
Indirect immunofluorescence.
A7r5 cells were washed three times in PBS, fixed with ice-cold methanol (MeOH) 5 min at 4°C, and again washed three times in PBS. BAOECs were fixed with PFA/Triton as previously reported (19). The coverslips were incubated with supplier recommended concentrations of primary antibodies against specific proteins of interest at 4°C overnight. Following this incubation, coverslips were rinsed with PBS and incubated with species-specific conjugated secondary antibodies for 45 min at 37°C. Secondary antibody-only controls were carried out to exclude nonspecific staining. Cells were imaged using a Zeiss LSM 510 Meta confocal microscope or a Zeiss LSM 800 confocal microscope with a ×40 oil-immersion lens at room temperature. Staining for pERK and pElk-1 after eNOS knockdown was imaged using a Nikon eclipse TE 2000-U fluorescence microscope. Settings were maintained for all images in each assay. For quantitative analysis, the fluorescent intensity of each cell was determined using the freehand tool in ImageJ. Background was subtracted, and intensities were compared relative to controls for the same assay. Statistical significance was determined by one-way ANOVA with post hoc Tukey honestly significant difference analysis.
RESULTS
Knockdown of eNOS Abolishes Heparin Responses in A7r5 VSMCs
Our previous study identified cGMP induction and PKG activation requirements for heparin effects on VSMC proliferation (28). These heparin effects include decreased cell proliferation along with decreased levels of active ERK and phosphorylated Elk (13, 28, 48, 49). The rapid time frame and similar endothelial cGMP induction suggested the possibility that eNOS was involved. To address the hypothesis that eNOS was required for the heparin response, we first determined the presence of eNOS in the A7r5 VSMCs and the ability to knock down the eNOS levels. A7r5 cells were stained with an eNOS antibody (Fig. 1A). Staining for eNOS suggested the presence of the enzyme. In addition, eNOS from A7r5 cells was consistently visualized by Western blotting of whole cell lysates (see, e.g., Fig. 2). To determine whether eNOS levels could be significantly decreased in VSMCs, A7r5 cells were transfected with small interfering RNA (siRNA) targeting eNOS to downregulate its expression. eNOS levels were significantly reduced in eNOS knocked down cells 48 h after transfection compared with control siRNA transfected cells (see Fig. 1A). These data indicate the sequence specific eNOS siRNA resulted in decreased staining by the antibody confirming the presence of eNOS in the control A7r5 cells.
Fig. 1.
Knockdown (KD) of endothelial nitric oxide synthase (eNOS) eliminates heparin responses in A7r5 cells. A: A7r5 cells transfected with siRNA against eNOS or control siRNA were treated with or without 200 μg/mL heparin for 10 min followed by fixation with ice-cold MeOH. N/T, not treated. Cells were stained for eNOS. Fluorescence intensity of at least 100 cells from 3 separate experiments was analyzed for each condition. ***P < 0.005. Scale bars = 50 μm. B: identical cells were prepared as in A. At least 50 cells per condition in each of 3 individual experiments were analyzed for pERK. ***P < 0.0001. C: eNOS siRNA-transfected A7r5 cells and control siRNA-transfected A7r5 cells were treated with 200 µg/mL heparin for 10 min followed by 1.5 µg/mL PDGF stimulation. Cells were stained for pELK. ***P < 0.0001, heparin/PDGF compared with PDGF. The graph represents data from 3 independent experiments with at least 50 cells analyzed per condition in each experiment.
Fig. 2.
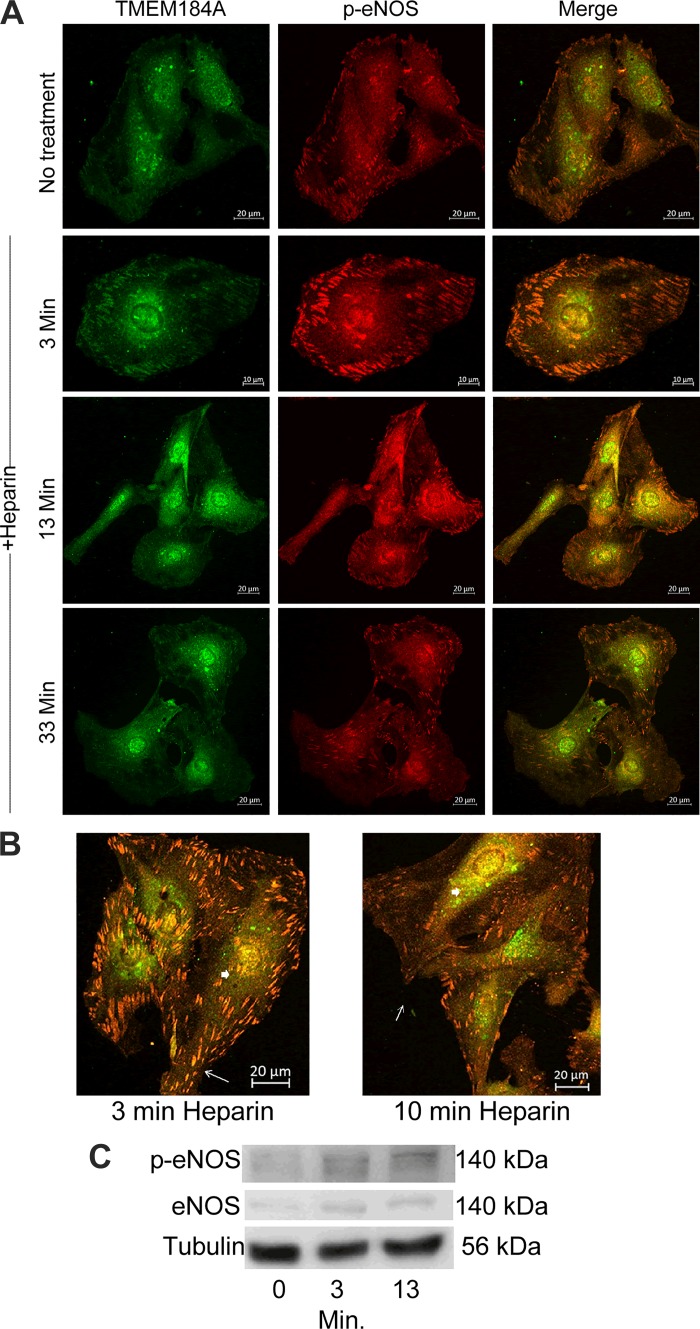
Endothelial nitric oxide synthase (eNOS) colocalizes with transmembrane protein 184A (TMEM184A) before and after heparin treatment. A: A7r5 cells were incubated with 200 μg/mL heparin for the indicated times and fixed and stained for p-eNOS (red) and TMEM184A (green). Images are representative of 2 separate experiments. Scale bars: no treatment = 20 μm; 3 min = 10 µm; 13 and 33 min = 30 µm. B: A7r5s treated as in A taken at a higher magnification show colocalization between p-eNOS and TMEM184A at focal adhesions (arrows) and perinuclear regions (arrowheads). Scale bars = 20 µm. C: A7r5 cells were not treated, or treated with heparin for 3 or 13 min, harvested as described, placed into sample buffer, and separated on SDS polyacrylamide gels. Western blots were developed using the CST rabbit eNOS antibody or p-Ser 1177 eNOS antibody. The blots are representative of at least 3 separate experiments.
To confirm that eNOS was required for heparin signaling, we investigated the effect of siRNA-mediated knockdown of eNOS on ERK and Elk-1 activity. In A7r5s transfected by control siRNA, heparin attenuated basal level phosphorylation of ERK (Fig. 1B). However, heparin had no effect on pERK levels in A7r5 cells treated with eNOS specific siRNA (Fig. 1B). Addition of PDGF induced increased phosphorylation of Elk-1 (Fig. 1C). Heparin-induced effects on Elk-1 (Fig. 1C) phosphorylation were diminished in A7r5s transfected with eNOS siRNA constructs, while cells treated with control siRNA constructs remained sensitive to heparin. These results support the hypothesis that eNOS plays an important role in heparin responses mediated by the MAPK pathway in VSMCs.
Phosphorylated eNOS Colocalizes With TMEM184A in Vascular Cells
TMEM184A, the vascular cell receptor for heparin, has been implicated in membrane trafficking (7) and found to colocalize with caveolin-1 in untreated cells (48), suggesting that TMEM184A might play a role in regulation of activity or translocation of eNOS by interacting with eNOS in caveolae. In immunofluorescent assays in which we costained the cells for TMEM184A and p-Ser 1177 eNOS with or without heparin treatment, after heparin treatment an elevated amount of p-Ser 1177 eNOS resided together with TMEM184A at focal adhesion sites and spread on cell membranes. We expect that these locations are similar to the cav-1 locations in which TMEM184A and eNOS have both been identified previously. There is an increase in focal adhesion p-Ser 1177 eNOS after incubation with heparin for 3 min but only some relocalization to the perinuclear region compared with the TMEM184A and p-Ser 1177 eNOS colocalization without heparin treatment (Fig. 2A). After 13 min, the level of colocalized p-Ser 1177 eNOS and TMEM184A at the cell membrane was obviously reduced, but it increased in the perinuclear region (see also Fig. 2B). After 30 min, there was still evident accumulation of colocalization of the two proteins at the perinuclear region. However, the signal intensities of the two proteins in the cytosol and focal adhesion sites dropped close to the basal level without heparin treatment. Heparin-induced increases in p-Ser 1177 eNOS could also be observed by Western blotting, while staining for total eNOS did not change after heparin treatment. (Fig. 2C).
Heparin-Induced Increases in p-Ser 1177 eNOS Are Dependent on TMEM184A
To investigate how eNOS is involved in the VSMC heparin signaling pathway, we examined whether heparin alters phosphorylation of eNOS at Ser 1177, which promotes eNOS activation. We then asked whether TMEM184A mediates this phosphorylation. A7r5s and stable knockdowns for TMEM184A were incubated with or without heparin at 37°C, for various times. Incubations were stopped by fixation, and the fixed cells were subsequently stained with p-eNOS (Ser 1177) antibody. The results show that in A7r5s heparin treatment caused increased eNOS phosphorylation at Ser 1177 as early as 3 min, which remained at 13 min (Fig. 3). The level of p-Ser 1177 eNOS rapidly reached a maximum at 3 min heparin, as in Fig. 3 (also seen in Fig. 2). In TMEM184A stable knockdown A7r5 cells, heparin did not induce elevation of p-Ser 1177 eNOS after 3 min or 13 min of treatment. Heparin effects on p-Ser 1177 were not observed in stable knockdown A7r5 cells (Fig. 3). However, it appeared that basal levels of p-Ser 1177 eNOS in stable knockdown A7r5 cells were somewhat higher than in A7r5 cells (Fig. 3). Our published data with the stable knockdown cells indicate more intense growth factor signaling. Our data here suggest higher levels of basal p-Ser 1177 eNOS in these cells. Even though the cells were seeded identically and processed together, the differing growth of the cells led us to avoid statistical comparisons of the TMEM184A knockdown cells with the control cells.
Fig. 3.
Transmembrane protein 184A (TMEM184A) knockdown (KD) A7r5 cells do not show heparin-induced increase in p-Ser 1177 endothelial nitric oxide synthase (eNOS). A: A7r5 cells and stable TMEM184A A7r5 knockdown cells were treated with 200 μg/mL heparin for the indicated times followed by fixation and staining for p-eNOS. Images are representative of 3 independent experiments. Scale bars = 50 μm. B: A7r5 cells and TMEM184A stable knockdown cells were treated with heparin as in A. At least 50 cells per condition from 3 separate experiments were evaluated for p-eNOS. ***P < 0.0001, for heparin vs. no heparin.
TMEM184A Associates With eNOS
To determine whether TMEM184A associates with eNOS, vascular cell proteins were immunoprecipitated with eNOS antibodies, and Western blots were analyzed using TMEM184A INT antibodies. TMEM184A staining was observed by TMEM184A INT antibodies when eNOS antibodies were used, but not in the case of affinity beads alone without any antibody (Fig. 4A). Note that some nonspecific proteins present in beads only and IP samples were stained in the blot and that the goat TMEM184A INT antibodies exhibit background protein staining in Western blots as previously shown (48) and do bind TMEM184A in blots as well as immunofluorescence (IF) (48). An arrow in Fig. 4A identifies the TMEM184A specific staining. This eNOS and TMEM184A association is true in both A7r5 VSMC and in BAOEC.
Fig. 4.
Transmembrane protein 184A (TMEM184A) interacts with endothelial nitric oxide synthase (eNOS) and αV integrin. A: membrane protein samples of bovine aortic endothelial cells (BAOECs) and A7r5 lysates were incubated with the NOS3 antibody (sc-8331) and precipitated using EZview affinity beads, or the lysates were incubated with beads without antibodies (B) and Western blots were developed using TMEM184A (internal domain) antibody. IB, immunoblot. The BAOEC blot is representative of 2 separate experiments. The BAOEC blot shown also contained a sample of whole cell lysate. B: A7r5 cells were harvested for immunoprecipitation (IP). Cell samples were incubated with TMEM184A (NTD, NH2-terminal domain) antibody and precipitated using EZview affinity beads or beads and cell lysates without antibody (B) and blots developed using integrin αV antibody. The blot is representative of 2 separate experiments.
TMEM184A Associates With αV Integrin
Data in Figs. 2 and 3 suggest that heparin-induced activation of VSMC eNOS occurs at focal adhesions. Both heparin and laminar shear stress can suppress proliferation of endothelial cells and VSMCs (1, 62), and downregulate p-ERK. Integrins, which are involved in mechanosensing, are heparin binding proteins (4). Evidence indicates some integrins are important upstream receptors that can mediate eNOS phosphorylation (18, 37). The similarities of shear stress and heparin in eNOS activation along with heparin and integrin interactions suggested the possibility that one or more integrins were involved in heparin effects on eNOS activation. The ectodomain of α5β1 integrin binds to heparin with high affinity (21). Heparin also binds to αvβ3 integrin (21). To examine whether TMEM184A, which is the receptor of heparin, associates with integrin αV, we immunoprecipitated VSMC proteins with antibodies against TMEM184A and analyzed the associated proteins by Western blotting with antibodies against integrin αV. The result indicates that TMEM184A associates with integrin αV in vascular cells (Fig. 4B). Similar experiments with antibodies against integrin β1 did not show any staining indicating that β1 integrins do not stably associate with TMEM184A under these conditions (data not shown).
Ca2+ Is Required in the Heparin-Signaling Pathway
Results from Figs. 2 and 3 indicate that heparin treatment activates eNOS by increasing the level of p-Ser 1177. Ca2+ is important in activation of eNOS. eNOS activation requires eNOS-calmodulin binding, which can be triggered by intracellular calcium transients (40). In addition, Ca2+/CaM complexes interact with and regulate a large number of proteins including CaMKII. CaMKII can catalyze phosphorylation of eNOS, at Ser 1177 which activates eNOS by enhancing affinity between calmodulin and eNOS (12). To investigate the hypothesis that heparin’s effects on eNOS are dependent on Ca2+ level and activity of CaMKII, we first treated A7r5 cells with EGTA (to chelate extracellular Ca2+) (Fig. 5, A and B). The results show that EGTA abolished heparin’s effects on eNOS phosphorylation, which indicates that extracellular Ca2+ is necessary for heparin treatment to result in eNOS activation. We also evaluated eNOS phosphorylation in EGTA-treated VSMC by Western blotting (Fig. 5C). These results also indicate that EGTA-treated cells did not exhibit increased p-Ser 1177 eNOS after heparin treatment. To confirm further that this is important in the previously identified heparin-induced VSMC signaling, EGTA-treated VSMC were examined for the ability of heparin to cause decreased ERK activation. Consistent with the EGTA treatment effects on p-Ser 1177 eNOS, EGTA treatment also blocked heparin-induced decreases in ERK phosphorylation (Fig. 5D). Next, KN-93, a CaMKII inhibitor, was employed (Fig. 6) to determine whether CaMKII activity was required for the heparin effect. The results in Fig. 6 reveal that inhibiting CaMKII reduced the ability of heparin treatment to activate eNOS. This implies that CaMKII is involved in heparin-induced activation of eNOS but suggests that calcium has effects on eNOS activation that go beyond the activation of CaMKII, consistent with the requirement of calcium-calmodulin for direct involvement in eNOS activity.
Fig. 5.
EGTA abolished heparin-induced activation of endothelial nitric oxide synthase (eNOS). A: A7r5 cells preincubated for 30 min with or without EGTA were treated for 13 min with or without 200 μg/mL heparin followed by fixation with ice-cold MeOH and staining for p-eNOS. Images are representative of 3 independent experiments. Scale bars = 50 μm. B: A7r5 cells were treated with heparin and EGTA as in A. In each experiment, at least 50 cells per condition were examined for p-eNOS. ***P < 0.0001, heparin treated vs. without heparin. C: A7r5 cells preincubated for 30 min with or without EGTA were then treated with or without heparin for 3 or 13 min and analyzed by Western blotting with antibodies against p-Ser 1177 eNOS and actin (produced in mouse) and developed with Cy3 tagged anti-rabbit secondary antibodies and Alexa 488 tagged anti-mouse secondary antibodies. D: less confluent cells treated as in C were analyzed by Western blotting with antibodies against pERK and tubulin with fluorescent secondary antibodies.
Fig. 6.
KN-93 treatment decreased heparin-induced activation of endothelial nitric oxide synthase (eNOS). A: A7r5 cells preincubated for 30 min with or without KN-93 were treated with or without 200 μg/mL heparin for 13 min followed by fixation with ice-cold MeOH and staining for p-Ser 1177 eNOS. Images are representative of 3 independent experiments. Scale bars = 50 μm. B: A7r5 cells were treated with heparin and KN-93 as in A. At least 50 cells per condition were examined for p-eNOS in each repeat. ***P < 0.0001, heparin vs. without heparin and KN-93 heparin vs. control heparin.
TRPV4 Inhibitors Reduce Heparin-Induced eNOS Phosphorylation
Results above indicate that extracellular Ca2+ is required in heparin-induced activation of eNOS. TRPV4 can play a role in mechanosignaling (5, 16) where it generates a calcium wave. It has recently been shown to be involved with NO regulation (39), responses to hypoxia (43), and barrier control (3, 45). To determine whether a heparin-induced calcium wave is originated through TRPV4 channels, we used RN1734 (3, 63), an inhibitor of the TRPV4 membrane Ca2+ channel (Fig. 7, A and B). In control cells without inhibitor, heparin caused a more than twofold increase in p-Ser 1177 eNOS. Results showed that RN1734 completely blocked heparin’s upregulation of eNOS activity. Another recently identified TRPV4 inhibitor, GSK2193874 (3, 14, 39, 45), was also examined. Again, heparin induced about a twofold increase in p-Ser 1177 eNOS in control but not inhibitor-treated cells. A small (~1.3-fold) heparin upregulation of eNOS activity occurred with the GSK inhibitor compared with the control cells in GSK2193874. The GSK2193874 alone cells had a higher basal level of phosphorylated eNOS. (Fig. 7, C and D). Western blot analysis of RN1734-treated samples provides evidence consistent with that in IF staining (Fig. 7E). Figure 7F illustrates that RN1734 blocked heparin effects on pERK as well. Overall, the TRPV4 inhibitor results imply that the eNOS-activating Ca2+ wave in response to heparin is mediated, at least partially, through TRPV4.
Fig. 7.
Transient receptor potential cation channel subfamily V member 4 (TRPV4) inhibitors decreased heparin-induced activation of endothelial nitric oxide synthase (eNOS). A and B: A7r5 cells preincubated for 30 min with or without RN1734 were treated with or without 200 μg/mL heparin followed by fixation and staining for p-eNOS. Images are representative of 3 independent experiments. Scale bars = 50 μm. C and D: A7r5 cells were treated with GSK and heparin as in A and B. At least 50 cells per condition were examined for p-eNOS in each repeat. **P < 0.0001, heparin vs. without heparin. E: A7r5 cells treated as in A and B were harvested for Western blotting, stained with antibodies against p-eNOS, total eNOS (5880), and tubulin, and blots were developed with secondary fluorescently tagged antibodies as in Fig. 5. F: less confluent A7r5 cells treated as in A and B were harvested for Western blotting; stained with antibodies against p-eNOS, pERK, and tubulin, and developed with fluorescently tagged antibodies as in Fig. 5.
Heparin-Induced eNOS Phosphorylation in Endothelial Cells
The focus of the present study was to examine a role for eNOS in the well-characterized heparin responses in VSMC where our previous studies identified heparin-induced increases in cGMP signaling (28). However, eNOS has been best characterized in endothelial cells. In addition, we have identified TMEM184A heparin interactions in endothelial cells (19). Therefore, we examined whether heparin treatment of BAOECs would result in increased eNOS phosphorylation similar to that found in the VSMCs. First, we confirmed that heparin treatment of cultured primary endothelial cells would result in increased phosphorylation of eNOS at Ser 1177. We compared this to identical cells treated instead with VEGF, a growth factor that induces eNOS phosphorylation in treated endothelial cells (Fig. 8A). Both heparin and VEGF consistently induced phosphorylation of eNOS in endothelial cells. To confirm that the signal system in endothelial cells is similar to that in VSMCs, we evaluated EGTA-treated cells similarly to the experiments in Fig. 5. While the overall heparin-induced phosphorylation did not appear as intense as the response in Fig. 8A, heparin did induce eNOS phosphorylation in control BAOEC (twofold increase at 3 min and 1.5 at 10 min), while in EGTA-treated cells there was no response to heparin (Fig. 8B). These results are similar in level to those seen in the VSMCs and are reproducible. In addition, RN1734-treated endothelial cells were also subjected to treatment with heparin. In this experiment, the heparin treatment of BAOECs also induced about a twofold increase in control cells. There was no response to heparin in RN1734-treated endothelial cells, despite a response in the control cells without RN1734 (Fig. 8C). As in VSMCs, these results support involvement of TRPV4 in eNOS responses to heparin.
Heparin-Induced eNOS Phosphorylation in Endothelial Cells Localized Similarly to That in the A7r5 Cells
Our data in Fig. 4 indicated that IP of eNOS in cultured endothelial cells resulted in pull down of TMEM184A and that αV integrin associated with the TMEM184A. Immunofluorescence staining of untreated BAOEC for eNOS and αV integrin suggested overlap in staining (see Fig. 9A). Immunofluorescence images of heparin-treated BAOECs indicated small puncta of activity rather than the larger VSMC p-Ser 1177 clusters which appeared to increase in heparin-treated cells. The eNOS phosphorylation in VSMC occurs primarily in what appear to be focal adhesion sites. These are similar to p-Ser 1177 eNOS puncta in BAOEC stained for αV integrin but do not always colocalize with it (Fig. 9B). Merged images clearly show increasing p-eNOS staining. The αV integrin intensity appears to augment the signal in heparin-treated BAOECs. Staining for αV integrin, TMEM184A, and total eNOS in heparin-treated cells indicates that some of the eNOS and TMEM184A move away from initial locations in the first 3 min, similar to the joint movement of TMEM184A and eNOS observed in VSMC. However, the overall eNOS staining pattern does not change dramatically. By 10 min, the TMEM184A staining appears somewhat diminished, while eNOS staining still is more diffusely located than in the untreated cells (Fig. 9C). Zoomed images of several cells showing only the TMEM184A (red) and total eNOS (green) staining confirm that overlap is limited to a small percentage of the total eNOS stain. Together these results (Figs. 8 and 9) suggest heparin-induced effects on eNOS in BAOECs are the same as those observed in VSMC, except that a smaller percentage of the endothelial eNOS is responsive to the heparin signal.
Fig. 9.
A: bovine aortic endothelial cells (BAOECs) were stained for αV integrin (red) and total endothelial nitric oxide synthase (eNOS; 5880) (green). B: BAOECs were grown on coverslips, treated with 200 μg/mL heparin for 3 or 10 min or left untreated, (fixed as noted in experimental procedures) and stained for p-Ser 1177 eNOS (green) and αV integrin (white). C: BAOECs were treated with heparin and stained with αV integrin (white), transmembrane protein 184A (TMEM184A; NH2-terminal domain; red), and total eNOS (5880, green). Scale bars = 20 µm. Zoomed sections of merge-3 images were altered to remove the αV integrin channel for clarity of the limited colocalization of TMEM184A and total eNOS. Scale bars on zoomed images are 10 µm.
DISCUSSION
The results presented here examined the hypothesis that eNOS has an essential role in heparin’s anti-proliferation responses in VSMCs. In addition, the data confirm a similar response in BAOECs. Our group previously reported that cGMP-dependent protein kinase, which is a downstream factor of eNOS, is involved in heparin-induced downregulation of MAPK activation in VSMCs (28). Our finding that the heparin receptor TMEM184A colocalizes with caveolin-1, which also associates with eNOS, supports the hypothesis that eNOS plays a role in heparin’s anti-proliferative signaling pathway (48). Published work by others (discussed below) provides evidence for calcium-induced activation of eNOS and also that TRPV4 activation in endothelial cells results in increased intracellular calcium and eNOS activation. In the present report, we investigated whether eNOS is required for the heparin response and whether the TRPV4 to CaMKII pathway activating eNOS is involved in the heparin response.
We report here that knockdown of eNOS abolishes heparin effects in VSMCs. Specifically, the ability of heparin treatment to decrease ERK and Elk-1 activation is eliminated. This finding provides evidence supporting the hypothesis of eNOS involvement in heparin responses.
To further support the hypothesis of eNOS involvement, we determined that in response to heparin treatment, eNOS’s activity and subcellular translocation are altered. In VSMCs, phosphorylation of eNOS on Ser 1177 was induced by heparin treatment as early as 3 min, and it dropped to basal levels after 30 min of heparin treatment. These data are consistent with earlier results indicating that heparin treatment can preserve NOS activity in damaged tissue (35). Crucially, we demonstrate here, using stably knocked down TMEM184A cells, that eNOS activation upon heparin treatment is dependent on heparin receptor TMEM184A. As we have shown, the stable knockdown cells do not respond to heparin (48).
Our work also reveals that phosphorylated eNOS is initially found at cell membrane and focal adhesion sites upon heparin treatment and subsequently moves with TMEM184A to the perinuclear region. This result coupled with coimmunoprecipitation suggests that TMEM184A may be an eNOS binding partner that serves to regulate the activity and/or translocation of p-eNOS from the regions adjacent to the cell surface to perinuclear compartments. These results are consistent with published evidence on TMEM184A, which implicated TMEM184A in membrane trafficking (7, 8). TMEM184A is predicted to be a multipass transmembrane protein that was originally identified in SK11 Sertoli cells and is involved in membrane trafficking (7, 8). A potential endosomal targeting domain was reported along with colocalization with VAMP-containing perinuclear and peripheral endosomes (8), and studies in exocrine cells suggest involvement in vesicle trafficking for secretion (7).
Others report that under quiescent conditions, eNOS is anchored in caveolae by cotranslational myristoylation and posttranslational palmitoylation (53). It is possible that TMEM184A facilitates the dissociation from cav-1 and subsequent internalization of the activated eNOS after heparin treatment. Detachment from caveolae is necessary for activation, (24) and the following internalization of p-Ser 1177 eNOS is required for the consequent deactivation and desensitization of eNOS. Some reports provide evidence that internalized eNOS can be localized to Golgi or to cytosol (22) suggesting that interaction with other players such as TMEM184A may direct eNOS traffic to specific intracellular locations. A recent report detailed the production of cell-free caveolae and their interactions (34). In this system, there was no direct interaction of eNOS with the cell-free caveolae suggesting that eNOS interactions with caveolae and cav-1 might involve other proteins. One report indicated that heparan sulfate proteoglycans (HSPGs) were required for caveolae internalization and eNOS activation in response to catestatin (25). In our TMEM184A stable knockdown cells, heparin did not induce increased eNOS phosphorylation or perinuclear localization (see Fig. 3). However, whether TMEM184A plays a direct role in the eNOS internalization or only facilitates phosphorylation remains to be determined.
Our immunofluorescence data indicate a likely focal adhesion location for the activation of eNOS in response to heparin. There are many reports indicating interactions between integrin and cav-1. For example, β1 integrin/cav-1 mechanosignaling complexes respond to shear stress and lead to RhoA and actin remodeling in endothelial cells (64). β1 integrin endocytosis is dependent on cav-1 in myofibroblasts (57). The ectodomain of α5β1 integrin binds to heparin with high affinity in endothelial cells (KD = 15.5 nM) (21). Heparin binds to αvβ3 integrin in endothelial cells (21). There is also evidence suggesting that integrin controls localization of cholesterol-enriched membrane microdomains (52) at the plasma membrane of epithelial cells to regulate cellular processes (54). Based on our previous report that TMEM184A partly colocalizes with cav-1 (48), it is possible that TMEM184A also interacts with integrin in caveolae or lipid rafts. Heparin’s anti-inflammatory and antiproliferation signaling pathway shares some players with the laminar shear stress responses.
Integrin is vital in the mechanosensing complex, and it is an upstream factor of Akt, eNOS and ERK1/2. There are published results showing that upregulation of HSPG synthesis induced by heparin in endothelial cells is dependent on the interaction of heparin with integrin while arginine-glycine-aspartate (RGD) peptide abolishes the effect (40). This upregulation is also associated with the phosphorylation of focal adhesion proteins. Therefore, we hypothesized that one or more integrins might interact with TMEM184A to modulate eNOS’s activity in response to heparin.
In addition to the localization of p-Ser 1177 eNOS at sites that appear to be focal adhesions, immunoprecipitation with anti-TMEM184A antibodies pulled αV integrin along, although similar results were not seen using probes for β1 integrin, which has been suggested for shear stress involvement. Published evidence suggests that the eNOS pathway in endothelial cells has important effects at focal adhesions including cGMP-dependent protein kinase regulation of migration and focal adhesion sites (58) and NO-induced modulation of focal adhesions (29). Our results suggest that some eNOS activation also occurs in heparin-treated endothelial cells. Therefore, it will be important to determine the mechanism by which αV integrin associates with TMEM184A and whether heparin effects on eNOS through TMEM184A involve the integrin interactions.
eNOS is regulated through various cellular events including posttranslational modification, calcium wave stimulation, and association or dissociation with its binding proteins. In addition, shear stress induces phosphorylation of endothelial eNOS through a PI3-kinase Akt pathway (9, 26). We evaluated the possibility that Akt was activated and then phosphorylated eNOS, but we found no evidence of changes in Akt activity in the period consistent with heparin effects (data not shown). Because heparin elevated eNOS activity shortly after treatment, we hypothesized that calcium stimulation might be involved in eNOS activation in response to heparin. Intracellular Ca2+ increases can cause calmodulin binding to eNOS and hence facilitate electron transfer from the reductase domain to the oxygenase domain of the NOS to synthesize NO (40). Heparin is a competitive antagonist of IP3 receptors (55), inhibiting the inositol (1,4,5)-trisphosphate (IP3) receptor, which is a classic intracellular Ca2+ release channel and a principle signaling protein involved in gap junction-mediated propagation of Ca2+ waves (55). Heparin treatment results in intracellular Ca2+ release and has been reported to activate PLCγI and CaMKII, as well as inducing NO production (40) since PLCγI produces IP3 and induces Ca2+ release. Besides facilitating electron transfer, Ca2+/calmodulin complexes can also activate CaMKII to phosphorylate eNOS. Studies reported here found that EGTA inhibited heparin-induced eNOS activation, which suggests that Ca2+ is essential for heparin’s effect on eNOS. EGTA eliminated extracellular Ca2+ from the cell media to reduce Ca2+ concentration suggesting that extracellular Ca2+ is required for initial eNOS activation (Figs. 5 and 8). We cannot exclude the possibility that eNOS was also activated by intracellular Ca2+ release in the longer term. We used CaMKII inhibitor KN-93 and found evidence suggesting that CaMKII is involved in eNOS’s activation by heparin because KN-93 decreased, but did not abolish, the activation. It is likely that both calcium/calmodulin binding to eNOS and CaMKII phosphorylation are involved in the response.
Considering that eNOS activation happens rapidly in response to heparin treatment, and eliminating extracellular Ca2+ blocked immediate heparin-induced responses, we decided to explore transmembrane Ca2+ channels. A TRPV4/ Ca2+-mechanism of eNOS activation was recently identified in endothelial cells (39). There is an ATP-dependent TRPV4 activation coupled with an eNOS feedback mechanism in the endothelial system that limits the extent of activation. A following study identified a role for TRPV4 in fluid shear stress in endothelial cells with a specific localization in small clusters containing β1 integrins at the basal membrane (5). We used TRPV4 inhibitors GSK2193874 and RN1734. The results we obtained using RN1734, a commonly employed inhibitor, showed a reversal of heparin’s effect on eNOS, consistent with TRPV4 playing a role in heparin-induced eNOS activation in both VSMC and endothelial cells. The inhibitor GSK2193874 only reduced such upregulation and resulted in increased basal eNOS phosphorylation. The reason for somewhat different responses to the two TRPV4 inhibitors is not clear but could possibly be due to TRPV4-interacting proteins differently influencing effects of the two inhibitors. Overall, our results support the idea that TRPV4 plays a role in heparin’s activation of eNOS through facilitating calcium transients required for eNOS phosphorylation. Together, our results suggest that TMEM184A association with exogenous heparin results in activation of TRPV4 channels allowing calcium entry and activation of eNOS including CaMKII-dependent phosphorylation (see Fig. 10). The initial activation of eNOS appears to occur in focal adhesions, but additional studies will be required to determine whether integrins play a role in the signaling pathway. Additional studies will also be required to determine how the heparin-TMEM184A-TRPV4 activation mechanism works, but this system is consistent with the rapid activation of eNOS identified in our studies.
Fig. 10.
Model for the signal steps between transmembrane protein 184A (TMEM184A) and endothelial nitric oxide synthase (eNOS) phosphorylation. This model indicates likely interactions between TMEM and transient receptor potential cation channel subfamily V member 4 (TRPV4). The mechanism for this interaction is unknown and represented by dashes. The remaining connections shown have been established by others, and our studies indicate roles for these in the response to heparin.
Immunoprecipitation in endothelial cells using heparin-receptor MAbs identified the target protein as TMEM184A (48). In cell lines including RAOSMCs and BAOECs, the putative heparin receptor TMEM184A colocalizes with cav-1, which is one of the most important eNOS-associating proteins that regulates activity of eNOS (48). Here we report that heparin interactions with TMEM184A result in activation of eNOS through Ca2+-dependent phosphorylation at Ser 1177. While the majority of these results were obtained in VSMC, data in Figs. 8 and 9 indicate that a similar pathway is operating in BAOECs suggesting that both cell types containing the TMEM184A receptor induce similar changes in a pool of eNOS activity in response to heparin treatment as summarized in Fig. 10. These results provide additional evidence linking mechanosignaling and heparin-induced signaling pathways. In addition, they suggest intriguing next steps in our exploration of heparin signaling mechanisms.
In addition to facilitating Ca2+-induced phosphorylation, it is possible that after heparin treatment, TMEM184A acquires the ability to interfere with eNOS’s binding with its binding partners such as cav-1 and subsequently activates eNOS. Alternatively, TMEM184A might alter eNOS’s activity by regulating its subcellular translocation and life cycle as suggested above since TMEM184A has been reported to be involved in membrane trafficking (8). Additional studies will be necessary to examine the possible roles for TMEM184A in trafficking of eNOS. What is now clear is that heparin interactions with TMEM184A play a role in activation of eNOS through increases in intracellular Ca2+ and Ca2+-induced phosphorylation of eNOS as summarized in Fig. 10.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL54269 to (to L. J. Lowe-Krentz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. and L.J.L.-K. conceived and designed research; Y.L., L.M.T.-A., K.A.S., and G.O.C. performed experiments; Y.L., L.M.T.-A., K.A.S., G.O.C., and L.J.L.-K. analyzed data; Y.L., L.M.T.-A., and L.J.L.-K. interpreted results of experiments; Y.L. and L.J.L.-K. prepared figures; Y.L. and L.J.L.-K. drafted manuscript; Y.L., L.M.T.-A., and L.J.L.-K. edited and revised manuscript; Y.L., L.M.T.-A., K.A.S., G.O.C., and L.J.L.-K. approved final version of manuscript.
REFERENCES
- 1.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ Res 86: 185–190, 2000. doi: 10.1161/01.RES.86.2.185. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Díaz J, Vara E, Torres-Melero J, García C, Hernández J, Balibrea JL. Local production of oxygen free radicals and nitric oxide in rat diaphragm during sepsis: effects of pentoxifylline and somatostatin. Eur J Surg 163: 619–625, 1997. [PubMed] [Google Scholar]
- 3.Arredondo Zamarripa D, Noguez Imm R, Bautista Cortés AM, Vázquez Ruíz O, Bernardini M, Fiorio Pla A, Gkika D, Prevarskaya N, López-Casillas F, Liedtke W, Clapp C, Thébault S. Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: diabetic milieu makes a difference. Sci Rep 7: 13094, 2017. [Erratum in Sci Rep 8: 9652, 2018.] doi: 10.1038/s41598-017-13621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballut L, Sapay N, Chautard E, Imberty A, Ricard-Blum S. Mapping of heparin/heparan sulfate binding sites on αvβ3 integrin by molecular docking. J Mol Recognit 26: 76–85, 2013. doi: 10.1002/jmr.2250. [DOI] [PubMed] [Google Scholar]
- 5.Baratchi S, Knoerzer M, Khoshmanesh K, Mitchell A, McIntyre P. Shear stress regulates TRPV4 channel clustering and translocation from adherens junctions to the basal membrane. Sci Rep 7: 15942, 2017. doi: 10.1038/s41598-017-16276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bârzu T, Molho P, Tobelem G, Petitou M, Caen J. Binding and endocytosis of heparin by human endothelial cells in culture. Biochim Biophys Acta 845: 196–203, 1985. doi: 10.1016/0167-4889(85)90177-6. [DOI] [PubMed] [Google Scholar]
- 7.Best D, Adams IR. Sdmg1 is a component of secretory granules in mouse secretory exocrine tissues. Dev Dyn 238: 223–231, 2009. doi: 10.1002/dvdy.21827. [DOI] [PubMed] [Google Scholar]
- 8.Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development 135: 1415–1425, 2008. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 10.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285: H1113–H1122, 2003. doi: 10.1152/ajpheart.00302.2003. [DOI] [PubMed] [Google Scholar]
- 11.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. FASEB J 16: 500–508, 2002. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 12.Cai H, Liu D, Garcia JG. CaM kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res 77: 30–34, 2008. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]
- 13.Castellot JJ Jr, Cochran DL, Karnovsky MJ. Effect of heparin on vascular smooth muscle cells. I. Cell metabolism. J Cell Physiol 124: 21–28, 1985. doi: 10.1002/jcp.1041240105. [DOI] [PubMed] [Google Scholar]
- 14.Cheung M, Bao W, Behm DJ, Brooks CA, Bury MJ, Dowdell SE, Eidam HS, Fox RM, Goodman KB, Holt DA, Lee D, Roethke TJ, Willette RN, Xu X, Ye G, Thorneloe KS. Discovery of GSK2193874: an orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med Chem Lett 8: 549–554, 2017. doi: 10.1021/acsmedchemlett.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiodelli P, Bugatti A, Urbinati C, Rusnati M. Heparin/heparan sulfate proteoglycans glycomic interactome in angiogenesis: biological implications and therapeutical use. Molecules 20: 6342–6388, 2015. doi: 10.3390/molecules20046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan MA, Johnson GP, Stavenschi E, Riffault M, Labour MN, Hoey DA. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci Rep 8: 3824, 2018. doi: 10.1038/s41598-018-22174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 18.Erkens R, Suvorava T, Kramer CM, Diederich LD, Kelm M, Cortese-Krott MM. Modulation of local and systemic heterocellular communication by mechanical forces: a role of endothelial nitric oxide synthase. Antioxid Redox Signal 26: 917–935, 2017. doi: 10.1089/ars.2016.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farwell SL, Kanyi D, Hamel M, Slee JB, Miller EA, Cipolle MD, Lowe-Krentz LJ. Heparin decreases in tumor necrosis factor α (TNFα)-induced endothelial stress responses require transmembrane protein 184a and induction of dual specificity phosphatase 1. J Biol Chem 291: 5342–5354, 2016. doi: 10.1074/jbc.M115.681288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasciano S, Patel RC, Handy I, Patel CV. Regulation of vascular smooth muscle proliferation by heparin: inhibition of cyclin-dependent kinase 2 activity by p27(kip1). J Biol Chem 280: 15682–15689, 2005. doi: 10.1074/jbc.M411458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faye C, Moreau C, Chautard E, Jetne R, Fukai N, Ruggiero F, Humphries MJ, Olsen BR, Ricard-Blum S. Molecular interplay between endostatin, integrins, and heparan sulfate. J Biol Chem 284: 22029–22040, 2009. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa XF, González DR, Puebla M, Acevedo JP, Rojas-Libano D, Durán WN, Boric MP. Coordinated endothelial nitric oxide synthase activation by translocation and phosphorylation determines flow-induced nitric oxide production in resistance vessels. J Vasc Res 50: 498–511, 2013. doi: 10.1159/000355301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand 168: 81–88, 2000. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 24.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res 43: 532–541, 1999. doi: 10.1016/S0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 25.Fornero S, Bassino E, Ramella R, Gallina C, Mahata SK, Tota B, Levi R, Alloatti G, Gallo MP. Obligatory role for endothelial heparan sulphate proteoglycans and caveolae internalization in catestatin-dependent eNOS activation. BioMed Res Int 2014: 783623, 2014. doi: 10.1155/2014/783623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem 274: 30101–30108, 1999. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 27.García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272: 25437–25440, 1997. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 28.Gilotti AC, Nimlamool W, Pugh R, Slee JB, Barthol TC, Miller EA, Lowe-Krentz LJ. Heparin responses in vascular smooth muscle cells involve cGMP-dependent protein kinase (PKG). J Cell Physiol 229: 2142–2152, 2014. doi: 10.1002/jcp.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goligorsky MS, Abedi H, Noiri E, Takhtajan A, Lense S, Romanov V, Zachary I. Nitric oxide modulation of focal adhesions in endothelial cells. Am J Physiol Cell Physiol 276: C1271–C1281, 1999. doi: 10.1152/ajpcell.1999.276.6.C1271. [DOI] [PubMed] [Google Scholar]
- 30.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry 41: 15845–15853, 2002. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 31.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des 20: 3503–3513, 2014. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horstman DJ, Fischer LG, Kouretas PC, Hannan RL, Rich GF. Role of nitric oxide in heparin-induced attenuation of hypoxic pulmonary vascular remodeling. J Appl Physiol (1985) 92: 2012–2018, 2002. doi: 10.1152/japplphysiol.00664.2001. [DOI] [PubMed] [Google Scholar]
- 33.Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol 18: 227–234, 1998. doi: 10.1161/01.ATV.18.2.227. [DOI] [PubMed] [Google Scholar]
- 34.Jung W, Sierecki E, Bastiani M, O’Carroll A, Alexandrov K, Rae J, Johnston W, Hunter DJB, Ferguson C, Gambin Y, Ariotti N, Parton RG. Cell-free formation and interactome analysis of caveolae. J Cell Biol 217: 2141–2165, 2018. doi: 10.1083/jcb.201707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouretas PC, Kim YD, Cahill PA, Myers AK, To LN, Wang YN, Wallace RB, Kron IL, Hannan RL. Heparin preserves nitric oxide activity in coronary endothelium during ischemia-reperfusion injury. Ann Thorac Surg 66: 1210–1215, 1998. doi: 10.1016/S0003-4975(98)00811-X. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 272: 30455–30462, 1997. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Kassab GS. Integrins mediate mechanical compression-induced endothelium-dependent vasodilation through endothelial nitric oxide pathway. J Gen Physiol 146: 221–232, 2015. doi: 10.1085/jgp.201411350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manduteanu I, Voinea M, Antohe F, Dragomir E, Capraru M, Radulescu L, Simionescu M. Effect of enoxaparin on high glucose-induced activation of endothelial cells. Eur J Pharmacol 477: 269–276, 2003. doi: 10.1016/j.ejphar.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Marziano C, Hong K, Cope EL, Kotlikoff MI, Isakson BE, Sonkusare SK. Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J Am Heart Assoc 6: e007157, 2017. doi: 10.1161/JAHA.117.007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medeiros VP, Paredes-Gamero EJ, Monteiro HP, Rocha HA, Trindade ES, Nader HB. Heparin-integrin interaction in endothelial cells: downstream signaling and heparan sulfate expression. J Cell Physiol 227: 2740–2749, 2012. doi: 10.1002/jcp.23018. [DOI] [PubMed] [Google Scholar]
- 41.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 272: 25907–25912, 1997. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 42.Miller EC, Capps BE, Sanghani RR, Clemmons DR, Maile LA. Regulation of igf-I signaling in retinal endothelial cells by hyperglycemia. Invest Ophthalmol Vis Sci 48: 3878–3887, 2007. doi: 10.1167/iovs.07-0014. [DOI] [PubMed] [Google Scholar]
- 43.Naik JS, Walker BR. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflugers Arch 470: 633–648, 2018. doi: 10.1007/s00424-018-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottlinger ME, Pukac LA, Karnovsky MJ. Heparin inhibits mitogen-activated protein kinase activation in intact rat vascular smooth muscle cells. J Biol Chem 268: 19173–19176, 1993. [PubMed] [Google Scholar]
- 45.Pairet N, Mang S, Fois G, Keck M, Kühnbach M, Gindele J, Frick M, Dietl P, Lamb DJ. TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS One 13: e0196055, 2018. doi: 10.1371/journal.pone.0196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, Sung HY, Lee JY, Kim HJ, Ahn JH, Jo I. B56α subunit of protein phosphatase 2A mediates retinoic acid-induced decreases in phosphorylation of endothelial nitric oxide synthase at serine 1179 and nitric oxide production in bovine aortic endothelial cells. Biochem Biophys Res Commun 430: 476–481, 2013. doi: 10.1016/j.bbrc.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Patton WA 2nd, Granzow CA, Getts LA, Thomas SC, Zotter LM, Gunzel KA, Lowe-Krentz LJ. Identification of a heparin-binding protein using monoclonal antibodies that block heparin binding to porcine aortic endothelial cells. Biochem J 311: 461–469, 1995. doi: 10.1042/bj3110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugh RJ, Slee JB, Farwell SL, Li Y, Barthol T, Patton WA, Lowe-Krentz LJ. Transmembrane protein 184A is a receptor required for vascular smooth muscle cell responses to heparin. J Biol Chem 291: 5326–5341, 2016. doi: 10.1074/jbc.M115.681122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pukac LA, Carter JE, Ottlinger ME, Karnovsky MJ. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. J Cell Physiol 172: 69–78, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 50.Ramadoss J, Pastore MB, Magness RR. Endothelial caveolar subcellular domain regulation of endothelial nitric oxide synthase. Clin Exp Pharmacol Physiol 40: 753–764, 2013. doi: 10.1111/1440-1681.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reilly CF, Kindy MS, Brown KE, Rosenberg RD, Sonenshein GE. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem 264: 6990–6995, 1989. [PubMed] [Google Scholar]
- 52.Riff JD, Callahan JW, Sherman PM. Cholesterol-enriched membrane microdomains are required for inducing host cell cytoskeleton rearrangements in response to attaching-effacing Escherichia coli. Infect Immun 73: 7113–7125, 2005. doi: 10.1128/IAI.73.11.7113-7125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson LJ, Michel T. Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proc Natl Acad Sci USA 92: 11776–11780, 1995. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA. Integrin regulation of caveolin function. J Cell Mol Med 11: 969–980, 2007. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleem H, Tovey SC, Riley AM, Potter BV, Taylor CW. Stimulation of inositol 1,4,5-trisphosphate (IP3) receptor subtypes by adenophostin A and its analogues. PLoS One 8: e58027, 2013. doi: 10.1371/journal.pone.0058027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo J, Lee JY, Sung MS, Byun CJ, Cho DH, Lee HJ, Park JH, Cho HS, Cho SJ, Jo I. Arsenite acutely decreases nitric oxide production via the ROS-protein phosphatase 1-endothelial nitric oxide synthase-Thr(497) signaling cascade. Biomol Ther (Seoul) 22: 510–518, 2014. doi: 10.4062/biomolther.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121: 2360–2371, 2008. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smolenski A, Poller W, Walter U, Lohmann SM. Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase I. J Biol Chem 275: 25723–25732, 2000. doi: 10.1074/jbc.M909632199. [DOI] [PubMed] [Google Scholar]
- 59.Thourani VH, Brar SS, Kennedy TP, Thornton LR, Watts JA, Ronson RS, Zhao ZQ, Sturrock AL, Hoidal JR, Vinten-Johansen J. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 278: H2084–H2093, 2000. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- 60.Trane AE, Hiob MA, Uy T, Pavlov D, Bernatchez P. Caveolin-1 scaffolding domain residue phenylalanine 92 modulates Akt signaling. Eur J Pharmacol 766: 46–55, 2015. doi: 10.1016/j.ejphar.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 61.Trane AE, Pavlov D, Sharma A, Saqib U, Lau K, van Petegem F, Minshall RD, Roman LJ, Bernatchez PN. Deciphering the binding of caveolin-1 to client protein endothelial nitric-oxide synthase (eNOS): scaffolding subdomain identification, interaction modeling, and biological significance. J Biol Chem 289: 13273–13283, 2014. doi: 10.1074/jbc.M113.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueba H, Kawakami M, Yaginuma T. Shear stress as an inhibitor of vascular smooth muscle cell proliferation. Role of transforming growth factor-beta 1 and tissue-type plasminogen activator. Arterioscler Thromb Vasc Biol 17: 1512–1516, 1997. doi: 10.1161/01.ATV.17.8.1512. [DOI] [PubMed] [Google Scholar]
- 63.Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389: 490–494, 2009. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Yang B, Radel C, Hughes D, Kelemen S, Rizzo V. p190 RhoGTPase-activating protein links the β1 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arterioscler Thromb Vasc Biol 31: 376–383, 2011. doi: 10.1161/ATVBAHA.110.217794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zapella PD, da-Silva AM, da-Costa-Maia JC, Terenzi HF. Serine/threonine protein phosphatases and a protein phosphatase 1 inhibitor from Neurospora crassa. Braz J Med Biol Res 29: 599–604, 1996. [PubMed] [Google Scholar]
- 66.Zheng J, Zhai K, Chen Y, Zhang X, Miao L, Wei B, Ji G. Nitric oxide mediates stretch-induced Ca2+ oscillation in smooth muscle. J Cell Sci 129: 2430–2437, 2016. doi: 10.1242/jcs.180638. [DOI] [PubMed] [Google Scholar]