Abstract
NAV 3 is a tumor suppressor of unknown function in leiomyomas. The objective of this study is to assess NAV3 expression and its potential role in human uterine leiomyomas. NAV3 protein expression was examined in patient leiomyoma and patient-matched myometrial tissue samples by Western blot and immunohistochemistry. NAV3 mRNA and protein expression was assessed in leuprolide acetate– and cetrorelix-treated cell line leiomyoma samples. RNAseq analysis of placebo-treated leiomyoma compared with myometrium demonstrated the presence of transcripts encoding for several neuronal proteins. For NAV3, RNA sequence analysis demonstrated decreased expression in leiomyoma as compared with myometrium (0.86 ± 0.03 fold). Presence of NAV3 mRNA was also decreased in leiomyoma surgical samples (0.43 fold ± 0.05, p = 0.026) compared with patient-matched myometrium. Confirmatory qRT-PCR results on immortalized leiomyoma and myometrial cell lines similarly demonstrated a decrease in expression of NAV3 in leiomyomas (0.28 ± 0.02, p = 0.00075). Immunohistochemical analysis demonstrated a significant decrease in NAV 3 protein in leiomyomas (H-score 154.7 ± 6.2) as compared with myometrium (H-score; 312.5 ± 14.7, p < 0.0001). Leuprolide acetate–treated leiomyoma cells demonstrated an increase in NAV 3 mRNA expression (1.53 ± 0.13, p < 0.0001). Similarly, Western blot analysis on leuprolide-treated leiomyoma cells showed a non-significant increase in NAV 3 protein expression (1.26 ± 0.09, p = 0.063). NAV 3, a tumor suppressor in numerous cancers, is decreased in leiomyoma cells and tissue compared with myometrium, and increased by GnRH analog treatment, suggesting that NAV3 may mediate steroid hormone–independent leiomyoma regulation by GnRH analogs.
Electronic supplementary material
The online version of this article (10.1007/s43032-019-00096-3) contains supplementary material, which is available to authorized users.
Keywords: Leiomyoma, Myometrium, NAV3, GnRH, Leuprolide acetate, Cetrorelix, Tumor suppressor gene
Introduction
Many factors potentially contribute to the pathology of leiomyomas, including hormonal, metabolic, dietary, and environmental factors. There has been increasing evidence suggesting that tumor suppressors may also play a role [1–4]. Tumor progression may be influenced by dysregulation of oncogenes or inactivation of these tumor suppressors. A recent study found the loss of tumor suppressor RE1 suppressing transcription factor/neuron-restrictive silencing factor (REST/NRSF), and the ensuing depression of GPR10 plays a role in the pathogenesis of uterine leiomyomas [1]. Additionally, the p53 network was found to be inactivated as a result of recurrent chromosomal translocations in leiomyomas with clonal chromosomal aberrations [2]. The transcriptional target of p53, cyclin G1, was elevated in leiomyoma as compared with patient-matched myometrium in a study performed by Baek and colleagues [3]. In addition, several tumor suppressor genes are abnormally hypermethylated in leiomyomas compared with adjacent myometrium [4]. The discovery of novel tumor suppressor genes may provide a greater understanding of the complex signaling pathways that underlie the development and progression of tumors.
NAV3 is a tumor suppressor gene which is expressed in both the central and peripheral nervous system; however, it is most commonly present in pyramidal neurons and cerebral frontal cortex tissue in the brain. It consists of 40 exons and is situated at chromosome band 12q21 [5]. NAV3 expression is altered in neural cancers and numerous other cancer-types [6–13]. In glial tumors, those with grade IV glioblastoma had significantly more NAV3 deletions than tumors with grades I, II, or III. In addition, NAV3 amplification showed better prognosis than those with normal NAV3 copy numbers [6]. Notably, NAV3 has been found to have altered expression in non-neural tumors as well. NAV3 copy number loss and corresponding absence of protein was found in 21% of the basal cell carcinoma and in 25% of squamous cell carcinoma specimens examined [7]. In colon cancer cells, NAV3 deletion and chromosome 12 polysomy were detected in 70% of microsatellite stable carcinomas (CRC) and 30% of adenomas. Additionally, Cohen and colleagues analyzed more than 2500 breast and lung cancer patients and found that decreased NAV3 was associated with shorter survival [13].
The mechanism of action of NAV3 is unknown; however, studies of NAV3 in colon cells demonstrated that NAV3 silencing induced an upregulation of interleukin 23 receptor (IL23R) and gonadotropin releasing hormone receptor (GnRHR) [8, 9]. It is known that leiomyoma cells have increased GnRHR activated signaling, resulting in activated β-catenin which has been postulated to contribute to tumor growth [14, 15]. Additionally, increased GnRHR activated signaling also increased T cell factors which contribute to inflammation and fibroid volume [16, 17]. The fact that NAV3 is related to GnRHR in other tissues provided the possibility that there was a relationship of NAV3 and GnRHR in the leiomyoma, a tissue which is known to have increased GnRHR signaling.
Prior to this study, NAV3 had been described primarily in relation to neuronal tissue and numerous tumors. The presence and function of this tumor suppressor had never been examined in leiomyomas. Through RNA sequencing we identified several neuronal genes which were aberrantly expressed in fibroids compared with myometrium. We chose to focus specifically on NAV3 because this neuronal gene was downregulated in leiomyoma in contrast to the other neuronal genes which were largely upregulated in leiomyoma. Additionally, we sought to examine NAV3 in the setting of fibroids because of its presence in numerous other tumor types, and because of its correlation with GnRHR pathways which are known to be upregulated in fibroids. We hypothesized that, as a tumor suppressor, NAV3 expression would be decreased in leiomyoma compared with patient-matched myometrium. A secondary aim of this study was to elucidate potential mechanisms involved in the NAV3 pathway. NAV3 gene silencing in colorectal tissue induces an upregulation in the GnRHR. Because leiomyomas express both GnRH and GnRHR and their expression is affected by GnRH agonists and antagonists such as leuprolide acetate (LA) and cetrorelix, we sought to examine the effect of these treatments on NAV3 expression in leiomyoma [18]. Given that NAV3 had not prior been examined in leiomyoma tissue, we were unsure if the initial stimulation of GnRH receptor (agonist) might have a different impact compared with the antagonist. Since both medications are clinical tools for leiomyoma treatment [19–21], we examined the impact of treatment with both agents on NAV3. We hypothesize that exposure to these medications would increase NAV3 expression.
Materials and Methods
Clinical Trial and Patient Tissue Collection
National Institutes of Health (NIH), National Institute of Child Health and Disease Branch (NICHD), performed a randomized control trial including 22 premenopausal women with symptomatic uterine leiomyomata [22, 23]. Women were assigned into one of three arms: CDB-2914 10 mg (ulipristal acetate 10 mg), CDB-2914 20 mg, or placebo for the equivalent of 3 menstrual cycles [22]. The same group published a second, randomized, double-blind, placebo-controlled, phase IIb study in 42 premenopausal women in which symptomatic uterine leiomyomata were randomized to receive placebo, CBD2914 10 mg, or 20 mg for 12 weeks (NCT00290251) [23]. Only the placebo myometrium and fibroid tissue samples from these studies were used to assess the presence of NAV3. Our laboratory performed RNA sequence (RNAseq) analysis on placebo-treated patient-matched leiomyoma and normal myometrium samples from the ulipristal (CBD-2914) clinical trials (Malik and Catherino, unpublished).
Tissue Culture
Studies were conducted with patient-matched myometrial and leiomyoma cells that had been immortalized using the human papillomavirus (HPV 16, E6/E7) [22, 23]. Cells were plated in 6-well plates at an initial concentration of 2 × 104 cells/well. Growth media was defined as Dulbecco’s Modified Eagle Medium (DMEM)/F12 media (Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS, Hyclone), as well as Normacin (Invivo Gen). Cell growth was maintained at 37 °C and 5% CO2 until 30% confluence had been achieved. Once the monolayer cultures reached 30% confluence, they were subsequently serum-starved for 24 h. Cells were then treated with either LA or cetrorelix at concentrations ranging from 10−9–10−6 M. The cells were exposed to fresh media supplemented with specific concentrations of LA or cetrorelix every other day. At the end of 120 h, the cells were at 85% confluence. After specified time points, the cells were collected for RNA or protein extraction for further analysis.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted from patient tissue and cell lines using the Ambion RNA Mini kit (Thermo Fisher Scientific) under the manufacturer’s protocol. DNAse I treatment was subsequently carried out using the Ambion Turbo DNA free-kit (Thermo Fisher Scientific). RNA was quantitated using the Nanodrop (Thermo-Fisher Scientific), aliquoted, and then stored at − 80 °C. Total RNA was converted into cDNA using the Bio-Rad iScript synthesis kit in Bio-Rad thermocycler. cDNA was diluted to 1:20 with diethylpyrocarbonate (DEPC)–treated water and stored at − 80 °C. Quantitative real-time PCR (qRT-PCR) was performed to determine the gene expression of NAV3 in placebo patient myometrium and leiomyoma tissue, and LA- and cetrorelix-treated myometrium and leiomyoma cell samples. Primer sequences for NAV3 were obtained from the published literature and are listed in supplemental Table 1 [8, 12]. Reactions were performed using a SYBR Green qRT-PCR, Master Mix reagent kit (BioRad), according to manufacturer’s protocol. Relative quantitation of target gene expression was calculated by the comparative cycle threshold (Ct) method [24]. The Ct values of each gene were normalized to 18S, and the resultant ratios were used to express fold difference of gene expression.
Protein Extraction and Western Blot
Protein was isolated using RIPA Lysis and Extraction Buffer (Pierce Biotechnology) containing Halt Protease Inhibitor Cocktail (Pierce Biotechnology), following our lab protocols [18]. Western blotting was performed with a SDS-PAGE electrophoresis system. Briefly 20–35-μg protein samples were resuspended in a reduced sample buffer, then electrophoresed on 4–15% Tris gels. Proteins were transferred to nitrocellulose membranes which were subsequently blocked with 5% nonfat milk in TBST (0.05% Tween 20 in 1x Tris-buffered saline) for 1 h. Nitrocellulose membranes were then probed with primary antibodies against anti-rabbit NAV3 (Sigma Aldrich N4288; 1:600, Abcam ab69868 1:200) overnight at 4 °C. A horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (Santa Cruz Biotechnology; sc-2004; 1:10,000) was then added, and secondary antibodies were detected through autoradiography using enhanced chemiluminescence. For normalization anti-rabbit Cox IV (Santa Cruz Biotechnology; sc-2414;1:1500) was used as an internal standard.
Immunohistochemistry
Rabbit anti-NAV3 antibody (Sigma Aldrich; HPA-32111; 1:200) was used to identify NAV3 in placebo leiomyoma and patient-matched myometrial samples using IHC in 14 patients. Tissues were embedded in paraffin and sectioned for IHC staining. Slides were deparaffinized in xylene and rehydrated in a series of sequential ethanol baths. Antigen retrieval was performed using 1x sodium citrate buffer (pH = 6.0, Thermo Fisher Scientific) and heated to 95 °C for a 45-min incubation in a vegetable steamer. Sections were blocked for 1 h in 1X TBS containing 3%/BSA/0.1% Tween-20 along with the appropriate non-immune serum. Next, tissue sections were incubated with 0.3% H2O2 in PBS for 1 h to inactivate tissue peroxidases, followed by a permeabilization step with 0.1% BSA. For NAV3 detection, tissue sections, after H2O2 treatment, underwent overnight incubation at 4 °C with anti-NAV3 primary antibody (Sigma Aldrich; HPA-32111; 1:200). After primary antibody incubation, the slides were washed and incubated in biotinylated anti-rabbit/mouse IgG (Vector Labs) followed by Elite™ ABC reagent (avidin-biotin peroxidase complex, Vector Labs). The peroxidase was then developed with the addition of DAB peroxidase substrate (3, 3′-diaminobenzidine). For positive controls, midbrain, thalamus, and hypothalamus samples were used. IHC images were acquired on Axioskop light microscope using AxioVision System software.
Statistical Analysis
For qRT-PCR data, the results are reported as mean ± SEM. For each result the average expression of three replicates was calculated before relative quantification, using normalization against the housekeeping gene (18S), was performed. For Western blot analysis, calculations were performed using Image Lab software v5.1 (Bio-Rad). Data are presented as mean fold difference between relative density units of treated and untreated leiomyoma samples, or leiomyoma and myometrial samples, and were corrected for the internal controls. The Wilcoxon signed rank test was used for nonparametric statistical evaluation. Statistical p values below 0.05 were considered significant.
For IHC, results were analyzed qualitatively and quantitatively using the H-score method. Triplicate images for each slide were de-identified and presented to six physicians who underwent general training on interpretation of IHC. Each image was graded from 0 to 4 on investigated parameters. Averages, SD, and H-scores were calculated for each individual slide and for total myometrial and leiomyoma slides. The H-score for each slide was assigned using the following formula:
where “Ʃ” equals the total of the numbers recorded by each of the observers for each of the slides evaluated. A final score gives a range from 0 to 400 for each respective slide. Data was analyzed using the Student t test in the GraphPad program.
Results
NAV3 RNA Sequencing Data Analysis
RNASeq data analysis (Malik and Catherino, unpublished) was employed in the discovery phase to note differential gene expression between fibroid and patient-matched myometrium. The presence of NAV3 was examined in leiomyoma and myometrium, using only placebo-treated leiomyoma and myometrial patient samples from the ulipristal acetate trial [ 22, 23]. The RNASeq analysis demonstrated expression of transcripts encoding neuronal proteins including NAV3, and also demonstrated the presence of several transcripts encoding other neuronal proteins (Supplementary Table 2). The presence of neuronal genes in leiomyoma was an unexpected finding, as neuronal gene products were not historically described in uterine tissue. Additionally, most neuronal transcripts were found to be increased in leiomyoma compared with myometrium, with the exception of NAV3. Unlike the other neuronal transcripts, unpaired analysis of placebo patient data demonstrated decreased expression (0.86 ± 0.03 fold) of NAV3 transcripts in leiomyoma as compared with myometrium.
NAV3 mRNA Expression in Cell Line and Patient Tissue Samples
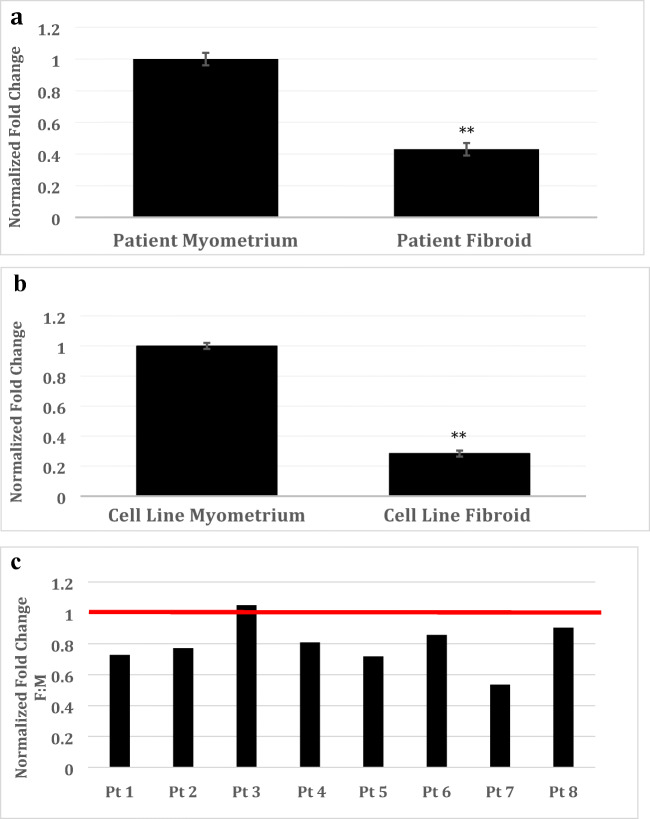
We then performed confirmatory studies on the expression of NAV3 using qRT-PCR, on patient tissue samples and immortalized leiomyoma and myometrial cell lines. As observed in Fig. 1A, leiomyoma patient tissue samples demonstrated a greater than 2-fold downregulation in NAV-3 transcripts compared with myometrium (n = 10, 0.43 ± 0.05-fold, p < 0.01). Immortalized leiomyoma cell lines demonstrated a greater than 3-fold downregulation (0.28 ± 0.02-fold, p < 0.0001) of NAV3 transcripts when compared with myometrium cells (Fig. 1B; 0.28 ± 0.02-fold, p < 0.0001).
Fig. 1.
NAV3 mRNA and protein expression in untreated cell line and surgical samples. Relative expression of NAV3 mRNA was decreased in leiomyoma cells as compared against myometrial cells from patient tissue (n = 14 patient-matched leiomyoma and myometrium pairs) (A). The same was found in tissue samples from cell line leiomyoma as compared against myometrium (B). Results are presented as fold change with non-targeting controls (NTC) as the reference, mean ± SEM of three independent experiments. *Statistically significant difference, p < 0.05. In the majority of patients, NAV3 protein was decreased in leiomyomas with the exception of one patient, in which the result found similar protein expression in both leiomyoma and myometrial samples. NAV3 Western data are presented for each individual patient to best illustrate this heterogeneity. Data shown represent mean ± standard error of mean (SEM) fold. Results were normalized to CoxIV (p = 0.0009) (C)
NAV3 Protein Expression in Patient Tissue Samples
As demonstrated in Fig. 1C, a statistically significant decrease in NAV3 protein was observed in leiomyoma tissue as compared with patient-matched myometrium (n = 8, 0.78 ± 0.15, p < 0.001). Given the heterogeneity of the patient population, there were some outliers resulting in a skew of the data. In the majority of patients, NAV3 was decreased in leiomyoma (0.53 ± 0.14 to 0.90 ± 0.14-fold) with the exception of one patient, in which no significant difference was observed in NAV3 protein amount. NAV3 Western data is presented for each individual patient to best illustrate this heterogeneity in Fig. 1C.
NAV 3 Protein Expression by Immunohistochemistry in Patient Tissue Samples
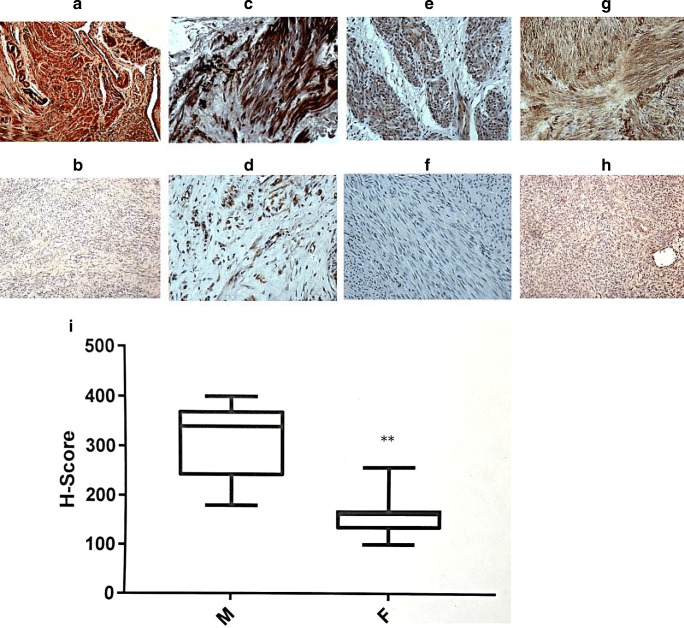
For spatial distribution of NAV3 we performed IHC analysis. As NAV3 had not been previously studied in uterine tissue its cellular location is unknown. Oil immersion microscopy localized NAV3 to the cytoplasm of myometrial cells in tissue samples (Supplemental Fig. 1). As observed in Fig. 2A–H, IHC demonstrated a qualitative decrease in NAV3 protein in leiomyoma as compared with myometrium microscopically by visual examination in morphologic appearance and staining intensity (n = 14). Four representative paired patient samples are presented and demonstrated an increased staining intensity in myometrial samples (A, C, E, G) compared with patient-matched fibroid samples (B, D, F, H). As observed in Fig. 2I, results from unpaired analysis (n = 14) were quantitated using the H-score method and demonstrated a statistically significant decrease (p < 0.0001) in NAV3 expression in leiomyoma (L; 154.7 ± 6.2) patient samples compared with myometrium (M; 312.5 ± 14.7).
Fig. 2.
NAV3 protein expression by immunohistochemistry in leiomyoma and adjacent myometrial surgical specimens. Representative sample of leiomyoma surgical specimen and the adjacent myometrium in four representative patient pairs: patient 1 myometrium (A), patient 1 leiomyoma (B); patient 2 myometrium (C), patient 2 leiomyoma (D); patient 3 myometrium (E), patient 3 leiomyoma (F); patient 4 myometrium (G), patient 4 leiomyoma (H). Images were obtained at × 20 magnification, demonstrating more prominent NAV3 staining (brown staining) in myometrial specimens as compared with leiomyoma specimens. Specimens (n = 14 patient-matched leiomyoma and myometrial pairs) were quantitated using the H-score method, and demonstrated a quantitative decrease in leiomyoma compared with myometrium (H-score 154.7 ± 6.2 vs 312.5 ± 14.7, P < 0.0001)(I)
NAV3 mRNA and Protein Expression in Leuprolide Acetate–Treated Cell Line Leiomyoma Samples
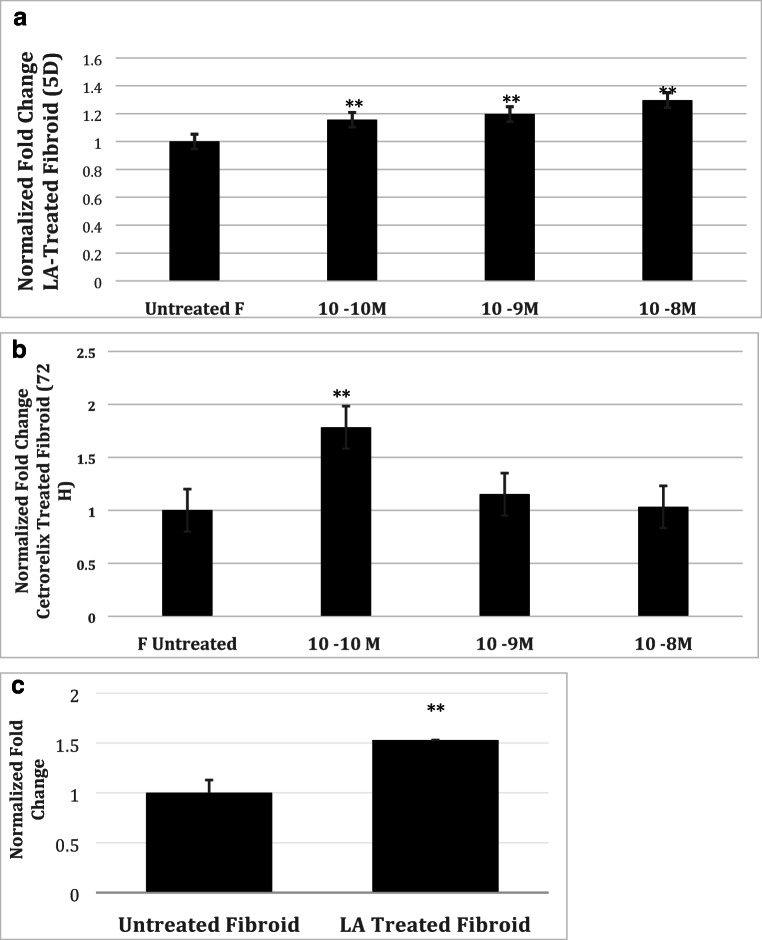
In order to determine potential mechanisms involved in the NAV3 pathway in leiomyomas, we sought to examine the effect of GNRH agonist treatments on NAV3 expression. As observed in Fig. 3C, LA-treated leiomyoma cells demonstrated an increase in NAV3 mRNA expression (1.53 ± 0.13-fold, p < 0.0001). Similarly, Western blot analysis on LA-treated leiomyoma cells at 120 h demonstrated a concentration-dependent increase in NAV 3 protein expression compared with untreated tissue (1.30 ± 0.07 fold change at 10−8 M, 1.20 ± 0.07 fold change at 10−9 M, 1.15 ± 0.07 fold change at 10−10 M, p = 0.063, Fig. 3A). At 24, 48, and 72 h of treatment there were no significant changes in NAV3 concentration (data not shown).
Fig. 3.
NAV3 mRNA and protein expression in leuprolide acetate– and cetrorelix-treated leiomyoma cells. NAV3 mRNA expression was found to be increased in treated leiomyoma compared against untreated leiomyoma with 5 days of LA treatment (A). Western blot analysis of NAV3 expression in treated leiomyoma cells (× 2 biologic replicates). Five-day treatment with LA increases NAV3 expression in leiomyoma (B). 72-h treatment with cetrorelix increases NAV3 protein expression in leiomyoma at 10−10 M concentration (C). Results were normalized to CoxIV. Data shown represent mean fold ± SD
NAV3 Protein Expression in Cetrorelix-Treated Cell Line Leiomyoma Samples
We then hypothesized that treatment with a pure GnRH antagonist would have a similar effect. As observed in Fig. 3B, Western blot analysis of untreated leiomyoma cells and cetrorelix-treated leiomyoma cells revealed an expected increase in NAV3 protein; however, this effect was most pronounced at lower concentrations (1.78 ± 0.40-fold change at 10−10 M, p = 0.017).
NAV3 Protein Expression in Cetrorelix-Treated Myometrial Cell Line
To examine if treatment with GnRH antagonists had the same effect on NAV3 protein concentration in the myometrium compared with NAV3 in the leiomyoma, we examined the protein expression of cetrorelix-treated myometrium compared with untreated myometrium. Results demonstrated that cetrorelix largely had no effect on NAV3 in the myometrium (0.917 ± 0.16 at 10−9 M and 0.958 ± 0.16 at 10−10 M p = 0.44 (data not shown)). This suggests that GnRH antagonist medications preferentially correct the aberrant NAV3 expression in leiomyoma and exert no effect on the adjacent myometrium.
Discussion
In the present study we demonstrated that NAV3, a neuronal tumor suppressor, is present in myometrial and leiomyoma tissue. Furthermore, we confirmed the hypothesis that, as a tumor suppressor, NAV3 mRNA and protein were decreased in leiomyoma compared with myometrium by qRT-PCR, Western blotting, and IHC. In an attempt to elucidate the mechanism of action and function of NAV3 in leiomyoma, attention was turned to prior studies performed in other tissue. Studies performed in colon cells, glioblastoma multiforme cells, and keratinocytes suggest that NAV 3 gene silencing by targeted siRNA induced an upregulation in GnRHR pathways (fold change > 14 in all CRC cell lines examined) [8, 9]. We postulated that decreased or aberrant NAV3 expression in leiomyomas may induce an upregulation of GnRHR as it does in other tissues. Because leiomyomas express both GnRH and GnRHR, and their expression is affected by GnRH agonists such as leuprolide acetate (LA) and antagonists such as cetrorelix, we sought to examine this hypothesis by assessing the effect of these treatments on NAV3 expression in leiomyoma [16–21]. Our results showed that treatment with both resulted in increased NAV3 mRNA and protein expression in leiomyoma, potentially restoring the tumor-suppressive functionality of NAV3 in leiomyomas.
It is known that in a typical leiomyoma cell, GnRHR related pathway signaling is present and functional which contributes, in part, to increased tumor volume. Upregulated GnRHR signaling results in this increased tumor volume via mediators such as β-catenin, and T cell factors. Upregulated GnRHR results in upregulation of T cell factors which increase inflammation, resulting in increased fibroid volume [14–16]. Additionally, upregulated GnRHR activates β-catenin. The role of B catenin in fibroid development is supported by a murine study in which selective overexpression of constitutively activated β-catenin in uterine mesenchyme during embryonic development and in adults, gives rise to leiomyoma-like tumors in the uterus of all female mice. This study supports that signaling by WNT/β-catenin seems to play a role in somatic stem cell function in the myometrium and uterine leiomyoma tissue [25].
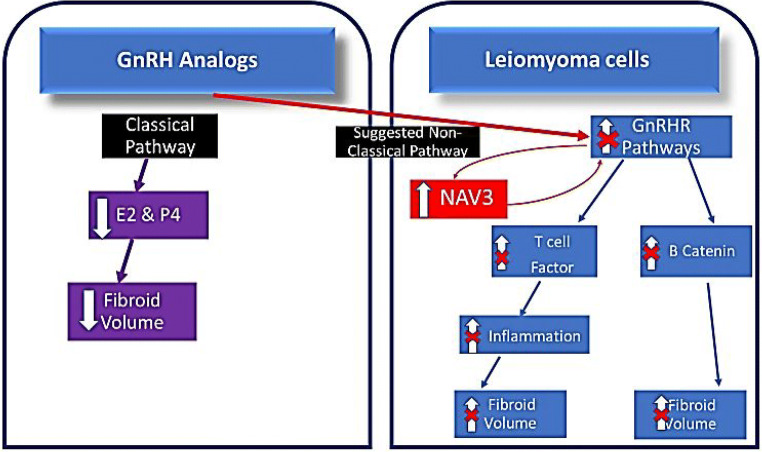
Based on our findings we propose a mechanism for potential NAV3 functioning in the fibroid (Fig. 4). With LA or cetrorelix treatment, GnRH receptors become downregulated over time [18, 26]. Once these receptors are downregulated, GnRHR-mediated processes decrease, potentially contributing to a decrease in leiomyoma volume [26]. When analogs bind to their receptor, in addition to downregulating these GnRHR pathways, binding also results in increased NAV3. Based on NAV3 functioning in other tissues, the increase in NAV3 may affect the GnRHR pathway signaling through the mechanism of a feedback loop, ultimately resulting in decreased GnRHR-mediated tumor growth. It is currently unknown if NAV3 is a byproduct of GnRHR pathway signaling or if it is a mediator. NAV3 silencing and/or overexpression studies are needed to better characterize the effect of NAV3 on these downstream components of the GnRHR pathways in the leiomyoma. Additionally, assessing the impact of NAV3 overexpression on extracellular matrix proteins will delineate if NAV3 is involved in the development of the leiomyoma phenotype.
Fig. 4.
Proposed GnRH-analog effect on NAV3 and resultant effect on leiomyoma development. It has prior been established that in a typical leiomyoma cell, GnRHR related pathway signaling is upregulated. This results in activated b-catenin contributing to tumor growth. Additionally, activated GnRHR pathways in the leiomyoma include upregulation of T cell factors which increase inflammation and also contribute to fibroid volume. With GnRH analog treatment GnRH receptors become downregulated, resulting in decreased GnRHR-mediated processes. We discovered that GnRH analogs increase NAV3 expression in fibroids. Based on NAV3 functioning in other tissues, the increase in NAV3 may affect the GnRHR pathway signaling through the mechanism of a feedback loop, ultimately resulting in decreased GnRHR-mediated tumor growth. It is currently unknown if NAV3 is a byproduct of GnRHR pathway signaling or if it is a mediator. Currently, the most commonly accepted mechanism of action of GnRH analogs on leiomyoma is through the pituitary-mediated decrease in estrogen and progesterone. This represents the canonical or known pathway. Our results highlight the potential hormone-independent mechanisms, for how GnRH analogs decrease fibroid volume, by downregulated GnRHR pathways which may be mediated by an increase in NAV3, (non-canonical pathway)
Currently, the most commonly accepted mechanism of action of GnRH analogs on leiomyoma is through the pituitary-mediated decrease in estrogen and progesterone. This represents the canonical or known pathway. Our results highlight the potential gonadal hormone-independent mechanisms, for how GnRH analogs decrease fibroid volume, by downregulated GnRHR pathways which may be mediated by an increase in NAV3 (non-canonical pathway).
Based on our hypothesis, GnRHR pathways contribute to leiomyoma growth and NAV3 may mediate this process. In addition to uterine tumors, there is evidence suggesting that GnRHR/GnRH signaling pathways contribute to the development of numerous extra pituitary tumors [17, 27–36]. For example, in ovarian cancer, there is an extensive presence (80%) of GnRH binding sites on ovarian cancer biopsy samples, supporting the involvement of GnRH signaling and a possible autocrine loop [35]. GnRHR has also been identified in 80% of malignant prostate tumors, and hormone-responsive prostate cancers are successfully treated with GnRH-a [36].
NAV3 may also play a role in cell-cell matrix adhesion and in actin cytoskeleton tethering. Members of the neuron navigator family contain various functional domains including calponin homology domain, four coiled-coil domains and cytoskeletal interacting domains [37]. These domains were shown to be involved in a variety of cellular processes, including microtubule dynamics and cell migration. In animal studies members of this family were shown to affect cell migration by adjusting microtubule dynamics [38, 39]. Additionally, animal studies have found members of the neuron navigator family to regulate the cytoskeleton [40]. The actin cytoskeleton plays a role in cell migration and is known to be an aberrant pathway in leiomyomas. Studies on leiomyomas have shown altered communication between the external mechanical environment and reorganization of the actin cytoskeleton mediated by RhoA [41]. Therefore, in addition to potentially affecting GnRHR pathways, NAV3 may also contribute to leiomyoma pathology by adjusting microtubule dynamics, cell migration, and actin cytoskeleton tethering. Additional studies on NAV3 functioning in leiomyoma are required to assess these hypotheses as they are currently based on NAV3 functioning in other tissues.
In summary, NAV3, a tumor suppressor in numerous cancers, is decreased in leiomyoma cells compared with myometrium, and increased by GnRH agonist and antagonist treatment. Currently, the most commonly accepted mechanism of action of GnRH agonist and antagonist treatment on leiomyoma is through the pituitary-mediated decrease in estrogen and progesterone. This study not only identifies a novel tumor suppressor in leiomyomas but also suggests that NAV3 may mediate hormone-independent effects of GnRH-a treatment on leiomyoma. NAV3 is increased upon GnRH treatment, which may result in the downregulation of GnRHR pathways, ultimately decreasing tumor size. Additionally, NAV3 may also play a role in cell-cell matrix adhesion and the actin cytoskeleton. Further studies are required to confirm a direct effect of NAV3 on the GnRHR in leiomyoma, and to elucidate the upstream and downstream regulators of NAV3 expression in leiomyoma tissue.
Electronic supplementary material
(DOCX 458 kb)
Acknowledgments
The generous gift of all placebo and ulipristal-treated human tissue was provided by Dr. Lynnette Nieman, as part of clinical trial NCT00290. Technical assistance by the Biomedical Instrumentation Center was critical for completion of the project.
References
- 1.Varghese BV, Koohestani F, McWilliams M, Colvin A, Gunewardena S, Kinsey WH, et al. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci. 2013;110:2187–2192. doi: 10.1073/pnas.1215759110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids—their relationship to cytogenetic subgroups. Int J Cancer. 2012;131:1528–1536. doi: 10.1002/ijc.27424. [DOI] [PubMed] [Google Scholar]
- 3.Baek WK, Kim D, Jung N, Yi YW, Kim JM, Cha SD, Bae I, Cho CH. Increased expression of cyclin G1 in leiomyoma compared with normal myometrium. Am J Obstet Gynecol. 2003;188:634–639. doi: 10.1067/mob.2003.140. [DOI] [PubMed] [Google Scholar]
- 4.Navarro A, Yin P, Monsivais D, Lin SM, Du P, Wei JJ, et al. Genome-wide DNA methylation indicates silencing of tumor suppressor genes in uterine leiomyoma. PLoS One. 2012;7:e33284. doi: 10.1371/journal.pone.0033284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coy JF, Wiemann S, Bechmann I, Bächner D, Nitsch R, Kretz O, Christiansen H, Poustka A. Pore membrane and/or filament interacting like protein 1 (POMFIL1) is predominantly expressed in the nervous system and encodes different protein isoforms. Gene. 2002;290:73–94. doi: 10.1016/S0378-1119(02)00567-X. [DOI] [PubMed] [Google Scholar]
- 6.Bleeker FE, Lamba S, Rodolfo M, Scarpa A, Leenstra S, Vandertop WP, et al. Mutational profiling of cancer candidate genes in glioblastoma, melanoma and pancreatic carcinoma reveals a snapshot of their genomic landscapes. Hum Mutat. 2009;30:E451–E459. doi: 10.1002/humu.20927. [DOI] [PubMed] [Google Scholar]
- 7.Maliniemi P, Carlsson E, Kaukola A, Ovaska K, Niiranen K, Saksela O, Jeskanen L, Hautaniemi S, Ranki A. NAV3 copy number changes and target genes in basal and squamous cell cancers. Exp Dermatol. 2011;20:926–931. doi: 10.1111/j.1600-0625.2011.01358.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson E, Ranki A, Sipilä L, Karenko L, Abdel-Rahman WM, Ovaska K, et al. Potential role of a navigator gene NAV3 in colorectal cancer. Brit J Cancer. 2012;106:517. doi: 10.1038/bjc.2011.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson E, Krohn K, Ovaska K, Lindberg P, Häyry V, Maliniemi P, et al. Neuron navigator 3 alterations in nervous system tumors associate with tumor malignancy grade and prognosis. Genes Chromosom Cancer. 2013;52:191–201. doi: 10.1002/gcc.22019. [DOI] [PubMed] [Google Scholar]
- 10.Karenko L, Hahtola S, Päivinen S, Karhu R, Syrjä S, Kähkönen M, Nedoszytko B, Kytölä S, Zhou Y, Blazevic V, Pesonen M, Nevala H, Nupponen N, Sihto H, Krebs I, Poustka A, Roszkiewicz J, Saksela K, Peterson P, Visakorpi T, Ranki A. Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue. Cancer Res. 2005;65:8101–8110. doi: 10.1158/0008-5472.CAN-04-0366. [DOI] [PubMed] [Google Scholar]
- 11.Hahtola S, Burghart E, Puputti M, Karenko L, Abdel-Rahman WM, Väkevä L, Jeskanen L, Virolainen S, Karvonen J, Salmenkivi K, Kinnula V, Joensuu H, Peltomäki P, Klein CA, Ranki A. Cutaneous T-cell lymphoma-associated lung cancers show chromosomal aberrations differing from primary lung cancer. Genes Chromosom Cancer. 2008;47:107–117. doi: 10.1002/gcc.20513. [DOI] [PubMed] [Google Scholar]
- 12.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Dvashi H, Ben-Chetrit N, Russell R, Carvalho S, Lauriola M, Nisani S, et al. Navigator-3, a modulator of cell migration, may act as a suppressor of breast cancer progression. EMBO Mol Med. 2015;7:299–314. [DOI] [PMC free article] [PubMed]
- 14.Ou G, Weaver VM. Tumor-induced solid stress activates β-catenin signaling to drive malignant behavior in normal, tumor-adjacent cells. BioEssays. 2015;37:1293–1297. doi: 10.1002/bies.201500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanwar PS, Lee HJ, Zhang L, Zukerberg LR, Taketo MM, Rueda BR, Teixeira JM. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275:5479–5495. doi: 10.1111/j.1742-4658.2008.06677.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheng KW, Leung PC. The expression, regulation and signal transduction pathways of the mammalian gonadotropin-releasing hormone receptor. Can J Physiol Pharmacol. 2000;78:1029–1052. doi: 10.1139/y00-096. [DOI] [PubMed] [Google Scholar]
- 18.Britten JL, Malik M, Levy G, Mendoza M, Catherino WH. Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production. Fertil Steril. 2012;98:1299–1307. doi: 10.1016/j.fertnstert.2012.07.1123. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y, Nakamura G, Matsuguchi H, Nozaki M, Sano M, Nakano H. Efficacy of a low-dose leuprolide acetate depot in the treatment of uterine leiomyomata in Japanese women. Fertil Steril. 1992;58:66–71. doi: 10.1016/S0015-0282(16)55138-3. [DOI] [PubMed] [Google Scholar]
- 20.Felberbaum RE, Germer U, Ludwig M, Riethmüller-Winzen H, Heise S, Buttge I, Bauer O, Reissmann T, Engel J, Diedrich K. Treatment of uterine fibroids with a slow-release formulation of the gonadotrophin releasing hormone antagonist Cetrorelix. Hum Reprod. 1998;13:1660–1668. doi: 10.1093/humrep/13.6.1660. [DOI] [PubMed] [Google Scholar]
- 21.Engel JB, Audebert A, Frydman R, Zivny J, Diedrich K. Presurgical short term treatment of uterine fibroids with different doses of cetrorelix acetate: a double-blind, placebo-controlled multicenter study. Eur J Obstet Gynecol Reprod Biol. 2007;134:225–232. doi: 10.1016/j.ejogrb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril. 2011;95:767–772. doi: 10.1016/j.fertnstert.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono M, Yin P, Navarro A, Moravek MB, Druschitz SA, Gottardi CJ, et al. Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil Steril. 2014;101:1441–1449. doi: 10.1016/j.fertnstert.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiesel LA, Rody A, Greb RR, Szilagyi A. Clinical use of GnRH analogues. Clin Endocrinol. 2002;56:677–687. doi: 10.1046/j.1365-2265.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 27.Everest HM, Hislop JN, Harding T, Uney JB, Flynn A, Millar RP, et al. Signaling and antiproliferative effects mediated by GnRH receptors after expression in breast cancer cells using recombinant adenovirus. Endocrinol. 2001;142:4663–4672. doi: 10.1210/endo.142.11.8503. [DOI] [PubMed] [Google Scholar]
- 28.Grundker C, Schlotawa L, Viereck V, Emons G. Protein kinase C-independent stimulation of activator protein-1 and c-Jun N-terminal kinase activity in human endometrial cancer cells by the LHRH agonist triptorelin. Eur J Endocrinol. 2001;145:651–658. doi: 10.1530/eje.0.1450651. [DOI] [PubMed] [Google Scholar]
- 29.Kraus S, Naor Z, Seger R. Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor. Arch Med Res. 2001;32:499–509. doi: 10.1016/S0188-4409(01)00331-9. [DOI] [PubMed] [Google Scholar]
- 30.Kang SK, Choi KC, Yang HS, Leung PC. Potential role of gonadotrophin-releasing hormone (GnRH)-I and GnRH-II in the ovary and ovarian cancer. Endocr Relat Cancer. 2003;10:169–177. doi: 10.1677/erc.0.0100169. [DOI] [PubMed] [Google Scholar]
- 31.Yin H, Cheng KW, Hwa HL, Peng C, Auersperg N, Leung PC. Expression of the messenger RNA for gonadotropin-releasing hormone and its receptor in human cancer cell lines. Life Sci. 1998;62:2015–2023. doi: 10.1016/S0024-3205(98)00173-8. [DOI] [PubMed] [Google Scholar]
- 32.Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun. 1992;189:289–295. doi: 10.1016/0006-291X(92)91556-6. [DOI] [PubMed] [Google Scholar]
- 33.Imai A, Ohno T, Iida K, Fuseya T, Furui T, Tamaya T. Gonadotropin-releasing hormone receptor in gynecologic tumors. Frequent expression in adenocarcinoma histologic types. Cancer. 1994;74:2555–2561. doi: 10.1002/1097-0142(19941101)74:9<2555::AID-CNCR2820740925>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Imai A, Ohno T, Iida K, Fuseya T, Furui T, Tamaya T. Presence of gonadotropin-releasing hormone receptor and its messenger ribonucleic acid in endometrial carcinoma and endometrium. Gynecol Oncol. 1994;55:144–148. doi: 10.1006/gyno.1994.1264. [DOI] [PubMed] [Google Scholar]
- 35.Imai A, Ohno T, Ohsuye K, Tamaya T. Expression of gonadotropin-releasing hormone receptor in human epithelial ovarian carcinoma. Ann Clin Biochem. 1994;31:550–555. doi: 10.1177/000456329403100604. [DOI] [PubMed] [Google Scholar]
- 36.Choi S, Lee AK. Efficacy and safety of gonadotropin-releasing hormone agonists used in the treatment of prostate cancer. Drug Healthc Patient Saf. 2011;3:107–119. doi: 10.2147/DHPS.S24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muley PD, McNeill EM, Marzinke MA, Knobel KM, Barr MM, Clagett-Dame M. The atRA-responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation. Develop Neurobiol. 2008;68(13):1441–1453. doi: 10.1002/dneu.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt KL, Marcus-Gueret N, Adeleye A, Webber J, Baillie D, Stringham EG. The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development. 2009;136(4):563–574. doi: 10.1242/dev.016816. [DOI] [PubMed] [Google Scholar]
- 39.Stringham E, Pujol N, Vandekerckhove J, Bogaert T. unc-53 controls longitudinal migration in C. elegans. Development. 2002;129(14):3367–3379. doi: 10.1242/dev.129.14.3367. [DOI] [PubMed] [Google Scholar]
- 40.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10(7):765. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 41.Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 31(1):57–65. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 458 kb)