Abstract
Aims
Mammalian target of rapamycin (mTOR) inhibitors used in drug-eluting stents (DES) to control restenosis have been found to delay endothelialization and increase incidence of late-stent thrombosis through mechanisms not completely understood. We revealed that mTOR inhibition (mTORi) upregulated the expression of cell growth suppressor IRF-1 in primary human arterial endothelial cells (HAEC). This study aimed to examine how mTOR-regulated IRF-1 expression contributes to the suppressive effect of mTORi on arterial endothelial proliferation.
Methods and Results
Western blotting, quantitative PCR, and a dual-luciferase reporter assay indicated that mTOR inhibitors rapamycin and torin 1 upregulated IRF-1 expression and increased its transcriptional activity. IRF-1 in turn contributed to the suppressive effect of mTORi by mediating HAEC apoptosis and cell cycle arrest in part through upregulation of caspase 1 and downregulation of cyclin D3, as revealed by CCK-8 assay, Annexin V binding assay, measurement of activated caspase 3, BrdU incorporation assay, and matrigel tube formation assay. In a mouse model of femoral artery wire injury, administration of rapamycin inhibited EC recovery, an effect alleviated by EC deficiency of IRF-1. Chromatin immunoprecipitation assay with HAEC and rescue expression of wild type or dominant-negative IRF-1 in EC isolated from Irf1−/− mice confirmed transcriptional regulation of IRF-1 on the expression of CASP1 and CCND3. Furthermore, mTORi activated multiple PKC members, among which PKCζ was responsible for the growth-inhibitory effect on HAEC. Activated PKCζ increased IRF1 transcription through JAK/STAT-1 and NF-κB signaling. Finally, overexpression of wild type or mutant raptor incapable of binding mTOR indicated that mTOR-free raptor contributed to PKCζ activation in mTOR-inhibited HAEC.
Conclusions
The study reveals an IRF-1-mediated mechanism that contributes to the suppressive effects of mTORi on HAEC proliferation. Further study may facilitate the development of effective strategies to reduce the side effects of DES used in coronary interventions.
Keywords: endothelium, cell proliferation, transcription factor, drug-eluting stents, mTOR
1. Introduction
Endovascular implantation of drug-eluting stents (DES) represents one of the most effective therapies for treating coronary artery lesions. Compared with conventional bare metal stents (BMS), DES significantly alleviates in-stent restenosis through suppression of smooth muscle cell (SMC) proliferation and migration. However, due to the non-specific inhibition of both SMC and endothelial cells (EC) lining the artery, DES may promote adverse arterial responses such as delayed endothelialization, thus increasing the incidence of late-stent thrombosis, which has been observed as long as five years after placement. Though relatively uncommon, when present it significantly raises the risk of heart attack and mortality [1–3]. This highlights the necessity to develop strategies to specifically promote endothelial proliferation and thereby enhance endothelialization of the stented artery.
Rapamycin and its analogue everolimus, are the predominant anti-proliferation agents used in DES. Rapamycin (also known as sirolimus), was isolated as a natural anti-inflammatory macrolide antibiotic [4]. Its known target, mammalian target of rapamycin (mTOR), is an evolutionarily conserved serine/threonine kinase [5]. It interacts with several proteins to form two complexes, mTOR complex 1 (mTORC1) and mTORC2. In addition to mTOR, mTORC1 contains raptor and pras40, and controls protein synthesis, cell proliferation and growth through phosphorylation of downstream effectors S6 kinase 1 and 4E-binding protein-1 (4EBP-1). Rictor, mSin1 and protor1/2 are specific to mTORC2, which is responsible for the regulation of cytoskeletal organization and cell survival through activation of the AGC kinase subfamily such as protein kinase Cα (PKCα), protein kinase B (AKT), and serum- and glucocorticoid-induced kinase 1 (SGK1).
Consistent with the involvement of mTOR signaling in regulating physiological and pathological cellular proliferation, mTOR inhibitors cause cell cycle arrest [6, 7] or apoptosis [8, 9] through different mechanisms, and have been widely applied in the intervention of diseases. In addition to prevention of coronary artery in-stent restenosis, rapamycin has been used as an immunosuppressant in autoimmune diseases or for treatment of cancer.
Rapamycin, binds to FK-506-binding protein 12 (FKBP12) inside the cell and acutely inhibits mTORC1 by disrupting the association between mTOR and raptor [10]. It has been recognized that chronic treatment with rapamycin also disrupts mTOR2 signaling [11]. In addition to rapamycin, a new class of ATP-competitive mTOR inhibitors, such as torin 1, which inhibit both mTOR complexes have been synthesized and approved for clinical trials for cancer [12].
Interferon regulatory factor 1 (IRF-1) was originally identified as a type I interferon-activating transcription factor. IRF-1 protein is constitutively expressed at a low level in the cells with a short half-life; however, upon a variety of stimuli such as cytokines, infections and IFNs, its expression is dramatically upregulated, mainly at the transcriptional level [13]. Studies in tumor cells have identified IRF-1 as a growth suppressor that targets a set of genes associated with regulation of cell cycle and apoptosis. For example, IRF-1 was reported to upregulate expression of growth-suppressive genes such as p21 [14, 15], p27 [15], caspase1 [15, 16], and p53 upregulated modulator of apoptosis (PUMA) [17], and to downregulate growth-promoting genes such as cyclin D1 [18], CDK4 [18], CDK2 [19] and surviving [14]. However, the role of IRF-1 in eliciting EC suppression caused by mTOR inhibition (mTORi) has never been reported, and could have implications for understanding and preventing stent failure.
Recently, we reported that mTORi upregulated IRF-1 expression in cytokine-stimulated primary human arterial endothelial cells (HAEC) [20]. Here, we have tested the hypothesis that IRF-1 upregulation mediates the anti-proliferative effects of mTORi on HAEC. We reveal that mTORi activated PKCζ, which transactivated IRF1 through STAT-1 and NF-κB pathways. Increased IRF-1 in turn elicited apoptosis and cell cycle suppression in HAEC by regulating caspase 1 and cyclin D3 expression, respectively. These results may elucidate new targets to reduce the unintended effects of rapamycin on endothelium associated with complications of DES.
2. Methods
Detailed methods and materials are available in the online supplementary file.
2.1. Cell culture and treatment
Primary HAEC were purchased from the American Type Culture Collection (Manassas, VA) and maintained in Endothelial Growth Medium-2 (Lonza) supplemented with 10% fetal bovine serum (FBS) (Gibco). Experiments were performed with cells from passage 4 to 9. Unless otherwise noted, HAEC without serum starvation were incubated with 1–10nM mTOR inhibitors rapamycin (Sigma-Aldrich) or torin 1 (Cell Signaling Technology) for 2h for real-time PCR to measure mRNA, for 4h for Western blotting to detect protein level, or for 16h for BrdU incorporation assay to examine cell cycle, CCK-8 assay to measure cell proliferation and flow cytometry analyses to detect apoptosis.
2.2. Cell transfection
siRNA or plasmid was transfected into HAEC using Lipofectamine 2000 (ThermoFisher Scientific) or 4D-Nucleofector system (Lonza) according to the manufacturers’ instructions. At 48–96h post-transfection, HAEC were treated and analyzed.
2.3. Western blotting
HAEC were lysed and protein concentrations measured. Proteins were separated with SDS-PAGE and transferred to a PVDF membrane (Millipore). After blocking, incubation with primary and HRP-conjugated secondary antibodies, the target protein bands were visualized with ECL and a digital gel image analysis system (Tanon, Shanghai, China). For phosphorylated protein detection, the same membrane was reprobed for total protein after incubation with stripping buffer (ThermoFisher Scientific). Representative images from at least three independent experiments are shown in the figures.
2.4. Detection of mTOR-bound and mTOR-free raptor
Raptor in the whole HAEC cell lysate before and after immunoprecipitation with anti-mTOR antiboby (Abcam) was analyzed with Western blotting. mTOR-bound raptor was calculated by subtraction of mTOR-free raptor from total raptor.
2.5. Quantification of mRNA
The procedure was performed as previously described [20]. Briefly, total RNA was extracted using TRIzol reagent (Takara Biotechnology). 1μg of total RNA was reverse transcribed into cDNA using 1st Strand cDNA Synthesis Kit (Yeasen Biotech) followed by real-time PCR employing SYBR Green (Yeasen Biotech) on a Light Cycler 480 Instrument II (Roche). Relative gene expression was normalized to GAPDH mRNA level with 2−ΔΔCt method.
2.6. CCK-8 assay
Cell viability was measured with Cell Counting Kit-8 (CCK-8) (MCE). Briefly, HAEC were seeded in a 96-well plate and grown to confluence. After treatment, 10μl WST-8 was added to each well followed by incubation at 37°C for 1h. Absorbance at 450nm and 600nm was measured on ELx800 (BioTek Instruments). Absorbance at 600nm served as reference. Cell count was calculated using a standard curve.
2.7. BrdU incorporation assay
After treatment, HAEC were incubated with BrdU for 24h in a CO2 incubator followed by staining with FITC-labeled antibody and 7AAD using a BrdU Flow Kit (BD Biosciences). Cells were acquired with FACSCalibur flow cytometer (BD) and FlowJo applied for post-acquisition analysis.
2.8. Detection of apoptosis
Apoptosis was evaluated by measuring either the amount of phosphatidylserine exposed to the outer layer of cell membrane with a FITC Annexin V Apoptosis Kit (BD Biosciences), or the caspase 3 activity with a Fluorescein Active Caspase 3 Staining Kit (BioVision). Cells were resolved by FACSCalibur flow cytometer (BD Biosciences) before data analyzed with FlowJo.
2.9. In vitro tube formation assay
After treatment, HAEC at a density of 6X104 cells per well were seeded in 24-well plates preloaded with 150μl of Matrigel (Corning) per well. After incubation for 12h, images were captured using a microscope (Olympus IX53) and tube formation quantified with Image J.
2.10. Luciferase activity assay
0.8μg of each pGL3 Firefly luciferase reporter plasmid together with 0.1μg phRL-TK (Promega) containing Renilla luciferase cistron as an internal control were co-transfected into HAEC seeded in a 24-well plate. 48h post-transfection, firefly and renilla luciferase activities were evaluated using Dual-luciferase Reporter Assay System (Promega) on a GloMax 20/20 Luminometer (Promega).
2.11. Chromatin immunoprecipitation assay
ChIP assay was performed as previously described [20] using the ChIP-IT Express kit (Active Motif). IRF-1 enrichment on CASP1 or CCND3 promoter region was quantified with real-time PCR and normalized to input DNA. In addition to the reported binding site on CASP1 promoter (−11/+10) [21], IRF-1 binding to another potential site on CASP1 promoter (−404/−393) and to two sites on CCND3 promoter (−154/−134, −544/−524) as predicted by Jaspar was studied.
2.12. Primary culture of aortic endothelial cells
The animal procedures for primary culture of aortic endothelial cells were approved by the institutional animal care and use committee (IACUC-1601043). Primary murine aortic endothelial cells were isolated from the global Irf1−/− mice (Jackson laboratory) and the wild type littermates using positive immuno-selection with a rat anti-mouse CD31 (BD) [22]. Endothelial cell identity was examined with uptake of Dil-Ac-LDL (Biomedical Technologies) and anti-CD31 labeling (Invitrogen).
2.13. Mouse model of femoral artery wire injury
The procedures for mouse model of femoral artery wire injury were approved by the institutional animal care and use committee (IACUC-1908027). Irf1fln/fln(EMMA), Flp1-transgenic mice (Jackson laboratory) and Tek2 promoter-driven Cre transgenic mouse line (Jackson laboratory) were used to generate Irf1flox/flox; Tek2Cre (Irf1EC−/−) mice with EC-specific deletion of IRF-1. The mouse model of femoral artery wire injury was performed as previously reported [23]. Rapamycin (S1039, Selleck) was administered by daily i.p. injection (2 mg/kg body weight/day), a dose relevant for patients treated with DES [24]. At 7 days following wire injury when ~80% of the EC in the femoral artery were recovered [23], the mice were sacrificed and arteries harvested for analysis of re-endothelialization by Evans blue assay and immunofluorescence staining of CD31 followed by quantification with OD620nm and Image Pro, respectively.
2.14. Statistical analyses
GraphPad Prism was employed for data presentation and statistical analysis. Data were shown as mean ± SEM. Student’s t test was used to compare the difference between two groups while ANOVA with Dunnett’s or Tukey’s post-hoc test was applied to analyze multiple groups. P≤0.05 was considered significant.
3. Results
3.1. mTORi suppressed HAEC proliferation by inducing apoptosis and inhibiting cell cycle
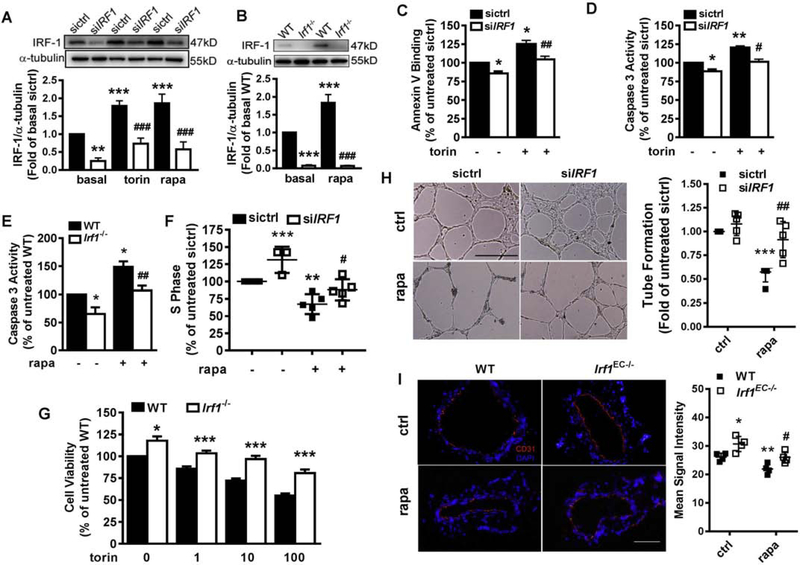
Inhibition of mTOR with rapamycin or torin 1 for 18h caused a dose-dependent decrease in cell viability, indicative of impaired HAEC proliferation, with a minimal effective dose at 0.1nM for rapamycin and 1nM for torin 1 (Fig 1A). Depletion of mTOR via transfection of siRNA also decreased HAEC viability by 21% (Fig 1B and 1C). Cell cycle arrest and apoptosis, two major functional endpoints associated with mTORi-induced cell growth suppression [6–9] were evaluated. A BrdU incorporation assay revealed that mTORi significantly decreased HAEC progression to S phase (Fig 1D, Suppl. Fig 1A). The minimal effective doses for rapamycin and torin 1 were 1nM and 100nM respectively, which led to a 40% and 35% reduction in the number of HAEC cells at S phase. Moreover, rapamycin or torin 1 increased HAEC apoptosis with a minimal effective dose of 10nM and 1nM, as evidenced by Annexin V binding assay (Fig 1E, Suppl. Fig 1B) and detection of caspase 3 activity (data not shown). These results confirm that mTORi promotes anti-proliferative effects in HAEC and establishes dosage and treatment times in the following experiments to treat EC for 18h with 1–10nM rapamycin for cell cycle study and 1–10nM torin 1 for apoptosis study.
Figure 1. mTORi suppressed HAEC proliferation concomitant with upregulation of IRF-1 expression.
(A) HAEC were treated with vehicle (ctrl), rapamycin (rapa) or torin 1(torin) at indicated dose for 18h prior to CCK-8 assay to measure cell viability. n=3; *p<0.05; **p<0.01; ***p<0.001 vs. control. (B) 96h post-transfection with control siRNA (sictrl) or MTOR-targeting siRNA (siMTOR), HAEC were subjected to CCK-8 assay (n=4) or Western blotting (C). (D) HAEC were treated with rapa or torin at indicated dose for 18h prior to BrdU incorporation assay to evaluate cell cycle progression. Shown are relative cell numbers at S phase (n=3). (E) HAEC were treated with rapa or torin at indicated dose for 18h prior to Annexin V binding assay to evaluate apoptosis (n=3–5). (F-G) HAEC were treated with rapa or torin at indicated dose for 18h (F), or at 10nM for indicated time (G) prior to Western blotting followed by quantification. Shown is representative image from at least three experiments. (H) HAEC were treated with rapa or torin at indicated dose for 2h prior to quantitative PCR to measure mRNA (n=3–4). (I) pGL3/IRF1 −998/+33 plasmid was constructed and promoter activity induced by 1h treatment with 10nM rapa or torin and quantified by dual-luciferase assay (n=6). *p<0.05; **p<0.01; ***p<0.001 vs. ctrl or sictrl (one way ANOVA with Dunnett’s test, A, D, E, F,G, H and I; or two-tailed paired t test, B).
3.2. mTORi induced IRF-1 expression
In a previous study, we found IRF-1 upregulated in mTOR-inhibited HAEC, either in the absence or presence of pro-inflammatory cytokine TNFα [20]. Here Western blot confirmed that rapamycin or torin1 upregulated IRF-1, which was both dose- and time-dependent (Fig 1F–G), with a plateau reached at 2–4h treatment with 1–10nM inhibitor.
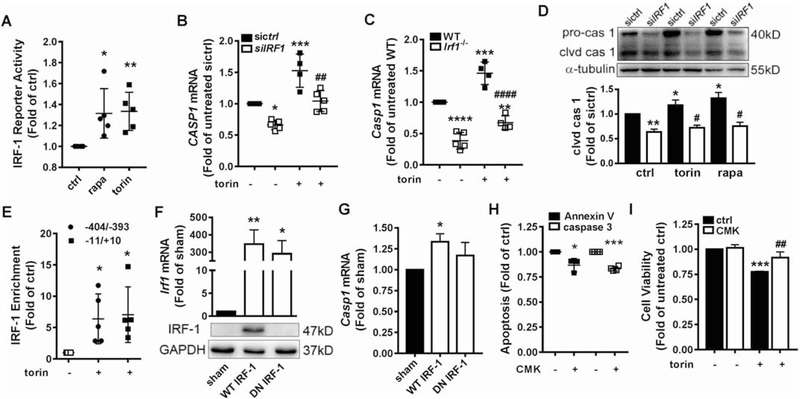
It was reported that IRF-1 has a short half-life and is regulated mainly at the transcriptional level.[13] Another study revealed that mTORi prevented protein degradation [25]. Therefore, we tested the effect of mTORi on IRF-1 degradation and transcription. Treatment with cycloheximide indicated that IRF-1 protein underwent similar degradation rate in the absence or presence of mTOR inhibitors (Suppl. Fig 2). Quantitative real-time PCR confirmed transactivation of IRF1 by mTORi. Treatment with torin 1 or rapamycin at 1nM for 2h increased IRF1 mRNA level to ~2 or ~1.5 fold of control, respectively (Fig 1H). An increase in the dose to 100nM did not elicit a further significant increase in the transcription (Fig 1H). Consistent with enhanced transcription, a dual-luciferase reporter assay confirmed that 1h treatment with rapamycin or torin 1 increased IRF1 promoter activity by about 30% (Fig 1I). It is noteworthy that although 2h treatment also increased luciferase activity, the increase was not as high as in 1h treatment (data not shown). The possible reason might be because longer treatment of torin or rapamycin inhibited the synthesis of luciferase. These data clearly demonstrated that mTORi upregulated IRF-1 expression through activating its transcription in HAEC, rather than affecting its degradation. These results demonstrate that 2–4h treatment with rapamycin or torin 1 at a dose of 1–10nM elicited a maximal effect on IRF-1 expression. Hence, those treatment conditions were applied in the following studies to elucidate the mechanism underlying mTORi-induced IRF1 transactivation.
3.3. IRF-1 mediated mTORi-induced endothelial apoptosis and cell cycle arrest
To examine the role of IRF-1 expression in mediating the suppressive effects of mTORi on endothelial proliferation, IRF-1 was suppressed by siRNA-mediated knockdown in HAEC. A role for IRF-1 was also assessed in primary EC isolated from wild type (WT) and Irf1−/− mice (Suppl. Fig 3). Depletion of IRF-1 in HAEC (Fig 2A, 2C–D, 2F) or mouse EC (Fig 2B and 2E, Suppl. Fig 4) partially rescued the cells from basal or mTORi-induced apoptosis while increasing the number of cells at S phase. In accordance with HAEC, IRF-1 in murine EC was also inducible by mTORi (Fig 2B). EC isolated from Irf1-null mice displayed a 17% increase in viability after 72h culture (Fig 2G). Deficiency of IRF-1 also alleviated the growth-suppressive effect induced by different doses of torin 1 (Fig 2G). A tube formation assay (Fig 2H) further confirmed the contribution of IRF-1 to mTORi-induced EC growth suppression. Finally, employing the clinically relevant wire injury model of the mouse femoral artery (Suppl. Fig 5A) confirmed the suppressive effect of rapamycin on EC growth in vivo. Deficiency of IRF-1 (Suppl. Fig 5B–C) enhanced EC recovery at day 7 post-surgery in the denuded artery whether or not rapamycin was administered, as evidenced by immunofluorescent staining of EC surface marker CD31 (Fig 2I) and Evans blue permeability assay (Suppl. Fig 5D). These results were associated with an alleviated hyperplasia (intima to media ratio) at day 21 post-injury (Suppl. Fig 5E). Together these results demonstrate a central role for IRF-1 in mediating the suppressive effects of mTORi on EC proliferation both in vitro and in vivo.
Figure 2. IRF-1 contributed to mTORi-induced endothelial apoptosis and cell cycle arrest.
(A-B) HAEC were transfected with control (sictrl) or IRF1-targeting siRNA (siIRF1) before treatment with 10nM rapa and torin at 10nM for 4h (A). Murine EC were isolated from Irf1−/− or wild type (WT) littermates followed by treatment with 10nM rapa for 2h (B). Western blotting confirmed depletion of IRF-1 in HAEC (A) and mouse EC (B). Shown are representative images (n=4). (C-D) After transfection of siRNA, HAEC were treated with or without 10nM torin for 18h, prior to flow cytometric analyses of apoptosis by measuring phosphatidylserine exposed to cell surface (n=4, C) or caspase 3 activity (n=5, D). (E) EC were treated with (n=3) or without (n=5) 50nM rapa for 18h, prior to flow cytometric analyses of caspase 3 activity. (F) After transfection of siRNA, HAEC were treated with (n=5) or without (n=3) 1nM rapa for 18h, prior to BrdU incorporation assay to evaluate cell cycle progression. (G) WT or Irf1−/− EC were treated with torin at indicated dose for 18h prior to CCK-8 assay to measure cell viability (n=3). *p<0.05; ***p<0.001 vs. WT treated with identical dose of torin (two-tailed paired t test). (H) After transfection of siRNA, HAEC were seeded into matrigel in the presence or absence of 1nM rapa, and tube formation was quantified as total tube length of the network after 12h. n=5; Shown are representative images. Scale=500μm. (I) 7 days after wire injury, the femoral artery of the EC-specific Irf1 knockout mice (Irf1flox/floxTek2Cre, Irf1EC−/−) or the wild type control (Irf1flox/flox, WT), administered with vehicle or rapa was sectioned for analysis of CD31 expression (n=3). Scale=100μm *p<0.05; **p<0.01; ***p<0.001 vs. untreated sictrl or WT; #p<0.05; ##p<0.01; ###p<0.001 vs. rapa/ torin-treated sictrl or WT (one way ANOVA with Tukey’s test, A, B, C, D, E, F, H and I).
3.4. IRF-1 promoted apoptosis in part through transcriptional activation of caspase 1
IRF-1 transcriptional activity was increased by ~30% in rapamycin or torin 1-treated HAEC as indicated by a dual-luciferase reporter assay (Fig 3A). CASP1 (encoding caspase 1), a gene target of IRF-1 [16] involved in apoptosis, was upregulated by mTORi (Fig 3B–D). In IRF1-depleted HAEC or Irf1−/− murine EC, torin 1-induced CASP1 transcription (Fig 3B–C) was significantly decreased. The same trend was observed in response to rapamycin treatment (Suppl. Fig 6). The amount of both pro-caspase-1 protein and its cleaved active form were decreased in HAEC depleted of IRF-1 (Fig 3D). Based on the literature [21] and predictions from the Jaspar software, we examined IRF-1 binding to two potential sites (−11/+10 and −404/−393) on CASP1 promoter with ChIP. The results demonstrated IRF-1 enrichment at both sites, which was enhanced by torin 1 treatment (Fig 3E). Expression of WT but not dominant-negative (DN) IRF-1 rescued Casp1 transcription in Irf1−/− murine EC (Fig 3F and 3G). Finally, caspase 1 was confirmed to contribute to mTORi-induced HAEC apoptosis (Fig 3H) and proliferation suppression (Fig 3I) as its specific inhibitor Ac-YVAD-CMK alleviated the effects. These data revealed that IRF-1 promoted apoptosis in mTOR-inhibited HAEC in part through positive upregulation of caspase 1.
Figure 3. IRF-1 promoted apoptosis in part through transcriptional activation of caspase 1.
(A) Dual-luciferase assay indicated that 1h treatment with 10nM rapa or torin increased IRF-1 transcriptional activity (n=5). (B-C) HAEC were transfected with control (sictrl) or IRF1-targeting siRNA (siIRF1) (B). Murine EC were isolated from Irf1−/− or wild type (WT) littermate (C). EC were treated with 10nM torin for 2h, prior to quantitative PCR to measure mRNA (n=4–5). (D) siRNA-transfected HAEC were treated with 10nM rapa or torin for 2h followed by Western blotting to detect pro-caspase 1 (pro-cas 1) and cleaved caspase 1 (clvd cas 1). Shown is representative blot (n=3). (E) HAEC were treated with or without 10nM torin for 2h. IRF-1 binding to CASP1 promoter region −404/−393 or −11/+10 was examined with ChIP followed by real time PCR with primers flanking these two sequences (n=3). (F) Irf1−/− EC were transfected with pcDNA (sham), pcDNA/WT IRF1 or pcDNA/dominant-negative (DN) IRF1. After 96h, Western blotting and quantitative PCR confirmed successful rescue of IRF-1 expression. The primary antibody for Western blotting is unable to detect the truncated DN IRF-1 (n=3–4). (G) Casp1 expression was increased by rescue of WT IRF-1 expression but not the DN form (n=3–4). (H) HAEC were treated with 5μM caspase 1 inhibitor Ac-YVAD-CMK (CMK) for 1h before incubation with 10nM torin for 18h. Apoptosis was evaluated by flow cytometric analysis of Annexin V binding and caspase 3 activity (n=3–4). (I) HAEC were treated with 5μM CMK for 1h before incubation with 10nM torin for 18h. Cell viability was measured by CCK-8 assay (n=3). ***p<0.001 vs. untreated. ##p<0.01 vs. torin-treated (one way ANOVA with Tukey’s test). *p<0.05; **p<0.01; ***p<0.001 vs. untreated sictrl or WT or sham; #p<0.05; ##p<0.01; ###p<0.001 vs. rapa/ torin-treated sictrl or WT (one way ANOVA with Dunnett’s test, A, E, F and G; one way ANOVA with Tukey’s test, B, C, D, I; two-tailed paired t test, H).
3.5. IRF-1 inhibited cell cycle progression in part through downregulation of cyclin D3
IRF-1 upregulation was accompanied by decreased expression of cyclin family members such as D3 and D1 in mTOR-inhibited HAEC (Fig 4A) or murine EC (Fig 4B). The minimal effective dose was 1nM for rapamycin and 100nM for torin 1 (Fig 4A–B), coinciding with those doses that elicited cell cycle arrest (Fig 1D). mTORi suppressed CCND3 transcription by ~10% in both HAEC (Suppl. Fig 7A) and murine EC (Suppl. Fig 7B). Deficiency of IRF-1 resulted in a higher level of both cyclin D3 protein (Fig 4C–D) and mRNA (Fig 4E–F), whereas rescue expression of WT IRF-1 but not DN IRF-1 in Irf1−/− mouse EC repressed its transcription (Fig 4G). It is noteworthy that although depletion of IRF-1 increased basal cyclin D1 expression, it failed to reverse the inhibitory effect elicited by mTORi in HAEC (Fig 4C), excluding the possibility that IRF-1 contributed to mTORi-induced cell cycle arrest through cyclin D1. IRF-1 binding to two potential sites on CCND3 promoter was examined with ChIP. The results demonstrated that torin 1 treatment significantly increased its interaction to −544/−524 (Suppl. Fig 7C). Furthermore, overexpression of cyclin D3 (Fig 4H) promoted HAEC progression to S phase (Fig 4I) and significantly increased cell proliferation (Fig 4J), indicating that IRF-1 inhibited cell cycle progression partially through downregulation of cyclin D3 expression.
Figure 4. IRF-1 inhibited cell cycle progression in part through downregulation of cyclin D3.
(A-B) HAEC (A) or murine (B) EC were treated with rapa or torin for 18h at indicated dose prior to Western blot analysis. (C) HAEC were transfected with siRNA, treated with or without 10nM rapa for 4h followed by Western blotting. Images were from the same blot. (D) EC isolated from WT or Irf1−/− mice, treated with torin at indicated dose for 18h followed by Western blotting. Shown is representative blot (n=3). (E) HAEC were transfected with siRNA, treated with 1nM rapa for 2h followed by quantitative PCR ( n=3). (F) EC isolated from WT or Irf1−/− mice, treated with 1nM rapa for 2h followed by quantitative PCR (n=3–4). (G) Ccnd3 expression was repressed by rescue of WT IRF-1 expression but not the DN form (n=3). (H) Western blotting confirmed successful overexpression of cyclin D3 after transfection of Rc/CMV-CCND3. (I) At 48h post transfection of Rc/CMV-CCND3, HAEC were treated with 1nM rapa for 18h followed by BrdU incorporation assay to evaluate cell cycle progression (n=4). (J) At 48h post transfection of Rc/CMV-CCND3, HAEC were treated with 1nM rapa for 18h followed by CCK-8 assay to evaluate cell viability (n=3–4). *p<0.05; ***p<0.001 vs. untreated sictrl or WT or sham; #p<0.05; ##p<0.01 vs. rapa-treated sictrl or WT (one way ANOVA with Tukey’s test, E, F, J; one way ANOVA with Dunnett’s test, G; one-tail paired t test, I).
3.6. mTORi activated STAT-1 and NF-κB
Previous studies implicated the JAK/STAT and NF-κB pathways in mediating IRF1 transcription and tumor cell apoptosis [26, 27]. Consistent with these studies, treatment of HAEC with rapamycin or torin 1 rapidly increased STAT-1 phosphorylation at Y701, which remained elevated through ~1h (Fig 5A). However, STAT-3 which was also involved in IRF1 transcription [26] was not activated (data not shown). Similarly, mTORi activated NF-κB p65 phosphorylation at S536, which peaked at ~2h treatment (Fig 5A). Consistent with these data, a dual-luciferase reporter assay showed that rapamycin and torin 1 increased the transcriptional activity of NF-κB (Fig 5B) and STAT-1 (Fig 5C) at 2h and 0.5h, respectively. Pharmacological inhibition of the activity of JAK, STAT-1, or NF-κB signaling using Ruxolitinib, Fludarabine, or BAY 11–7082 decreased basal and mTORi-induced IRF1 gene expression both at mRNA (Fig 5D, Suppl Fig 8A) and protein (Fig 5E–F) levels. Moreover, inhibition of STAT-1 and NF-κB resulted in a reduction in caspase 1 (Fig 5G) and an increase in cyclin D3 expression (Fig 5H), accompanying an attenuation in mTORi-induced apoptosis (Fig 5I, Suppl. Fig 8B) and cell proliferation suppression (Fig 5J). Inhibition of NF-κB but not STAT-1 also promoted cell progression to S phase (Suppl. Fig 8C). These findings confirmed that mTORi upregulated IRF-1 and suppressed HAEC growth in part through activation of STAT-1 and NF-κB.
Figure 5. mTORi activated STAT-1 and NF-κB.
(A) HAEC were treated with 10nM rapa or torin at indicated time followed by Western blot analysis. (B) HAEC were treated with 10nM rapa or torin for 2h (B) or 0.5h (C) followed by dual-luciferase assay to detect the transcriptional activity of NF-κB (B) or STAT-1 (C). n=3. (D) After pretreated with vehicle (ctrl), JAK, STAT-1 or NF-κB specific inhibitors Ruxolitinib (Ruxo, 1μM), Fludarabine (Flu, 17.5μM) or BAY 11–7082 (Bay, 5μM) for 1h, HAEC were incubated with 10nM torin for 2h before IRF1 mRNA was quantified with real-time PCR(n=3–5). (E-F) HAEC were treated with vehicle (ctrl), Bay (5μM) (E), Ruxo (1μM) or Flu (L: 3.5μM; H:17.5μM) (F) for 1h before incubation with 10nM rapa or torin for another 4h. HAEC lysates were subjected to Western blotting for analysis of IRF-1. (G-H) HAEC were treated with vehicle (ctrl), Bay (5μM) or Flu (17.5μM) for 1h before incubation with 10nM torin (G) or 1nM rapa (H) for 2h. CASP1 (G, n=3) or CCND3 (H, n=6) mRNA was quantified with real-time PCR. (I) HAEC were treated with vehicle (ctrl), Bay (5μM) or Flu (17.5μM) for 1h before incubation with 10nM torin for 18h. Caspase 3 activity was measure by flow cytometry (n=5). (J) HAEC were treated with vehicle (ctrl), Bay (5μM) or Flu (17.5μM) for 1h before incubation with 10nM torin for 18h. Cell viability was measured with CCK-8 assay (n=3). *p<0.05; **p<0.01 vs. ctrl (B, C); *p<0.05; **p<0.01; ***p<0.001 vs. torin/rapa-only treatment (D, G, H, I) (one way ANOVA with Dunnett’s test); **p<0.01 vs. untreated ctrl; ###p<0.001 vs. torin-only treatment (one way ANOVA with Tukey’s test, J).
3.7. PKCζ mediated mTORi-induced IRF-1 upregulation and cell proliferation suppression
Phosphorylation of p70S6K and 4EBP-1 are downstream of mTORC1 signaling, which is inhibited by treatment of rapamycin or torin 1 (Suppl. Fig 9A). After phosphorylation, p70S6K becomes activated and phosphorylates its substrate S6 protein of the 40S ribosomal subunit which is involved in translational regulation of mRNAs [28]. 4EBP-1 is a translation repressor that inhibits cap-dependent translation by binding to the translation initiation factor eIF4E. Phosphorylation of 4EBP-1 disrupts this interaction and results in activation of cap-dependent translation [28]. Since both rapamycin and torin 1 rapidly upregulated IRF-1, a role for inhibition of phosphorylation of p70S6K and 4EBP-1 in mTORi-induced IRF1 transactivation was investigated. However, either knockdown of RPS6KB1 (encoding p70S6K) or EIF4EBP-1 (encoding 4EBP-1), or overexpression of a WT or phosphorylation sites–mutant (T37AT46A) EIF4EBP-1 to mimic mTORi failed to increase IRF1 transcription (Suppl. Fig 9B–9C).
In our previous study we reported that chemical inhibitors or siRNA-mediated MTOR knockdown, significantly activated conventional PKC member PKCα.[20] In addition to PKCα, treatment with rapamycin or torin 1 led to activation of novel and atypical PKC member PKCδ (data not shown) and PKCζ (Fig 6A), which became more pronounced when HAEC were co-treated with cytokine TNFα, even at a low dose of 0.1ng/mL (data not shown).
Figure 6. PKCζ mediated mTORi-induced IRF-1 upregulation and cell growth suppression.
(A) HAEC were treated with 10nM rapa or torin for indicated time prior to Western blot analysis. (B) Western blotting confirmed successful knockdown of PKCζ by transfection of siRNA. (C) After transfection with control siRNA or PKCζ-targeting siRNA (siPKCζ), HAEC were treated with 1nM rapa or 100nM torin for 18h prior to CCK-8 assay to measure cell viability (n=3). (D) After transfection with control siRNA or siPKCζ, HAEC were treated with 10nM rapa or torin for 2h prior to real-time PCR to quantify IRF1 mRNA (n=3–5). (E) HAEC were pretreated with 1μM PKCζ inhibitor Pseudo-substrate inhibitor Myristoylated (PSI) followed by treatment with 10nM torin for 4h. Cell lysates were analyzed with Western blotting. (F-G) After transfection with siRNA, HAEC were treated with 10nM torin (F) or 1nM rapa (G) for 2h. CASP1 (F, n=3) or CCND3 (G, n=4) mRNA was quantified with real-time PCR. (H) After transfection of siRNA, HAEC were treated with 10nM torin for 18h. Caspase 3 activity was evaluated with flow cytometry (n=6). (I) After pretreatment with 1μM PSI for 1h, HAEC were incubated with 1nM rapa for 18h. BrdU assay was applied to evaluate cell cycle progression (n=5). *p<0.05; **p<0.01; ***p<0.001 vs. identically-treated sictrl or ctrl (two-tail unpaired t test, C; two-tail unpaired t test, D, F-I).
siRNA targeting PKCα, PKCδ, and PKCζ or their specific inhibitors, GF109203X, Rotterlin, and Pseudo-substrate inhibitor Myristoylated (PSI), were applied to further evaluate their role in mTORi-induced IRF-1 upregulation and HAEC growth suppression. Inhibition of only PKCζ, but not PKCα or PKCδ, significantly attenuated mTORi-induced cell growth suppression (Fig 6B–C, Suppl. Fig 10A–C). Consistently, knockdown of PKCζ (Fig 6D) or treatment with PSI (Suppl. Fig 10D) inhibited basal, rapamycin- or torin 1-induced IRF1 transcription. These data suggest that the PKCζ isoform regulates IRF-1 expression and plays a major role in mTORi-induced HAEC growth suppression.
Further supporting this observation, inhibition of PKCζ resulted in inactivation of STAT-1 and NF-κB (Fig 6E), accompanying caspase 1 downregulation and cyclin D3 upregulation in untreated (Suppl. Fig 11A–B) or mTOR-inhibited (Fig 6F–G) HAEC. Inhibition of PKCζ not only attenuated basal (Suppl. Fig 11C) and mTORi-induced apoptosis (Fig 6H), but also promoted cell cycle progression either in the absence (Suppl. Fig 11D) or presence of mTORi (Fig 6I). These data implicate PKCζ as a mediator of mTORi-induced IRF-1 upregulation and cell proliferation suppression through activation of STAT-1 and NF-κB.
3.8. mTOR-free raptor contributed to mTORi-induced PKCζ activation
Our previous study demonstrated that disruption of both mTORC1 and mTORC2 via dual knockdown of RPTOR (encoding raptor) and RICTOR (encoding rictor) resulted in distinct phenotypes from that elicited by mTORi via knockdown of mTOR itself or treatment with rapamycin or torin 1 [20]. Remarkable was that dual knockdown decreased, whereas mTORi increased PKCα phosphorylation. Similarly, in the current study PKCζ phosphorylation was dramatically diminished in HAEC transfected with siRNA against both RPTOR and RICTOR while the total PKCζ reprobed on the same blot was not significantly reduced (Fig 7A–7B). Consistent with the low PKCζ activity, STAT-1 and NF-κB phosphorylation were inhibited (Fig 7A) and IRF1 transcription decreased by 21% (Fig 7C) in these cells. In contrast, PKCζ phosphorylation was increased (Fig 7A–B) and IRF1 transcription elevated by 44% in MTOR-silenced HAEC (Fig 7C). The phosphorylated/ total STAT-1 also increased although total STAT-1 expression was decreased (Fig 7A).
Figure 7. Raptor contributed to mTORi-induced PKCζ activation independent of mTORC1.
(A) At 48h post transfection with control siRNA, siRNA against RPTOR and RICTOR (siRPT+RIC), or MTOR-targeting siRNA, HAEC were submitted to Western blot analysis (A). (B) Quantification of the ratio of phosphorylated PKCζ to total PKCζ (n=3). (C) At 48h post transfection, IRF1 mRNA was measured with real-time PCR (n=5). (D) At 48h post transfection with control siRNA, siRNA against RPTOR, or siRNA against RICTOR, HAEC were submitted to Western blot analysis. (E) HAEC were treated with rapa at 25nM (rapa-L) or 250nM (rapa-H), or with torin at 50nM (torin-L) or 500nM (torin-H) for 18h prior to Western blot analysis. (F) Immunoprecipitation assay confirmed interaction between mTOR and raptor. (G) The same whole cell lysates used for immunoprecipitation in (F), or the lysates collected after immunoprecipitation reaction, were analyzed with Western blotting. Shown were results from one independent experiment each with two batches of lysates from two separate plates of HAEC culture. (H) HAEC were transfected with vector (pRK-5), pRK-5/HA-RPTOR (WT) or pRK-5/HA-RPTOR mutant (Mut) along with sictrl or siMTOR. (I) Transfected HAEC were treated with or without 10nM rapa for 1h prior to Western blot analysis. All blots shown are representative images from at least three experiments. *p<0.05; ***p<0.001 vs. sictrl; ###p<0.001 vs. siMTOR (one way ANOVA with Tukey’s test, B-C).
Dual knockdown of RPTOR and RICTOR also decreased mTOR expression (Fig 7A), making the stoichiometric ratio among the three molecules similar to that in scrambled siRNA-transfected HAEC. Although knockdown of MTOR also inhibited raptor or rictor expression, the ratio of raptor or rictor to mTOR significantly increased in MTOR-silenced cells (Suppl. Fig 12), suggesting extra raptor or rictor (or mTOR-free raptor or rictor) may contribute to PKCζ activation. Consistently, depletion of raptor or rictor in HAEC decreased PKCζ phosphorylation (Fig 7D).
Since acute rapamycin treatment, which did not affect rictor binding to mTOR, was capable of activating PKCζ and inducing IRF-1 expression (Fig 1F–G, Fig 6A), it was possible that mTOR-free raptor but not mTOR-free rictor played a major role in these mTORi-induced effects. In support of this hypothesis, treatment of HAEC with rapamycin or torin 1 increased the raptor/ mTOR ratio by decreasing mTOR expression in the case of rapamycin treatment or by increasing raptor expression in the case of torin 1 treatment (Fig 7E). As reported in podocytes [29]. rapamycin treatment of HAEC also decreased rictor expression, leaving the rictor/ mTOR ratio unchanged. Furthermore, Western blot analysis of the lysate after depletion of mTOR-bound complex with immunoprecipitation confirmed that up to 65.2% of raptor remained in a mTOR-free state, whereas only 34.8% was found in the mTOR-bound complex (Fig 7F–G, Suppl. Fig. 14.).
To further test this possibility, WT raptor or the mutant unable to bind mTOR [30] was overexpressed in HAEC. As shown in Fig 7H–I, overexpression of both WT and mutant raptor increased PKCζ phosphorylation, confirming that raptor increased PKCζ activity independent of its interaction with mTOR. In the presence of siRNA (Fig 7H) or rapamycin (Fig 7I) to disrupt raptor interaction with mTOR, PKCζ phosphorylation also increased in raptor-overexpressed HAEC, further indicating that mTOR-free raptor contributed to PKCζ activation and IRF-1 upregulation.
4. Discussion
While significantly alleviating endovascular restenosis, application of mTOR inhibitors in DES are associated with cumulative incidence of late-stent thrombosis due to inhibition of arterial EC. Although mTOR is a well-recognized regulator of cellular growth and survival, how mTORi suppresses EC growth remains largely unknown. In this study, we reveal a mechanism by which mTORi activates PKCζ-mediated pathways to upregulate IRF-1, which in turn mediates a suppressive effect on EC proliferation by enhancing apoptosis and inhibiting cell cycle progression.
Considerable evidence from immune and tumor cells has indicated that mTORi suppressed cell cycle and/or promoted apoptosis presumably through disruption of mTORC1 and/or mTORC2. In early T-cell progenitors or B cells, disruption of mTORC1 or both mTOR complexes resulted in cell cycle abnormalities that were associated with the cyclin-CDK complex [6, 7]. In tumor cells, mTORi enhanced cisplatin-induced apoptosis by inhibiting the translation of p21 [8] or by inhibiting protein phosphatase 5 to activate apoptosis-signal regulating kinase 1 [9].
In the cardiovascular system, upregulation of mTORC1 activated p70S6K and increased SMC proliferation, and thus contributed to aldosterone-induced pulmonary arterial hypertension [31]. Consistently, inhibition of mTORC1 by rapamycin or by depletion of mTORC1-defining component raptor blocked PDGF-induced SMC proliferation and phenotypic conversion [32]. On the other hand, disruption of mTORC2 also accounted for long-term rapamycin treatment-induced inhibition of EC growth and migration through preventing the degradation of p27kip1 [25].
Consistent with these results, we demonstrate in both human and mouse arterial EC that mTORi suppressed proliferation in a dose-dependent manner through mechanisms involving cell cycle arrest and apoptosis. As observed in tumor cells [12], HAEC displayed different sensitivity to rapamycin and torin 1. At a dose as low as 0.1nM, rapamycin significantly inhibited HAEC proliferation by ~15%. A similar degree of inhibition was not exhibited by torin 1 below 10nM. A cytostatic effect on HAEC dominated in rapamycin treatment, in contrast to a cytotoxic effect in response to torin 1 treatment. At a low dose, rapamycin was capable of inhibiting cell cycle while torin 1 induced apoptosis. A relatively high dose was required for torin 1 to suppress cell cycle and for rapamycin to promote apoptosis. The dosage and time effects may in part be explained by a different mechanism of action, since torin 1 inhibits both mTOR1 and mTOR2. Our results imply that mTORi suppress HAEC growth primarily through the cytostatic effect, which is more sensitive to inhibition of mTORC1 alone. In contrast, inhibition of mTORC2 alone or both complexes sensitized HAEC to apoptosis. The cross-talk between cell cycle arrest and apoptosis [33] may explain why low-dose rapamycin or high-dose torin 1 protected HAEC from apoptosis. It is noteworthy that prolonged (24h) treatment with rapamycin was reported to disrupt the assembly of mTORC2 and inhibit Akt phosphorylation, which caused the suppression on tumor cell growth [11]. Whether apoptosis dominates in chronic treatment of rapamycin in HAEC warrants further investigation, given the chronically-releasing property of the drug in DES.
Although mTORi has been considered to function mainly through suppressing protein translation by inhibiting phosphorylation of downstream signaling molecules such as p70S6K, studies have also indicated that mTOR contributed to regulation of transcriptional programs. Rapamycin treatment inhibited transcription of a large number of genes involved in regulation of cell cycle, apoptosis and extracellular matrix production, through downregulation of critical transcriptional factors such E2F1 [34]. In another study, mTORi inhibited balloon catheter-induced SMC proliferation through upregulation of transcription factor KLF4 [35].
Studies in immune and tumor cells have established a central role for IRF-1 in the regulation of gene expression. We demonstrate that mTORi-induced growth inhibition coincided with upregulation of IRF-1. Deficiency of IRF-1 attenuated EC sensitivity to mTORi, confirming the involvement of IRF-1 in the regulation of mTOR on HAEC proliferation. Until now, the effect of mTORi on IRF-1 expression has not been well-studied. Moreover, results of the limited studies are conflicting, which might be attributed to the different type of cells and experimental conditions applied. In A549 cells, inactivation of mTOR by silencing of MTOR or short-term treatment with rapamycin for 0.5h (mRNA level) or 1h (protein level) significantly enhanced interferon-γ-induced IRF-1 expression [36]. In HUVEC cells, however, 24h treatment of rapamycin decreased TNFα-induced IRF-1 expression [37]. We have recently reported upregulation of IRF-1 expression by mTORi in both the absence and presence of low-dose TNFα in HAEC [20]. In this study, we further investigated the time- and dose-dependent effect of mTORi on the dynamics of IRF-1 expression. The results indicated that mTOR inhibitors upregulated IRF1 transcription and protein expression with a peak reached at 2–4h at a dose of ~10nM. mTORi with knockdown of MTOR also led to IRF-1 upregulation, although not as pronounced as that with rapamycin or torin 1 treatment. Moreover, the same regulatory effect by mTOR on IRF-1 expression existed in murine EC, confirming the evolutionarily conserved character of mTOR signaling.
Consistent with mTORi, IRF-1 suppressed cell growth through cell cycle arrest and apoptosis via upregulating cell growth-inhibiting genes (p21, caspase-1, PUMA, etc) [14–17] or downregulating growth-promoting genes (cyclin D1, CDK4, CDK2, survivin, etc) [14, 18, 19]. CASP1 is a recognized target gene of IRF-1 [16]. Its protein product caspase 1 is known to contribute to IRF-1-mediated apoptosis [38] and more recently, to pyroptosis [39]. In this study, we confirmed that caspase 1 was regulated by IRF-1 and contributed to apoptosis in mTOR-inhibited HAEC. Although cyclin D1 has been reported to be a key target for IRF-1-mediated tumor-suppressive effects [18], silencing of IRF1 failed to rescue its expression inhibited by mTORi in HAEC. Cyclin D3, another cyclin member promoting cell cycle progression, has been identified as a target of mTORi that accounted for cell cycle arrest in immune cells [6, 7]. Consistent with a previous study [8], mTORi itself might affect both transcription and translation of cyclin D3, as evidenced by a much more profound reduction in protein than in CCND3 mRNA. Although gene activation by IRF-1 has been widely studied, exactly how this transcription factor downregulates gene expression is less well-known. It has been suggested that IRF-1 may interfere with other transcription factor at the target gene promoter [19]. In the present study, we found that IRF-1 bound to CCND3 promoter and negatively regulated its transcription, which contributed to the cell cycle arrest induced by mTORi. However, whether IRF-1 interacts with other transcription factors at CCND3 promoter to downregulate its transcription remains to be investigated.
mTOR signaling is classically considered to function through mTORC1 and/or mTORC2. Rapamycin treatment acutely increased IRF-1 expression in HAEC, suggesting that disruption of mTORC1 is capable of upregulating IRF1 gene expression. However, inhibition of two classical mTORC1 downstream signaling pathways failed to recapitulate this phenomenon (Suppl. Fig 9). This was consistent with a previous study in A549 cells finding that regulation of IRF-1 by mTOR was independent of p70S6K.[36] Recently, emerging evidence has suggested that mTOR components or inhibitors regulate cellular processes independently of the mTOR complex [40, 41]. Our recent findings that silencing of MTOR or its pharmacological inhibition, and compound depletion of raptor and rictor led to distinct phenotypes in HAEC support a mTOR complex-independent mechanism. In this study we analyzed the cell lysates after depletion of mTOR complex and provided direct evidence for the existence of mTOR-free raptor in HAEC. Adding to this line of evidence, this study found that mTORi induced IRF1 transcription while dual depletion reduced it. Furthermore, results with overexpressed raptor mutant incapable of binding mTOR confirmed the involvement of mTOR-free raptor in PKCζ activation and IRF-1 upregulation. It is noteworthy that inhibition of mTOR complexes by rapamycin or torin 1 is mostly likely to synergize with IRF-1 in causing inhibition on HAEC proliferation although mTORi transactivated IRF1 independently of the disruption of mTOR complexes.
Protein kinase C (PKC), a large family of serine/ threonine kinases, are critical regulators of many cellular functions including gene expression, cell proliferation and differentiation, cell survival and apoptosis. Each PKC structurally consists of a regulatory domain and a catalytic domain. Depending on the regulatory domain and the requirement for activation, PKC are categorized into three groups. The conventional PKC (PKCα, βI, βII, and γ) have DAG- and Ca2+-binding domains. The novel PKC (PKCδ, ε, η, θ) contain DAG- but not Ca2+-binding domains. The atypical PKC (PKCζ and λ/ι) require neither Ca2+ nor DAG for their activity; instead, they are regulated predominantly by protein-protein interaction [42] and by 3-phosphoinositide-dependent protein kinase1 (PDK1)-mediated phosphorylation of critical threonine at the activation loop [43, 44].
PKC is known to regulate IRF-1 expression and activity [13]. mTORC2 has been traditionally considered to be responsible for the regulation of PKC activation. However, our recent study challenged this convention based on the fact that inhibiting mTOR to disrupt both mTORC1 and mTORC2 increased PKCα phosphorylation [20]. Consistent to our findings, study reported that sirolimus caused Ca2+-dependent activation of PKCα in EC [45]. Similarly, mTORi activated PKCδ and PKCζ. Interestingly, compound silencing of raptor and rictor dramatically decreased PKCζ (Fig 7A–B) and PKCα phosphorylation [20] concomitant with a reduction in IRF1 transcription (Fig 7C). Based on the observations from literature and our studies, we proposed that mTOR-free raptor contributed to PKC activation in mTOR-inhibited cells. Overexpression of mutant raptor incapable of binding mTOR with or without mTORi treatment further confirmed that raptor activated PKC in mTORC1-independent manner. Further studies examining whether mTORi or raptor activate PKC through alteration of the concentration of cytosolic DAG or Ca2+ or phosphorylation of PDK1 in the cell are ongoing.
Dysregulation of selected PKC isozymes has been implicated in various stages of different pathologies such as cancer, inflammatory diseases and vascular restenosis after interventional therapy [46]. In this study, we found that all three PKC members, PKCα, PKCδ, and PKCζ, regulated IRF-1 expression; however, only PKCζ was responsible for the suppressive effect of mTORi on HAEC. Consistent with the promoting role in SMC proliferation [47], PKCα is a pro-growth signal in HAEC cells (Suppl. Fig 10B). Its activation may be a part of the pro-survival response to mTORi. Although PKCδ promoted HAEC apoptosis (data not shown), its inhibition did not protect HAEC from growth suppression in this study (Suppl. Fig 10B), probably due to concomitant activation of other pro-survival signals.
Both tumor promoting [48] and suppressing [49] activities of PKCζ have been reported in several different tissues. Similarly, the role of PKCζ in vascular cell growth is unclear. In this study, we have provided evidence that through regulating IRF-1 expression, PKCζ suppressed HAEC growth by promoting apoptosis and cell cycle arrest. Similar to other PKC isoforms, PKCζ is essential for the adequate activation of NF-κB and JAK1/STAT-1 signaling [50], which are typical pathways to activate IRF1 transcription [26, 27]. Consistently, PKCζ activated NF-κB and JAK/STAT-1 which resulted in IRF1 transactivation in mTOR-inhibited HAEC. Our results were also consistent with previous studies reporting that mTORi increased the activation and nuclear localization of NF-κB in HUVEC [51] and STAT-1 in A549 cells [36]. It was reported that in SMC PKCζ is undetectable [52] while in EC its level relatively high [53]. Our preliminary experiment confirmed that PKCζ was expressed at a higher level in HAEC than in human aortic SMC (HASMC) (Suppl. Fig 13A). Furthermore, although knockdown of IRF-1 promoted HASMC growth both in the absence and presence of rapamycin (Suppl. Fig 13B–D), mTORi did not significantly induce its expression in HASMC (Suppl. Fig 13E). This may be related to low expression level of PKCζ and/ or possibly different signaling cascade operating in HASMC in response to mTORi. Additional studies in vitro and in vivo are needed to investigate the differential effect of PKCζ/ IRF-1 signaling on the growth of primary EC and SMC. This component of the signaling pathway may prove to be an alternative therapeutic target to inhibit SMC neointimal growth while enhancing EC proliferation for better outcome post-DES.
In summary, we demonstrate that mTOR-free raptor activates PKCζ in mTOR-inhibited arterial EC. Through IRF-1-mediated regulation of gene expression, PKCζ suppressed HAEC growth by promoting apoptosis and cell cycle arrest. Further study may facilitate the development of effective strategies to specifically attenuate the suppressive effect of mTORi on EC, and thus to reduce the severe negative side effects of delayed endothelialization of DES.
Supplementary Material
Highlights.
Raptor contributed to PKCζ activation in mTOR-inhibited human aortic endothelial cells independent of mTOR complex 1.
PKCζ activated the JAK/STAT-1 and NF-κB signaling pathways, resulting in upregulation of IRF-1.
IRF-1 mediated the suppressive effects of mTOR inhibition on arterial endothelial cells in part through transcriptional regulation of the expression of CASP1 (encoding caspase 1) and CCND3 (encoding cyclin D3).
Endothelial-specific deletion of IRF-1 was associated with enhanced endothelial recovery and reduced intimal hyperplasia in a mouse femoral artery injury model.
Acknowledgments
We would like to thank Drs. Yong Xu and Teng Wu from Nanjing Medical University for the valuable help with SMC study.
Funding
This work was supported by National Natural Science Foundation of China [81870355, 81670410 to C.S.], Natural Science Foundation of the Jiangsu Higher Education Institutions of China [18KJA310001 to C.S.], and NIH and National Institute of Allergy and Infectious Diseases [AI047294 to S.I.S].
Abbreviations
- BMS
bare metal stents
- DES
drug-eluting stents
- mTOR
mammalian target of rapamycin
- mTORi
mTOR inhibition
- HAEC
human arterial endothelial cells
- IRF-1
interferon regulatory factor 1
- SMC
smooth muscle cells
- 4EBP-1
4E-binding protein-1
- PKC
protein kinase C
- AKT
protein kinase B
- SGK1
serum- and glucocorticoid-induced kinase 1
- FKBP12
FK-506-binding protein 12
- STAT1
signal transducer and activator of transcription 1
- NF-κB
nuclear factor binding near the κ light-chain gene in B cells
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL, Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials, The American journal of medicine 119(12) (2006) 1056–61. [DOI] [PubMed] [Google Scholar]
- [2].Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, et al. , Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents, The New England journal of medicine 356(10) (2007) 1030–9. [DOI] [PubMed] [Google Scholar]
- [3].Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R, One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis, JACC. Cardiovascular imaging 2(5) (2009) 625–8. [DOI] [PubMed] [Google Scholar]
- [4].Vezina C, Kudelski A, Sehgal SN, Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle, The Journal of antibiotics 28(10) (1975) 721–6. [DOI] [PubMed] [Google Scholar]
- [5].Laplante M, Sabatini DM, mTOR signaling in growth control and disease, Cell 149(2) (2012) 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoshii T, Kasada A, Hatakeyama T, Ohtani M, Tadokoro Y, Naka K, et al. , Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells, Proceedings of the National Academy of Sciences of the United States of America 111(10) (2014) 3805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tamahara T, Ochiai K, Muto A, Kato Y, Sax N, Matsumoto M, et al. , The mTOR-Bach2 Cascade Controls Cell Cycle and Class Switch Recombination during B Cell Differentiation, Molecular and cellular biology 37(24) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, et al. , The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation, Cell 120(6) (2005) 747–59. [DOI] [PubMed] [Google Scholar]
- [9].Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, et al. , Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity, The Journal of biological chemistry 279(35) (2004) 36490–6. [DOI] [PubMed] [Google Scholar]
- [10].Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, et al. , Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function, Genes to cells : devoted to molecular & cellular mechanisms 9(4) (2004) 359–66. [DOI] [PubMed] [Google Scholar]
- [11].Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. , Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB, Molecular cell 22(2) (2006) 159–68. [DOI] [PubMed] [Google Scholar]
- [12].Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, et al. , Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2, Cancer research 70(2) (2010) 621–31. [DOI] [PubMed] [Google Scholar]
- [13].Watanabe N, Sakakibara J, Hovanessian AG, Taniguchi T, Fujita T, Activation of IFN-beta element by IRF-1 requires a posttranslational event in addition to IRF-1 synthesis, Nucleic acids research 19(16) (1991) 4421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pizzoferrato E, Liu Y, Gambotto A, Armstrong MJ, Stang MT, Gooding WE, et al. , Ectopic expression of interferon regulatory factor-1 promotes human breast cancer cell death and results in reduced expression of survivin, Cancer research 64(22) (2004) 8381–8. [DOI] [PubMed] [Google Scholar]
- [15].Egwuagu CE, Li W, Yu CR, Che Mei Lin M, Chan CC, Nakamura T, et al. , Interferon-gamma induces regression of epithelial cell carcinoma: critical roles of IRF-1 and ICSBP transcription factors, Oncogene 25(26) (2006) 3670–9. [DOI] [PubMed] [Google Scholar]
- [16].Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, et al. , An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes, Nature 376(6541) (1995) 596–9. [DOI] [PubMed] [Google Scholar]
- [17].Gao J, Senthil M, Ren B, Yan J, Xing Q, Yu J, et al. , IRF-1 transcriptionally upregulates PUMA, which mediates the mitochondrial apoptotic pathway in IRF-1-induced apoptosis in cancer cells, Cell death and differentiation 17(4) (2010) 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kroger A, Stirnweiss A, Pulverer JE, Klages K, Grashoff M, Reimann J, et al. , Tumor suppression by IFN regulatory factor-1 is mediated by transcriptional down-regulation of cyclin D1, Cancer research 67(7) (2007) 2972–81. [DOI] [PubMed] [Google Scholar]
- [19].Xie RL, Gupta S, Miele A, Shiffman D, Stein JL, Stein GS, et al. , The tumor suppressor interferon regulatory factor 1 interferes with SP1 activation to repress the human CDK2 promoter, The Journal of biological chemistry 278(29) (2003) 26589–96. [DOI] [PubMed] [Google Scholar]
- [20].Fan X, Chen X, Feng Q, Peng K, Wu Q, Passerini AG, et al. , Downregulation of GATA6 in mTOR-inhibited human aortic endothelial cells: effects on TNF-alpha-induced VCAM-1 expression and monocytic cell adhesion, American journal of physiology. Heart and circulatory physiology 316(2) (2019) H408–H420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cerretti DP, Hollingsworth LT, Kozlosky CJ, Valentine MB, Shapiro DN, Morris SW, et al. , Molecular characterization of the gene for human interleukin-1 beta converting enzyme (IL1BC), Genomics 20(3) (1994) 468–73. [DOI] [PubMed] [Google Scholar]
- [22].Sun C, Wu MH, Yuan SY, Nonmuscle myosin light-chain kinase deficiency attenuates atherosclerosis in apolipoprotein E-deficient mice via reduced endothelial barrier dysfunction and monocyte migration, Circulation 124(1) (2011) 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhuang T, Liu J, Chen X, Pi J, Kuang Y, Wang Y, et al. , Cell-Specific Effects of GATA (GATA Zinc Finger Transcription Factor Family)-6 in Vascular Smooth Muscle and Endothelial Cells on Vascular Injury Neointimal Formation, Arteriosclerosis, thrombosis, and vascular biology 39(5) (2019) 888–901. [DOI] [PubMed] [Google Scholar]
- [24].Camici GG, Steffel J, Amanovic I, Breitenstein A, Baldinger J, Keller S, et al. , Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents, European heart journal 31(2) (2010) 236–42. [DOI] [PubMed] [Google Scholar]
- [25].Moss SC, Lightell DJ Jr., Marx SO, Marks AR, Woods TC, Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1, The Journal of biological chemistry 285(16) (2010) 11991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Andersen P, Pedersen MW, Woetmann A, Villingshoj M, Stockhausen MT, Odum N, et al. , EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells, International journal of cancer 122(2) (2008) 342–9. [DOI] [PubMed] [Google Scholar]
- [27].Moschonas A, Kouraki M, Knox PG, Thymiakou E, Kardassis D, Eliopoulos AG, CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-kappaB and of NF-kappaB-mediated de novo synthesis of IRF-1, Molecular and cellular biology 28(20) (2008) 6208–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Richter JD, Sonenberg N, Regulation of cap-dependent translation by eIF4E inhibitory proteins, Nature 433(7025) (2005) 477–80. [DOI] [PubMed] [Google Scholar]
- [29].Vollenbroker B, George B, Wolfgart M, Saleem MA, Pavenstadt H, Weide T, mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes, American journal of physiology. Renal physiology 296(2) (2009) F418–26. [DOI] [PubMed] [Google Scholar]
- [30].Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. , mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery, Cell 110(2) (2002) 163–75. [DOI] [PubMed] [Google Scholar]
- [31].Aghamohammadzadeh R, Zhang YY, Stephens TE, Arons E, Zaman P, Polach KJ, et al. , Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30(7) (2016) 2511–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ha JM, Yun SJ, Kim YW, Jin SY, Lee HS, Song SH, et al. , Platelet-derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1, Biochemical and biophysical research communications 464(1) (2015) 57–62. [DOI] [PubMed] [Google Scholar]
- [33].Huang S, Liu LN, Hosoi H, Dilling MB, Shikata T, Houghton PJ, p53/p21(CIP1) cooperate in enforcing rapamycin-induced G(1) arrest and determine the cellular response to rapamycin, Cancer research 61(8) (2001) 3373–81. [PubMed] [Google Scholar]
- [34].Zohlnhofer D, Nuhrenberg TG, Neumann FJ, Richter T, May AE, Schmidt R, et al. , Rapamycin effects transcriptional programs in smooth muscle cells controlling proliferative and inflammatory properties, Molecular pharmacology 65(4) (2004) 880–9. [DOI] [PubMed] [Google Scholar]
- [35].Wang Y, Zhao B, Zhang Y, Tang Z, Shen Q, Zhang W, et al. , Kruppel-like factor 4 is induced by rapamycin and mediates the anti-proliferative effect of rapamycin in rat carotid arteries after balloon injury, British journal of pharmacology 165(7) (2012) 2378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fielhaber JA, Han YS, Tan J, Xing S, Biggs CM, Joung KB, et al. , Inactivation of mammalian target of rapamycin increases STAT1 nuclear content and transcriptional activity in alpha4- and protein phosphatase 2A-dependent fashion, The Journal of biological chemistry 284(36) (2009) 24341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang C, Qin L, Manes TD, Kirkiles-Smith NC, Tellides G, Pober JS, Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2, The Journal of experimental medicine 211(3) (2014) 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horiuchi M, Yamada H, Akishita M, Ito M, Tamura K, Dzau VJ, Interferon regulatory factors regulate interleukin-1beta-converting enzyme expression and apoptosis in vascular smooth muscle cells, Hypertension 33(1) (1999) 162–6. [DOI] [PubMed] [Google Scholar]
- [39].Wu D, Pan P, Su X, Zhang L, Qin Q, Tan H, et al. , Interferon Regulatory Factor-1 Mediates Alveolar Macrophage Pyroptosis During LPS-Induced Acute Lung Injury in Mice, Shock 46(3) (2016) 329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim K, Qiang L, Hayden MS, Sparling DP, Purcell NH, Pajvani UB, mTORC1-independent Raptor prevents hepatic steatosis by stabilizing PHLPP2, Nature communications 7 (2016) 10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, Xu M, Ding X, Yan C, Song Z, Chen L, et al. , Protein kinase C controls lysosome biogenesis independently of mTORC1, Nature cell biology 18(10) (2016) 1065–77. [DOI] [PubMed] [Google Scholar]
- [42].Moscat J, Diaz-Meco MT, Albert A, Campuzano S, Cell signaling and function organized by PB1 domain interactions, Molecular cell 23(5) (2006) 631–40. [DOI] [PubMed] [Google Scholar]
- [43].Dainichi T, Hayden MS, Park SG, Oh H, Seeley JJ, Grinberg-Bleyer Y, et al. , PDK1 Is a Regulator of Epidermal Differentiation that Activates and Organizes Asymmetric Cell Division, Cell reports 15(8) (2016) 1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, et al. , Regulation of protein kinase C zeta by PI 3-kinase and PDK-1, Current biology : CB 8(19) (1998) 1069–77. [DOI] [PubMed] [Google Scholar]
- [45].Habib A, Karmali V, Polavarapu R, Akahori H, Cheng Q, Pachura K, et al. , Sirolimus-FKBP12.6 impairs endothelial barrier function through protein kinase C-alpha activation and disruption of the p120-vascular endothelial cadherin interaction, Arteriosclerosis, thrombosis, and vascular biology 33(10) (2013) 2425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ding RQ, Tsao J, Chai H, Mochly-Rosen D, Zhou W, Therapeutic potential for protein kinase C inhibitor in vascular restenosis, Journal of cardiovascular pharmacology and therapeutics 16(2) (2011) 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Leszczynski D, Joenvaara S, Foegh ML, Protein kinase C-alpha regulates proliferation but not apoptosis in rat coronary vascular smooth muscle cells, Life sciences 58(7) (1996) 599–606. [DOI] [PubMed] [Google Scholar]
- [48].Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, et al. , Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer, The American journal of pathology 154(1) (1999) 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim JY, Valencia T, Abu-Baker S, Linares J, Lee SJ, Yajima T, et al. , c-Myc phosphorylation by PKCzeta represses prostate tumorigenesis, Proceedings of the National Academy of Sciences of the United States of America 110(16) (2013) 6418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moscat J, Rennert P, Diaz-Meco MT, PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways, Cell death and differentiation 13(5) (2006) 702–11. [DOI] [PubMed] [Google Scholar]
- [51].Minhajuddin M, Fazal F, Bijli KM, Amin MR, Rahman A, Inhibition of mammalian target of rapamycin potentiates thrombin-induced intercellular adhesion molecule-1 expression by accelerating and stabilizing NF-kappa B activation in endothelial cells, J Immunol 174(9) (2005) 5823–9. [DOI] [PubMed] [Google Scholar]
- [52].Grange JJ, Baca-Regen LM, Nollendorfs AJ, Persidsky Y, Sudan DL, Baxter BT, Protein kinase C isoforms in human aortic smooth muscle cells, Journal of vascular surgery 27(5) (1998) 919–26; discussion 926–7. [DOI] [PubMed] [Google Scholar]
- [53].Magid R, Davies PF, Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta, Circulation research 97(5) (2005) 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.