Abstract
Background:
Approximately 47 million people in the United States have been diagnosed with arthritis. Autologous platelet-rich plasma (PRP) injections have been documented to alleviate symptoms related to knee osteoarthritis (OA) in randomized controlled trials, systematic reviews, and meta-analyses. Autologous bone marrow aspirate concentrate (BMC) injections have also emerged as a treatment option for knee OA, with a limited clinical evidence base.
Purpose:
To compare the efficacy of BMC to PRP for the treatment of knee OA regarding pain and function at multiple time points up to 12 months after an injection. We hypothesized that BMC will be more effective in improving outcomes in patients with knee OA.
Study Design:
Randomized controlled trial; Level of evidence, 2
Methods:
A total of 90 participants aged between 18 and 80 years with symptomatic knee OA (Kellgren-Lawrence grades 1-3) were randomized into 2 study groups: PRP and BMC. Both groups completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and subjective International Knee Documentation Committee (IKDC) questionnaires before and 1, 3, 6, 9, and 12 months after a single intra-articular injection of leukocyte-rich PRP or BMC.
Results:
There were no statistically significant differences in baseline IKDC or WOMAC scores between the 2 groups. All IKDC and WOMAC scores for both the PRP and BMC groups significantly improved from baseline to 1 month after the injection (P < .001). These improvements were sustained for 12 months after the injection, with no difference between PRP and BMC at any time point.
Conclusion:
Both PRP and BMC were effective in improving patient-reported outcomes in patients with mild to moderate knee OA for at least 12 months; neither treatment provided a superior clinical benefit. Autologous PRP and BMC showed promising clinical potential as therapeutic agents for the treatment of OA, and while PRP has strong clinical evidence to support its efficacy, BMC has limited support. This study did not prove BMC to be superior to PRP, providing guidance to clinicians treating OA. It is possible that the results were affected by patients knowing that there was no control group.
Registration:
NCT03289416 (ClinicalTrials.gov identifier).
Keywords: platelet-rich plasma, bone marrow aspirate, bone marrow concentrate, regenerative medicine, osteoarthritis
In the United States, it is estimated that 47 million people have been diagnosed with arthritis; 4.3 million have been diagnosed with isolated osteoarthritis (OA) of the knee, and knee arthritis accounts for 20% of disability claims.22 The prevalence of the disease continues to climb and is expected to double by 2030. In addition, 4 of 5 patients with OA have movement limitations.22 Currently, there are no curative treatments for OA. Nonsurgical treatment options include weight loss, oral medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, injectables such as corticosteroids and hyaluronic acid (HA), physical treatments such as rehabilitative therapy, unloading braces, assistive devices (ie, canes or walkers), activity modification, and genicular nerve radiofrequency ablation.11,32,45,48 Surgical options include arthroscopic surgery, osteotomy, and total joint replacement. However, expectations are not always met with current surgical options, and not all patients with knee OA are candidates for surgical treatments. For these and other reasons, many patients choose nonoperative treatments, and there has been great interest in injections classified as regenerative. There is a current unmet clinical need to improve the quality of life of patients suffering from all stages of OA, and for these patients, injection therapies have a developing role.
Although commonly used for the treatment of knee OA, the clinical efficacy of HA and corticosteroid injections to treat knee OA has been questioned.1 This has led to the development of additional injection options, such as autologous blood– and bone marrow–derived products. Leukocyte-poor platelet-rich plasma (LP-PRP) has preclinical basic science, randomized controlled comparative clinical, and meta-analysis studies supporting its safety and efficacy.§ Bone marrow aspirate concentrate (BMC), a more cellular and protein-rich product,6 has also emerged as a treatment. A benchtop comparative study of the components of PRP and BMC has suggested that BMC contains a higher number of cells that will culture/differentiate, often referred to as stem cells, and a higher concentration of potentially advantageous chemokines.6 A higher cellular component of cells that can be grown in culture suggests a higher potential for tissue regeneration and anti-inflammatory effects; however, clinical superiority remains theoretical.18 The full clinical potential of BMC is unclear, but studies have shown promise with preclinical animal studies for cartilage repair,41 clinical case series for the treatment of OA,8,9,18 and clinical studies suggesting a role in open implantation for cartilage repair.4,15,16,41
Clinical comparisons of PRP and BMC are lacking. The main objective of this study was to compare, with validated patient-reported outcomes, the effectiveness of BMC to PRP for the treatment of knee OA after a single injection. We hypothesized that BMC will outperform PRP at multiple time points after an injection.
Methods
Participants
This study was approved by the affiliated hospital’s institutional review board, which oversees the facility where the study was performed. Participants aged between 18 and 80 years with evidence of knee OA were screened for eligibility in the study (N = 110). All participants were informed of the experimental procedures, risks, and benefits of the study and provided written informed consent before the screening appointment. Participants were instructed not to take any prescription over-the-counter NSAIDs for 3 weeks before the screening appointment. NSAID use was not monitored or regulated after treatment.
Patients were screened with a 4-view radiographic series of the knee (long-leg, lateral, sunrise, and bilateral Rosenberg views), and they were included in the study if they had pain or swelling of the knee of at least 4 months in duration and a Kellgren-Lawrence grade24,27 between 1 and 3 on the radiographic evaluation. The population studied is typical of patients with knee OA who typically inquire about orthobiologic injections. Exclusion criteria included patients with a major mechanical axis deviation of more than 50% into either compartment (varus or valgus), a corticosteroid injection within 3 months or an HA injection within 6 months, or a history of any of the following medical conditions: diabetes, autoimmune disorders, disorders requiring immunosuppression, rheumatoid arthritis, hemophilic arthropathy, infectious arthritis, Charcot knee, Paget disease of the femur or tibia, history of cancer, ongoing infectious diseases, or significant cardiovascular, renal, or hepatic diseases.
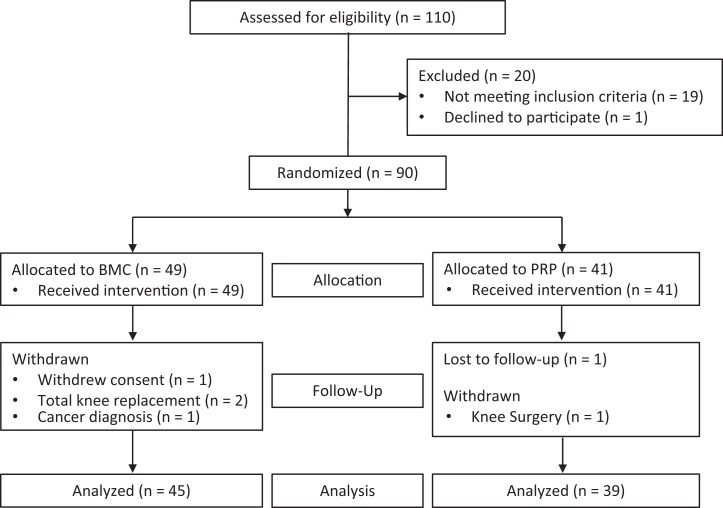
Participants were enrolled over the course of 4 years. There were 91 participants who met the inclusion and exclusion criteria; 1 declined to participate. A total of 90 participants were randomized using a computer-generated sequence into 2 groups: BMC (n = 49) and PRP (n = 41); enrollment was stopped at 90 participants. All participants received the allocated treatment (Figure 1). In the BMC group, 4 participants withdrew, and in the PRP group, 1 participant withdrew, while 1 was lost to follow-up, leaving 84 participants in the study. Participant characteristics are summarized in Table 1. There were no significant differences between the groups in these characteristics.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. BMC, bone marrow aspirate concentrate; PRP, platelet-rich plasma.
Table 1.
Participant Characteristicsa
| All (n = 84) | BMC (n = 45) | PRP (n = 39) | |
|---|---|---|---|
| Sex, male/female, n | 49/35 | 27/18 | 22/17 |
| Age, y | 54.1 ± 11.9 | 55.8 ± 11.3 | 52.2 ± 12.4 |
| Height, cm | 173.9 ± 11.7 | 175.2 ± 11.1 | 172.3 ± 12.3 |
| Weight, kg | 86.7 ± 20.5 | 89.5 ± 20.6 | 83.5 ± 20.3 |
| Body mass index, kg/m2 | 28.2 ± 5.7 | 27.7 ± 5.0 | 27.9 ± 5.8 |
| Kellgren-Lawrence grade | 1.8 ± 0.7 | 1.8 ± 0.7 | 1.9 ± 0.7 |
aValues are reported as mean ± SD unless otherwise indicated. BMC, bone marrow aspirate concentrate; PRP, platelet-rich plasma.
Patient-Reported Outcomes
Both groups completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)3 and subjective International Knee Documentation Committee (IKDC)19 questionnaires before any treatment. An a priori power analysis (G*Power 3.1.9.3) revealed that a sample size of 25 and 50 patients in each group was necessary to detect large effects using a power of 0.8 and alpha of 0.05 using the WOMAC and IKDC, respectively. Participants also completed the WOMAC and IKDC questionnaires at 1, 3, 6, 9, and 12 months after the allocated injection.
Injection Protocol
Group 1 (BMC group) received a single intra-articular injection of BMC. Group 2 (PRP group) received a single intra-articular injection of PRP. Because of the invasive nature of obtaining marrow aspirate, blinding of the participants and clinicians was not feasible. All injections and all associated procedures were performed in the clinic setting by either the first author (A.W.A.: 34 PRP, 40 BMC) or the last author (J.G.H.: 7 PRP, 9 BMC).
The harvest of blood for PRP production involved standard, antecubital venipuncture with a 60-mL syringe preloaded with 10 mL of sodium citrate anticoagulant. Blood was processed at the point of care with a dual-spin protocol/disposable (PurePRP; EmCyte) to make leukocyte-rich (LR-PRP), which is monocyte/lymphocyte rich and neutrophil poor. The blood was loaded into a first cylinder disposable and centrifuged for 1.5 minutes at 3800 rpm, according to the instructions for use. A 2-layer soft stack, that is, platelet plasma suspension above the red cell layer, was produced. The top platelet plasma suspension was aspirated off until red blood cells filled the aspiration pipe and was then loaded into a second disposable and centrifuged for 5 minutes at 3800 rpm, creating a platelet-poor plasma (PPP) top layer and platelet buffy coat at the bottom of the disposable. PPP was aspirated off, leaving approximately 7 mL of pure PRP. The plasma and platelet buffy coat were resuspended into the remaining plasma by swirling, and the final PRP, approximately 7 mL, was aspirated into the injection syringe.
For bone marrow harvest, two 30-mL syringes and a traditional 11-gauge, 11-cm Jamshidi needle (Ranfac) were prerinsed with heparin. The two 30-mL syringes were loaded with 5 mL of sodium citrate anticoagulant in each syringe. Aspiration was performed from a bone puncture at the posterior superior iliac spine with the Jamshidi needle. For bone marrow aspiration, participants were placed into the lateral decubitus position. The posterior superior iliac spine was localized with ultrasound and prepared with ChloraPrep (chlorhexidine gluconate; BD). The skin, subcutaneous tissues, and periosteum were anesthetized with 1% lidocaine, and no systemic analgesics or anxiolytics were required. The needle was used to puncture the posterior superior iliac spine, advanced 3 to 5 cm, and aspiration performed while withdrawing and rotating the needle. Aspiration was performed until the 30-mL mark was reached on the first syringe, and then the needle was advanced a second time in a divergent trajectory. Aspiration was repeated with the second 30-mL syringe while withdrawing and rotating the needle. Bone marrow was processed at the point of care with a dual-spin protocol/disposable (PureBMC; EmCyte). The bone marrow was loaded into a first cylinder disposable through a bone marrow aspiration filter, according to the instructions for use. It was then centrifuged for 2.5 minutes at 3800 rpm. This produced a 3-layer hard stack: platelet plasma suspension, early buffy coat, and red cell layer. The top plasma layer and 2-mL buffy coat were aspirated off and loaded into a concentrating accessory disposable. A second centrifuge was performed for 7 minutes at 3800 rpm, creating a PPP top layer and BMC buffy coat at the bottom of the disposable. PPP was aspirated off, leaving approximately 7 mL of plasma and the BMC buffy coat. The BMC buffy coat was reconstituted into the plasma by swirling, and the final BMC, approximately 7 mL, was loaded into the injection syringe. Intra-articular injections were performed as a standard sterile procedure with ultrasound guidance and a superolateral, parapatellar approach.23
This study was initiated before guidelines were issued for minimal reporting of biologic product studies33; the reporting standards were published near the conclusion of the study. For this reason, 4 participants, 3 in the BMC group and 1 in the PRP group, were selected to have cellular analysis of the product. For this analysis, a small sample (1 mL) of the whole blood or bone marrow aspirate and BMC or PRP was separated and sent to an independent laboratory for analysis (BSR Laboratories). The laboratory analysis included obtaining a complete blood count for all samples with the addition of flow cytometry for human CD34+ hematopoietic stem/progenitor analysis and colony-forming unit–fibroblast (CFU-F) for bone marrow aspirate and BMC after the cell culture protocol of the laboratory.
After the injection, participants were given follow-up care instructions that included no use of NSAIDs for at least 7 days and partial weightbearing on the limb for 2 to 3 days, followed by the initiation of a standard physical therapy program at 1 week after the injection for 4 weeks.
Statistical Analysis
Separate 2 (group) × 6 (time) mixed-design analyses of variance (ANOVAs) were used to detect differences between groups and among time points for the WOMAC and IKDC. If the Mauchly test of sphericity was statistically significant (P ≤ .05), the Huynh-Feldt adjustment was used to correct for the violation of sphericity. Statistical significance was set a priori at P < .05. All analyses were conducted using SPSS version 24.0 software (IBM).
Results
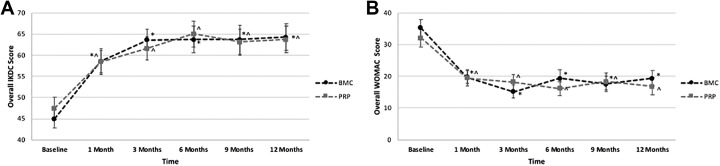
The mixed-design ANOVAs revealed a significant effect of time on the IKDC score (F3.79,310.88 = 31.928; P < .001; ηp 2 = 0.280), with no differences between the BMC and PRP groups. Both groups significantly improved from baseline to 1-month follow-up, and scores plateaued at 3 months, remaining consistent with no further significant improvement through 6, 9, and 12 months (Figure 2A and Table 2). For the BMC group, mean scores after 1 month improved by 30.0 points for the IKDC and 43.9 for the WOMAC, and for the PRP group, the scores improved by 23.2 points for the IKDC and 39.3 for the WOMAC.
Figure 2.
Overall (A) International Knee Documentation Committee (IKDC) scores and (B) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores versus time for the bone marrow aspirate concentrate (BMC) and platelet-rich plasma (PRP) treatment groups. *Significant difference from baseline for BMC (P < .05). ^Significant difference from baseline for PRP (P < .05).
Table 2.
IKDC and WOMAC Scoresa
| Baseline | 1 mo | 3 mo | 6 mo | 9 mo | 12 mo | |
|---|---|---|---|---|---|---|
| IKDC | ||||||
| BMC | 45.0 ± 14.2 | 58.5 ± 18.4b | 63.6 ± 16.7b | 63.7 ± 21.4b | 63.7 ± 22.9b | 64.3 ± 20.8b |
| 95% CI | 40.5-49.6 | 53.0-64.1 | 58.6-68.7 | 57.6-69.7 | 57.4-70.0 | 58.3-70.3 |
| ▵ from baseline, % | 30.0 | 41.3 | 41.6 | 41.6 | 42.9 | |
| PRP | 47.4 ± 16.6 | 58.4 ± 19.1b | 61.6 ± 17.5b | 65.0 ± 19.1b | 63.1 ± 19.2b | 63.7 ± 19.6b |
| 95% CI | 42.5-52.3 | 52.5-64.4 | 56.2-67.1 | 58.5-71.5 | 56.3-69.9 | 57.2-70.1 |
| ▵ from baseline, % | 23.2 | 30.0 | 37.1 | 33.1 | 34.4 | |
| WOMAC total | ||||||
| BMC | 35.3 ± 18.1 | 19.8 ± 14.3b | 15.2 ± 13.3b | 19.4 ± 18.6b | 17.6 ± 16.1b | 19.4 ± 16.2b |
| 95% CI | 29.9-40.6 | 15.2-24.3 | 11.0-19.4 | 14.5-24.3 | 12.7-22.6 | 14.5-24.3 |
| ▵ from baseline, % | 43.9 | 56.9 | 45.0 | 50.1 | 45.0 | |
| PRP | 32.1 ± 17.9 | 19.5 ± 16.3b | 18.2 ± 15.3b | 16.2 ± 13.6b | 18.4 ± 17.1b | 16.8 ± 16.9b |
| 95% CI | 26.4-37.9 | 14.6-24.4 | 13.7-22.8 | 11.0-21.5 | 13.1-23.7 | 11.5-22.1 |
| ▵ from baseline, % | 39.3 | 43.3 | 49.5 | 42.7 | 47.7 | |
| WOMAC pain | ||||||
| BMC | 7.0 ± 3.3 | 3.9 ± 3.4b | 3.2 ± 3.1b | 4.1 ± 4.0b | 3.2 ± 3.3b | 3.5 ± 3.1b |
| 95% CI | 6.0-8.1 | 2.9-4.9 | 2.3-4.1 | 3.1-5.1 | 2.2-4.2 | 2.5-4.4 |
| ▵ from baseline, % | 44.3 | 54.3 | 41.4 | 54.3 | 50.0 | |
| PRP | 6.2 ± 3.8 | 3.8 ± 3.3b | 3.5 ± 3.1b | 2.6 ± 2.7b | 3.5 ± 3.5b | 2.9 ± 3.1b |
| 95% CI | 5.1-7.3 | 2.8-4.9 | 2.5-4.5 | 1.5-3.7 | 2.4-4.5 | 1.9-3.9 |
| ▵ from baseline, % | 38.7 | 43.5 | 58.1 | 43.5 | 53.2 | |
| WOMAC stiffness | ||||||
| BMC | 3.8 ± 1.6 | 2.1 ± 1.5b | 1.8 ± 1.5b | 1.9 ± 1.7b | 2.1 ± 1.6b | 2.3 ± 1.6b |
| 95% CI | 3.3-4.4 | 1.7-2.5 | 1.3-2.2 | 1.4-2.4 | 1.6-2.6 | 1.8-2.8 |
| ▵ from baseline, % | 44.7 | 52.6 | 50.0 | 44.7 | 39.5 | |
| PRP | 3.4 ± 1.5 | 2.1 ± 1.4b | 1.9 ± 1.4b | 1.7 ± 1.4b | 1.8 ± 1.6b | 1.8 ± 1.5b |
| 95% CI | 2.9-3.9 | 1.6-2.6 | 1.5-2.4 | 1.2-2.3 | 1.3-2.3 | 1.3-2.3 |
| ▵ from baseline, % | 38.2 | 44.1 | 50.0 | 47.1 | 47.1 | |
| WOMAC function | ||||||
| BMC | 22.9 ± 13.2 | 12.9 ± 9.3b | 9.5 ± 9.3b | 12.5 ± 12.4b | 11.5 ± 11.1b | 12.8 ± 11.6b |
| 95% CI | 19.1-26.7 | 9.7-16.1 | 6.5-12.6 | 9.2-15.9 | 8.0-14.9 | 9.3-16.3 |
| ▵ from baseline, % | 43.7 | 58.5 | 45.4 | 49.8 | 44.1 | |
| PRP | 21.3 ± 12.5 | 12.7 ± 11.8b | 12.2 ± 11.4b | 11.2 ± 9.9b | 12.4 ± 12.2b | 11.3 ± 12.2b |
| 95% CI | 17.2-25.4 | 9.3-16.2 | 8.7-15.4 | 7.6-14.9 | 8.7-16.1 | 7.5-15.1 |
| ▵ from baseline, % | 40.4 | 42.7 | 47.4 | 41.8 | 46.9 | |
aValues are reported as mean ± SD unless otherwise indicated. BMC, bone marrow aspirate concentrate; IKDC, International Knee Documentation Committee; PRP, platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
bSignificant improvement from baseline (P < .05).
A significant effect of time was observed on all WOMAC subscales: WOMAC total (F3.88,318.09 = 29.165; P < .001; ηp 2 = 0.262), WOMAC pain (F4.18,342.65 = 24.735; P < .001; ηp 2 = 0.232), WOMAC stiffness (F4.18,342.88 = 32.306; P < .001; ηp 2 = 0.283), and WOMAC function (F4.00,327.80 = 25.389; P < .001; ηp 2 = 0.236). Both groups significantly improved on all WOMAC subscales from baseline to 1-month follow-up, with no further significant improvement in scores (Figure 2B and Table 2). Scores remained significantly improved between baseline and 3, 6, 9, and 12 months after both BMC and PRP injections.
Results from the composition analysis of the samples sent for independent review are presented in Table 3. The results of the cellular composition analysis showed an increase in platelet, white blood cell, CD34+, and CFU-F counts with concentration, with an expected decrease in the red blood cell count.
Table 3.
Results of Cellular Composition Analysisa
| Sample | Bone Marrow Aspirate | Bone Marrow Aspirate Concentrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RBC, ×106/mL | Platelet, ×106/mL | WBC, ×106/mL | CD34+, Cells/mL | CFU-F, Cells/mL | RBC, ×106/mL | Platelet, ×106/mL | WBC, ×106/mL | CD34+, Cells/mL | CFU-F, Cells/mL | |
| 1 | 3120 | 175 | 15.9 | 33,373 | 42 | 1020 | 492 | 41.6 | 90,305 | 194 |
| 2 | 2310 | 64 | 18.7 | 40,827 | 37 | 1250 | 337 | 46.6 | 112,558 | 86 |
| 3 | 3480 | 64 | 113,470 | 57 | 1130 | 410 | 309,910 | 136 | ||
| Whole Blood | Platelet-Rich Plasma | |||||||||
| RBC, ×106/mL | Platelet, ×106/mL | WBC, ×106/mL | Mono/Lym, ×106/mL | Gran, ×106/mL | RBC, ×106/mL | Platelet, ×106/mL | WBC, ×106/mL | Mono/Lym, ×106/mL | Gran, ×106/mL | |
| 4 | 4.03 | 238 | 8.5 | 1.1/2.0 | 5.3 | 0.12 | 1216 | 14.7 | 2.3/11.9 | 0.4 |
aCFU-F, colony-forming unit–fibroblast; Gran, granulocyte; Lym, lymphocyte; Mono, monocyte; RBC, red blood cell; WBC, white blood cell.
Discussion
The objective of this study was to compare the efficacy of PRP to BMC for the treatment of knee OA. This study showed significant improvement in WOMAC and IKDC scores for both the PRP and BMC groups as early as 4 weeks after the injection, and the improvement remained through to 12-month follow-up. The most important finding of this study is that BMC was not superior to PRP for the treatment of OA out to 12 months, contrary to our hypothesis. To our knowledge, this is the first direct clinical comparative study of PRP and BMC. For the BMC group, mean scores at 1-month follow-up improved by 30.0 points for the IKDC and 43.9 for the WOMAC, and for the PRP group, the scores improved by 23.2 points for the IKDC and 39.3 for the WOMAC. The improvement in WOMAC (minimal detectable change = 22.8) and IKDC (minimal detectable change = 13.7) scores was clinically significant for established values in OA.2,17
PRP has a pyramid of developmental work, starting with preclinical benchtop and animal studies, continuing with high level of evidence clinical trials, and ending with systematic reviews. In benchtop studies, PRP has clear mechanisms of action to improve the catabolic and inflammatory environment of OA.7,35,47 Animal studies have reflected benchtop principles, with histological and morphological findings documenting a beneficial effect on the synovium and cartilage.25,38,49
Clinical studies have established safety and efficacy for PRP, with comparison including HA and saline as controls.10,13,36,39,40,42,44 The body of evidence has progressed to meta-analyses and systematic reviews.5,14,29,30,46 One study for comparison, by Smith,44 involved United States Food and Drug Administration observations, employed stricter inclusion/exclusion criteria than the current study, and reported similar findings out to 12 months. Smith44 reported on 114 participants screened to yield 30 participants and then randomized into 2 groups. A series of 3 weekly injections of LP-PRP was compared with saline as a control; the WOMAC served as the primary efficacy outcome measure. No adverse events were reported, and at the conclusion, WOMAC scores for the participants receiving LP-PRP had improved by 78% from baseline, whereas scores for the placebo group had improved by only 7%.44 The current study illustrates similar improvement in WOMAC scores as reported by Smith. By contrast, however, the current study had less restrictive selection criteria, had a scenario more consistent with clinical practice, and studied a single injection instead of a series.
Systematic reviews and meta-analyses provide further evidence and are clarifying a consensus that PRP is an effective intra-articular treatment for knee OA.5,29–31,46 A 2017 systematic review of the literature found 29 well-designed studies including 26 evaluating knee OA and 3 evaluating hip OA.31 The compilation included 9 prospective randomized controlled trials (RCTs; 8 knee and 1 hip), 4 prospective comparative studies, 14 case series, and 2 retrospective comparative studies. As a control group, HA was used in 11 studies (7 RCTs, 2 prospective comparative studies, and 2 retrospective comparative studies). Only 2 RCTs, 1 for the knee and 1 for the hip, did not report the significant superiority of PRP compared with the control group that received HA. Also, 9 of 11 HA studies showed significantly better results in the PRP group.31 Conclusively, PRP has been shown consistently to be safe and effective for improvements in pain and function for patients with sustained benefit lasting ≥12 months.5,29–31,46 The current study further supports this conclusion. Additionally, in this study, BMC showed similar improvement as PRP out to 12 months.
Developing orthopaedic literature has produced opinions that LP-PRP is the preferred preparation for knee OA because variable results have been found with LR-PRP.12,28 Kon et al28 conducted a prospective comparative trial in 150 patients comparing a series of 3 LR-PRP injections to 2 different preparations of HA. The PRP group showed stronger and longer efficacy than the HA groups. The study involved 5-mL injection volumes, a 600% increase in platelets from baseline whole blood, and a 2-spin preparation method. Leukocyte values with differentials were not reported.28 These authors followed with an RCT comparing PRP to a high–molecular weight HA in 192 participants and did not find superior clinical improvement in the PRP group.12 In that study, platelets were increased by a factor of 4.6 and leukocytes by a factor of 1.1. Leukocyte differentials were not reported.12 Our study involved LR-PRP, which was prepared to be 7 mL, monocyte/lymphocyte rich, and neutrophil poor. The findings of the current study reaffirm leukocyte differential and product volume as 2 variables that affect PRP effectiveness and warrant further study. The volume reported by Smith44 in his RCT of LP-PRP was 3 to 8 mL.
Evidence firmly supporting the clinical use of BMC for knee OA is lacking. Overall, 2 studies from a single data registry cohort and an additional case series are the only studies supporting its clinical efficacy.8,9,26 Centeno et al8 reported on 840 procedures involving BMC, divided into 2 groups: 616 procedures performed on 518 patients in the first group and 224 procedures performed on 163 patients in the second group. The first group received an injection of hypertonic dextrose solution, followed 2 to 5 days later by an injection of bone marrow concentrate, PPP, and platelet lysate. The platelet lysate was created by storing buffy coat–based PRP at –20°C. The second group received the same injections as the first group in addition to a lipoaspirate point-of-care product injected with the BMC solution. The lipoaspirate component was prepared from 5 to 15 mL of lipoaspirate. Outcomes included the numerical pain rating scale score at an average of 6.5 months and a lower extremity functional scale (LEFS) score at an average of 6 months. Centeno et al reported moderate survey response rates, 66% and 74%, and included patients with symptomatic knee OA, determined by magnetic resonance imaging, broadly defined as any abnormality of the cartilage, bone, or meniscus. The response rate for the last available LEFS score was 33.3% at an average of 6.2 months for the first group and 40.6% at 5.7 months for the second group (by contrast, the current study had 95% of treated participants reporting at 12 months). The LEFS score was of a possible 80 points, and the study reported a respective increase of 7.9 and 9.8 in the 2 groups. The mean numerical pain rating scale score, of a possible 10, decreased from 4.0 to 2.6 and from 4.3 to 3.0 in the 2 groups. Adverse events were reported as 6.0% and 8.9% in the 2 groups.8
A single-blind, placebo-controlled study investigating BMC for knee OA involved participants with bilateral knee OA: one knee receiving BMC and the other knee receiving saline as a control. This study, by Shapiro et al,43 involved 25 participants. Similar to the current study, the harvest included 52 mL of bone marrow aspirate, 8 mL of anticoagulant citrate dextrose solution-A (ACDA), and an automated concentration system. The BMA technique used by Shapiro et al included bilateral iliac crests and small-volume aspirates, a harvest technique advocated by Hernigou et al20,21 to maximize cellular content. By contrast, the technique utilized in the present study involved a posterior superior iliac spine harvest and a divergent technique using 30-mL syringes and a traditional aspiration needle. A comparative study of techniques has suggested no difference in the cell composition of products with the Hernigou et al technique versus the divergent technique used in this study.34
The low CFU-F aspiration numbers in this study were concentrated by the centrifugation device. Several reasons may have contributed to the low number of CFU-F counts, including age and sex variability among participants and the traditional needle. The BMA needle used in this study has a design configuration with an open end, with a potential for peripheral blood contamination. Furthermore, marrow processing revealed a relatively high red blood cell count. Through 6 months, Shapiro et al43 found improvement in both groups but no significant difference between BMC and saline using Osteoarthritis Research Society International measures and the visual analog scale. Their study concluded that BMC cannot be recommended for the regular treatment of OA. Our outcomes similarly showed that a single BMC injection for mild to moderate knee OA was not superior to a single PRP injection for improvement in pain and function, questioning the value of the increased invasiveness of the BMC procedure when compared with PRP. We do not have any data to support whether PRP or BMC injections are superior to placebo. In clinical practice, we have noted trends regarding the reimbursement of biologic products. HA injections are covered by most insurance companies, PRP costs on average US $714,37 and BMC costs on average US $3000.
One limitation of this study was the lack of blinding. Neither the participants nor practitioners were blinded to the treatment, given the invasive nature of bone marrow aspiration; this could have introduced reporting bias in the patient-reported outcomes. A second limitation was that there was no control group, and the patients knew that there was no control group; therefore, their survey responses may have been biased. Previous studies have shown a dramatic placebo effect when analyzing the effects of biologics.43 An additional critique is that a comparative arm of steroids or HA was not provided and would have further improved this study’s real-world applicability. The length of follow-up was 12 months, and it is possible that a longer follow-up would show a difference between BMC and PRP outcomes. Our intent is to continue to follow this cohort for another year.
A third limitation was the lack of product sampling throughout the study. A recent consensus statement has developed a minimal reporting requirement for biologic studies33; however, this study was initiated before the consensus statement. While we tested 4 samples, it is hard to develop any comparison of the PRP to BMC product differences in cellular compositions. Also, our experience with this study highlighted variability when sending samples for culturing. There is a chance that viability data were affected by sending samples to a remote laboratory. Since this study’s conclusion, we have brought in house the ability to test samples with an automated hemocytometer, flow cytometry, microscopy, and culturing. This study included patients with essentially no joint space narrowing (Kellgren-Lawrence grade 1) and excluded patients with changes in underlying bone shapes (Kellgren-Lawrence grade 4); therefore, we cannot conclude whether either technique would be effective for severe OA. Additionally, the study was not powered to compare the results of the treatment for different Kellgren-Lawrence grades. Finally, we used LR-PRP, whereas subject leader experts have been advocating LP-PRP. Results may be different with LP-PRP.
Conclusion
This study demonstrated that both PRP and BMC were effective in improving patient-reported outcomes in patients with mild to moderate knee arthritis for at least 12 months. Through the 12-month period, neither treatment provided a superior clinical benefit.
Acknowledgment
The authors acknowledge Hillary Plummer for her assistance with preparing this article.
Footnotes
Final revision submitted October 21, 2019; accepted October 25, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: The Andrews Research & Education Foundation received funding and material support from EmCyte to support this study. A.W.A. has received educational and research support from Arthrex and CGG Medical; consulting fees from Arthrex, Bioventus, Ceterix, MicroAire, and Blue Belt Technologies; speaking fees from Arthrex and Smith & Nephew; royalties from Arthrex; and hospitality payments from Procedural Orthopedics. P.A.E. is employed by EmCyte. J.R.A. has received speaking fees from Arthrex and Halyard Health; has received consulting fees from Theralase Technologies, Bauerfeind, and Physiotherapy Associates/Select Medical; and has stock/stock options in FastHealth, Patient Connection, Connective Orthopaedics, and Contessa Health. J.G.H. has received consulting fees from Carticept Medical, Fujifilm SonoSite, and Ferring Pharmaceuticals; speaking fess from Ferring Pharmaceuticals; honoraria from Tenex Health, Avanos Medical, and Fidia Pharma; and educational support from Tenex Health. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Baptist Hospital (study No. 542260-1).
References
- 1. American Academy of Orthopaedic Surgeons. Summary of recommendations In: Jevsevar DS. ed. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd ed Rosemont, Illinois: American Academy of Orthopaedic Surgeons; 2013;21(9):571–576. [DOI] [PubMed] [Google Scholar]
- 2. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384–391. [DOI] [PubMed] [Google Scholar]
- 3. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 4. Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(suppl 2):2–11. [DOI] [PubMed] [Google Scholar]
- 5. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213–2221. [DOI] [PubMed] [Google Scholar]
- 6. Cassano JM, Kennedy JG, Ross KA, Fraser EJ, Goodale MB, Fortier LA. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc. 2018;26(1):333–342. [DOI] [PubMed] [Google Scholar]
- 7. Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;96(5):423–429. [DOI] [PubMed] [Google Scholar]
- 8. Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. [DOI] [PubMed] [Google Scholar]
- 11. Davis T, Loudermilk E, DePalma M, et al. Twelve-month analgesia and rescue, by cooled radiofrequency ablation treatment of osteoarthritic knee pain: results from a prospective, multicenter, randomized, cross-over trial. Reg Anesth Pain Med. 2019;44(14):499–506. [DOI] [PubMed] [Google Scholar]
- 12. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–1582. [DOI] [PubMed] [Google Scholar]
- 13. Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. [DOI] [PubMed] [Google Scholar]
- 14. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42(3):648–657. [DOI] [PubMed] [Google Scholar]
- 17. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee subjective knee form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891–902. [DOI] [PubMed] [Google Scholar]
- 18. Hauser RA, Orlofsky A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: a case series. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226–234. [DOI] [PubMed] [Google Scholar]
- 20. Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. [DOI] [PubMed] [Google Scholar]
- 21. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. [DOI] [PubMed] [Google Scholar]
- 22. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. [DOI] [PubMed] [Google Scholar]
- 23. Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84(9):1522–1527. [DOI] [PubMed] [Google Scholar]
- 24. Kellgren JH, Lawrence JS. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs. Vol 2 Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 25. Khatab S, van Buul GM, Kops N, et al. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am J Sports Med. 2018;46(4):977–986. [DOI] [PubMed] [Google Scholar]
- 26. Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505–1511. [DOI] [PubMed] [Google Scholar]
- 27. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. [DOI] [PubMed] [Google Scholar]
- 29. Lai LP, Stitik TP, Foye PM, Georgy JS, Patibanda V, Chen B. Use of platelet-rich plasma in intra-articular knee injections for osteoarthritis: a systematic review. PM R. 2015;7(6):637–648. [DOI] [PubMed] [Google Scholar]
- 30. Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49(10):657–672. [DOI] [PubMed] [Google Scholar]
- 31. Laver L, Marom N, Dnyanesh L, Mei-Dan O, Espregueira-Mendes J, Gobbi A. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage. 2017;8(4):341–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu CY, Li CD, Wang L, et al. Function scores of different surgeries in the treatment of knee osteoarthritis: a PRISMA-compliant systematic review and network-meta analysis. Medicine (Baltimore). 2018;97(21):e10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Joint Surg Am. 2017;99(10):809–819. [DOI] [PubMed] [Google Scholar]
- 34. Oliver K, Awan T, Bayes M. Single- versus multiple-site harvesting techniques for bone marrow concentrate: evaluation of aspirate quality and pain. Orthop J Sports Med. 2017;5(8):2325967117724398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osterman C, McCarthy MB, Cote MP, et al. Platelet-rich plasma increases anti-inflammatory markers in a human coculture model for osteoarthritis. Am J Sports Med. 2015;43(6):1474–1484. [DOI] [PubMed] [Google Scholar]
- 36. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. [DOI] [PubMed] [Google Scholar]
- 37. Piuzzi NS, Ng M, Kantor A, et al. What is the price and claimed efficacy of platelet-rich plasma injections for the treatment of knee osteoarthritis in the United States? J Knee Surg. 2019;32(9):879–885. [DOI] [PubMed] [Google Scholar]
- 38. Saito M, Takahashi KA, Arai Y, et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol. 2009;27(2):201–207. [PubMed] [Google Scholar]
- 39. Sanchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910–913. [PubMed] [Google Scholar]
- 40. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. [DOI] [PubMed] [Google Scholar]
- 41. Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25(12):1391–1400. [DOI] [PubMed] [Google Scholar]
- 42. Say F, Gurler D, Yener K, Bulbul M, Malkoc M. Platelet-rich plasma injection is more effective than hyaluronic acid in the treatment of knee osteoarthritis. Acta Chir Orthop Traumatol Cech. 2013;80(4):278–283. [PubMed] [Google Scholar]
- 43. Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O’Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45(1):82–90. [DOI] [PubMed] [Google Scholar]
- 44. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–891. [DOI] [PubMed] [Google Scholar]
- 45. Steadman JR, Briggs KK, Pomeroy SM, Wijdicks CA. Current state of unloading braces for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):42–50. [DOI] [PubMed] [Google Scholar]
- 46. Tietze DC, Geissler K, Borchers J. The effects of platelet-rich plasma in the treatment of large-joint osteoarthritis: a systematic review. Phys Sportsmed. 2014;42(2):27–37. [DOI] [PubMed] [Google Scholar]
- 47. van Buul GM, Koevoet WL, Kops N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362–2370. [DOI] [PubMed] [Google Scholar]
- 48. Vannabouathong C, Bhandari M, Bedi A, et al. Nonoperative treatments for knee osteoarthritis: an evaluation of treatment characteristics and the intra-articular placebo effect. A systematic review. JBJS Rev. 2018;6(7):e5. [DOI] [PubMed] [Google Scholar]
- 49. Yin WJ, Xu HT, Sheng JG, et al. Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in treating rabbit knee osteoarthritis. Med Sci Monit. 2016;22:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]