Abstract
Cutaneous malignant melanoma (MM) is the most malignant type of all skin neoplasms. There is wide variability in the characteristics of MM between patients of different races. The aim of the present study was to investigate the clinicopathological characteristics of patients with MM in central China and to assess the value of specific hematological and biochemical indices for predicting metastasis. The data of 167 patients with MM from the First Affiliated Hospital of Zhengzhou University (Henan, China) were retrospectively analyzed and compared with the data of patients with MM available from cBioPortal for Cancer Genomics. Following analysis of the clinicopathological characteristics of the 167 patients, the median overall survival time was 50 months, and the median disease-free survival time was 35 months. Albumin/D-dimer prognosis score (ADPS), lactate dehydrogenase, sex, T stage, tumor-node-metastasis stage, Breslow thickness, Clark level, histological type, growth phase, ulceration and metastasis were all significantly associated with prognosis. An ADPS of <341.01 was identified as an independent predictor of metastasis. The trial registration no. is 2018-LW-037 and this clinical trial was registered in the First Affiliated Hospital of Zhengzhou University Clinical Trial Registry in March 1, 2018.
Keywords: MM, metastasis, albumin/D-dimer, prognosis, cBioPortal
Introduction
Malignant melanoma (MM) is a highly aggressive cancer derived from neural crest melanocytes and occurs most frequently in the skin, digestive tract, eyes, genitals and nasal cavity (1–3) The prognosis of patients with MM is poor and the 5-year survival is reported to be <20% (4). Each year, ~20,000 cases of cutaneous MM are reported in China and the incidence is growing by 3–5% per year (5). The incidence of MM is lower in China compared with Western countries, but survival is shorter in Chinese patients (6,7). In addition, differences in incidence, etiology and clinical characteristics between different races are still poorly understood.
The development of prognostic markers of MM, such as serum lactic dehydrogenase (LDH) and the identification of new treatment targets; for example, melanogenesis, have improved the treatments available for patients and patient outcomes (8,9). Serum LDH appears to be an independent marker of stage IV MM and studies have suggested that serum LDH may be used to identify patients requiring complete mastectomy (1,2). However, to the best of our knowledge, it is unclear whether serum LDH can be used to predict the metastatic possibility in the early stages of MM.
In the present study, the prognostic value of commonly tested hematological and biochemical parameters were investigated, including serum albumin and prealbumin, LDH, total leukocyte count and serum D-dimer levels, a degradation product of fibrinolysis. In addition, the albumin/D-dimer ratio [serum albumin/D-dimer prognosis score (ADPS)] and the serum prealbumin/D-dimer ratio [serum prealbumin/D-dimer prognosis score (PDPS)] were determined. Serum albumin (10) and prealbumin levels (11) reflect the nutritional and inflammatory status of the patient. Malnourished patients with cancer often have poor immune function, drug tolerance and poor response to treatments (12). D-dimer has been found to be associated with malignancy and D-dimer levels are associated with tumor stage, tumor prognosis, lymph node involvement and survival of patients with several types of cancer, including esophageal squamous cell carcinoma (13), gastric cancer (14), breast cancer (15), colorectal cancer (16), lung cancer (17) and ovarian cancer (18). Moreover, cancer-associated inflammation is an important contributor to disease progression and survival, and systemic inflammation is associated with alterations in peripheral blood leukocytes (19). Therefore, in the present study, it was hypothesized that a combination of D-dimer levels, leukocyte count and albumin or prealbumin levels may be useful for predicting prognosis of patients with MM.
The aim of the present study was: i) To explore the characteristics of MM and the factors affecting prognosis in Chinese patients with MM; ii) analyze the similarities and differences in characteristics with patients with MM from other regions of the world; and iii) to determine the value of commonly tested hematological and biochemical parameters for predicting metastasis in patients with MM.
Patients and methods
Patients
The present study was approved by The Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China; trial registration no. 2018-LW-037). Written informed consent was obtained from all participants included in the study. A total of 176 patients at the First Affiliated Hospital of Zhengzhou University were collected. Patients with a history of other malignancies, autoimmune disease, chronic renal or hepatic disease, diabetes, thyroid disorders and taking anti-inflammatory drugs were excluded. A total of 167 patients were included in this study, including 74 men (44.3%) and 93 women (55.7%). All patients were diagnosed with cutaneous or mucosal MM according to the criteria of the American Joint Committee on Cancer, 8th edition (1) between April 2003 and April 2018. The age range of all patients was 22–92 years and the median age was 56.81 years. The demographic and clinical data of these patients was obtained from medical records for analysis; including personal data (age at diagnosis, sex, living habits, family history); blood test results (serum albumin and prealbumin, serum LDH, total leukocyte count, serum D-dimer); tumor-related data [tumor location and stage, presence of metastasis, Breslow level (20), Clark level (3), presence of ulceration]; treatment received (adjuvant therapy and type of surgery) and follow-up data (current status, survival). Table I summarizes the collected data.
Table I.
Clinicopathological characteristics of patients with MM from the experimental cohort.
| Variable | n | Mean ± standard deviation/proportion, % |
|---|---|---|
| OS, months | 167 | 35.9±31.6 |
| DFS, months | 167 | 26.9±28.8 |
| Age, years | 167 | 56.8±15.0 |
| ≤40 | 23 | 13.8% |
| 40–50 | 39 | 22.7% |
| 50–60 | 33 | 20.4% |
| 60–70 | 44 | 26.3% |
| 70–80 | 20 | 12.0% |
| >80 | 8 | 4.8% |
| Sex | 167 | |
| Male | 74 | 44.3% |
| Female | 93 | 55.7% |
| Overall survival status | 167 | |
| Alive | 85 | 50.9% |
| Deceased | 82 | 49.1% |
| Family history of tumor | 167 | |
| Yes | 24 | 14.4% |
| No | 97 | 58.1% |
| Unknown | 46 | 27.5% |
| T stage | 167 | |
| pT0 | 13 | 7.9% |
| pT1 | 42 | 25.1% |
| pT2 | 57 | 34.1% |
| pT3 | 36 | 21.5% |
| pT4 | 19 | 11.4% |
| TNM stage | 167 | |
| I or II | 64 | 38.3% |
| III | 38 | 22.8% |
| IV | 65 | 38.9% |
| Breslow, mm | 137 | |
| ≤1.00 | 42 | 30.7% |
| 1.01–2.00 | 48 | 35.0% |
| 2.01–4.00 | 27 | 19.7% |
| >4.00 | 20 | 14.6% |
| Clark | 138 | |
| 1 | 30 | 21.7% |
| 2 | 34 | 24.6% |
| 3 | 41 | 29.7% |
| 4 | 16 | 11.6% |
| 5 | 17 | 12.3% |
| Histological type | 167 | |
| ALM | 52 | 13.8% |
| NM | 24 | 22.7% |
| SSM | 33 | 20.4% |
| LMM | 22 | 26.3% |
| MCM | 24 | 12.0% |
| Unclassifiable | 12 | 4.8% |
| Growth phase | 130 | |
| Radial | 61 | 46.9% |
| Vertical | 69 | 53.0% |
| Anatomic region | 167 | |
| Trunk | 21 | 12.6% |
| Head/neck | 14 | 8.4% |
| Extremities | 97 | 58.1% |
| Mucosal | 24 | 14.4% |
| Unknown | 11 | 6.6% |
| Ulceration | 147 | |
| With | 88 | 52.7% |
| Without | 59 | 35.3% |
| Unknown | 20 | 13.0% |
| Metastasis | ||
| With | 104 | 62.3% |
| Without | 63 | 37.7% |
| Therapy | 167 | |
| Surgery | 59 | 35.3% |
| Adjuvant therapy without surgery | 14 | 8.4% |
| Adjuvant therapy with surgery | 88 | 52.7% |
| Without therapy | 6 | 3.6% |
| Albumin, g/l | 109 | 42.83±3.32 |
| Prealbumin, mg/l | 100 | 244.60±54.26 |
| D-dimer, ug/l | 105 | 0.208±0.209 |
| Leukocyte 109/l | 113 | 6.56±2.17 |
| LDH, U/l | 96 | 183.78±55.54 |
T0, no evidence of primary tumor; OS, overall survival; DFS, disease free survival; T, tumor; TNM, tumor node metastasis; ALM, acral lentiginous melanoma; MCM, mucosal melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; LDH, lactate dehydrogenase.
cBioPortal
The characteristics of the patients of the present study were compared with patients around the world registered in the cBioPortal for Cancer Genomics (cbioportal.org). cBioPortal combines a number of large-scale cancer genomics projects, including The Cancer Genome Atlas (https://www.cancer.gov/tcga) and The International Cancer Genome Consortium (https://icgc.org/), and catalogues data on genetic, epigenetic, gene expression and proteomic events. The portal also provides graphical summaries of gene-level data from multiple platforms, network visualization and analyses, survival analysis (21) patient-centric queries and software programmatic access (2). cBioPortal contained 12 studies with a total of 1,566 patients, focusing on melanoma of eye and skin (Table II). Following elimination of duplicate data, a total of 1,518 patients remained, including 97% of Caucasian patients from America and Europe and 3% of Asian or African patients (Fig. 1A).
Table II.
Results of tumor type and number of cases from cBioPortal.
| Author, year | Source | Cancer type | No. of cases | (Refs.) |
|---|---|---|---|---|
| Taylor et al, 2018; Sanchez-Vega et al, 2018; Liu et al, 2018; Hoadley et al, 2018; Gao et al, 2018; Ellrott et al, 2018 | TCGA, PanCancer Atlas | Uveal melanoma | 80 | (42–47) |
| 2018 | TCGA, provisionala | Uveal melanoma | 80 | |
| Liang et al, 2017 | TGEN, genome research, 2017 | Paired-exome sequencing of acral melanoma | 38 | (48) |
| Berger et al, 2012 | Broad/Dana Farber, nature 2012 | Cutaneous melanoma | 26 | (49) |
| 2017 | MSKCC, JCO precision oncology, 2017b | Next generation sequencing (NGS) of pre-treatment metastatic melanoma samples | 66 | |
| Hodis et al, 2012 | Broad, cell, 2012 | Skin cutaneous melanoma | 121 | (50) |
| Taylor et al, 2018; Sanchez-Vega et al, 2018; Liu et al, 2018; Hoadley et al, 2018; Gao et al, 2018; Ellrott et al, 2018 | TCGA, PanCancer Alas | Skin cutaneous melanoma | 448 | (42–47) |
| 2018 | TCGA, provisional | Skin cutaneous melanomaa | 479 | |
| Krauthammer et al, 2012 | Yale, nat genet 2012 | Skin cutaneous melanoma | 91 | (51) |
| Van Allen et al, 2014 | Broad, cancer discov 2014 | Skin cutaneous melanoma | 78 | (52) |
| Hugo et al, 2016 | UCLA, cell 2016 | Whole-exome sequences of pretreatment melanoma tumors | 39 | (53) |
| Shain et al, 2015 | Broad institute, nat genet 2015 | Desmoplastic melanoma | 20 | (54) |
Raw data at the National Cancer Institute (https://www.cancer.gov/tcga).
Next generation sequencing (NGS) of pre-treatment. TCGA, The Cancer Genome Atlas; MSKCC, Memorial Sloan-Kettering Cancer Center; UCLA, University of California, Los Angeles.
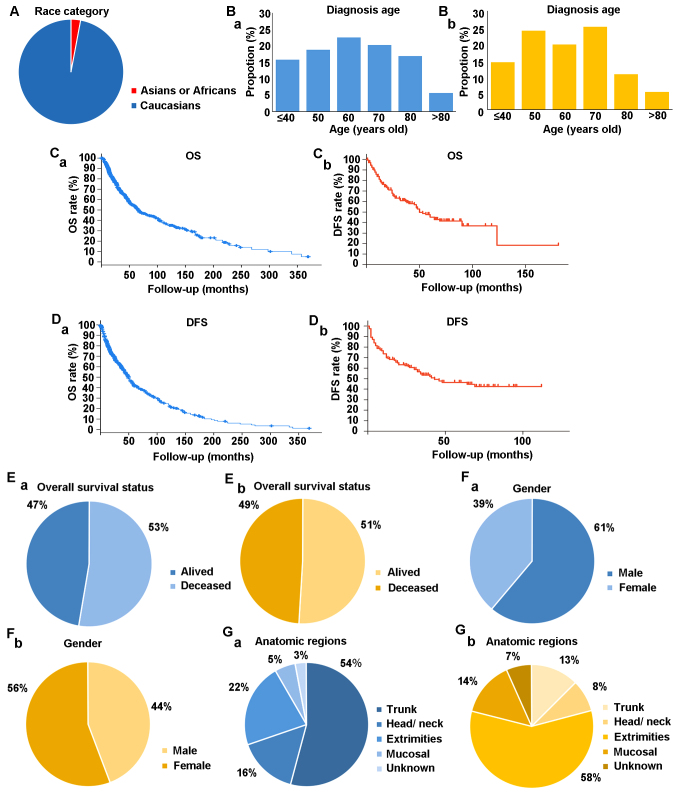
Figure 1.
Comparison of clinical characteristics between the cBioPortal cohort and the experimental cohort. (A) Categorization of patients in cBioPortal by race. (Ba) Categorization of patients in cBioPortal by age. (Bb) Categorization of patients in the experimental cohort by age at diagnosis. (Ca) OS from cBioPortal. (Cb) OS of experimental cohort. (Da) DFS from cBioPortal. (Db) DFS from the experimental cohort. (Ea) Overall survival status from cBioPortal cohort. (Eb) OS from the experimental cohort. (Fa) Sex of patients from the cBioPortal cohort. (Fb) Sex of patients from the experimental cohort. (Ga) Anatomic regions of MM for patients with MM of the cBioPortal cohort. (Gb) Anatomic regions of MM for patients with MM of the experimental cohort. OS, overall survival; DFS, disease free survival; MM, malignant melanoma.
Statistical analysis
Data were analyzed using Microsoft Excel 2007 (Microsoft Corporation), SPSS version 20.0 (IBM Corp.) and GraphPad Prism version 5 (GraphPad Software Inc.). Quantitative variables were presented as either mean ± standard deviation or median and interquartile range, depending on the normality of the distribution. Normally distributed quantitative variables were analyzed using a Student's t test, data with unequal variance were analyzed using a Welch's t-test. Non-normally distributed continuous data were compared using a Mann-Whitney U test. Qualitative variables were reported as frequencies and percentages and compared using a χ2 test or the Fisher's exact test. Receiver operating characteristic (ROC) analysis was used to identify the ideal cutoff value of ADPS for distinguishing between patients with and without metastasis. The Youden index [(specificity + sensitivity)-1] was calculated as a measure of overall efficacy. The area under curve (AUC) was used to assess the predictive value of ADPS, serum prealbumin/D-dimer prognosis score (PDPS) and D-dimer. Regression tree analysis was used to measure the sensitivity, specificity and accuracy of the indicator for predicting metastasis in patients with MM. Variables that were significantly associated with tumor metastasis were analyzed using multivariate Cox regression analysis to identify independent predictors of metastasis. Kaplan-Meier curves were constructed for survival analysis and a log-rank test was used to determine the differences in survival rate. In Kaplan-Meier survival analysis of prognostic factors for OS, the prognostic factors with multigroups in T stage, TNM stage, Breslow thickness, Clark level, histological type and anatomic region were analyzed by pooled over strata in log-rank test. P<0.05 was considered to indicate a statistically significant difference. In the comparison of MM patients with and without metastasis, each numerical data was limited by the mean difference and divided into higher group and lower group. Regarding other statistic analyses, MM patients were divided into low or high group according to the ADPS value (341.01), the ideal cutoff value by ROC analysis, whereas other quantitative data were grouped by mean value.
Results
Clinical characteristics of patients with MM
A total of 85 patients (50.9%) were still alive at the end of the study whereas 82 patients (49.1%) had died. Median overall survival (OS) was 50 months (range, 0–181 months; Fig. 1C) and median disease-free survival (DFS) was 35 months (range, 0–124 months; Fig. 1D). The proportion of patients with tumor node metastasis (TNM) stage I/II, III or IV were 64/167 (38.3%), 38/167 (22.8%) and 65/167 (38.9%), respectively. Data on Breslow thickness were available for 137 patients; the Breslow thickness was <1 mm in 42/137 patients (30.7%), 1.01–2.00 mm in 48/137 patients (35.0%), 2.01–4.00 mm in 27/137 patients (19.7%) and >4.00 mm in 20/137 patients (14.6%). Data on the Clark level were available for 138 patients; 30/138 patients (21.7) were classed as level 1, 34/138 patients (21.7%) were classed as level 2, 41/138 patients (29.7%) were classed as level 3, 16/138 patients (11.6%) were classed as level 4 and 17/138 patients (12.3%) were classed as level 5. The primary lesion was on the trunk in 21/167 patients (12.6%), in the head/neck region in 14/167 patients (8.4%), in the extremities in 97/167 patients (58.1%), in the mucosa in 24/167 patients (37.8%) and at unknown locations in 11/167 patients (6.6%). Ulcerative melanoma was observed in 88 out of 167 patients (52.7%) and nonulcerative melanoma was observed in 59 out of 167 patients (35.3%). The mean total serum Albumin, D-dimer and LDH were 42.83±3.32 g/l, 0.208±0.209 ug/l and 183.78±55.54 U/l, respectively. The clinicopathological characteristics of patients and results of blood tests are described in Table I.
Comparison of characteristics of Chinese patients with MM with the cBioPortal data
The proportion of male patients was higher in the cBioPortal cohort than in the experimental cohort (61% vs. 44%; Fig. 1Fa and Fb). Age at diagnosis of patients with MM ranged from 40–70 years in both the cBioPortal cohort and in experimental cohort (Fig. 1Ba and Bb). MM was most commonly diagnosed in patients aged 50–60 years in the cBioPortal cohort (Fig. 1Ba); whereas, in the experimental cohort MM was most commonly diagnosed in patients aged 60–70 years (Fig. 1Bb). Median OS was 66.43 months (range, 0.36–369.65 months) in the cBioPortal cohort vs. 50 months (range, 0–181 months) in the experimental cohort. Median DFS was 49.21 months (range, 0.46–386.50 months) in the cBioPortal cohort vs. 35 months (range, 0–112 months) in experimental cohort. The 3-, 5- and 10-year survival rates were 59.1, 47.7 and 31.6%, respectively, in the cBioPortal cohort vs. 59.0, 43.0 and 13.5% in the experimental cohort (Fig. 1C and D). OS was 47% in the cBioPortal cohort vs. 49% in experimental cohort (Fig. 1E). In the cBioPortal cohort, the most common locations of melanoma were the trunk (54%), followed by the extremities (22%), head and neck (16%) and mucosa (5%) (Fig. 1Ga), whereas in the experimental cohort the most common locations of melanoma were the extremities (58.1%), followed by the mucosa (14%) and the trunk (13%) (Fig. 1Gb). The reasons for these differences remain unclear; however, a possible explanation may be due to differences in lifestyle factors.
Melanoma treatment
Patients in the experimental cohort received a variety of treatments, including combinations of surgery, chemotherapy, immunotherapy and biotherapy. A total of 88/167 patients (52.7%) received surgery and adjuvant therapy, whereas 59/167 patients (35.3%) received only surgery and 14/167 patients (8.4%) received only adjuvant therapy (Fig. 2); treatment details were not available for 6/167 (3.6%) patients (Table I). Treatment selection for the patients in the experimental cohort was in accordance with the 1st edition of Consensus on the Diagnosis and Treatment of Melanoma in China (August, 2008) (1). Chemotherapeutic agents used included Taxol, Dacarbazine and platinum-type drugs. Immunotherapeutic agents used included thymopentin, and recombinant human interferon g and/or interleukin-2. There were a total of 15 different combinations of treatments; the most common treatment was surgery alone, followed by surgery plus immunotherapy and then surgery plus bioimmunotherapy (Fig. 2).
Figure 2.
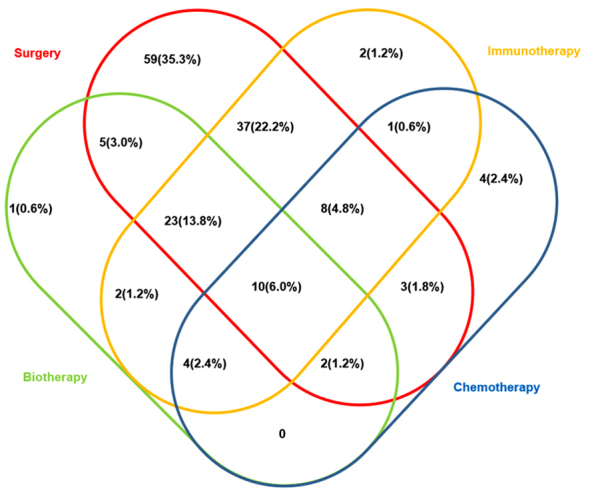
Venn diagram of treatments of patients with MM received in the experimental cohort. MM, malignant melanoma.
Comparison of patients with and without metastasis
The experimental patients were separated into two groups: Patients with metastasis (n=104) and those without metastasis (n=63). Mean serum albumin (P=0.145), prealbumin (P=0.752), LDH (P=0.150) and leukocyte count (P=0.224) did not significantly differ between the two groups. However, patients with metastasis had significantly higher D-dimer levels (0.237±0.217 µg/l vs. 0.151±0.134 µg/l; P<0.001), and a significantly lower ADPS (328.61±235.72 vs. 452.46±302.07; P<0.001) and PDPS (1,913.53±1,464.42 vs. 2,657.75±1,983.42; P<0.05) (Table III).
Table III.
Comparison between experimental cohort patients with and without metastatic MM.
| Variable | Metastatic, mean ± SD | Non-metastatich, mean ± SD or mean (n=%) | P-value |
|---|---|---|---|
| Albumin, g/ld | 42.12±4.35 | 42.99±2.90 | 0.145 |
| Prealbumin, mg/le | 246.90±57.58 | 243.70±50.70 | 0.752 |
| LDH, U/lf | 191.43±59.86 | 176.56±37.22 | 0.150 |
| D-dimer, ug/lf | 0.43±0.85 | 0.16±0.13 | <0.001 |
| Leukocyte, 109/lf | 6.77±4.58 | 5.93±1.97 | 0.224 |
| ADPSf | 290.46±241.84 | 434.35±276.99 | <0.001 |
| PDPSe | 1,789.50±1,449.42 | 2,521.04±1,809.78 | 0.018a |
| Sex | <0.001c | ||
| Male | 57 (54.8%) | 17 (25.8%) | |
| Female | 47 (45.2%) | 49 (74.2%) | |
| Breslowg | <0.001c | ||
| ≤1.00 | 12 (16.0%) | 29 (50.0%) | |
| 1.01–2.00 | 28 (37.3%) | 19 (32.8%) | |
| 2.01–4.00 | 18 (24.0%) | 8 (13.8%) | |
| >4.00 | 17 (22.7%) | 2 (3.4%) | |
| Clark levelg | <0.010a | ||
| 1 | 12 (16.0%) | 17 (29.8%) | |
| 2 | 14 (18.7%) | 19 (33.3%) | |
| 3 | 25 (33.3%) | 15 (26.3%) | |
| 4 | 11 (14.7%) | 4 (7.0%) | |
| 5 | 13 (17.3%) | 2 (3.5%) | |
| Anatomic regiong | 0.078 | ||
| Truck | 16 (17.0%) | 5 (7.9%) | |
| Head/neck | 10 (10.6%) | 4 (6.3%) | |
| Extremities | 51 (54.3%) | 47 (74.6%) | |
| Mucosal | 7 (18.1%) | 7 (11.1%) | |
| Ulcerationg | <0.001c | ||
| With | 69 (80.2%) | 19 (31.1%) | |
| Without | 17 (19.8%) | 42 (68.9%) | |
| T stageg | 0.003b | ||
| pT0 | 13 (7.8%) | 0 (0) | |
| pT1 | 11 (6.6%) | 31 (18.6%) | |
| pT2 | 38 (22.8%) | 19 (11.4%) | |
| pT3 | 25 (15.0%) | 11 (6.6%) | |
| pT4 | 17 (10.2%) | 2 (1.2%) | |
| Histological typeg | <0.001c | ||
| ALM | 21 (13.5%) | 31 (20.0%) | |
| NM | 23 (14.8%) | 1 (0.6%) | |
| SSM | 17 (11.0%) | 16 (10.3%) | |
| LMM | 14 (9.0%) | 8 (5.1%) | |
| MCM | 17 (11.0%) | 7 (4.5%) | |
| Growth phaseg | <0.001c | ||
| Radial | 15 (20.3%) | 46 (82.1%) | |
| Vertical | 59 (79.7%) | 10 (17.9%) |
P<0.05
P<0.01
P<0.001.
Mean ± SD, P-value based on Welch's t-test
Mean ± SD, P-value based on Student's t-test
Mean ± SD, P-value based on Mann-Whitney U test
P-value based on χ2 test
Unresectable melanoma. SD, standard deviation; MM, malignant melanoma; LDH, lactate dehydrogenase; ADPS, serum albumin/D-dimer prognosis score; PDPS, serum prealbumin/D-dimer prognosis score; T, tumor; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; MCM, mucosal melanoma.
ROC analysis
ROC analysis showed an ADPS of 341.01 to be the ideal cutoff value for differentiating between patients with and without metastasis and the AUC for ADPS was 0.773 (Fig. 3). For prediction of metastasis, ADPS had 93.8% sensitivity, 59.6% specificity, 54.6% accuracy and a Youden index of 53.40%. The AUC for PDPS was 0.547. For prediction of metastasis, PDPS had 31.3% sensitivity, 84.7% specificity, 9.4% accuracy and a Youden index of 16.0%. The AUC for D-dimer was 0.447. For prediction of metastasis, D-dimer had 31.35% sensitivity, 75.5% specificity, 10.6% accuracy and a Youden index of 6.9% (Table IV).
Figure 3.
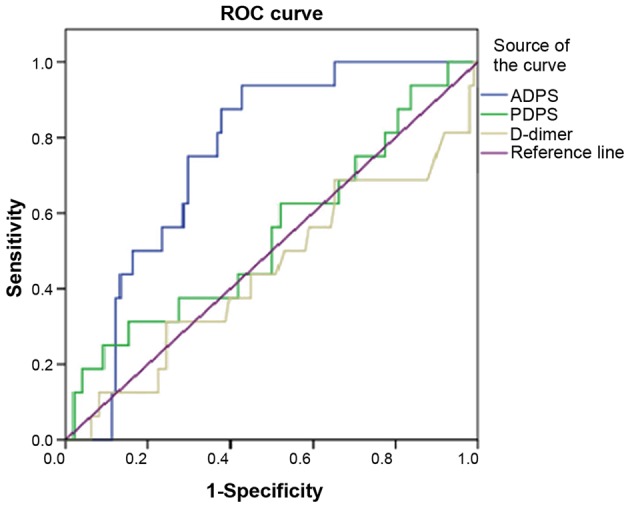
ROC curve of patients with MM in the experimental cohort. ROC, Receiver operating characteristic; MM, malignant melanoma; ADPS, serum albumin/D-dimer prognosis score; PDPS, serum prealbumin/D-dimer prognosis score.
Table IV.
ROC analysis of metastasis in patients with MM.
| AUC | SEa | Cut off value | Acurrency ratio, % | Sensitivity, % | Specificity, % | LB | UB | Youden index (%) | |
|---|---|---|---|---|---|---|---|---|---|
| ADPS | 0.773 | 0.045 | 457.38 | 54.60 | 93.8 | 59.6 | 0.686 | 0.861 | 53.40 |
| PDPS | 0.547 | 0.082 | 3,456.61 | 9.40 | 31.30 | 84.70 | 0.386 | 0.707 | 16.00 |
| D-dimer | 0.447 | 0.084 | 0.238 | 10.60 | 31.35 | 75.50 | 0.282 | 0.611 | 6.85 |
Non-parametric. MM, malignant melanoma; SE, standard error; AUC, area under the curve; ROC, receiver operating characteristic; LB, 95% confidence interval lower bound; UB, 95% confidence interval upper bound.
Univariate and multivariable Cox regression analyses for metastasis of patients with MM
Univariate Cox regression analyses were performed to screen the potential predictors of metastasis for patients with MM. A total of 9 clinicopathological predictors for overall survival were ascertained through multivariate Cox regression analysis and 3 variables were shown to be independently associated with metastasis; including ADPS Group [hazard ratio (HR)=8.534; 95% confidence interval (CI)= 3.109–23.425], ulceration (HR=4.287; 95% CI=2.204–8.953) and vertical growth phase (HR=2.324; 95% CI=1.067–5.063). In contrast, sex, Breslow thickness, Clark level, histological type, anatomic location and T stage were not shown to be independent predictors of metastasis (Tables V and VI).
Table V.
Univariate Cox regression analyses of metastasis in patients with MM.
| Univariate analysis | |||
|---|---|---|---|
| Variables | HR | 95% CI | P-value |
| ADPS group | 13.611 | 6.707–27.622 | 0.000 |
| Ulceration | 5.257 | 3.036–9.102 | 0.000 |
| Growth phase | 5.475 | 3.074–9.752 | 0.000 |
| Sex | −0.580 | 0.339–0.737 | 0.000 |
| Breslow | 1.749 | 1.415–2.162 | 0.000 |
| Clark level | 1.507 | 1.261–1.801 | 0.000 |
| T stage | 1.423 | 1.272–1.592 | 0.000 |
| Histological type | |||
| ALM | −0.114 | 0.054–0.242 | 0.000 |
| NM | −0.556 | 0.273–1.131 | 0.105 |
| SSM | −0.167 | 0.077–0.361 | 0.000 |
| LMM | −0.207 | 0.093–0.462 | 0.000 |
| MCM | −0.226 | 0.104–0.492 | 0.000 |
| Anatomic region | |||
| Trunk | −0.311 | 0.142–0.682 | 0.004 |
| Head/neck | −0.303 | 0.126–0.729 | 0.008 |
| Extremities | −0.189 | 0.096–0.375 | 0.000 |
| Mucosal | −0.259 | 0.118–0.568 | 0.001 |
ADPS, serum albumin/D-dimer prognosis score; T, tumor; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; MCM, mucosal melanoma.
Table VI.
Multivariate Cox regression analyses of metastasis in patients with MM.
| Multivariate analysis | ||||
|---|---|---|---|---|
| Variables | Coefficient | HR | 95% CI | P-value |
| ADPS group | 2.144 | 8.534 | 3.109–23.425 | 0.000 |
| Ulceration | 1.567 | 4.792 | 2.204–8.953 | 0.000 |
| Growth phase | 0.843 | 2.324 | 1.067–5.063 | 0.034 |
| Sex | −0.535 | 0.586 | 0.314–1.092 | 0.092 |
| Breslow | 0.339 | 1.404 | 0.739–2.666 | 0.300 |
| Clark level | 0.047 | 1.048 | 0.820–1.339 | 0.708 |
| T stage | 0.498 | 1.645 | 0.857–3.158 | 0.134 |
| Histological type | −0.109 | 0.896 | 0.653–1.230 | 0.498 |
| Anatomic region | 0.035 | 1.035 | 0.718–1.493 | 0.853 |
MM, malignant melanoma; HR, hazard ratio; CI, confidence interval; ADPS, serum albumin/D-dimer prognosis score; T, tumor; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; MCM, mucosal melanoma.
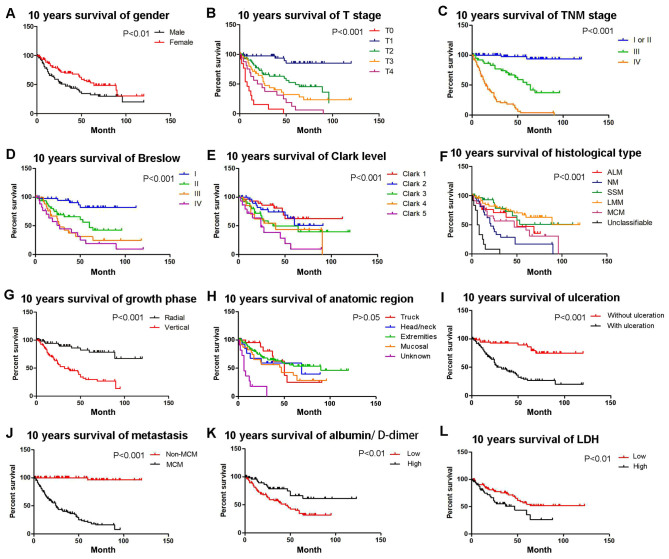
Kaplan-Meier survival analysis of prognostic factors for OS
A 10-year Kaplan-Meier survival analysis was performed and a Log-rank test was used to compare the survival curves of two or more groups. Survival of patients with high ADPS was significantly longer compared with patients with low ADPS. The OS was significantly worse for MM patients with ulcerated melanoma (compared with patients without ulceration; P<0.001; Fig. 4I), MM patients with metastasis (compared with patients without metastasis; P<0.001; Fig. 4J), female patients (compared with male patients; P=0.0062; Fig. 4A), Clark level >3 (compared with Clark level 3 and 4; P<0.001; Fig. 4E) and patients with vertical growth phase (compared with radial; P<0.001; Fig. 4G). The Kaplan-Meier survival curves also demonstrated significant difference in distinct TNM stage (multiple comparison; P<0.001; Fig. 4C) and Breslow thickness (multiple comparison; P<0.001; Fig. 4D). The median OS by stage was as follows: T0 stage, 8 months; T1 stage, 108 months; T2 stage, 60 months; T3 stage, 27 months; and T4 stage, 19 months (multiple comparison; P<0.001; Fig. 4B). Median overall survival by histological type was as follows: Acral lentiginous melanoma, 82 months; nodular melanoma, 21 months; superficial spreading melanoma, 74 months; Lentigo malignant melanoma, 50 months; Mucosal melanoma, 47 months; and indeterminate types, 6 months (multiple comparison; P<0.001; Fig. 4H). Anatomic region of the MM was not associated with OS (multiple comparison; P=0.171; Fig. 4F; Table VII).
Figure 4.
Kaplan-Meier analyses of 10-year OS for the entire cohort of patients according to different stratums by prognostic factors. OS of patients based on (A) sex (P<0.01), (B) T stage (compared with T0, T1, T2, T3 and T4; P<0.001), (C) TNM stage (compared with TNM stage I/II, III and IV; P<0.001), (D) Breslow thickness (compared with Breslow I, II, III and IV; P<0.001), (E) Clark level (compared with Clark level 1, 2, 3, 4 and 5; P<0.001), (F) histological type (compared with ALM, NM, SSM, LMM, MCM and unclassifiable; P<0.001), (G) growth phase (P<0.001), (H) anatomic region (compared with trunk, head/neck, extremities, mucosal and unknown; P>0.05), (I) ulceration (P<0.001), (J) metastasis (P<0.001), (K) albumin/D-dimer (P<0.01), (L) LDH levels (P<0.01). OS, overall survival; T, Tumor; TNM, tumor node metastasis; LDH, lactate dehydrogenase; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; MCM, mucosal melanoma.
Table VII.
Kaplan-Meier survival analysis of prognostic factors for OS.
| Variables | Median OS, months | df | Log-rank analysis, P value |
|---|---|---|---|
| Sex | 1 | 0.0062b | |
| Male | 32 | ||
| Female | 64 | ||
| T stage | 4 | <0.001c | |
| pT0 | 8 | ||
| pT1 | 108 | ||
| pT2 | 60 | ||
| pT3 | 27 | ||
| pT4 | 19 | ||
| TNM stage | 2 | <0.001c | |
| I or II | 120 | ||
| III | 60 | ||
| IV | 14 | ||
| Breslow, mm, n=137 | 3 | <0.001c | |
| ≤1.00 | 98 | ||
| 1.01–2.00 | 60 | ||
| 2.01–4.00 | 27 | ||
| >4.00 | 24 | ||
| Clack level, n=138 | 4 | <0.001c | |
| 1 | 83 | ||
| 2 | 60 | ||
| 3 | 37 | ||
| 4 | 40 | ||
| 5 | 24 | ||
| Histological type | 5 | <0.001c | |
| ALM | 82 | ||
| NM | 21 | ||
| SSM | 74 | ||
| LMM | 50 | ||
| MCM | 47 | ||
| Unclassifiable | 6 | ||
| Growth phase | 1 | <0.001c | |
| Radial | 98 | ||
| Vertical | 32 | ||
| Anatomic region | 3 | 0.171 | |
| Trunk | 48 | ||
| Head/neck | 69 | ||
| Extremities | 89 | ||
| Mucosal | 45 | ||
| Ulceration, n=147 | 1 | <0.001c | |
| With | 79 | ||
| Without | 27 | ||
| Metastasis | 1 | <0.001c | |
| With | 24 | ||
| Without | 113 | ||
| ADPS group | 0.0035b | ||
| Low | 24 | 1 | |
| High | 99 | ||
| LDH group | 1 | 0.010a | |
| Low | 89 | ||
| High | 37 |
P<0.05
P<0.01
P<0.001. n=167 unless otherwise specified. OS, overall survival; df, degree of freedom; T, tumor; TMN, tumor node metastasis; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; MCM, mucosal melanoma; ADPS, serum albumin/D-dimer prognosis score; LDH, lactate dehydrogenase.
Comparison between patients with low and high ADPS
ADPS was calculated for 124 patients. There were 24 patients with low ADPS (<341.01) and 99 patients with high ADPS (≥341.01). Patients with high ADPS level were more likely to have a longer OS (P=0.016), DFS (P=0.001) and earlier TNM stage (TNM stages I and II) of MM (24.2% vs. 17.4%; P=0.008), whereas these patients were less likely to have an ulcerated melanoma (19.9% vs. 20.6%; P<0.001) or a metastatic melanoma (17.4% vs. 24.2%; P=0.002; Table VIII).
Table VIII.
Comparison between patients with high ADPS level and low ADPS.
| Low ADPS level | High ADPS level | P-value | |
|---|---|---|---|
| OSc, mean ± SD | 26.96±23.67 | 41.34±30.65 | 0.139 |
| DFSc, mean ± SD | 15.46±17.13 | 34.88±26.03 | 0.733 |
| TNM staged, n (%) | 1.000 | ||
| I or II | 25 (18.9%) | 32 (24.2%) | |
| III | 20 (15.2%) | 12 (9.1%) | |
| IV | 32 (24.2%) | 11 (8.3%) | |
| Sexd, n (%) | 0.644 | ||
| Male | 30 (25.6%) | 21 (17.9%) | |
| Female | 36 (56.4%) | 30 (25.6%) | |
| Breslowd | 0.335 | ||
| ≤1.00 | 20 (17.1%) | 17 (14.5%) | |
| 1.01–2.00 | 19 (16.2%) | 21 (17.9%) | |
| 2.01–4.00 | 16 (13.7%) | 8 (6.8%) | |
| >4.00 | 11 (9.4%) | 5 (4.3%) | |
| Clark leveld | 0.713 | ||
| 1 | 18 (14.9%) | 9 (7.4%) | |
| 2 | 15 (12.4%) | 15 (12.4%) | |
| 3 | 17 (14.0%) | 16 (13.2%) | |
| 4 | 7 (5.8%) | 6 (5.0%) | |
| 5 | 9 (7.4%) | 9 (7.4%) | |
| Anatomic regiond | 0.132 | ||
| Truck | 16 (10.9%) | 5 (3.4%) | |
| Head/neck | 10 (6.8%) | 4 (2.7%) | |
| Extremities | 51 (34.7%) | 47 (32.0%) | |
| Mucosal | 7 (4.8%) | 7 (4.8%) | |
| Ulcerationd | 0.001a | ||
| With | 68 (51.9%) | 26 (19.9%) | |
| Without | 10 (7.6%) | 27 (20.6%) | |
| Metastasisd | 0.002b | ||
| With | 53 (40.2%) | 23 (17.4%) | |
| Without | 24 (18.2%) | 24 (18.2%) | |
| T staged | 0.706 | ||
| pT0 | 4 (3.0%) | 0 | |
| pT1 | 21 (15.9%) | 16 (12.1%) | |
| pT2 | 22 (16.6%) | 23 (17.4%) | |
| pT3 | 19 (14.4%) | 11 (8.3%) | |
| pT4 | 11 (8.3%) | 5 (11.0%) | |
| Histological typed | 0.294 | ||
| ALM | 26 (22.2%) | 16 (13.7%) | |
| NM | 1 (0.8%) | 6 (5.1%) | |
| SSM | 15 (12.8%) | 14 (12.0%) | |
| LMM | 6 (5.1%) | 13 (11.1%) | |
| MCM | 11 (9.4%) | 6 (5.1%) | |
| Unclassifiable | 3 (2.7%) | 0 | |
| Growth phased | 0.065 | ||
| Radial | 22 (19.8%) | 26 (23.4%) | |
| Vertical | 40 (36.0%) | 23 (20.7%) |
P<0.001
P<0.01
P-value based on t-test.
P-value based on χ2 test. SD, standard deviation; ADPS, serum albumin/D-dimer prognosis score; OS, overall survival; DFS, disease free survival; TNM, tumor node metastasis; T, tumor; ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma; LMM, lentigo malignant melanoma; MCM, mucosal melanoma.
Discussion
Due to the low incidence of MM in China there is still no standard approach for diagnosis and treatment, and this is partly due to a lack of reliable indicators of metastasis in the early stages of disease. The aim of the present study was to determine the prognostic value of specific hematological and biochemical parameters routinely assessed at admission to hospital, and their predictive value for prognosis of patients with MM.
Prognostic markers of MM, such as serum LDH, and the identification of new treatment targets including melanogenesis, serve important roles in the treatment and prognosis of MM (9). Melanogenesis is a highly regulated multistep biochemical process of melanin production by melanocytes (22,23). Previous studies have indicated that melanin pigment increases the resistance of melanoma cells to different types of therapy including chemo- or radiotherapy (22). Regarding the role of melanin pigment in chemoresistance of melanoma cells, it was reported that melanogenesis can generate cytotoxic, genotoxic or mutagenic intermediates, which influence tumor microenvironment and tumor immunity (24). Furthermore, studies have shown that melanogenesis reduces OS and DFS in patients with MM (23).
Serum albumin is synthesized in the liver and participates in numerous biological functions in the body, including maintenance of plasma osmotic pressure and regulation of the dynamic balance between tissue fluid and blood vessels (25,26). Serum albumin is also essential for the transport of a number of substances, including hormones, long-chain fatty acids to the liver, unconjugated bilirubin, metals and ions (27,10). Decreases in plasma albumin levels can result in decreased activity of enzymes essential for metabolism of organisms (28). Serum albumin levels can be used to assess nutritional and inflammatory status (28). It has also been demonstrated that malnourished cancer patients often have reduced immune function, poor drug tolerance and poor response to treatments (12,29).
D-dimer is a by-product of fibrinolysis and increases in the levels of D-dimers suggest the presence of coagulation and fibrinolysis (30). Plasma D-dimer levels are useful for monitoring the state of thrombotic diseases and disseminated intravascular coagulation (13). Several studies have shown that the levels of D-dimer are associated with tumor stage, tumor prognosis, lymph node involvement and survival of patients with ovarian cancer (18). In addition, increased D-dimer levels are associated with the degree of malignancy of tumors. Tumor cells or necrotic tissues stimulate the generation and release of coagulation-promoting substances, which activate exogenous coagulation factors resulting in abnormal coagulation and thus activation of the plasminogen activator (30,31). Locally synthesized fibrinolytic enzymes degrade the extracellular matrix and facilitate tumor invasion (14).
In the present study, significant differences in D-dimer levels, ADPS and PDPS were observed in patients with metastasis compared with those without metastasis (Table III). ROC analysis and multivariate Cox regression analysis showed that ADPS was an independent predictor of metastasis for patients with MM. Previous studies reported that albumin and D-dimer served as independent prognostic predictors of prognosis of patients with miliary tuberculosis (32) and the postoperative survival of patients with esophageal squamous cell carcinoma (13). In other studies investigating MM, low serum albumin levels are predictors of morbidity and mortality in patients with MM (33) and elevated D-dimer levels indicated a poor prognosis (32). However, to the best of our knowledge, the present study is the first to show ADPS as an independent predictor of MM metastasis.
In the present study, the highest incidence of MM was observed in individuals aged between 40–70 years, similar to the reports from other studies with Chinese cohorts (32). Statistical analysis showed ulceration and vertical growth phase were independent risk factors for tumor metastasis in patients with MM and previous studies have also identified ulceration as an independent prognostic factor in patients with melanoma (34). In certain Chinese studies >4 mm tumor thickness and clinical stage III and IV were also found to be significant predictive factors (5).
In the experimental cohort used in the present study, surgery was the most common treatment, followed by surgery combined with immunotherapy, and surgery combined with bioimmunotherapy. To the best of our knowledge, it is unclear if surgery is the best choice to completely remove all lesions in patients with MM with locally advanced or early disease and in patients with distant MM metastasis. However, previous studies have shown that patients with MM with distant metastasis may still benefit from surgery (35,36). Patients with stage IV MM are usually treated with systemic biologics and/or chemotherapy; however, treatment for patients with metastatic MM has been a challenge as aggressive treatments, including combination of immunotherapy with other therapies, have failed to show satisfactory efficacy (37,38).
The cohort of the present study was compared with the cohort of patients with MM in cBioPortal and significant differences were found, including differences in OS, DFS, age at diagnosis and anatomic locations of metastasis between the two groups of patients. There may be several reasons for these differences, for example the sample size of the experimental cohort in the present study was small, and Caucasians comprised a majority of the patients in the cBioPortal cohort, whereas all the patients in the present study were Chinese. Melanoma is more common in light-skinned individuals, as individuals lacking protective melanin pigments are more susceptible to cutaneous melanoma compared with darker-skinned individuals (22). Therefore lower quantities of protective melanin pigment may increase the susceptibility of Caucasians to damage caused by ultraviolet radiation (22,24). Previous epidemiological and experimental data have indicated that ultraviolet light, through its mutagenic activity, is the most likely cause of cutaneous melanoma in Caucasian patients (39). MM in Caucasian patients also predominantly occurs on the trunk and the most common type is the superficial spreading type (40); however, in Chinese patients, MM occurs most frequently on the extremities or the mucosa (41). Moreover, the majority of Caucasian patients are diagnosed at stage I (20), whereas the majority of Chinese patients are diagnosed at stage II or III (41). The reasons for these ethnic differences should be a focus of future studies.
The present study has certain limitations. First, the sample size was small, and all patients were recruited from a single institution. However, the First Affiliated Hospital of Zhengzhou University is a major referral center for MM treatment in central China, and the study population can be considered representative of this region. Second, some patients were lost to follow-up as a number of patients failed to comply with regular follow-up due to economic reasons or lack of awareness.
Overall, there are significant differences between patients with MM from central China and those from other parts of the world. ADPS may be a useful predictor of MM metastasis in the early stages of disease.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- OS
overall survival
- DFS
disease free survival
- MM
malignant melanoma
- LDH
lactate dehydrogenase
- ADPS
serum albumin/D-dimer prognosis score
- PDPS
serum prealbumin/D-dimer prognosis score
- AUC
area under the curve
- HR
hazard ratio
- CI
confidence interval.
Funding
The present study was supported by grants from Science and Technology Project of Henan (grant no. 182102310085) and the Scientific Research Team Construction Foundation of Henan Province (grant no. TD2011010).
Availability of data and materials
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
GSL and LBL designed the study. KS analyzed and interpreted the patient data, and was a major contributor in writing the manuscript. XRZ, ZYL, NS, LSG and YW analyzed and interpreted the patient data. XC, ZWZ, BHX and SXY performed the histological examination of the samples. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by The Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Written informed consent was obtained from all individual participants included in the study. The trial registration no. is 2018-LW-037 and this clinical trial was registered in the First Affiliated Hospital of Zhengzhou University Clinical Trial Registry in March 2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, et al. Melanoma staging: Evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JF, Soong SJ, Balch CM, Gershenwald JE, Ding S, Coit DG, Flaherty KT, Gimotty PA, Johnson T, Johnson MM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: An analysis of patients in the multi-institutional American joint committee on cancer melanoma staging database. J Clin Oncol. 2011;29:2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temam S, Mamelle G, Marandas P, Wibault P, Avril MF, Janot F, Julieron M, Schwaab G, Luboinski B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005;103:313–319. doi: 10.1002/cncr.20775. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Luo X, Huang H, Zhai Z, Shen Z, Lin H. Clinical characteristics of malignant melanoma in southwest China: A single-center series of 82 consecutive cases and a meta-analysis of 958 reported cases. PLoS One. 2016;11:e0165591. doi: 10.1371/journal.pone.0165591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28:96–102. doi: 10.1016/j.sder.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Hao M, Zhao G, Du X, Yang Y, Yang J. Clinical characteristics and prognostic indicators for metastatic melanoma: Data from 446 patients in north China. Tumor Biol. 2016;37:10339–10348. doi: 10.1007/s13277-016-4914-4. [DOI] [PubMed] [Google Scholar]
- 8.Iacono D, Basile D, Gerratana L, Vitale MG, Pelizzari G, Cinausero M, Poletto E, Puglisi F, Fasola G, Minisini AM. Prognostic role of disease extent and lymphocyte-monocyte ratio in advanced melanoma. Melanoma Res. 2019;29:510–515. doi: 10.1097/CMR.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 9.Daneshmandi S, Wegiel B, Seth P. Blockade of lactate dehydrogenase-a (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers (Basel) 2019;11:E450. doi: 10.3390/cancers11040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: From bench to bedside. Mol Aspects Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Mittman N, Avram MM, Oo KK, Chattopadhyay J. Prealbumin as an important predictor for survival and nutritional status in hemodialysis and peritoneal dialysis patients. In: Avram MM, editor. Improving Prognosis for Kidney Disorders. Springer; Dordrecht: 2002. pp. 61–67. [DOI] [Google Scholar]
- 12.Fan L, Wang X, Chi C, Wang Y, Cai W, Shao X, Xu F, Pan J, Zhu Y, Shangguan X, et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate. 2017;77:1233–1241. doi: 10.1002/pros.23381. [DOI] [PubMed] [Google Scholar]
- 13.Liu DQ, Li FF, Jia WH. Cumulative scores based on plasma D-dimer and serum albumin levels predict survival in esophageal squamous cell carcinoma patients treated with transthoracic esophagectomy. Chin J Cancer. 2016;35:11. doi: 10.1186/s40880-015-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao D, Wang Z, Cheng Y, Zhang H, Guo Q, Song Y, Zhu K, Li K, Liu D, Dang C. D-dimer: Not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One. 2014;9:e101125. doi: 10.1371/journal.pone.0101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batschauer AP, Figueiredo CP, Bueno EC, Ribeiro MA, Dusse LM, Fernandes AP, Gomes KB, Carvalho MG. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21:1267–1272. doi: 10.1093/annonc/mdp474. [DOI] [PubMed] [Google Scholar]
- 16.Kilic M, Yoldas O, Keskek M, Ertan T, Tez M, Gocmen E, Koc M. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis. 2008;10:238–241. doi: 10.1111/j.1463-1318.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 17.Altiay G, Ciftci A, Demir M, Kocak Z, Sut N, Tabakoglu E, Hatipoglu ON, Caglar T. High plasma D-dimer level is associated with decreased survival in patients with lung cancer. Clin Oncol (R Coll Radiol) 2007;19:494–498. doi: 10.1016/j.clon.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Mirshahi SS, Pujade-Lauraine E, Soria C, Mirshahi M, Fretault J, Bernadou A, Soria J. D-dimer and CA-125 levels in patients with ovarian cancer during antineoplastic therapy. Prognostic significance for the success of anti-cancer treatment. Cancer. 1992;69:2289–2292. doi: 10.1002/1097-0142(19920501)69:9<2289::AID-CNCR2820690914>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuurman MS, de Waal AC, Thijs EJM, van Rossum MM, Kiemeney LALM, Aben KKH. Risk factors for second primary melanoma among Dutch patients with melanoma. Br J Dermatol. 2017;176:971–978. doi: 10.1111/bjd.15024. [DOI] [PubMed] [Google Scholar]
- 21.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brożyna AA, Jóźwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget. 2016;7:17844–17853. doi: 10.18632/oncotarget.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brozyna AA, Jozwicki W, Carlson JA, Slominski AT. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum Pathol. 2013;44:2071–2074. doi: 10.1016/j.humpath.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Kim TK, Brożyna AA, Janjetovic Z, Brooks DL, Schwab LP, Skobowiat C, Jóźwicki W, Seagroves TN. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(Suppl 3):193–199. doi: 10.1007/s11739-012-0802-0. [DOI] [PubMed] [Google Scholar]
- 26.Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, Kattan J. The Therapeutic use of human albumin in cancer patients' management. Crit Rev Oncol Hematol. 2017;120:203–209. doi: 10.1016/j.critrevonc.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Xu H, Wu C, Wang W, Jin W, Gao H, Li H, Zhang S, Xu J, Zhang W, et al. Prognostic value of γ-glutamyltransferase-to-albumin ratio in patients with pancreatic ductal adenocarcinoma following radical surgery. Cancer Med. 2019;8:572–584. doi: 10.1002/cam4.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda S, Takeuchi H, Kawakubo H, Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T, Kitagawa Y. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: Comparison with the glasgow prognostic score. Ann Surg Oncol. 2015;22:302–310. doi: 10.1245/s10434-014-3857-5. [DOI] [PubMed] [Google Scholar]
- 29.Forrest LM, Mcmillan DC, Mcardle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desch A, Gebhardt C, Utikal J, Schneider SW. D-dimers in malignant melanoma: Association with prognosis and dynamic variation in disease progress. Int J Cancer. 2017;140:914–921. doi: 10.1002/ijc.30498. [DOI] [PubMed] [Google Scholar]
- 31.Kwon HC, Oh SY, Lee S, Kim SH, Han JY, Koh RY, Kim MC, Kim HJ. Plasma levels of prothrombin fragment F1+2, D-dimer and prothrombin time correlate with clinical stage and lymph node metastasis in operable gastric cancer patients. Jpn J Clin Oncol. 2008;38:2–7. doi: 10.1093/jjco/hym157. [DOI] [PubMed] [Google Scholar]
- 32.Deng W, Yu M, Ma H, Hu LA, Chen G, Wang Y, Deng J, Li C, Tong J, Wang DX. Predictors and outcome of patients with acute respiratory distress syndrome caused by miliary tuberculosis: A retrospective study in Chongqing, China. BMC Infect Dise. 2012;12:121. doi: 10.1186/1471-2334-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta M, Savage P, Lovato J, Schwartz GG. Serum calcium, albumin and tumor stage in cutaneous malignant melanoma. Future Oncol. 2016;12:2205–2214. doi: 10.2217/fon-2016-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Zhang N. Clinical and prognostic factors in 98 patients with malignant melanoma in China. J Int Med Res. 2017;45:1369–1377. doi: 10.1177/0300060517708922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raigani S, Cohen S, Boland GM. The role of surgery for melanoma in an era of effective systemic therapy. Curr Oncol Rep. 2017;19:17. doi: 10.1007/s11912-017-0575-8. [DOI] [PubMed] [Google Scholar]
- 36.Saranga-Perry V, Ambe C, Zager JS, Kudchadkar RR. Recent developments in the medical and surgical treatment of melanoma. Ca Cancer J Clin. 2014;64:172–185. doi: 10.3322/caac.21224. [DOI] [PubMed] [Google Scholar]
- 37.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 39.Slominski AT, Carlson JA. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clinic Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang K, Xu Y, Joseph R, Bagaria SP, Misra S, Chen Y. Comparative analysis of acral melanoma in Chinese and caucasian patients. Ann Surg Oncol. 2019;26:S158–S159. doi: 10.1155/2020/5169051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YS, Chen XX, Yang SX, Wu LS, Zhao JY, Li XY, Tu P, Li H. Preliminary exploration of the clinical features of Chinese patients with skin malignancies and premalignancies: A retrospective study of 1420 cases from peking university first hospital. J Eur Acad Dermatol Venereol. 2013;27:1114–1119. doi: 10.1111/j.1468-3083.2012.04673.x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, Schumacher SE, Wang C, Hu H, Liu J, et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell. 2018;33:676–689.e673. doi: 10.1016/j.ccell.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173:321–337.e310. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416.e411. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304.e296. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Q, Liang WW, Foltz SM, Mutharasu G, Jayasinghe RG, Cao S, Liao WW, Reynolds SM, Wyczalkowski MA, Yao L, et al. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep. 2018;23:227–238.e223. doi: 10.1016/j.celrep.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellrott K, Bailey MH, Saksena G, Covington KR, Kandoth C, Stewart C, Hess J, Ma S, Chiotti KE, McLellan M, et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 2018;6:271–281.e277. doi: 10.1016/j.cels.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, Craig DW, Adkins J, Cuyugan L, Manojlovic Z, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res. 2017;27:524–532. doi: 10.1101/gr.213348.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E, Ghosh P, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genetics. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, Place CS, Taylor-Weiner A, Whittaker S, Kryukov GV, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shain AH, Garrido M, Botton T, Talevich E, Yeh I, Sanborn JZ, Chung J, Wang NJ, Kakavand H, Mann GJ, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genetics. 2015;47:1194–1199. doi: 10.1038/ng.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.