Abstract
Background:
In recent years, norepinephrine has attracted increasing attention for the management of maternal hypotension during elective cesarean section with spinal anesthesia. Intermittent bolus is a widely used administration paradigm for vasopressors in obstetric anesthesia in China. Thus, in this randomized, double-blinded study, we compared the efficacy and safety of equivalent bolus norepinephrine and phenylephrine for rescuing maternal post-spinal hypotension.
Methods:
In a tertiary women's hospital in Nanjing, China, 102 women were allocated with computer derived randomized number to receive prophylactic 8 μg norepinephrine (group N; n = 52) or 100 μg phenylephrine (group P; n = 50) immediately post-spinal anesthesia, followed by an extra bolus of the same dosage until delivery whenever maternal systolic blood pressure became lower than 80% of the baseline. Our primary outcome was standardized maternal cardiac output (CO) reading from spinal anesthesia until delivery analyzed by a two-step method. Other hemodynamic parameters related to vasopressor efficacy and safety were considered as secondary outcomes. Maternal side effects and neonatal outcomes were collected as well.
Results:
Compared to group P, women in group N had a higher CO (standardized CO 5.8 ± 0.9 vs. 5.3 ± 1.0 L/min, t = 2.37, P = 0.02) and stroke volume (SV, standardized SV 73.6 ± 17.2 vs. 60.0 ± 13.3 mL, t = 4.52, P < 0.001), and a lower total peripheral resistance (875 ± 174 vs. 996 ± 182 dyne·s/cm5, t = 3.44, P < 0.001). Furthermore, the incidence of bradycardia was lower in group N than in group P (2% vs. 14%, P = 0.023), along with an overall higher standardized heart rate (78.8 ± 11.6 vs. 75.0 ± 7.3 beats/min, P = 0.049). Other hemodynamics, as well as maternal side effects and neonatal outcomes, were similar in two groups (P > 0.05).
Conclusions:
Compared to equivalent phenylephrine, intermittent bolus norepinephrine provides a greater CO for management of maternal hypotension during elective cesarean section with spinal anesthesia; however, no obvious maternal or neonatal clinical advantages were observed for norepinephrine.
Keywords: Norepinephrine, Phenylephrine, Cardiac output, Cesarean section
Introduction
Maternal hypotension is a common complication during cesarean section with spinal anesthesia, which can possibly result from a synergy of reduced venous return, reduced cardiac output (CO), or decreased peripheral vascular resistance. It usually leads to adverse maternal outcomes such as nausea, vomiting, and dizziness. Besides, compromised placental perfusion raises the concerns of fetal acidosis, hypoxia, and even postnatal neurological injury.[1] Thus, effective prevention or treatment of maternal hypotension is of great clinical significance.
Phenylephrine is the current gold-standard vasopressor in obstetric anesthesia to rescue maternal hypotension. It has α-adrenergic receptor agonist property; however, at the usual clinical dose, it is devoid of β-adrenergic receptor activity, which comes with undesired side effects, such as dose-dependent depression of heart rate (HR) companied by a decrease of CO. Unlike phenylephrine, norepinephrine has weak β receptor agonistic properties, other than α-receptor agonism property. Our previous work systematically discussed its feasibility as a substitution of phenylephrine based on available limited clinical trials and suggested it is a promising alternative for phenylephrine in obstetric anesthesia.[2,3]
Although prophylactic infusion to prevent spinal hypotension is recommended,[4] intravenous bolus is still the favorite medication paradigm for most anesthesiologists in China.[5] Besides, use of intermittent intravenous norepinephrine bolus seems feasible to prevent spinal induced hypotension in obstetric patients without presence of obvious side effects.[6] In this study, we observed the hemodynamics as well as the efficacy and safety of equivalent norepinephrine and phenylephrine for rescuing maternal post-spinal hypotension when a bolus was injected. Maternal hemodynamics, as well as maternal side effects, neonatal Apgar scores, and umbilical cord blood gas analysis were recorded. We hope this study can provide new evidence about the efficacy and safety of norepinephrine for post-spinal hypotension.
Methods
Ethical approval
This study was approved by the Clinical Research Ethics Committee of Women's Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, Nanjing, China (No. 2018-79) and was conducted between June and July 2018. All enrolled subjects provided written informed consent after recruitment.
Inclusion criteria
The inclusion criteria were American Society of Anesthesiologists I or II, primiparity, singleton, term pregnancy, elective cesarean section, and scheduled for spinal anesthesia. Eligible subjects were invited to participate in the study immediately after entering the operating room.
Exclusion criteria
Subjects with one or more of the following characteristics were excluded from the study: younger than 18 or older than 45 years of age; weight less than 50 kg or more than 100 kg; height shorter than 140 cm or taller than 180 cm; multiparous women; twin gestation; suspected fetal compromise; concurrent with pre-existing hypertension or pregnancy-related hypertension; comorbid with pregnancy-related diabetes; comorbid with cardiovascular or cerebrovascular disease; diagnosed with depression or anxiety during pregnancy; occurrence of intra-operative shivering; patients taking monoamine oxidase inhibitors, serotonin reuptake inhibitor, or tricyclic antidepressant; failure in spinal anesthesia.
Randomization assignment
Using the random allocation protocol, a random number was generated via the online software QuickCalcs# (GraphPad Inc., San Diego, CA, USA), and eligible subjects were allocated to receive norepinephrine (group N) or phenylephrine (group P). Just before the spinal anesthesia administration, the group assignment number was allocated to determine which vasopressor would be given. If one specific subject was excluded from the study and lost in follow up, this number would be automatically allocated to the next enrolled subject. The patient and the anesthesiologist on duty were blinded to the group allocation.
Intra-operative monitor and patient management
The antecubital vein was routinely cannulated with an 18-G indwelled needle. CO, stroke volume (SV), and total peripheral resistance (TPR) were monitored using a Cheetah transthoracic impedance NICOM monitor (Cheetah Medical, Israel). After entering the patient's age, height, weight, and sex, the monitor was connected to the patient with four sensor pads encircling the heart located at the bilateral clavicles and lower thorax walls, respectively. Meanwhile, monitor (BSM 2351K, NIHON KOHDEN, Tomioka, Japan) was attached to detect the electrocardiogram, blood pressure (BP), and pulse oximetry. The mean value of three repeated measures of each parameter was obtained as baseline value.
Thereafter, parturients were placed in the left lateral position for spinal anesthesia by the anesthesiologist on duty. After skin sterilization and regional infiltration with 2 mL 2% lidocaine, a 25-G pencil-point needle was inserted at the L3-4 vertebral interspace and 15 mg 0.5% ropivacaine was intrathecally injected. After spinal anesthesia, the parturient was replaced to the supine position with a wedge pillow under the right hip for a lateral approximately 30° tilt. Meanwhile, lactated Ringer solution was rapidly coloaded with the flow slowed to a maintenance rate only after delivery.
Immediately after intrathecal injection, 8 μg norepinephrine or 100 μg phenylephrine was bolus-injected according to group allocation. If hypotension was observed thereafter, an extra bolus of 8 μg norepinephrine or 100 μg phenylephrine was injected in line with the group allocation. Our primary outcome was maternal CO post-spinal anesthesia. Other hemodynamic parameters related to vasopressor efficacy and safety were considered secondary outcomes, including systolic BP (SBP), HR, SV, TRP, incidence of requirement for extra bolus, time to first extra bolus, and frequency of extra bolus, incidence of bradycardia, bradycardia comorbid with hypotension, and hypertension. Maternal side effects and neonatal outcomes including Apgar score and blood gas analysis were collected as well.
The dose of norepinephrine and phenylephrine were chosen according to a previous random allocation graded dose-response study, which determined the equivalent dose of norepinephrine to be 8 μg (95% confidence interval, 6–10 μg) compared to 100 μg of phenylephrine when used to rescue the first episode of hypotension.[7] Either norepinephrine or phenylephrine was prepared by a specific study member and diluted using normal saline solution to 8 or 100 μg/mL, respectively.
In the study, maternal hypotension and hypertension were defined as SBP <80% or >120% of the baseline value. Bradycardia was defined as HR <60 beats/min. Sole HR <60 beats/min without hypotension was managed expectantly. However, if HR <50 beats/min or HR <60 beats/min comorbid with hypotension, the parturient was treated with 0.5 mg atropine, an anticholinergic drug. We also recorded the total amount of intravenous fluid given until delivery, as well as blood loss throughout the surgery. Furthermore, sensory anesthesia level was determined via ice, if adequate (T5) the surgery was permitted to start. During the anesthesia and operation, all the parturients breathed air spontaneously, additional oxygen was given only when the pulse oximeter was lower than 95%. The study endpoint was set at delivery; afterward, hemodynamic management was at the discretion of the anesthesiologist on duty.
Sample size calculation
We calculated the sample size according to CO. In the pilot study, 8 μg norepinephrine or 100 μg phenylephrine was immediately bolus-injected after spinal anesthesia, at 2-min post-injection, and we obtained a CO value 7.5 ± 1.1 L/min for norepinephrine and 6.6 ± 1.5 L/min for phenylephrine, respectively. When α was set at 0.05, β at 0.10, and the power of test (1-β), at 0.90, a minimum of 46 subjects per group was required to detect a statistical significance. Considering potential dropouts or missing data, the sample size was increased to 50 patients.
Statistical analysis
Data are expressed as the mean ± standard error of mean, median (interquartile range), median (minimum to maximum) or number (percentage) in this study. Inter-group univariate data were tested for normality distribution with the D’Agostino-Pearson omnibus normality test followed with non-paired t test or Mann-Whitney test accordingly. Nominal data between groups were compared with Chi-square test or Fisher exact test. Of note, serial hemodynamic data between groups were analyzed using a two-step summary measure.[8] This was due to the variation of number of data points among parturients; therefore, it was not adequate to compare mean values in one particular time point for serial variables. Thus, we calculated area under the curve of each parturient, which was then divided by the number of recording points of this specific subject to derive one standardized value. Furthermore, the standardized data were compared by routine inter-group analysis using a t test or Mann-Whitney test. Statistical analysis was performed with GraphPad Prism v.7.0 software (GraphPad Inc.). A P value <0.05 was considered to be statistically significant.
Results
A detailed flow chart of parturients enrollment, allocation, follow-up, and analysis is shown in Figure 1. A total of 160 parturients were initially enrolled in this study, and after strict exclusion and follow-up, 52 and 50 parturients were finally allocated to groups N and P, respectively. Table 1 shows the parturient characteristics, with no significant differences observed between groups. The varying surgical time among subjects led to the varied number of hemodynamic recordings after spinal anesthesia being obtained. Umbilical artery (UA) blood gas analysis was not performed in four and five subjects in groups N and P, respectively, due to insufficient blood, inadequate anticoagulation, or equipment failure, whereas umbilical venous (UV) blood gas analysis was not performed in three and four subjects in groups N and P, respectively.
Figure 1.
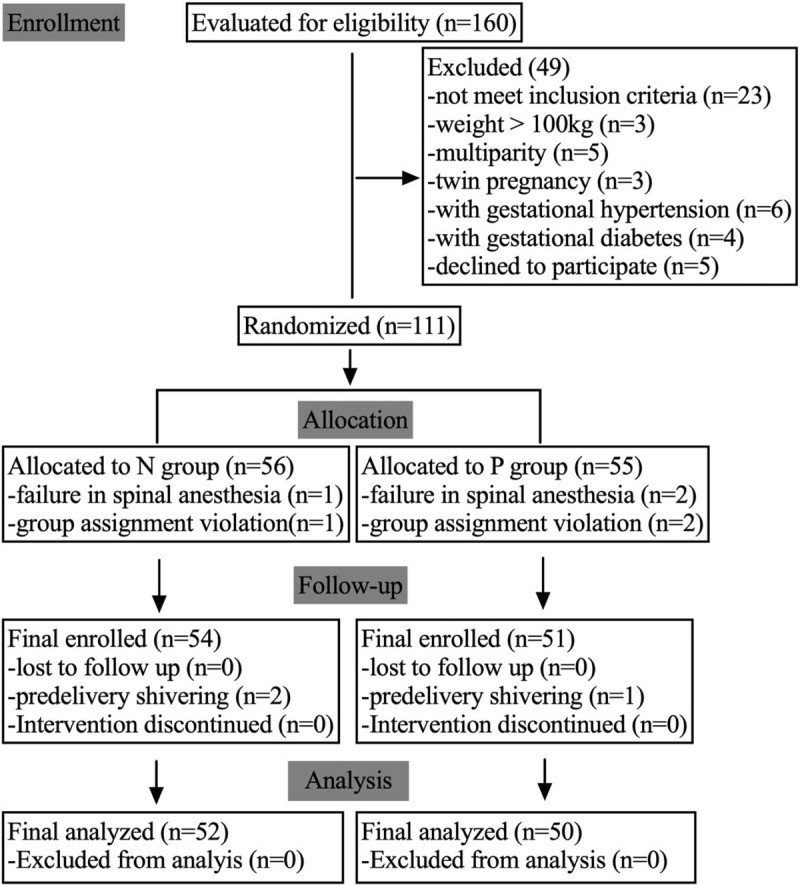
Flow chart of parturient enrollment, allocation, follow-up, and analysis.
Table 1.
Parturient characteristics and surgical times of all patients.

Baseline hemodynamic parameters including SBP, HR, CO, SV, and TPR were comparable between the two groups as shown in Table 2. The incidence of bradycardia, defined as HR <60 beats/min was lower in group N compared with that in group P (2% vs. 14%, χ2 = 5.14 df = 1, P = 0.023). Consistently, standardized HR was higher in group N compared to group P (standardized HR 78.8 ± 11.6 vs. 75.0 ± 7.3 beats/min, t = 1.99, P = 0.049). Additionally, no significant differences were observed in standardized SBP and other hemodynamic parameters. Maternal side effects, including nausea, vomiting, and dizziness were not different between the groups.
Table 2.
Maternal hemodynamic data, side effects, and drug administration.

Figure 2 shows the changes in CO, SV, and TPR in the first 10 min post-spinal anesthesia, which was the greatest time point for data available for most patients. Compared with group P, women in group N had a higher CO (standardized CO 5.8 ± 0.9 vs. 5.3 ± 1.0 L/min, t = 2.37, P = 0.02), SV (standardized SV 73.6 ± 17.2 vs. 60.0 ± 13.3 mL, t = 4.52, P < 0.001), and a lower TPR (875 ± 174 vs. 996 ± 182 dyne·s/cm5, t = 3.44, P < 0.001). However, as presented in Figure 3, when compared with baseline values, CO increased in group N but decreased in group P (4% vs. −5.7%, P = 0.0029), with significant statistical difference also observed for HR (−6.7% vs. −12.1%, P = 0.0036), SV (2.0% vs. −2.2%, P < 0.0001), and TPR (−35.0% vs. −27.8%, P < 0.001).
Figure 2.
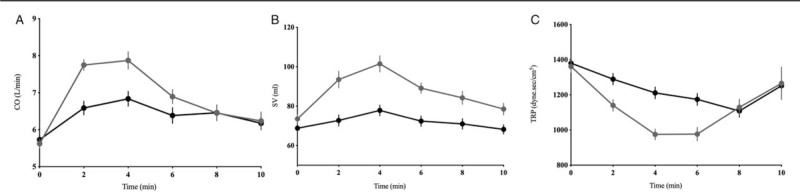
Hemodynamic trends including CO (A), SV (B), and TPR (C) during the observational period are depicted in parturients receiving norepinephrine and phenylephrine in the first 10 min post-anesthesia. Norepinephrine treatment is associated with a higher CO, SV, and lower TPR compared to phenylephrine. Horizontal coordinates in A–C mean minutes post-spinal anesthesia. CO: Cardiac output; SV: Stroke volume; TPR: Total peripheral resistance.
Figure 3.
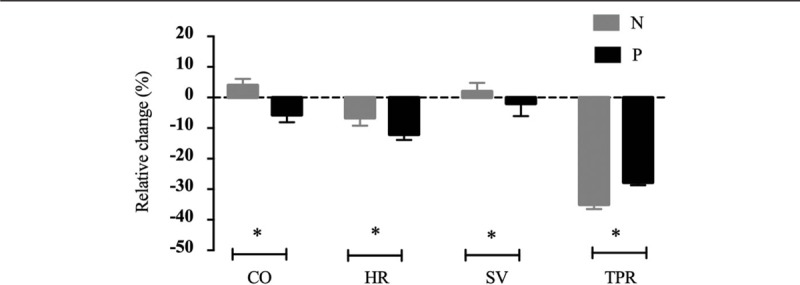
Hemodynamic variables including HR, CO, SV, and TPR at baseline and post-analgesia are presented in parturients receiving norepinephrine (gray) and phenylephrine (black). Norepinephrine treatment is associated with an increase of CO and SV, reduced decrease of HR, and a greater decrease of TPR compared to phenylephrine. Data are shown as mean ± standard error, and intergroup comparison is performed with non-paired t test. ∗P < 0.05. CO: Cardiac output; HR: Heart rate; SV: Stroke volume; TPR: Total peripheral resistance.
The neonatal outcomes are shown in Table 3. All Apgar scores at 1 and 5 min were greater than 7, and no neonate had a UA or UV pH less than 7.2. Glucose content was higher in group N compared to group P in both UA (3.5 [1.1–5.3] vs. 3.3 [2.1–5.3], P = 0.046) and UV (4.4 [2.5–7.7] vs. 4.2 [3.1–6.2], P = 0.04). Besides, UA and UV PO2 were higher in group N than in group P; however, no statistically significant differences were observed. Similarly, the other blood gas analysis variables, including PCO2, HCO3−, base excess (BEecf), glucose, and lactate were similar between the two groups.
Table 3.
Neonatal outcomes of the two groups.

Discussion
This study showed a phenylephrine equivalent bolus of norepinephrine provided a greater CO, HR, SV, and a lower TPR to manage post-spinal hypotension; however, both showed a similar efficacy and safety for other maternal hemodynamics parameters and neonatal outcomes.
A typical cardiovascular response to spinal anesthesia is the prominent decrease of TPR, while BP is maintained by a synergy of CO and TPR; therefore, the body increases SV and HR as a compensatory mechanism. However, due to multiple and intricate reasons, such as sympathetic blockade, peripheral vascular dilation, and reduced venous return, the compensation is usually insufficient to maintain maternal BP in the absence of appropriate intervention. Thus, the use of vasopressors is recommended to counter the decrease in arteriolar vasodilation and systemic vascular resistance,[9] with commonly used ephedrine, phenylephrine, or norepinephrine, all of which have an α-adrenergic effect.
Phenylephrine is commonly used; however, as a pure α agonist, it shows a dose-dependent negative chronotropic response, in turn decreasing CO and harming the fetus in certain circumstances.[10] Meanwhile, norepinephrine has a weak β1 adrenergic effect, rendering it less likely to decrease HR and theoretically offer a better maintenance of CO. In a previous study, Ngan Kee et al[11] compared the efficacy of computer-controlled variable infusion of 0 to 5 μg/min norepinephrine or 0 to 100 μg/min phenylephrine in maintaining maternal SBP near the baseline value. Their study found that norepinephrine provided a higher CO compared to phenylephrine with superiority mainly deriving from a better HR rather than SV. Further, a study by Vallejo et al compared the effects of a fixed infusion rate of 0.05 μg·kg−1·min−1 norepinephrine vs. 0.1 μg·kg−1·min−1 phenylephrine on maternal hemodynamics, especially the requirement of an extra bolus.[12] However, they did not find a significant difference in CO, HR, SV, and systemic vascular resistance between the two groups.
Our study differs from these studies as it showed a superiority of CO post norepinephrine bolus, which might be obtained from the synergy of HR and SV, but not HR alone. Several reasons may cause such discrepancy. First, the detection method of cardiovascular function is different. Ngan Kee et al's study[11] applied non-invasive suprasternal Doppler to track the CO and SV trend, requiring a more subjectively determination of the aortic valve cross-sectional area location. Vallejo et al[12] adopted a Nexfin non-invasive hemodynamic monitor to reflect hemodynamic alteration, which is a kind of pulse contour analysis method more suited for trend analysis. In contrast, the NICOM applied in our study may provide sequential quantitative values automatically updated every one minute, rendering it possible to calculate relative changes and perform more precise intergroup comparison. Second, the dosing regimen and hemodynamic management objectives are different. Ngan Kee et al[11] and Vallejo et al[12] infused norepinephrine or phenylephrine continuously to maintain SBP near 100% or 100% to 120% of the baseline values, respectively. However, in our study, norepinephrine or phenylephrine was bolus-injected to achieve an SBP higher than 80% of the baseline values. Finally, Ngan Kee et al compared norepinephrine with phenylephrine according to an estimated potency ratio of 20:1,[11] while a ratio of 2:1 was adopted in Vallejo et al's study,[12] and 13:1 in ours. Despite the differences, both Ngan Kee et al's study and ours consistently showed a higher CO advantage of norepinephrine compared to phenylephrine.
Other than the statistical CO superiority, we observed a similar efficacy of intermittent bolus norepinephrine and phenylephrine in maintaining maternal SBP as shown in Table 2. Besides, one observed significant different side effect is bradycardia, which has an incidence 2% for norepinephrine and 14% for phenylephrine. This, combined with a higher HR with norepinephrine throughout the observational period shown in Figure 2B, collectively suggests an obvious HR advantage compared to phenylephrine. Nevertheless, other than HR, we did not find other evidence of adverse maternal outcomes between the groups. The incidence of maternal side effects, including nausea, vomiting, and dizziness, is small and comparable.
On the other hand, one important objective of hemodynamic management is to ensure adequate uteroplacental blood flow, which is highly correlated with CO rather than BP.[13,14] However, the evidence for this is indirect and it remains to be confirmed whether a global measure of CO correlates with regional uteroplacental perfusion. Besides, CO superiority of norepinephrine in our study is relative, other studies including this study, have consistently shown excellent neonatal outcomes of phenylephrine even with large doses or in the presence of bradycardia.[15,16] Apgar score is comparable in the two groups. No neonatal Apgar score <7 at 1 min or Apgar score <9 at 5 min were reported. A higher UA or UV glucose content was observed in group N compared to group P, because this was not associated with a difference in pH, BE, or blood lactate, it might be plausibly resulted from the maternal glucose content difference and followed placental transfer, rather the effect of vasopressors. Collectively, the absence of obvious clinical advantages questioned the CO superiority observed herein. We suggest further work be performed to determine the CO advantage of norepinephrine in conditions where uteroplacental perfusion is restricted such as in fetal compromise or preeclampsia.
As shown in Figure 3, HR decreased below the baseline to a less extent with norepinephrine in both groups. Norepinephrine has both direct positive chronotropic and reflective negative chronotropic actions, and the overall effect on HR presents to be widely variable.[17] In rescuing general anesthesia-induced hypotension, low doses of bolus norepinephrine (5 μg) and phenylephrine (50 μg) caused an equivalent increase of mean arterial pressure and SV, as well as a significant equivalent decrease of HR.[18] Ngan Kee et al also observed a lesser decrease of HR in response to norepinephrine compared to phenylephrine; however, absolute change compared to the baseline was not explored in his study.[11] Types of anesthesia, different populations, and volume conditions may underlie such HR reaction discrepancy.
Norepinephrine provides a higher SV compared to phenylephrine when bolus was injected as shown in Figure 3. In our study, patients in both groups fasted for a similar duration and underwent a standard anesthesia procedure, fluid management, and patient positioning at the time of vasopressor injection, thus the effect of volume status on SV was excluded with the observed SV discrepancy largely resulting from the action of specific vasopressor. Norepinephrine causes an arterial and venous vasoconstriction, thus improving venous return and cardiac preload.[19,20] Although the exact action of norepinephrine on preload still needs to be validated in the context of obstetric spinal anesthesia, a combination with the β1 agonism derived inotropic effect, it might partially explain the increased SV compared to phenylephrine.
Furthermore, norepinephrine restored TPR less effectively compared to phenylephrine. This lower TPR may lead to a higher SV with norepinephrine, and an inferior α-adrenergic mediated vasoconstriction action cannot be excluded. Although the potency ratio for norepinephrine to phenylephrine is approximately 13:1, in terms of SBP maintenance, such values are much higher with regard to the restoration of TPR in the context of obstetric spinal anesthesia.
In summary, we observed a greater CO and a lower incidence of bradycardia with norepinephrine compared to phenylephrine when an intermittent bolus of each was injected for the management of maternal hypotension during elective cesarean section with spinal anesthesia. However, such CO advantages require careful interpretation as no obvious maternal or neonatal clinical advantages are observed.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang X, Mao M, Zhang SS, Wang ZH, Xu SQ, Shen XF. Bolus norepinephrine and phenylephrine for maternal hypotension during elective cesarean section with spinal anesthesia: a randomized, double-blinded study. Chin Med J 2020;133:509–516. doi: 10.1097/CM9.0000000000000621
References
- 1.Macarthur A, Riley E. Obstetric anesthesia controversies: vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin 2007; 45:115–132. doi: 10.1097/AIA.0b013e31802b8d53. [DOI] [PubMed] [Google Scholar]
- 2.Xu S, Shen X, Liu S, Yang J, Wang X. Efficacy and safety of norepinephrine versus phenylephrine for the management of maternal hypotension during cesarean delivery with spinal anesthesia: a systematic review and meta-analysis. Medicine 2019; 98:e14331.doi: 10.1097/MD.0000000000014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Shen X, Liu S, Yang J, Xu S. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. BioMed Res Int 2018; 2018:1869189.doi: 10.1155/2018/1869189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsella SM, Carvalho B, Dyer RA, Fernando R, Mcdonnell N, Mercier FJ, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018; 73:71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 5.Allen TK, Muir HA, George RB, Habib AS. A survey of the management of spinal-induced hypotension for scheduled cesarean delivery. Int J Obstet Anesth 2009; 18:356–361. doi: 10.1016/j.ijoa.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Onwochei DN, Ngan Kee WD, Fung L, Downey K, Ye XY, Carvalho JCA. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg 2017; 125:212–218. doi: 10.1213/ane.0000000000001846. [DOI] [PubMed] [Google Scholar]
- 7.Ngan Kee WD. A random-allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology 2017; 127:934–941. doi: 10.1097/aln.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 8.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990; 300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer RA, Biccard BM. Ephedrine for spinal hypotension during elective caesarean section: the final nail in the coffin? Acta Anaesthesiol Scand 2012; 56:807–809. doi: 10.1111/j.1399-6576.2012.02719.x. [DOI] [PubMed] [Google Scholar]
- 10.Langesaeter E, Rosseland LA, Stubhaug A. Continuous invasive blood pressure and cardiac output monitoring during cesarean delivery: a randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology 2008; 109:856–863. doi: 10.1097/ALN.0b013e31818a401f. [DOI] [PubMed] [Google Scholar]
- 11.Ngan Kee WD, Lee SW, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology 2015; 122:736–745. doi: 10.1097/aln.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 12.Vallejo M, Attaallah A, Elzamzamy O, Cifarelli D, Phelps A, Hobbs G, et al. An open-label randomized controlled clinical trial for comparison of continuous phenylephrine versus norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth 2017; 29:18–25. doi: 10.1016/j.ijoa.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Stewart A, Fernando R, Mcdonald S, Hignett R, Jones T, Columb M. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg 2010; 111:1230–1237. doi: 10.1213/ANE.0b013e3181f2eae1. [DOI] [PubMed] [Google Scholar]
- 14.Robson SC, Boys RJ, Rodeck C, Morgan B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br J Anaesth 1992; 68:54–59. doi: 10.1093/bja/68.6.635-c. [DOI] [PubMed] [Google Scholar]
- 15.Ngan Kee W, Khaw K, Tan P, Ng F, Karmakar M. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2009; 111:506–512. doi: 10.1097/ALN.0b013e3181b160a3. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2002; 97:1582–1590. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson-Myrthil N. Vasopressor use in adult patients. Cardiol Rev 2012; 20:153–158. doi: 10.1097/CRD.0b013e31824e2294. [DOI] [PubMed] [Google Scholar]
- 18.Poterman M, Vos J, Vereecke H, Struys M, Vanoverschelde H, Scheeren T, et al. Differential effects of phenylephrine and norepinephrine on peripheral tissue oxygenation during general anaesthesia: a randomised controlled trial. Eur J Anaesthesiol 2015; 32:571–580. doi: 10.1097/EJA.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 19.Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012; 40:3146–3153. doi: 10.1097/CCM.0b013e318260c6c3. [DOI] [PubMed] [Google Scholar]
- 20.Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011; 39:689–694. doi: 10.1097/CCM.0b013e318206d2a3. [DOI] [PubMed] [Google Scholar]


