Abstract
Background
Acute pancreatitis in pregnancy (APIP) is a life‐threatening disease for both mother and fetus. To date, only three patients with recurrent hypertriglyceridemia‐induced APIP (HTG‐APIP) have been reported to carry rare variants in the lipoprotein lipase (LPL) gene, which encodes the key enzyme responsible for triglyceride (TG) metabolism. Coincidently, all three patients harbored LPL variants on both alleles and presented with complete or severe LPL deficiency.
Methods
The entire coding regions and splice junctions of LPL and four other TG metabolism genes (APOC2, APOA5, GPIHBP1, and LMF1) were analyzed by Sanger sequencing in a Han Chinese patient who had experienced two episodes of HTG‐APIP. The impact of a novel LPL missense variant on LPL protein expression and activity was analyzed by transient expression in HEK293T cells.
Results
A novel heterozygous LPL missense variant, p.His210Leu (c.629A > T), was identified in our patient. This variant did not affect protein synthesis but significantly impaired LPL secretion and completely abolished the enzymatic activity of the mutant protein.
Conclusion
This report describes the first identification and functional characterization of a heterozygous variant in the LPL that predisposed to recurrent HTG‐APIP. Our findings confirm a major genetic contribution to the etiology of individual predisposition to HTG‐APIP.
Keywords: HTG‐APIP, lipoprotein lipase (LPL) gene, missense variant, recurrent acute pancreatitis in pregnancy
The present study describes the first identification and functional characterization of a heterozygous variant in the LPL that predisposed to recurrent HTG‐APIP. Our findings not only underscore the importance of genetic defects in predisposing to HTG and/or acute pancreatitis but also shed new light on the complexity underlying the etiology of these phenotypes.

1. INTRODUCTION
Acute pancreatitis in pregnancy (APIP) is a life‐threatening disease for both mother and fetus, with an incidence of 1 in 1,000–10,000 pregnancies (Igbinosa, Poddar, & Pitchumoni, 2013; Zhang et al., 2013). Over the past decades, significant improvements in diagnosis and management have reduced the mortality rate from 37% to 3.3% for mothers and from 60% to 11.6% for the fetus, respectively (Ducarme, Maire, Chatel, Luton, & Hammel, 2014; Fan et al., 2018; Ramin, Ramin, Richey, & Cunningham, 1995; Tang, Xu, Song, Mei, & Zhang, 2018; Vilallonga, Calero‐Lillo, Charco, & Balsells, 2014; Xu, Wang, & Zhang, 2015). Gallstones are the leading cause of APIP worldwide while hypertriglyceridemia (HTG) ranks second in China and third in Western countries, respectively (Luo et al., 2018; Papadakis, Sarigianni, Mikhailidis, Mamopoulos, & Karagiannis, 2011). Compared to biliary APIP, HTG‐induced APIP (HTG‐APIP) often has a more severe disease course, is associated with a poorer outcome, and causes more maternal and fetal deaths (Deng, Wang, Wu, Tang, & Chen, 2014; Liu, Lun, Lv, Hou, & Wang, 2016; Luo et al., 2018; Sun, Fan, & Wang, 2013; Tang et al., 2018).
A normal triglyceride (TG) level is less than 1.7 mmol/L (150 mg/dl). During the late stages of gestation, the maternal TG concentration normally increases two‐ to fourfold due to a high‐fat diet, lack of exercise, increase in estrogen, and other factors (Alvarez, Montelongo, Iglesias, Lasuncion, & Herrera, 1996; Basaran, 2009). However, the TG level in HTG‐APIP patients can increase to more than 11.3 mmol/L (1,000 mg/dl), the threshold for defining severe HTG (Garg, Garg, Hegele, & Lewis, 2019), which may be determined by genetic factors associated with TG metabolism. In this regard, variants in the lipoprotein lipase (LPL) gene (OMIM# 609708), which encode the key enzyme responsible for TG metabolism, have been reported in a dozen of HTG‐APIP patients (Bartha et al., 2009; Gilbert, Rouis, Griglio, de Lumley, & Laplaud, 2001; Goldberg & Hegele, 2012; Hieronimus, Benlian, Bayer, Bongain, & Fredenrich, 2005; Keilson, Vary, Sprecher, & Renfrew, 1996; Liu et al., 2016; Ma et al., 1993, 1994; Murugasu et al., 1998; Suga et al., 1998; Xie et al., 2015). Of these patients, only three had experienced a recurrence of HTG‐APIP. Interestingly, all three of these recurrent HTG‐APIP patients harbored LPL variants on both alleles and exhibited either no or very low‐plasma post‐heparin LPL activity (Liu et al., 2016; Murugasu et al., 1998; Suga et al., 1998). Herein, we report the first identification and functional characterization of a heterozygous variant in the LPL predisposing to recurrent HTG‐APIP.
2. MATERIALS AND METHODS
2.1. Ethical compliance
This study was approved by the Ethics Committee of Jinling Hospital. Informed consent was obtained from all participants.
2.2. Patient
The patient is of Han Chinese origin. She had been pregnant twice and had suffered from HTG‐APIP during each pregnancy.
The patient experienced her first episode of acute pancreatitis at 40(+1) weeks of gestation in 2012 (at the age of 26), with a fasting TG level of 12.55 mmol/L (1,104.2 mg/dl) (Figure 1). She underwent emergency cesarean section and a healthy baby girl was delivered with an Apgar score of 10. About 200 ml chylous ascites were drained from her abdominal cavity during the cesarean delivery. The severity of the pancreatitis was mild in accordance with the Atlanta criteria (Banks et al., 2013). The patient was successfully managed with fasting, and was discharged 11 days after delivery without any complications.
Figure 1.
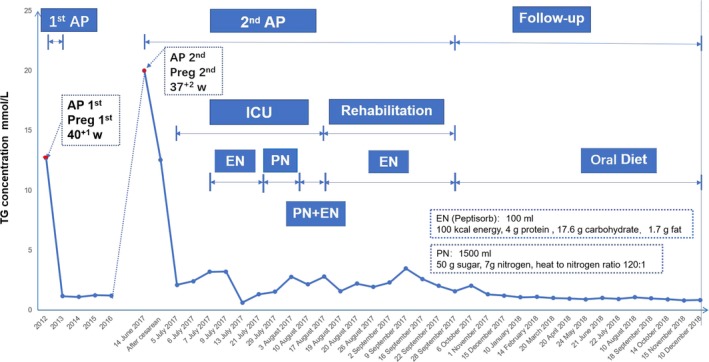
Timeline of the patient's two episodes of hypertriglyceridemia‐induced acute pancreatitis during pregnancy and her triglyceride (TG) levels. Note that (i) only some of the treatment procedures adopted have been illustrated and (ii) the patient was transferred to the Surgical Intensive Care Unit (SICU) at Nanjing on the 5 July 2017. AP, acute pancreatitis; EN, enteral nutrition; PN, parenteral nutrition
The second attack of acute pancreatitis occurred at 37(+2) weeks of pregnancy in 2017 (at the age of 31), with an even higher fasting TG level, namely 20.1 mmol/L (1,777.9 mg/dl), being observed (Figure 1). Again she underwent cesarean section; a healthy baby boy was delivered with an Apgar score of 10. Once again, chylous ascites (300 ml) were drained from the abdominal cavity. The patient was treated with fasting, gastrointestinal decompression, inhibition of acid and enzyme secretion, and fluid therapy in an intensive care unit but had a persistent fever (38.5°C–4°C) beginning 7 days after delivery. She was therefore transferred to the Surgical Intensive Care Unit (SICU) at Nanjing, the largest acute pancreatitis therapy center in Southern China, for further treatment at 22 days after delivery, by which time her fasting serum TG level had decreased to a borderline high level (i.e., 2.1 mmol/L (185.94 mg/dl)).
In our center, the patient was diagnosed with severe acute pancreatitis exacerbated by infected pancreatic necrosis (Figure 2a). She was treated twice with computer tomography‐guided percutaneous peritoneal drainage (Figure 2b), 10 days of tigecycline administration, and one procedure of endoscopic removal of necrotic pancreatic tissue, followed by enteral nutrition and parenteral nutrition. By 66 days after delivery, the patient's physical condition had improved remarkably and she was started on an oral fluid diet. Two days later, the patient was transferred to our rehabilitation center for further recovery. After 40 more days, the patient was discharged from our hospital, with the peripancreatic exudation and necrosis being greatly reduced (Figure 2c).
Figure 2.
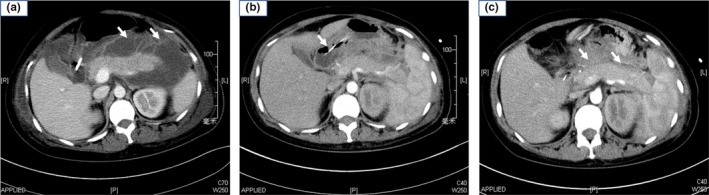
Computer tomography images of the patient taken during her second episode of hypertriglyceridemia‐induced acute pancreatitis during pregnancy. (a) Image taken upon the patient's admission to our service. Arrows indicate walled‐off pancreatic necrosis. (b) Image showing puncture and drainage of accumulated fluid in the affected pancreas under computer tomography guidance. Arrow indicates the puncture needle. (c) Image taken at discharge from our hospital. Peripancreatic fluid collection and necrosis were greatly reduced (arrows)
Finally, it is important to note that the patient's serum TG level was in the normal range (ranging from 0.8 to 1.3 mmol/L) without taking medications between the two episodes of acute pancreatitis and from the end of 2017 to the end of 2018 (Figure 1).
2.3. Plasma lipid profile analysis
A blood sample was taken from the patient after fasting for 12 hr. Serum TG and other lipid levels were measured enzymatically on an automatic analyzer (Hitachi High‐Tech, 7600–120).
2.4. Variant screening
Genomic DNA was extracted from peripheral blood cells using the Gentra Puregene Blood kit (Qiagen) according to the manufacturer's instructions. All exons and splicing junctions of the LPL as well as the four other canonical TG metabolism genes, namely APOC2 (OMIM# 608083), APOA5 (OMIM# 606368), LMF1 (OMIM# 611761), and GPIHBP1 (OMIM# 612757), were amplified from genomic DNA, and analyzed by Sanger sequencing (Chen et al., 2019). The identified variants were confirmed by independent PCR amplification and sequencing.
2.5. Reference sequence and variant nomenclature
NM_000237.3 and NC_000008.11 were employed as the LPL mRNA and genomic reference sequences, respectively. Nomenclature for the description of LDL variants was in accordance with the recommendations of the Human Genome Variation Society (HGVS) (den Dunnen et al., 2016).
2.6. In silico analysis
The Combined Annotation‐Dependent Depletion (CADD) tool (Kircher et al., 2014) and the PP3 rule established by VarSome (Kopanos et al., 2019) were employed to predict the pathogenicity of the LPL missense variant detected. CADD integrates more than 60 diverse annotations into a single measure (C‐score) for each variant, with a C‐score of >20 being regarded as evidence of pathogenicity. VarSome's PP3 rule was based upon a combined consideration of predictions from 10 in silico programs, namely DANN, MutationTaster, dbNSFP.FATHMM, MetaSVM, MetaLR, GERP, LRT, MutationAssessor, PROVEAN, and SIFT.
2.7. Analysis of LPL mass and activity in the patient's plasma
A blood sample was collected from the patient after overnight fasting and 10 min after an intravenous injection of heparin (60 IU/kg body weight). Blood plasma was then prepared by centrifugation at 400 g for 30 min. Plasma LPL mass was determined with a human LPL Elisa kit (TSZ Biological Trade). Plasma total lipase activity and plasma hepatic lipase activity were both determined using a Free Fatty Acid (FFA) assay kit (Wako kit, NEFA‐HR(2)). In the case of the plasma hepatic lipase activity assay, the sample was pretreated with 1 M NaCl and incubated for 60 min at 4°C to inactivate the LPL. Plasma LPL activity was then calculated by subtracting total lipase activity from hepatic lipase activity. All assays were performed in triplicate.
2.8. Cell culture and transfection
Human wild‐type and mutant LPL cDNAs were synthesized and cloned into the pcDNA3.1 vector by GeneArt Gene Synthesis (Vigene Biosciences), and confirmed by DNA sequencing. HEK293T (ATCC, CRL‐3216) cells, which have no endogenous LPL expression, were cultured in DMEM medium with 10% FBS and 1% PS at 37°C in a humidified chamber supplemented with 5% CO2. Transfection was performed using Lipofectamine 3,000 (Thermo, L3000015) in 6‐well plates (Costar, 3,516) according to the manufacturer's instructions. Six hours after transfection, the medium was changed into fresh DMEM with 2% FBS and the cells were then cultured for 48 hr before LPL mass and activity analysis. In the case of heparin treatment, the medium was further changed to a 500‐µl heparin‐DMEM mixture (the ratio of DMEM and heparin is 500:8, heparin is 20 units/ml) per well, and the cells were cultured for an additional 30 min.
2.9. Analysis of LPL mass and activity in transfected cell medium and lysate
The transfected cell medium was centrifuged to remove cells and cell debris, and stored at −20°C for later analysis. The transfected cells were treated by RIPA Lysis Buffer (Beyotime, China, P0013) and centrifuged at 4°C for 10 min at 12,000 g; the resulting supernatant was stored at −20°C for later analysis.
The expression of LPL protein in cell medium and lysate was analyzed by Western blot. The antibodies used were as listed below: 1:200 primary rabbit LPL antibodies (Cell Signaling Technology), 1:5000 primary mouse GAPDH antibody (Santa Cruz Biotechnology), 1:2000 secondary anti‐mouse, and 1:5000 anti‐rabbit HRP‐conjugated IgGs (Santa Cruz Biotechnology). Band intensities were quantified using the ImageJ software.
LPL activity in cell medium and lysate was determined using the abovementioned FFA assay (Wako kit, NEFA‐HR(2)).
3. RESULTS
3.1. Genetic findings
We analyzed the entire coding regions and proximal flanking intronic sequences of the LPL, APOC2, APOA5, GPIHBP1, and LMF1 in the patient by means of Sanger sequencing. We focused on the rare coding variants that resulted in missense, frameshift, or nonsense mutations and rare intronic variants that altered canonical splice sites. Rare variants are defined here as having an allele frequency of <1% in the East Asian population according to the gnomAD database (Lek et al., 2016). We identified only one such variant, c.629A > T, in exon 5 of the LPL, which was predicted to result in a missense variant, p.His210Leu (Figure 3a). This variant is absent from the gnomAD database, has not been previously reported in the literature, and was also not found in any of our other 28 Chinese HTG‐APIP patients.
Figure 3.
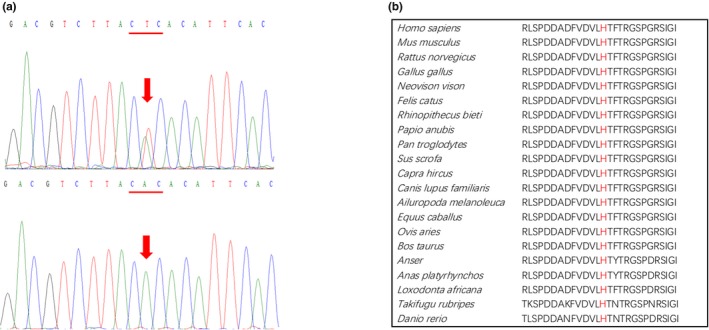
The novel LPL missense variant found in the patient. (a) Sanger sequencing electropherogram showing the heterozygous A > T substitution at position c.629 of the LPL (indicated by arrow) that would be predicted to change the CAC codon for histidine at position p.210 (underlined) to a CTC codon specifying leucine (i.e., p.His210Leu). Upper panel, patient. Lower panel, a healthy control. (b) Alignment of LPL amino acid sequences from a range of vertebrates illustrating the strict evolutionary conservation of histidine at residue 210
3.2. Pathogenicity prediction
The histidine residue at LPL amino acid position 210 is highly conserved from zebrafish (Danio rerio) to humans (Figure 3b). Moreover, leucine, an aliphatic and uncharged residue, is significantly different from histidine, a polar and positively charged residue, in terms of physicochemical properties. Taken together, these observations suggest that the p.His210Leu missense variant is highly likely to affect LPL protein structure and function. Indeed, it was predicted to be pathogenic by both CADD (C‐score, 26.2) and VarSome (in accordance with its PP3 rule).
3.3. Analysis of LPL mass and activity in the patient's plasma
Plasma LPL mass and activity were measured when the patient was admitted to our center. Her LPL mass was determined to be 185.7 ± 12.49 U/L, approximately half of the mean value of 10 normal controls (382 ± 75.62 U/L). Her LPL activity was 0.071 ± 0.025 mEq L−1 hr−1, approximately 60% of the mean value of 10 normal controls (0.118 ± 0.055 mEq L−1 hr−1).
3.4. In vitro functional characterization of the LPL missense variant
The LPL wild‐type and p.His210Leu mutant coding sequences were transiently expressed in HEK293T cells. We first compared LPL expression levels in cell lysates and media of wild‐type and mutant transfected cells with heparin treatment by means of Western blotting. Exogenous heparin triggers the release of cell surface‐bound LPL into the medium. Significantly higher LPL expression was found in the cell lysate but significantly lower LPL expression was noted in the medium of the mutant transfected cells as compared to those of the wild‐type transfected cells (Figure 4). These results indicated that the p.His210Leu variant greatly reduced LPL secretion. We then compared LPL expression levels in the cell lysates of wild‐type and mutant transfected cells without heparin treatment, finding no significant difference (Figure 5). This latter finding indicated that the p.His210Leu variant has no effect on LPL synthesis.
Figure 4.
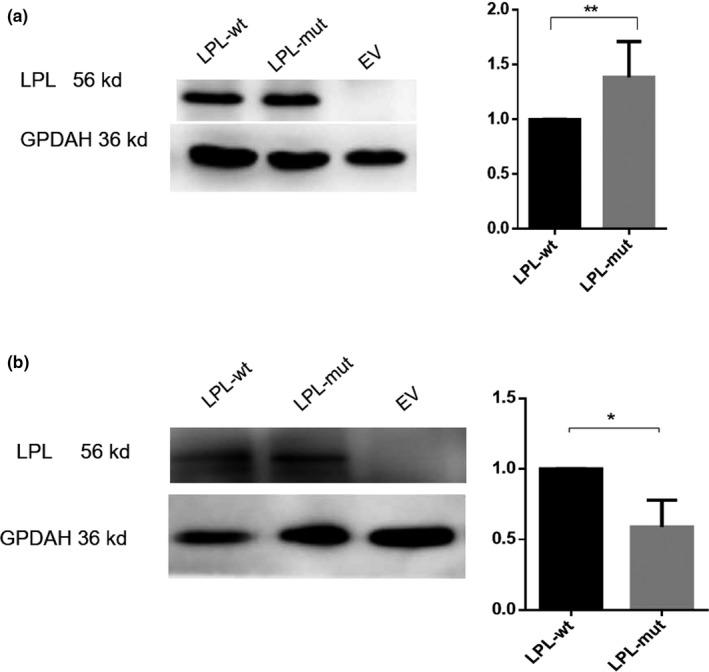
Western blot analysis of LPL expression in cell lysates (a) and media (b) of transfected human embryonic kidney (HEK293T) cells treated with heparin, whose role was to release LPL into the cell medium. In each panel, a representative blot is shown on the left while the LPL expression level in the p.His201Leu mutant vector‐transfected cells relative to that in the wild‐type vector‐transfected cells is shown on the right. Results were the average taken from three independent experiments. EV, empty vector; mut, mutant; wt, wild‐type. GPDAH was used as a loading control. *p < .05; **p < .01
Figure 5.
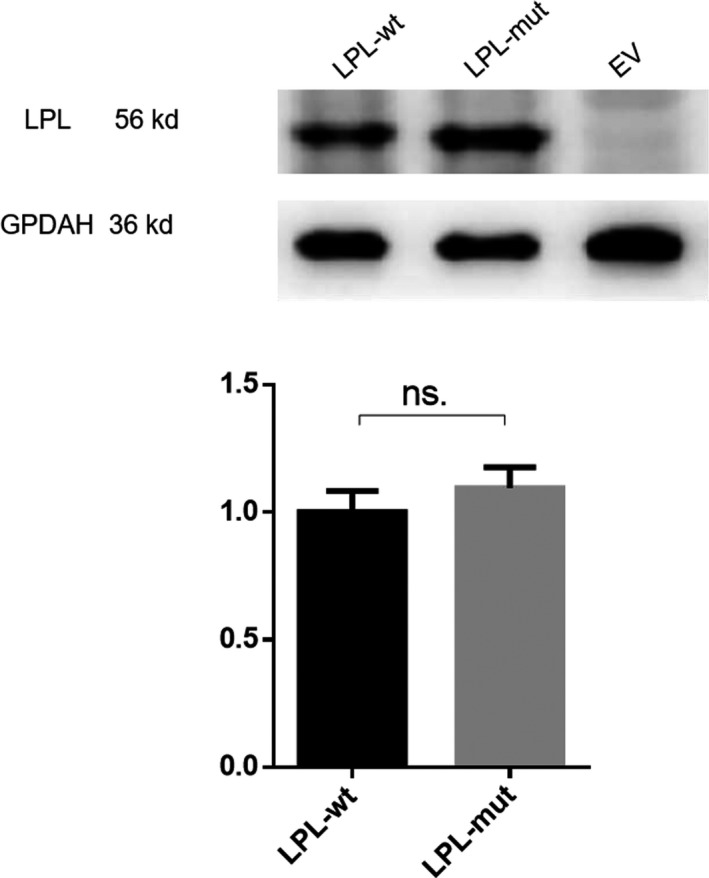
Western blot analysis of LPL expression in cell lysates of transfected HEK293T cells without heparin treatment. A representative blot is shown on the left. The LPL expression level in the p.His201Leu mutant vector‐transfected cells relative to that in the wild‐type vector‐transfected cells is shown on the right. Results were the average taken from three independent experiments. EV, empty vector; mut, mutant; ns, not significant; wt, wild‐type. GPDAH was used as a loading control
By contrast, LPL activity was not detectable in either the cell lysate or the medium of mutant vector‐transfected cells with heparin treatment. This indicated that the p.His210Leu variant completely abolished the enzymatic activity of the mutant LPL protein. For comparison, LPL activities in the cell lysate and medium of wild‐type transfected cells with heparin treatment were 0.35 ± 0.24 mEq L−1 hr−1 and 0.33 ± 0.19 mEq L−1 hr−1.
4. DISCUSSION
In the present study, we identified a novel heterozygous LPL missense variant, p.His210Leu (c.629A > T), in a Chinese patient with recurrent HTG‐APIP. The pathogenicity of this variant was supported by predictions made using CADD and VarSome, then demonstrated by virtue of the significantly reduced LPL mass and activity in the patient's plasma (compared to that of normal controls), and finally confirmed by in vitro functional analyses. The in vitro analysis demonstrated that the p.His210Leu missense variant did not affect protein synthesis but significantly impaired LPL secretion and completely abolished enzymatic activity of the mutant protein. It should be noted that two other missense variants, p.His210Asp (c.628C > G) and p.His210Arg (c.629A > G), have been reported in LPL amino acid residue 210. p.His210Asp also caused a complete loss of LPL activity when tested in vitro (Holzl et al., 2000). However, in vitro protein analysis was not performed on this variant and no data on plasma LPL mass in the carrier were available. Therefore, it remains unclear whether the complete loss of enzyme activity caused by the p.His210Asp missense variant is due to its impact on enzymatic activity or alternatively whether it exerts its effects on protein synthesis and/or secretion. p.His210Arg was not functionally characterized in vitro; however, the patient, who was homozygous for this variant, exhibited a LPL activity deficiency but a LPL mass value within the normal range (Ariza et al., 2018). In short, all three missense variants caused a complete or almost complete functional loss of the affected allele, highlighting the importance of the evolutionarily strictly conserved residue 210 for LPL structure and function. In this regard, it is pertinent to note that based upon the LPL crystal structure, “the active site cleft is lined by the hydrophobic side chains of W82, V84, W113, Y121, Y158, L160, A185, P187, F212, I221, F239, V260, V264, and K265, which would form van der Waals interactions with (and stabilize) the hydrophobic tails of lipid substrates in the active site” (Birrane et al., 2019). The spatial proximity of residue 210 to these active site‐defining residues or structures speaks for itself.
As mentioned earlier, only three patients with recurrent HTG‐APIP have so far been reported to carry rare LPL variants (Liu et al., 2016; Murugasu et al., 1998; Suga et al., 1998). Coincidentally, all three patients had severe LPL deficiency and were each found to have two affected LPL alleles. One patient, who had complete LPL deficiency, was homozygous for a nonsense variant, p.Trp409* (c.1227G > A) (Suga et al., 1998); one patient, with almost complete LPL deficiency, was homozygous for a missense variant, p.Cys291Tyr (c.872G > A) (Murugasu et al., 1998); and the third patient, whose post‐heparin LPL activity was <17% that of normal controls, was compound heterozygous for p.Glu269Lys (c.805G > A) and p.Leu279Val (c.835C > G) (Liu et al., 2016). The p.Trp409* homozygous patient, when not pregnant, managed to maintain a mild HTG state with a plasma TG level ranging from 5.6 to 11.3 mmol/L by ingesting a low‐fat diet (Suga et al., 1998); after discharge, the compound heterozygous [p.Glu269Lys and p.Leu279Val] patient maintained a consistently elevated TG level (ranging from 7 to 11 mmol/L) by following a lipid‐lowering drug regimen (Liu et al., 2016); finally, in the case of the p.Cys291Tyr homozygous patient, her TG level at 4 weeks postpartum (25.5 mmol/L) exhibited no significant decrease by comparison to that measured at 18 weeks of gestation (29.6 mmol/L) (Murugasu et al., 1998). By contrast, our patient was characterized by a much higher post‐heparin LPL activity (i.e., ~50% of normal controls), harbored a heterozygous LPL missense variant that led to the complete functional loss of the affected allele, but exhibited normal TG levels in the follow‐up periods without taking any medication. This notwithstanding, it is clear that LPL variants, whether heterozygous, compound heterozygous, or homozygous, can predispose to recurring HTG‐APIP. In this regard, it is also worth mentioning that the development of severe HTG in pregnancy could also be ascribed to polygenic inheritance (Dron et al., 2019). Under the pregnancy metabolic setting, a combination of variants in many genes may raise plasma TG with various mechanisms related or not to LPL.
In our patient, her second episode of HTG‐APIP was associated with a higher peak TG level, an increase in chylous ascites, and more severe pancreatitis as compared to the first episode experienced. It also appears that TG levels in all three previously reported HTG‐APIP patients tended to be higher in later episodes of the disease than the earlier ones (Liu et al., 2016; Murugasu et al., 1998; Suga et al., 1998). These findings are consistent with recent observations suggesting that (a) an episode of acute pancreatitis may predispose to the risk of developing more severe metabolic derangements (Singh et al., 2018) and (b) more severe HTG may result in more severe disease (Jo et al., 2019).
There is one final point to make. Our patient bore two healthy children owing to timely cesarean section. It follows that early diagnosis and clinical intervention are vitally important to acute pancreatitis patients caused by gestational HTG and should help to improve the prognosis of the fetus.
5. CONCLUSIONS
The present study describes the first identification and functional characterization of a heterozygous variant in the LPL that predisposed to recurrent HTG‐APIP. Our findings not only underscore the importance of genetic defects in predisposing to HTG and/or acute pancreatitis but also shed new light on the complexity underlying the etiology of these phenotypes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Qi Yang, Xiao‐Lei Shi, and Wei‐Qin Li conceived and designed the study. Xiao‐Lei Shi performed the experiments. Qi Yang, Xiao‐Lei, Na Pu, Xiao‐Yao Li, Wei‐Wei Chen, Jing Zhou, Gang Li, and Zhi‐Hui Tong collected patient data. Xiao‐Lei Shi, Qi Yang, and Jian‐Min Chen drafted the paper. Claude Férec, David N. Cooper, and Wei‐Qin Li critically revised the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
This study was supported by the Key Research and Development Program Foundation of Jiangsu Province of China (BE2015685 and BE2016749) and the National Natural Science Foundation of China (Nos. 81570584, 81670588, and 81870441).
Shi X‐L, Yang Q, Pu N, et al. Identification and functional characterization of a novel heterozygous missense variant in the LPL associated with recurrent hypertriglyceridemia‐induced acute pancreatitis in pregnancy. Mol Genet Genomic Med. 2020;8:e1048 10.1002/mgg3.1048
Xiao‐Lei Shi and Qi Yang equally contributed to this work.
Contributor Information
Qi Yang, Email: yangqi_nj@163.com.
Wei‐Qin Li, Email: njzy_pancrea@163.com.
REFERENCES
- Alvarez, J. J. , Montelongo, A. , Iglesias, A. , Lasuncion, M. A. , & Herrera, E. (1996). Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. Journal of Lipid Research, 37(2), 299–308. [PubMed] [Google Scholar]
- Ariza, M. J. , Rioja, J. , Ibarretxe, D. , Camacho, A. , Díaz‐Díaz, J. L. , Mangas, A. , … Ferrando Vela, J. (2018). Molecular basis of the familial chylomicronemia syndrome in patients from the National Dyslipidemia Registry of the Spanish Atherosclerosis Society. Journal of Clinical Lipidology, 12(6), 1482–1492.e3. 10.1016/j.jacl.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Banks, P. A. , Bollen, T. L. , Dervenis, C. , Gooszen, H. G. , Johnson, C. D. , Sarr, M. G. , … Vege, S. S. (2013). Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut, 62(1), 102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- Bartha, I. , Dinya, T. , Seres, I. , Paragh, G. , Ross, C. , Hayden, M. R. , … Vargha, G. (2009). Acute hypertriglyceridemic pancreatitis during pregnancy due to homozygous lipoprotein lipase gene mutation. Clinica Chimica Acta, 400(1–2), 137–138. 10.1016/j.cca.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Basaran, A. (2009). Pregnancy‐induced hyperlipoproteinemia: Review of the literature. Reproductive Sciences, 16(5), 431–437. 10.1177/1933719108330569 [DOI] [PubMed] [Google Scholar]
- Birrane, G. , Beigneux, A. P. , Dwyer, B. , Strack‐Logue, B. , Kristensen, K. K. , Francone, O. L. , … Meiyappan, M. (2019). Structure of the lipoprotein lipase‐GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proceedings of the National Academy of Sciences, 116(5), 1723–1732. 10.1073/pnas.1817984116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.‐W. , Yang, Q. I. , Li, X.‐Y. , Shi, X.‐L. , Pu, N. A. , Lu, G.‐T. , … Li, W.‐Q. (2019). Identification of a novel and heterozygous LMF1 nonsense mutation in an acute pancreatitis patient with severe hypertriglyceridemia, severe obesity and heavy smoking. Lipids in Health and Disease, 18(1), 68 10.1186/s12944-019-1012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen, J. T. , Dalgleish, R. , Maglott, D. R. , Hart, R. K. , Greenblatt, M. S. , McGowan‐Jordan, J. , … Taschner, P. E. M. (2016). HGVS recommendations for the description of sequence variants: 2016 update. Human Mutation, 37(6), 564–569. 10.1002/humu.22981 [DOI] [PubMed] [Google Scholar]
- Deng, Y. Y. , Wang, R. , Wu, H. , Tang, C. W. , & Chen, X. Z. (2014). Etiology, clinical features and management of acute recurrent pancreatitis. Journal of Digestive Diseases, 15(10), 570–577. 10.1111/1751-2980.12180 [DOI] [PubMed] [Google Scholar]
- Dron, J. S. , Wang, J. , Cao, H. , McIntyre, A. D. , Iacocca, M. A. , Menard, J. R. , … Hegele, R. A. (2019). Severe hypertriglyceridemia is primarily polygenic. Journal of Clinical Lipidology, 13(1), 80–88. 10.1016/j.jacl.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Ducarme, G. , Maire, F. , Chatel, P. , Luton, D. , & Hammel, P. (2014). Acute pancreatitis during pregnancy: A review. Journal of Perinatology, 34(2), 87–94. 10.1038/jp.2013.161 [DOI] [PubMed] [Google Scholar]
- Fan, S. J. , Xiang, J. X. , Xiao, M. , Wang, F. H. , Lin, X. J. , Zhou, X. H. , … Liu, L. (2018). Influence of acute pancreatitis in pregnancy on pregnancy outcomes and neonates. Zhongguo Dang Dai Er Ke Za Zhi = Chinese Journal of Contemporary Pediatrics, 20(4), 274–278. 10.7499/j.issn.1008-8830.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, A. , Garg, V. , Hegele, R. A. , & Lewis, G. F. (2019). Practical definitions of severe versus familial hypercholesterolaemia and hypertriglyceridaemia for adult clinical practice. The Lancet Diabetes & Endocrinology, 7(11), 880–886. 10.1016/s2213-8587(19)30156-1 [DOI] [PubMed] [Google Scholar]
- Gilbert, B. , Rouis, M. , Griglio, S. , de Lumley, L. , & Laplaud, P. (2001). Lipoprotein lipase (LPL) deficiency: A new patient homozygote for the preponderant mutation Gly188Glu in the human LPL gene and review of reported mutations: 75 % are clustered in exons 5 and 6. Annales De Genetique, 44(1), 25–32. 10.1016/S0003-3995(01)01037-1 [DOI] [PubMed] [Google Scholar]
- Goldberg, A. S. , & Hegele, R. A. (2012). Severe hypertriglyceridemia in pregnancy. Journal of Clinical Endocrinology and Metabolism, 97(8), 2589–2596. 10.1210/jc.2012-1250 [DOI] [PubMed] [Google Scholar]
- Hieronimus, S. , Benlian, P. , Bayer, P. , Bongain, A. , & Fredenrich, A. (2005). Combination of apolipoprotein E2 and lipoprotein lipase heterozygosity causes severe hypertriglyceridemia during pregnancy. Diabetes & Metabolism, 31(3 Pt 1), 295–297. 10.1016/S1262-3636(07)70197-0 [DOI] [PubMed] [Google Scholar]
- Holzl, B. , Kraft, H. G. , Wiebusch, H. , Sandhofer, A. , Patsch, J. , Sandhofer, F. , & Paulweber, B. (2000). Two novel mutations in the lipoprotein lipase gene in a family with marked hypertriglyceridemia in heterozygous carriers. Potential interaction with the polymorphic marker D1S104 on chromosome 1q21‐q23. Journal of Lipid Research, 41(5), 734–741. [PubMed] [Google Scholar]
- Igbinosa, O. , Poddar, S. , & Pitchumoni, C. (2013). Pregnancy associated pancreatitis revisited. Clinics and Research in Hepatology and Gastroenterology, 37(2), 177–181. 10.1016/j.clinre.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Jo, S. I. , Chang, J. H. , Kim, T. H. , Kim, C. W. , Kim, J. K. , & Han, S. W. (2019). Subsets associated with developing acute pancreatitis in patients with severe hypertriglyceridemia and the severity of pancreatitis. Pancreatology, 19(6), 795–800. 10.1016/j.pan.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Keilson, L. M. , Vary, C. P. , Sprecher, D. L. , & Renfrew, R. (1996). Hyperlipidemia and pancreatitis during pregnancy in two sisters with a mutation in the lipoprotein lipase gene. Annals of Internal Medicine, 124(4), 425–428. 10.7326/0003-4819-124-4-199602150-00007 [DOI] [PubMed] [Google Scholar]
- Kircher, M. , Witten, D. M. , Jain, P. , O'Roak, B. J. , Cooper, G. M. , & Shendure, J. (2014). A general framework for estimating the relative pathogenicity of human genetic variants. Nature Genetics, 46(3), 310–315. 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanos, C. , Tsiolkas, V. , Kouris, A. , Chapple, C. E. , Albarca Aguilera, M. , Meyer, R. , & Massouras, A. (2019). VarSome: The human genomic variant search engine. Bioinformatics, 35(11), 1978–1980. 10.1093/bioinformatics/bty897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek, M. , Karczewski, K. J. , Minikel, E. V. , Samocha, K. E. , Banks, E. , Fennell, T. , … Exome Aggregation Consortium (2016). Analysis of protein‐coding genetic variation in 60,706 humans. Nature, 536(7616), 285–291. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Lun, Y. , Lv, W. , Hou, X. , & Wang, Y. (2016). A Chinese patient with recurrent pancreatitis during pregnancy induced by hypertriglyceridemia associated with compound heterozygosity (Glu242Lys and Leu252VaL) in the lipoprotein lipase gene. Journal of Clinical Lipidology, 10(1), 199–203.e1. 10.1016/j.jacl.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Luo, L. , Zen, H. , Xu, H. , Zhu, Y. , Liu, P. , Xia, L. , … Lv, N. (2018). Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Archives of Gynecology and Obstetrics, 297(2), 333–339. 10.1007/s00404-017-4558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Liu, M. S. , Ginzinger, D. , Frohlich, J. , Brunzell, J. D. , & Hayden, M. R. (1993). Gene‐environment interaction in the conversion of a mild‐to‐severe phenotype in a patient homozygous for a Ser172–>Cys mutation in the lipoprotein lipase gene. The Journal of Clinical Investigation, 91(5), 1953–1958. 10.1172/jci116414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Ooi, T. C. , Liu, M. S. , Zhang, H. , McPherson, R. , Edwards, A. L. , … Hayden, M. R. (1994). High frequency of mutations in the human lipoprotein lipase gene in pregnancy‐induced chylomicronemia: Possible association with apolipoprotein E2 isoform. Journal of Lipid Research, 35(6), 1066–1075. [PubMed] [Google Scholar]
- Murugasu, C. G. , Armstrong, G. , Creedon, G. , Cavanna, J. S. , Galton, D. J. , & Tomkin, G. H. (1998). Acute hypertriglyceridaemic pancreatitis in a pregnant Indian: A new lipoprotein lipase gene mutation. Journal of the Royal Society of Medicine, 91(4), 205–207. 10.1177/014107689809100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis, E. P. , Sarigianni, M. , Mikhailidis, D. P. , Mamopoulos, A. , & Karagiannis, V. (2011). Acute pancreatitis in pregnancy: An overview. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 159(2), 261–266. 10.1016/j.ejogrb.2011.07.037 [DOI] [PubMed] [Google Scholar]
- Ramin, K. D. , Ramin, S. M. , Richey, S. D. , & Cunningham, F. G. (1995). Acute pancreatitis in pregnancy. American Journal of Obstetrics and Gynecology, 173(1), 187–191. 10.1016/0002-9378(95)90188-4 [DOI] [PubMed] [Google Scholar]
- Singh, R. G. , Pendharkar, S. A. , Cervantes, A. , Cho, J. , Miranda‐Soberanis, V. , & Petrov, M. S. (2018). Abdominal obesity and insulin resistance after an episode of acute pancreatitis. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 50(10), 1081–1087. 10.1016/j.dld.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Suga, S. , Tamasawa, N. , Kinpara, I. , Murakami, H. , Kasai, N. , Onuma, T. , … Suda, T. (1998). Identification of homozygous lipoprotein lipase gene mutation in a woman with recurrent aggravation of hypertriglyceridaemia induced by pregnancy. Journal of Internal Medicine, 243(4), 317–321. 10.1046/j.1365-2796.1998.00306.x [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Fan, C. , & Wang, S. (2013). Clinical analysis of 16 patients with acute pancreatitis in the third trimester of pregnancy. International Journal of Clinical and Experimental Pathology, 6(8), 1696–1701. [PMC free article] [PubMed] [Google Scholar]
- Tang, M. , Xu, J. M. , Song, S. S. , Mei, Q. , & Zhang, L. J. (2018). What may cause fetus loss from acute pancreatitis in pregnancy: Analysis of 54 cases. Medicine (Baltimore), 97(7), e9755 10.1097/md.0000000000009755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilallonga, R. , Calero‐Lillo, A. , Charco, R. , & Balsells, J. (2014). Acute pancreatitis during pregnancy, 7‐year experience of a tertiary referral center. Cirugía Española, 92(7), 468–471. 10.1016/j.ciresp.2013.12.016 [DOI] [PubMed] [Google Scholar]
- Xie, S. L. , Chen, T. Z. , Huang, X. L. , Chen, C. , Jin, R. , Huang, Z. M. , & Zhou, M. T. (2015). Genetic variants associated with gestational hypertriglyceridemia and pancreatitis. PLoS ONE, 10(6), e0129488 10.1371/journal.pone.0129488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q. , Wang, S. , & Zhang, Z. (2015). A 23‐year, single‐center, retrospective analysis of 36 cases of acute pancreatitis in pregnancy. International Journal of Gynaecology and Obstetrics, 130(2), 123–126. 10.1016/j.ijgo.2015.02.034 [DOI] [PubMed] [Google Scholar]
- Zhang, D. L. , Huang, Y. , Yan, L. , Phu, A. , Ran, X. , & Li, S. S. (2013). Thirty‐eight cases of acute pancreatitis in pregnancy: A 6‐year single center retrospective analysis. Journal of Huazhong University of Science and Technology [Medical Sciences], 33(3), 361–367. 10.1007/s11596-013-1125-8 [DOI] [PubMed] [Google Scholar]


