Abstract
Sake lees (Sake-kasu) are the sediments of Japanese sake brewing process from fermented rice with Aspergillus oryzae and yeasts. Sake lees contain various enzymes and metabolites derived from the Sake starter culture, and expected to add aroma, flavor and softness to sausages. We investigated the effects of Sake lees supplementation on fermented dry sausage characteristics over an aging period of 35 days. Sake lees supplementation significantly accelerated sarcoplasmic and myofibrillar protein decomposition and increased peptide and free amino acid content compared to untreated sausage meat. Sake lees significantly acidified the sausages, enhanced their sour taste, and influenced their acceptability. Sake lees supplementation also significantly improved the hardness of the final product and conferred a preferable flavor to it. These results suggest that the various enzymes and compounds in Sake lees improve the flavor and texture of fermented dry sausages.
Keywords: Food science, Food analysis, Microbiology, Sake lees, Fermented dry sausage, Protein degradation, Protease, Aroma
Food science, Food analysis, Microbiology, Sake lees, Fermented dry sausage, Protein degradation, Protease, Aroma.
1. Introduction
Fermented dry sausage is a traditional meat product widely consumed in European countries, which is characterized by a unique flavor and a sour taste. The fermented dry sausage is prepared by grinding raw meat, seasoning and curing it, packing it into casings, drying it, and fermenting it either with natural microflora or commercial starter cultures. Present-day, commercial starter cultures such as Lactobacillus plantarum have replaced the natural microflora used in fermentation. Fermentation is a traditional method of meat preservation, which suppresses the growth of pathogenic and spoilage bacteria due to the acidity (pH 4.59–5.94) fostered by the lactic acid production by beneficial bacteria (Aro Aro et al., 2010). This practice ensures the safety and prolongs the shelf life of fermented meat products such as sausages.
Proteolysis and lipolysis are the most important biochemical changes that occur during the aging of fermented dry sausages (Aro Aro et al., 2010; Casquete et al., 2011). Exogenous enzymes from the starter cultures and endogenous proteolytic and lipolytic enzymes catalyze the steps in fermentation process (Díaz et al., 1997). The peptide, free amino acid and fatty acid by-products account for the characteristic flavor and taste of fermented sausages (Casquete et al., 2011). However, the characteristic unpleasant odor and tangy taste of fermented sausages could reduce consumer acceptability. Moreover, the dry and hard texture of fermented sausage might make mastication difficult for the elderly and people with problematic oral and dental conditions (Lee et al., 2004). Therefore, it would be beneficial to develop a fermented sausage with a pleasant flavor and odor and a favorable texture as it would appeal to range of consumers.
Sake lees (Sake-kasu) are the white paste derived from the filtered residue of Japanese Sake (rice wine) mash. Sake lees are a rich source of proteins, peptides, amino acids, carbohydrates, fiber, fat, ash, and vitamins (Tsutsui et al., 1998). Consuming sake lees as toasted cakes and soup has a long history. Nowadays, Sake lees are added to pickled vegetables and fish to impart a fruity aroma and flavor in them. They are also used as flavoring agents for beverages, confectionery and various processed foods (Tsutsui et al., 1998). Sake lees contain various enzymes and metabolites derived from molds and yeasts such as Aspergillus oryzae and Saccharomyces cerevisiae used in Japanese Sake brewing. Aspergillus oryzae produces acid proteases and acid carboxypeptidases (Kiyono et al., 2013). Saccharomyces cerevisiae generates carboxypeptidases, proteinases, and aminopeptidases (Van Den Hazel et al., 1996). Sake lees and their constituents have been reported to improve hepatic lipid accumulation (Kubo et al., 2017), reduce blood pressure (Saito et al., 1994), mitigate hyperalgesia (Shimizu et al., 2020), prevent allergic rhinitis-like symptoms (Kawamoto et al., 2011), and suppress acute alcohol-induced liver injury (Izu et al., 2006). Several studies reported reductions in the viable counts of aerobic bacteria in raw ham treated with Sake lees (Matsuoka et al., 2006) and altered texture in squid meat cured with Sake lees based on sensory tests, texturometry, and protein decomposition analyses (Shimomura et al., 1992). Despite the well-established biotechnological and nutritional advantages of Sake lees, their applications in food processing have seldom been explored.
We hypothesized that the incorporation of Sake lees in fermented sausage could accelerate protein degradation, enhance the availability of flavor components such as amino acids, and impart a Japanese Sake aroma that might improve palatability. Sake lees supplementation in dry sausage processing could add value to the final product and repurpose a plentiful industrial by-product. The aim of this study was to evaluate the effects of Sake lees supplementation on physicochemical properties and sensory attributes of fermented sausages during the aging process.
2. Materials and methods
2.1. Materials
Beef and pork thigh meat were purchased from Sasaki Cattle & Meat Suppliers Co., Ltd. (Hokkaido, Japan) and Yamasa Meat (Hokkaido, Japan), respectively. Sake lees and starter culture (Lactobacillus plantarum + Staphylococcus carnosus) (Rowu-Ferm; Indasia Gewürzwerk GmbH, Georgsmarienhütte, Germany) were purchased from Tanaka Sake Brewing Co. Ltd. (Hokkaido, Japan) and Ono Shoji Co. Ltd. (Chiba, Japan), respectively.
2.2. Sausage production and sample preparation
Beef and pork thigh meat (each 10 kg) were cut into cubes 5–6 cm thick and cured for 1 d at 4 °C in 2% (w/w) sodium chloride, 0.01% (w/w) sodium nitrite, 0.5% (w/w) glucose, 0.3% (w/w) onion powder, 0.2% (w/w) garlic powder, 0.6% (w/w) coarsely ground black pepper, and 0.4% (w/w) coarsely ground white pepper. Pork back fat (5 kg) was cut into cubes 2 cm thick and cured under the same conditions and using the same ingredients as the beef and pork thigh meat. The cured meats and fat were stored at -30 °C until further processing. Semi-frozen cured beef and pork (2 kg each) were either mixed with 200 g Sake lees or left untreated. The cured meats were ground using a silent cutter (OMS-780KS; OHMICHI Co. Ltd., Gunma, Japan) with 540 g cured back fat and 3.6 g starter culture dissolved in 30 mL distilled water. The batter was then stuffed into fibrous casings (4.3 cm × 25 cm; VISKASE® Co. Inc., IL, USA). The sausages were smoked in a smokehouse (SUB-800C; OHMICHI Co. Ltd., Gunma, Japan) at 20 °C for 1 h and dried at 20 °C for 5 h. The sausages were then dried and aged in an aging chamber (Bio TRON; NK Systems, Osaka, Japan) for 35 d, under the following conditions: 20 °C and 90% relative humidity (RH) (days 0–7); 15 °C and 90% RH (days 8–13); 15 °C and 88% RH (day 14); and 15 °C and 85% RH (days 15–35). When the sausage weight had declined to <60% of its original at day 0, the sausages were vacuum-packaged and aged until day 35. Sausage samples with or without Sake lees were randomly taken for analysis on days 0, 7, 14, 21, 28, and 35.
2.3. Microbial analysis
Microbiological analyses were performed on the samples. Ten grams sausage was homogenized in 90 mL sterilized water in a stomacher (Exnizer 400; Organo Corp., Tokyo, Japan). Serial dilutions of the homogenate were prepared and cultured in selective media to enumerate specific bacterial species by the viable plate count method. Total aerobic and facultative anaerobic bacterial counts were enumerated on Pearlcore® plate count agar (Eiken Chemical Co. Ltd., Tochigi, Japan), while lactic acid bacteria (LAB) and staphylococci were cultured on Man, Rogosa and Sharpe (MRS) agar (CM 0361; Oxoid, Hampshire, England) and Pearlcore® mannitol salt agar (MSA) (Eiken Chemical Co. Ltd., Tochigi, Japan), respectively. Coliforms and salmonella were cultured on Chromocult® coliform agar (Merck KGaA, Darmstadt, Germany) and deoxycholate hydrogen sulfide lactose (DHL) agar (Merck, Darmstadt, Germany), respectively. The coliform and DHL agar plates were incubated under aerobic conditions at 37 °C for 24 h. All other agar plates were incubated under aerobic conditions at 37 °C for 48 h. The colonies were visually counted and recorded as decadic logarithm of colony forming units per gram sausage sample (log10 CFU/g).
The presence of Staphylococcus aureus in the sausages was determined by a tube coagulase test according to the method of Mizobuchi et al. (1994), with slight modifications. Yellow bacterial colonies harvested from the day-35 sausage sample homogenates were cultured on MSA agar and inoculated into rabbit plasma (Eiken Chemical Co. Ltd., Tochigi, Japan) to detect coagulation.
2.4. pH determination
The pH of the homogenate prepared for microbiological analysis was measured in triplicate with a calibrated portable pH meter (F-51; Horiba Ltd., Kyoto, Japan) according to the method of Aro Aro et al., 2010.
2.5. Residual nitrite analysis
Samples of untreated fermented sausages and those supplemented with Sake lees were analyzed for residual nitrite content according to the analytical method prescribed by the Japanese Food Safety Standard (Standard Methods of Analysis in Food Safety Regulation, Food Additive Edition (2003), Japan Food Hygiene Association) and AOAC Official Method 973.31, with a slight modification. The absorbance of the test solution was measured in a spectrophotometer (V-630 BIO; Jasco Corp., Tokyo, Japan) at 540 nm.
2.6. Instrumental color evaluation
The effect of Sake lees supplementation on the color properties of the fermented sausages samples were evaluated by spectrophotometry (Chroma Meter Minolta CM-2600D; Minolta, Tokyo, Japan) using a D65 light source and a standard 10° observer angle relative to the untreated sausage samples. The Commission Internationale de'l Eclairage (CIE) L* (lightness), a* (redness), and b* (yellowness) values were measured on both surfaces of three untreated- and three Sake lees treated sausage sections 5 mm thick.
2.7. Characterization of proteins
2.7.1. Protein extraction and fractionation
Sarcoplasmic and myofibrillar proteins were extracted from fermented sausages according to the method of Toldrá et al. (1993), with modifications. Four grams of minced meat was excised from the center of the fermented sausages, mixed with 40 mL of 0.03 M potassium phosphate buffer (pH 8.0), and homogenized on ice using a Physcotron (Niti-On Co., Ltd., Chiba, Japan). The homogenate was centrifuged at 10,000 × g and 0 °C for 20 min. The supernatant was passed through a Milpap filter paper (Azumi Filter Paper Co. Ltd., Osaka, Japan). The filtrate was stored as the sarcoplasmic protein fraction. The precipitate was homogenized in 40 mL of 8 M urea containing 0.1% β-mercaptoethanol and was centrifuged at 10,000 × g and 0 °C for 20 min. The supernatant was passed through a Milpap filter paper and the filtrate was stored as the myofibrillar protein fraction. Both fractions were separated using a dialyzing membrane with distilled water for 6 h followed by 0.05 M ethylenediaminetetraacetic acid (EDTA) and 0.05 M tris hydrochloride (Tris-HCl) buffer (pH 8.0) overnight. After dialysis, the sarcoplasmic and myofibrillar fraction concentrations were determined by the improved Lowry method (1951) using a DC protein assay kit (Bio-Rad Laboratories, Inc., California, USA). Then the sarcoplasmic and myofibrillar protein fractions were diluted in 0.05 M EDTA and 0.05 M Tris-HCl buffer (pH 8.0) to final concentration of 0.5 mg/mL and 1.0 mg/mL, respectively. One milliliter from each diluted fraction was mixed with an equal volume of sample buffer (2.5 mM Tris-HCl containing 0.02% sodium dodecyl sulfate (SDS), 0.01% β-mercaptoethanol, 0.1% glycerol, and 0.005% bromophenol blue) and incubated at 95 °C for 2 min. Sake lees protein was prepared as a control by extraction under the same conditions as the sausage samples. The protein content was adjusted to 0.5 mg/mL as previously described (Shimomura et al., 1992).
2.7.2. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE (Laemmli, 1970) was used with modifications to separate the proteins from the aforementioned sarcoplasmic and myofibrillar fractions. Separating and stacking gels (12.5% and 4% polyacrylamide, respectively) were prepared and the protein fractions were loaded onto them, along with a protein standard (pre-stained MP-0120 protein marker, low range; Sigma-Aldrich Corp., St. Louis, MO, USA) in each electrophoretic run. Electrophoresis was conducted in a Bio-Rad Power Pac HC™ system (Bio-Rad Laboratories, Hercules, CA, USA) at 10 mA for the stacking gel and 20 mA for the separating gel. The gels were stained for 1 h in 0.2% Coomassie Brilliant Blue R-250 containing 50% methanol and 10% acetic acid. The gels were de-stained for 12 h in a mixture of 5% methanol and 7.5% acetic acid. The resultant protein fraction bands were scanned and their densities were quantified using Image J 1.52a software (National Institute of Health, Bethesda, MD, USA).
2.8. Peptide content
The peptide content of the fermented sausages supplemented with Sake lees was measured as previously described (Aro Aro et al., 2010), with slight modifications. Five grams of sausage obtained on days 0, 7, 14, 21, 28, and 35 of the aging period was homogenized with 50 mL ultrapure water (Milli-Q, Merck KGaA, Darmstadt, Germany) using a Physcotron. The homogenate was centrifuged at 10,000 × g and 4 °C for 20 min, and the supernatant was filtered using a filter paper (No. 5C; Toyo Roshi Kaisha Ltd., Tokyo, Japan). Four grams filtrate was mixed with an equal volume of 4% trichloroacetic acid (TCA), incubated at 37 °C, and filtered again. This filtrate served as the 2% TCA soluble fraction.
A 0.5 mL fraction of the 2% TCA soluble fraction was mixed with 2.4 mL distilled water and 1.0 mL Lowry reagent (2% sodium carbonate in 0.1 N sodium hydroxide; 2.7% sodium potassium tartrate; 1% copper (II) sulfate pentahydrate; 100/1/1, v/v/v) and incubated at 37 °C for 15 min. The mixture was combined with 0.1 mL of 1 N Folin-Ciocalteu reagent (SAJ, Tokyo, Japan) and incubated at 37 °C for 30 min. The absorbance of the mixture was measured in a spectrophotometer (V-630 BIO; JASCO Applied Science, MD, USA) at 660 nm. The peptide content was calculated on days 0, 7, 14, 21, 28, and 35 of the aging period by interpolation from a bovine serum albumin (BSA) standard curve. The peptide content was recorded as mg/100 g sausage.
2.9. Free amino acid content
For free amino acid analysis, samples were prepared as previously described by Aro Aro et al., 2010, with slight modifications. The 2% TCA soluble fraction containing a known peptide concentration (Subsection 2.8) was used as the sample solution. The sample solution was processed in a high-speed amino acid analyzer (L-8800F; Hitachi High-Tech Science Co., Tokyo, Japan) by the ninhydrin method (Moore and Stein, 1948). The total and free amino acid contents were calculated on days 0, 7, 14, 21, 28, and 35 of the aging period by interpolation from a standard curve for amino acid mixture type H (Wako Pure Chemical Industries Ltd., Osaka, Japan) and reported as mg/100 g sausage.
2.10. Sensory analysis
Sensory analysis was conducted according to the guidelines of the Declaration of Helsinki, and our protocol was approved by the Obihiro University of Agriculture and Veterinary Medicine Ethics Review Committee on Research Involving Human Subjects (approval number: 2019-07). Informed consent was obtained from the panelists via a sensory questionnaire.
Sixty-one untrained panelists performed a two-point discrimination test for fermented sausage hardness and sourness and a two-point preference test for fermented sausage odor and total acceptability. Untreated and Sake lees-supplemented sausages aged for 35 d were used in sensory analysis. Water was provided to the panelists to rinse their mouths between samples.
2.11. Statistical analysis
All data except those for the sensory analysis were expressed as the means ± SE of three independent experiments performed in triplicate. Data were analyzed using the F-test and Student's t-test, and P < 0.05 was considered statistically significant. For the sensory analysis data, a binomial test was performed with P < 0.05 and P < 0.01 as the acceptance limits. One-sided- and two-sided tests were used for the two-point discrimination and preference assays, respectively. Data were analyzed using JMP® v. 13.0 (SAS Institute Inc., Cary, NC, USA).
3. Results and discussion
3.1. Microbiological analysis
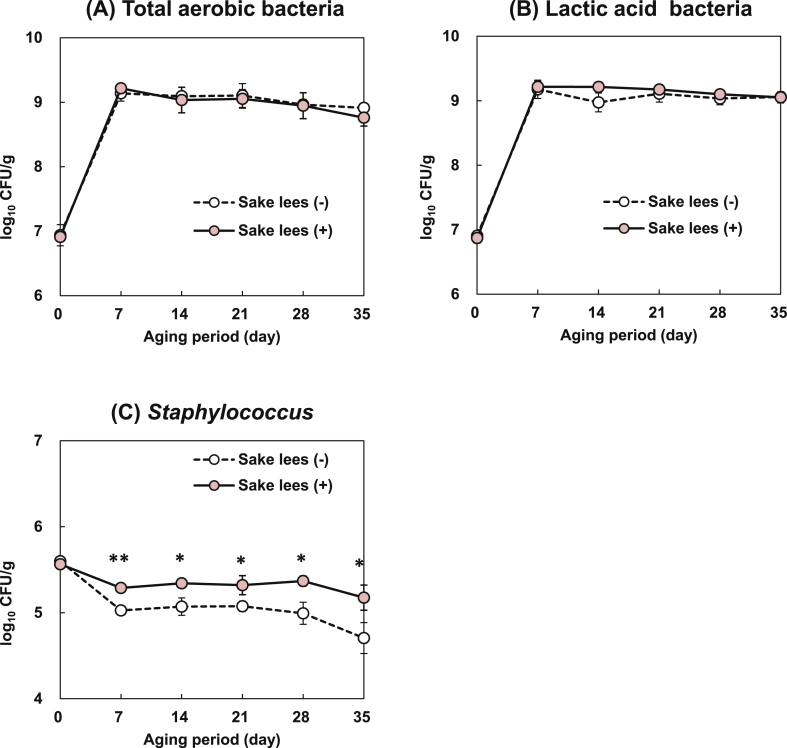
Total aerobic bacteria, LAB, and Staphylococcus counts in fermented sausages are shown in Figure 1. The initial total aerobic bacterial counts were 6.94 ± 0.09 and 6.91 ± 0.02 log10 CFU/g sausage without (-) and with (+) Sake lees, respectively (Figure 1A). By day 7 of the aging period, the aerobic bacterial counts had increased to 9.14 ± 0.07 and 9.22 ± 0.03 log10 CFU/g sausages (Sake lees (-) and (+), respectively) and remained nearly constant until day 35. Throughout the rest of the aging period, Sake lees supplementation did not affect the total aerobic bacterial count in the fermented sausages. Aerobic bacteria play important roles in sausage quality and may contribute to the development of the characteristic flavor (Montel et al., 1998) (Figure 1).
Figure 1.
Counts of (A) total aerobic bacteria, (B) lactic acid bacteria, and (C) Staphylococcus in fermented sausages with (+) or without (-) Sake lees supplementation during the aging period. Data are means ± SE of three independent experiments performed in triplicate. *P < 0.05, **P < 0.01 vs. Sake lees (-) group.
The LAB counts were 6.91 ± 0.01 and 6.87 ± 0.04 log10 CFU/g sausage at day 0 and 9.18 ± 0.08 and 9.22 ± 0.05 log10 CFU/g sausage by day 7 (Sake lees (-) and (+), respectively) (Figure 1B). By day 14, the LAB count was higher in the Sake lees (+) sausages than Sake lees (-) sausages. Nevertheless, the difference was not significant (P = 0.057). LAB is known to develop flavor and taste while enhancing the shelf life and microbial safety of fermented sausages due to the rapid acidification ability (Leroy and De Vuyst, 2004). The relatively higher LAB counts in Sake lees (+) suggest that this material fosters LAB growth, and the development, quality and safety of fermented sausages.
The Staphylococcus counts on day 0 were 5.60 ± 0.01 and 5.56 ± 0.01 log10 CFU/g sausage for Sake lees (-) and (+), respectively. From days 7 to day 35, a significantly higher (P < 0.05) Staphylococcus counts were observed in Sake lees (+) than the Sake lees (-) (Figure 1C). In contrast, the negative coagulase test results for the day 35 samples confirmed that the Sake less supplemented sausages were free of S. aureus endotoxin. As shown in Figure 1C, the Staphylococcus count was significantly higher in the Sake lees (+) than the Sake lees (-) sausages from day 7 onwards. However, this evaluation might have indicated the growth and development of the Staphylococcus carnosus, starter culture in both Sake lees (-) and (+) sausages. Therefore, Sake lees inhibited the growth of pathogenic S. aureus and facilitated the proliferation of coagulase-negative Staphylococcus (CNS). Coagulase negative Staphylococcus has a large variety of subdominant metabolic activities such as the production of flavor and aroma components in fermented meat (Sánchez Mainar et al., 2017). Thus, the measured increase in CNS caused by Sake lees supplementation may have contributed to the development of the sausage taste and flavor.
Table 1 shows the coliform and Salmonella counts in the fermented sausages with (+) and without (-) Sake lees during the aging period. These parameters are indicators of meat contamination. The coliform counts in the Sake lees (+) and Sake lees (-) sausage samples were <300 CFU/g in both Sake lees (-) and (+) sausages at day 0. However, they gradually decreased to <30 CFU/g between days 7 and 14. From day 21 onwards, no coliforms were detected (Table 1). Escherichia coli was not detected in Sake lees (-) or (+) sausages throughout the aging period. The Salmonella counts in the sausage samples at day 0 were <30 CFU/g and <300 CFU/g in the Sake lees (-) and (+) sausages, respectively. They were <30 CFU/g for Sake lees (-) and (+) sausages between days 7 and 14. No salmonellae were detected from day 21 onwards Sake lees supplementation did not markedly affect the coliform or Salmonella counts in the fermented sausages. Throughout the aging period, the coliform and Salmonella counts for the Sake lees (-) and (+) sausages remained within the food safety standards thresholds (≤100 CFU/g (E. Coli) and negative/zero (Salmonella) in uncooked meat products) prescribed by the Japanese Food Sanitation Act. The aforementioned results suggested that the sausages supplemented with Sake lees were prepared hygienically and fit for human consumption (Table 1).
Table 1.
Coliform, Escherichia coli, and Salmonella counts in fermented sausages with (+) or without (-) Sake lees supplementation during the aging period.
| Sake lees | Aging period (day) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | ||
| Coliforms | - | <300 | <30 | <30 | ND | ND | ND |
| + | <300 | <30 | <30 | ND | ND | ND | |
| Escherichia coli | - | ND | ND | ND | ND | ND | ND |
| + | ND | ND | ND | ND | ND | ND | |
| Salmonella | - | <30 | <30 | <30 | ND | ND | ND |
| + | <300 | <30 | <30 | ND | ND | ND | |
Data are presented as <300, <30 CFU/g, and ND (not detected) and were obtained by a conservative estimate of three independent experiments per aging period.
3.2. pH
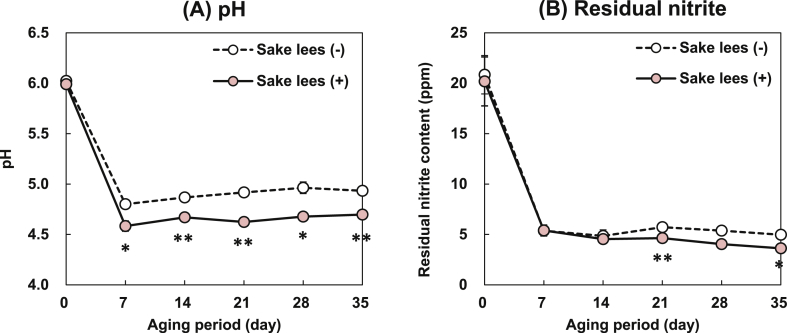
pH variation in the fermented sausages during the aging period is an important parameter that determines the microbial growth and development. The initial pH values of the sausages (day 0) with (+) or without (-) Sake lees supplementation were 6.02 ± 0.01 and 5.99 ± 0.02, respectively (Figure 2A). By day 7, however, the pH had decreased in the Sake lees (-) and (+) sausages (4.80 ± 0.02 and 4.58 ± 0.05, respectively) and continued to decline until day 35. Zhang et al. (2017) showed that inoculation of sausage meat with a LAB starter culture caused a pH reduction of fermented sausages, as the LAB proliferated and produced lactic acid. Increases in sausage meat LAB counts were observed in this study by day 7 (Figure 1B). Thus, the observed pH reduction may have been the result of LAB-mediated lactic acid generation. Previous reports showed that Salmonella and E. coli were eliminated from fermented sausages at the low pH (5.66) created by fermented sausages treated with LAB starter culture (Cenci-Goga et al., 2012). Similarly, the pH reduction observed in the present study (Figure 2A) might have sufficed to suppress the growth of coliforms, E. coli, and Salmonella throughout the aging period (Table 1). Koji mold (A. oryzae) used in Sake production is also present in Sake lees (Kitagaki and Kitamoto, 2013). It might have contributed to the relatively low pH of Sake lees (+) sausages. Zapelena et al., (1999) reported low pH levels in dry fermented sausages treated with protease from A. oryzae and starter culture compared to those treated with the starter culture alone. The substantial decrease in pH observed after day 7 in Sake lees-supplemented sausages might have been the result of enzymes derived from the A. oryzae present in the Sake lees (Figure 2).
Figure 2.
Changes in (A) pH and (B) residual nitrite content in fermented sausages with (+) or without (-) Sake lees supplementation during the aging period. Data are means ± SE of three independent experiments performed in triplicate. *P < 0.05, **P < 0.01 vs. Sake lees (-) group.
3.3. Residual nitrite content
Figure 2B shows the residual nitrite content in the fermented sausages with (+) and without (-) Sake lees during the aging period. Šojić et al. (2019) reported that nitrite has antimicrobial properties and is a curing agent for processed sausages. However, nitrite reacts with certain biogenic amines to form volatile N-nitrosamines (Wei et al., 2009) that were shown to induce tumors in several organs of experimental animal species (Lijinsky, 1999). Therefore, the residual nitrite level in the foodstuff must be ≤70 ppm to comply with the safety standards of the Food Sanitation Act of Japan. On day 0, the nitrite levels were 20.84 ± 1.90 and 20.18 ± 2.43 ppm in Sake lees (-) and (+) fermented sausages, respectively, were the highest for the entire aging period. By day 7, however, nitrite content had decreased to 5.37 ± 0.48 and 5.39 ± 0.54 ppm in Sake lees (-) and (+) sausages, respectively. The nitrite levels continued to decline in both sausage types from day 14 onwards. An earlier study showed that the residual nitrite content in cured meat was decreased by the addition of Lactobacillus plantarum to the product (Darmadji and Izumimoto, 1994), due to its nitrite reductase activity (Paik and Lee, 2014). The reduction in the residual nitrite level and the LAB count in sausages during the aging period in this study also exhibited a trend similar to the previous findings. Moreover, nitrite was markedly lower in Sake lees (+) sausage than Sake less (-) sausage by days 21 and 35. Further, previous reports showed that staphylococci also possessed nitrite reductase and/or nitrate reductase activity and were known to be highly efficient nitrate-reducing organisms (Gøtterup et al., 2008). The higher staphylococci count (Figure 1C) and the lower residual nitrite level observed in Sake lees (+) sausages might suggest residual nitrite might have been degraded by staphylococcal nitrite reductase activities compared to Sake lees (-) sausages.
3.4. Instrumental color evaluation
As shown in Table 2, L* (lightness), a* (redness), and b* (yellowness) did not significantly differ between the Sake lees (-) and (+) sausages throughout the aging period (P ≥ 0.211). Previous reports showed that the L*a*b* systems provide the best correlations between visual and objective assessments of color in meat products (Ferreira et al., 1994). Our results have suggested that the Sake lees supplementation did not affect visual color of sausages (Table 2).
Table 2.
Instrumental color evaluation (CIE lab L*, a*, and b*) of fermented dry sausage with (+) or without (-) Sake lees supplementation during the aging period.
| Sake lees | Aging period (day) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | ||
| L* | - | 47.97 ± 1.63 | 50.07 ± 1.91 | 48.96 ± 2.43 | 44.93 ± 3.07 | 41.97 ± 2.12 | 40.91 ± 2.44 |
| + | 48.77 ± 1.20 | 52.03 ± 2.20 | 50.13 ± 2.22 | 45.50 ± 1.73 | 43.04 ± 1.79 | 42.25 ± 1.44 | |
| a* | - | 7.41 ± 0.64 | 10.12 ± 0.19 | 9.23 ± 0.44 | 10.60 ± 0.96 | 9.60 ± 1.22 | 9.38 ± 0.91 |
| + | 8.00 ± 0.32 | 9.46 ± 0.42 | 9.95 ± 0.87 | 9.39 ± 1.04 | 8.84 ± 0.98 | 8.89 ± 1.15 | |
| b* | - | 7.15 ± 0.74 | 10.12 ± 0.19 | 6.74 ± 1.22 | 5.79 ± 0.18 | 7.04 ± 0.69 | 5.99 ± 1.04 |
| + | 7.37 ± 0.14 | 9.46 ± 0.42 | 8.40 ± 0.75 | 6.93 ± 0.75 | 8.70 ± 1.02 | 6.81 ± 0.78 | |
Data are means ± SE of three independent experiments performed in triplicate. L*, lightness; a*, redness; b*, yellowness.
3.5. Characterization of proteins
We performed SDS-PAGE analyses to evaluate the effects of Sake lees supplementation on protein degradation and to identify the sarcoplasmic and myofibrillar fraction protein patterns in Sake lees (-) and (+) sausages on days 0, 7, and 35 (Figures 3A and 3B). Figure 3A shows the number of signals above the 110-kDa band had decreased in the Sake lees (+) and (-) samples by days 7 and 35 compared to day 0, for the sarcoplasmic fraction. Signals for the protein fractions ~110 kDa in size were observed in the Sake lees (+) sausage samples on days 7 and 35 but were absent in the Sake lees (-) sausage samples on the same days. Moreover, a ~33 kDa signal was detected only in the Sake lees (+) sausage on day 35. In the Sake lees (+) sausages, there was a strong myoglobin (MYG) signal (17 kDa) broken considerably at day 7 (Figure 3A). The intensities of the myosin heavy chain (MHC) signals (220 kDa) gradually decreased during the aging process (Figure 3B) and were higher for the Sake lees (+) sausage than the Sake lees (-) sausage on days 0, 7, and 35. The intensities of the actin (A) signals (45 kDa) were also lower for the Sake lees (+) sausage than Sake lees (-) sausage on days 7 and 35. Therefore, we can suggest that the changes occurred in the patterns of sarcoplasmic and myofibrillar protein in the sausages supplemented with Sake lees might be attributed to the fact that this material contains A. oryzae which encodes up to 134 different proteases (Machida et al., 2005; Kobayashi et al., 2007). Shimomura et al. (1992) reported that the proteins such as MHC and actin in squid muscle preserved in Sake lees extract for 2 d had decomposed to fragments of relatively lower molecular size. No such degradation was observed after the squid muscle had been soaked in precooked Sake lees extract, which suggested active enzymes in the raw Sake lees may have decomposed the MHC and actin in the squid muscle (Shimomura et al., 1992). Thus, the present study suggests that raw Sake lees supplementation was a source of active enzymes that could have accelerated protein degradation and fermentation in the dry sausages (Figure 3).
Figure 3.
Molecular weight fractions of proteins, peptides and total amino acids in fermented sausages with (+) or without (-) Sake lees supplementation during the aging period. Representative SDS-PAGE images of (A) sarcoplasmic and (B) myofibrillar proteins on days 0 and 35. M, protein standard marker; S, Sake lees sample; MHC, myosin heavy chain; A, actin; MYG, myoglobin. (C) Peptide and (D) total free amino acid content in the sausages. Data are means ± SE of three independent experiments performed in triplicate. **P < 0.01 vs. Sake lees (-) group. Total amino acid content is expressed as the sum of 17 amino acids (Thr, threonine; Ser, serine; Gly, glycine; Ala, alanine; Lys, lysine; Pro, proline; Asp, aspartic acid; Glu, glutamic acid; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; His, histidine; Arg, arginine; Tyr, tyrosine; Cys, cysteine).
3.6. Peptide and free amino acid content
Since we have observed a prominent protein degradation in the sausages supplemented with Sake lees, we measured the peptide and free amino acid contents during the aging period. As shown in Figure 3C, the peptide content in Sake lees (+) sausages (473.9 ± 24.4 mg/100 g) was significantly higher than that of the Sake lees (-) (324.0 ± 15.0 mg/100 g) on day 0 (P < 0.01). Saito et al. (1994) reported that both Sake and Sake lees contain various peptides. Therefore, the relative difference in peptide content between Sake lees (-) and (+) sausages on day 0 may be explained by the inherent peptide content of the Sake lees itself. The peptide content of the Sake lees (-) and (+) sausages on day 7 had dramatically increased to 694.7 ± 39.8 mg/100 g and 1,038.6 ± 18.6 mg/100 g, respectively, and the difference between the Sake lees (-) and (+) sausages was also significant (P < 0.01). Between days 14 and 35, the peptide content was significantly higher in the Sake lees (+) sausages than the Sake lees (-) sausage. As shown in Figures 3A and 3B, the presence of several sarcoplasmic and myofibrillar protein fraction signals suggested considerable protein decomposition in the sausages supplemented with Sake lees. Various proteases derived from A. oryzae had been reported to be present in Sake lees (Machida et al., 2005; Kobayashi et al., 2007) and further the acid proteases expressed by A. oryzae were reported to be stable at pH 2.5–6.5 (Vishwanatha et al., 2009). Therefore, the acid proteases in the Sake lees may have degraded the proteins into peptides throughout the fermented sausage aging period.
The total free amino acid content was significantly higher in the Sake lees (+) sausages than the Sake lees (-) sausages throughout the aging period (P < 0.01; Figure 3D). On day 0, the free amino acid content in the Sake lees (+) sausages was 1.7 times higher than it was in the Sake lees (-) sausages. On the other hand, the free amino acid content in the Sake lees (+) sausages had increased by 2.2–2.4-fold compared with the Sake lees (-) sausages between day 7 and 35. It was reported that Sake lees contain intrinsic amino acids (Tsutsui et al., 1998). However, Sake lees supplementation was expected to accelerate free amino acid production in fermented sausages as this material contains microbial proteases (Figure 3D).
Table 3 categorizes 17 amino acids identified in the sausage samples on day 0 and 35 into four taste groups (sweet, umami and sour, bitter, and other). For the Sake lees (+) and Sake lees (-) sausages, the levels of all amino acids except Arg had increased by day 35 relative to their levels at day 0. On day 35, the Asp content was 3.39-fold higher in the Sake lees (+) sausages than the Sake lees (-) sausages. Asp is generated by aspartic protease, carboxypeptidase, and aminopeptidase from A. oryzae (Kiyono et al., 2013; Rao et al., 1998) and by carboxypeptidase, proteinase, and aminopeptidase from S. cerevisiae (Van Den Hazel et al., 1996). It was previously reported that Sake lees contain the aforementioned enzymes (Kiyono et al., 2013). Thus, the Sake lees might have participated in the protein degradation and the observed increases in Asp and other amino acids in the supplemented sausages. The increases in Asp and other free amino acids may have contributed to the sour and umami taste of Sake lees-supplemented sausages. In contrast, only Arg decreased in the Sake lees (+) and Sake lees (-) sausages by day 35 compared to day 0. Various lactic acid bacteria metabolize arginine to citrulline and ammonia via the arginine deiminase pathway (Poolman, 1993). The Lactobacillus plantarum starter culture used in the present study may have reduced the Arg content in the sausage meat. The levels of the other 16 amino acids had increased in the Sake lees (+) and (-) sausages by day 35. In addition, the total levels of the amino acids belonging to each taste category (sweet, umami and sour, bitter, and other) had increased especially in the Sake lees (+) sausages (Table 3).
Table 3.
Amino acid content associated with various taste classes in the fermented sausages with (+) or without (-) Sake lees supplementation on days 0 and 35 of the aging period.
| Taste | Amino acid | Day 0 |
Day 35 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sake lees |
Sake lees |
||||||||
| (-) | (+) | Ratio (+/-) | P value (-) vs (+) | (-) | (+) | Ratio (+/-) | P value (-) vs (+) | ||
| Sweet | Thr | 11.03 ± 0.82 | 19.17 ± 0.54 | 1.74 | 0.0012 | 79.10 ± 6.48 | 168.57 ± 6.23 | 2.13 | 0.0006 |
| Ser | 9.24 ± 0.43 | 17.54 ± 0.28 | 1.90 | <0.0001 | 49.77 ± 2.08 | 126.78 ± 2.80 | 2.55 | <0.0001 | |
| Gly | 14.92 ± 0.93 | 17.62 ± 0.74 | 1.18 | 0.0851 | 61.73 ± 3.19 | 109.87 ± 3.48 | 1.78 | 0.0005 | |
| Ala | 31.71 ± 1.40 | 45.86 ± 1.40 | 1.45 | 0.0020 | 165.86 ± 4.55 | 295.14 ± 7.58 | 1.78 | 0.0001 | |
| Lys | 16.93 ± 0.75 | 27.29 ± 0.48 | 1.61 | 0.0003 | 109.54 ± 4.46 | 302.29 ± 3.94 | 2.76 | <0.0001 | |
| Pro | 4.97 ± 0.36 | 8.40 ± 0.38 | 1.69 | 0.0029 | 34.63 ± 1.31 | 67.00 ± 5.78 | 1.93 | 0.0008 | |
| Total | 88.79 ± 1.94 | 135.88 ± 1.67 | 1.53 | <0.0001 | 500.63 ± 17.52 | 1,069.64 ± 19.83 | 2.14 | <0.0001 | |
| Umami and sour | Asp | 3.23 ± 0.31 | 9.24 ± 0.50 | 2.86 | 0.0005 | 38.07 ± 4.00 | 128.94 ± 6.33 | 3.39 | 0.0003 |
| Glu | 26.97 ± 4.80 | 41.78 ± 4.09 | 1.55 | 0.0787 | 219.79 ± 26.01 | 462.33 ± 38.01 | 2.10 | 0.0062 | |
| Total | 30.20 ± 5.11 | 51.01 ± 4.22 | 1.69 | 0.0348 | 257.86 ± 29.98 | 591.27 ± 44.34 | 2.29 | 0.0034 | |
| Bitter | Val | 8.18 ± 0.50 | 19.01 ± 0.32 | 2.32 | <0.0001 | 86.93 ± 4.10 | 191.92 ± 2.69 | 2.21 | <0.0001 |
| Met | 5.38 ± 0.37 | 10.07 ± 0.30 | 1.87 | 0.0006 | 49.52 ± 2.07 | 113.76 ± 1.67 | 2.30 | <0.0001 | |
| Ile | 5.43 ± 0.41 | 13.04 ± 0.17 | 2.40 | <0.0001 | 59.75 ± 3.27 | 152.65 ± 2.24 | 2.55 | <0.0001 | |
| Leu | 10.75 ± 0.75 | 24.25 ± 0.41 | 2.26 | <0.0001 | 149.05 ± 5.38 | 333.12 ± 2.24 | 2.23 | <0.0001 | |
| Phe | 9.72 ± 0.21 | 22.31 ± 0.71 | 2.30 | <0.0001 | 98.40 ± 4.45 | 200.59 ± 1.62 | 2.04 | <0.0001 | |
| His | 6.29 ± 0.25 | 9.80 ± 0.02 | 1.56 | 0.0002 | 34.78 ± 0.93 | 66.30 ± 1.70 | 1.91 | <0.0001 | |
| Arg | 18.51 ± 2.12 | 28.56 ± 1.65 | 1.54 | 0.0201 | 7.93 ± 0.37 | 23.57 ± 0.35 | 2.97 | <0.0001 | |
| Total | 64.27 ± 3.85 | 127.04 ± 3.31 | 1.98 | 0.0002 | 486.36 ± 17.54 | 1,081.90 ± 8.38 | 2.22 | <0.0001 | |
| Other | Tyr | 7.58 ± 0.71 | 15.02 ± 0.85 | 1.98 | 0.0026 | 41.06 ± 8.80 | 100.49 ± 15.68 | 2.45 | 0.0298 |
| Cys | 3.13 ± 0.10 | 4.93 ± 0.20 | 1.58 | 0.0014 | 10.07 ± 0.31 | 18.89 ± 0.71 | 1.88 | 0.0003 | |
| Total | 193.97 ± 6.18 | 333.88 ± 3.92 | 1.72 | <0.0001 | 1,295.97 ± 41.46 | 2,862.19 ± 58.08 | 2.21 | <0.0001 | |
The amino acid content is expressed in mg/100 g fermented sausage. Data are means ± SE of three independent experiments performed in triplicate. Seventeen amino acids were categorized into the taste groups sweet, umami and sour, bitter, and other. Thr, threonine; Ser, serine; Gly, glycine; Ala, alanine; Lys, lysine; Pro, proline; Asp, aspartic acid; Glu, glutamic acid; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; His, histidine; Arg, arginine; Tyr, tyrosine; Cys, cysteine.
3.7. Sensory analysis
To determine whether the microbial and physicochemical changes in the sausages caused by Sake lees supplementation affected its sensory attributes, two-point discrimination and preference tests were performed by 61 panelists (Figure 4). The two-point discrimination assay revealed a significant difference in hardness between the Sake lees (-) and Sake lees (+) sausages (P < 0.01). Most panelists rated Sake lees (-) sausages as “hard” compared to the Sake less (+) sausages (Figure 4A). This result is consistent with previous reports whose sensory evaluation showed that Sake lees treatment in reduced squid meat hardness (Shimomura et al., 1992). Myofibrillar proteins such as MHC and actin associated with meat texture were degraded by Sake lees supplementation during the aging period. This process may have accounted for the comparatively soft texture of the Sake lees-supplemented sausages (Figure 3B). However, Sake lees supplementation also enhanced the sour taste of fermented sausages (Figure 4A). As shown in Figure 2A, a significant decrease in pH was observed in the Sake lees (+) sausages during the aging period. The relatively higher Asp and Glu contents in the Sake lees (+) sausages may also have contributed to their sour and umami taste. The observed increased in these amino acids may be explained by accelerated protein degradation and, therefore, augmented Asp and Glu production (Table 3). In the two-point (odor) preference assay, two-thirds of the panelists preferred Sake lees (+) sausages and reported significant differences between them and the Sake lees (-) sausages (Figure 4B). Sake lees may contain volatile flavor components such as isobutanol, isoamyl acetate, ethyl acetate, ethyl caproate, and others found in Japanese Sake (Kang et al., 2016). For this reason, Sake lees supplementation could have contributed a fruity aroma to the fermented sausages. However, the Sake lees (+) sausages had lower overall acceptability than the Sake lees (-) sausages possibly because the former had relatively higher sourness (Figure 4B). Over 85% of the panelists felt that the Sake lees (+) sausages were sour compared to the Sake lees (-) sausages (Figure 4).
Figure 4.
Binomial sensory analysis of fermented sausages with (+) or without (-) Sake lees supplementation. (A) Two-point discrimination for hardness and sourness (one-sided test). (B) Two-point preference test for odor and overall acceptability (two-sided test). Histograms represent the number of panelists (n = 61) preferring one type of sausage over the other. *P < 0.05, **P < 0.01 vs. Sake lees (-) group.
4. Conclusion
In this study, dry sausages were supplemented with Sake lees during fermentation. The effects of this treatment were evaluated based on the microbiological, physicochemical, and sensory properties of the sausages over a 35-d aging period. Sake lees supplementation accelerated protein degradation and increased the peptide and free amino acid content (especially Asp, which contributes to umami and sour tastes) in the sausages throughout the aging period. Moreover, Sake lees supplementation also improved hardness and imparted a preferred odor to the sausage. Various enzymes derived from Sake koji, yeast, and other constituents of the Sake lees may have contributed to the flavor and texture of sausages. Thus, we can conclude that Sake lees is a hygienic ingredient and functional food that can effectively improve the texture, odor, and safety of fermented dry sausages. On the other hand, Sake lees increased the sourness of the sausages and reduced their overall acceptability. For this reason, further research is needed to improve the sour taste resulted by Sake lees addition, by optimizing the amount of Sake lees supplement and/or adding a suitable taste-masking agents to the sausage.
Declarations
Author contribution statement
Nana Mikami: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yoshiro Tsukada: Conceived and designed the experiments; Performed the experiments.
Samanthi W. Pelpolage: Analyzed and interpreted the data; Wrote the paper.
Kyu-Ho Han, Michihiro Fukushima, Kenichiro Shimada: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Aro Aro J.M., Nyam-Osor P., Tsuji K., Shimada K., Fukushima M., Sekikawa M. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010;119:279–285. [Google Scholar]
- Casquete R., Benito M.J., Martín A., Ruiz-Moyano S., Córdoba J.J., Córdoba M.G. Role of an autochthonous starter culture and the protease EPg222 on the sensory and safety properties of a traditional Iberian dry-fermented sausage “salchichón”. Food Microbiol. 2011;28:1432–1440. doi: 10.1016/j.fm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Cenci-Goga B.T., Rossitto P.V., Sechi P., Parmegiani S., Cambiotti V., Cullor J.S. Effect of selected dairy starter cultures on microbiological, chemical and sensory characteristics of swine and venison (Dama dama) nitrite-free dry-cured sausages. Meat Sci. 2012;90:599–606. doi: 10.1016/j.meatsci.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Darmadji P., Izumimoto M. Effects of chitosan and nitrite on the properties of fermented meat. Anim. Sci. Technol. 1994;65:636–646. [Google Scholar]
- Díaz O., Fernandez M., De Fernando G.D., De la Hoz L., Ordoñez J.A. Proteolysis in dry fermented sausages: the effect of selected exogenous proteases. Meat Sci. 1997;46:115–128. doi: 10.1016/s0309-1740(97)00013-2. [DOI] [PubMed] [Google Scholar]
- Ferreira V.L.P., Fernandes S.V., Yotsuyanagi K. The colour of chicken and pork meat loaf with added cured bovine blood as evaluated by the Rab, Hunter Lab, L’, a*, b’ and X Y Z CIE systems. Rev. Esp. Cienc. Tecnol. Aliment. 1994;34:311–322. [Google Scholar]
- Gøtterup J., Olsen K., Knøchel S., Tjener K., Stahnke L.H., Møller J.K. Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Sci. 2008;78:492–501. doi: 10.1016/j.meatsci.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Izu H., Shobayashi M., Manabe Y., Goto K., Iefuji H. Sake yeast suppresses acute alcohol-induced liver injury in mice. Biosc. Biotech. Biochem. 2006;70:2488–2493. doi: 10.1271/bbb.60216. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Kaneoke M., Ohkouchi K., Amano Y., Takaoka Y., Kume K. Sake lees fermented with lactic acid bacteria prevents allergic rhinitis-like symptoms and IgE-mediated basophil degranulation. Biosc. Biotech. Biochem. 2011;75:140–144. doi: 10.1271/bbb.100541. [DOI] [PubMed] [Google Scholar]
- Kang H.R., Hwang H.J., Lee J.E., Kim H.R. Quantitative analysis of volatile flavor components in Korean alcoholic beverage and Japanese sake using SPME-GC/MS. Food Sci. Biotechnol. 2016;25:979–985. doi: 10.1007/s10068-016-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagaki H., Kitamoto K. Breeding research on sake yeasts in Japan: history, recent technological advances, and future perspectives. Annu. Rev. Food Sci. Technol. 2013;4:215–235. doi: 10.1146/annurev-food-030212-182545. Review. [DOI] [PubMed] [Google Scholar]
- Kiyono T., Hirooka K., Yamamoto Y., Kuniishi S., Ohtsuka M., Kimura S. Identification of pyroglutamyl peptides in Japanese rice wine (sake): presence of hepatoprotective pyroGlu-Leu. J. Agric. Food Chem. 2013;61:11660–11667. doi: 10.1021/jf404381w. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Abe K., Asai K., Gomi K., Juvvadi P.R., Kato M. Genomics of Aspergillus oryzae. Biosc. Biotech. Biochem. 2007;71:646–670. doi: 10.1271/bbb.60550. [DOI] [PubMed] [Google Scholar]
- Kubo H., Hoshi M., Matsumoto T., Irie M., Oura S., Tsutsumi H. Sake lees extract improves hepatic lipid accumulation in high fat diet-fed mice. Lipids Health Dis. 2017;16:106. doi: 10.1186/s12944-017-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Weyant R.J., Corby P., Kritchevsky S.B., Harris T.B., Rooks R. Edentulism and nutritional status in a biracial sample of well-functioning, community-dwelling elderly: the Health, aging, and body composition study. Am. J. Clin. Nutr. 2004;79:295–302. doi: 10.1093/ajcn/79.2.295. [DOI] [PubMed] [Google Scholar]
- Leroy F., De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004;15:67–78. [Google Scholar]
- Lijinsky W. N-Nitroso compounds in the diet. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999;443:129–138. doi: 10.1016/s1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- Matsuoka A., Furukawa N., Yamanaka Y. Microbial and physicochemical properties of raw ham matured in Sake-lees paste. Nihon Yoton Gakkaishi. 2006;43:180–183. (Japanese) [Google Scholar]
- Mizobuchi S., Minami J., Jin F., Matsushita O., Okabe A. Comparison of the virulence of methicillin-resistant and methicillin-sensitive Staphylococcus aureus. Microbiol. Immunol. 1994;38:599–605. doi: 10.1111/j.1348-0421.1994.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Montel M.C., Masson F., Talon R. Bacterial role in flavour development. Meat Sci. 1998;49:S111–S123. [PubMed] [Google Scholar]
- Moore S., Stein W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948;176:367–388. [PubMed] [Google Scholar]
- Paik H.D., Lee J.Y. Investigation of reduction and tolerance capability of lactic acid bacteria isolated from kimchi against nitrate and nitrite in fermented sausage condition. Meat Sci. 2014;97:609–614. doi: 10.1016/j.meatsci.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Poolman B. Energy transduction in lactic acid bacteria. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 1993;12:125–147. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Rao M.B., Tanksale A.M., Ghatge M.S., Deshpande V.V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 1998;62:597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Wanezaki K., Kawato A., Imayasu S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosc. Biotech. Biochem. 1994;58:1767–1771. doi: 10.1271/bbb.58.1767. [DOI] [PubMed] [Google Scholar]
- Sánchez Mainar M., Stavropoulou D.A., Leroy F. Exploring the metabolic heterogeneity of coagulase-negative staphylococci to improve the quality and safety of fermented meats: a review. Int. J. Food Microbiol. 2017;247:24–37. doi: 10.1016/j.ijfoodmicro.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Nakatani Y., Kakihara Y., Taiyoji M., Saeki M., Takagi R., Yamamura K., Okamoto K. Daily administration of Sake Lees (Sake Kasu) reduced psychophysical stress-induced hyperalgesia and Fos responses in the lumbar spinal dorsal horn evoked by noxious stimulation to the hindpaw in the rats. Biosci. Biotechnol. Biochem. 2020 Jan;84(1):159–170. doi: 10.1080/09168451.2019.1662278. [DOI] [PubMed] [Google Scholar]
- Shimomura M., Shimosaka C., Matsumoto J. Changes in texture and proteins of squid meat cured in sake lees. Nippon. Shokuhin Kogyo Gakkaishi. 1992;39:418–424. (Japanese) [Google Scholar]
- Šojić B., Pavlić B., Ikonić P., Tomović V., Ikonić B., Zeković Z. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019;157:107879. doi: 10.1016/j.meatsci.2019.107879. [DOI] [PubMed] [Google Scholar]
- Standard methods of analysis in food safety regulation, food additive edition Japan food Hygiene association. 2003;7:142–148. (Japanese) [Google Scholar]
- Toldrá F., Rico E., Flores J. Cathepsin B, D, H and L activities in the processing of dry-cured ham. J. Sci. Food Agric. 1993;62:157–161. [Google Scholar]
- Tsutsui N., Yamamoto Y., Iwami K. Protein-nutritive assessment of sake lees obtained by brewing from liquefied rice. J. Nutr. Sci. Vitaminol. 1998;44:177–186. doi: 10.3177/jnsv.44.177. [DOI] [PubMed] [Google Scholar]
- Van Den Hazel H.B., Kielland-Brandt M.C., Winther J.R. Review: biosynthesis and function of yeast vacuolar proteases. Yeast. 1996;12:1–16. doi: 10.1002/(sici)1097-0061(199601)12:1<1::aid-yea902>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Vishwanatha K.S., Appu Rao A.G., Singh S.A. Characterisation of acid protease expressed from Aspergillus oryzae MTCC 5341. Food Chem. 2009;114:402–407. [Google Scholar]
- Wei F., Xu X., Zhou G., Zhao G., Li C., Zhang Y. Irradiated Chinese Rugao ham: changes in volatile N-nitrosamine, biogenic amine and residual nitrite during ripening and post-ripening. Meat Sci. 2009;81:451–455. doi: 10.1016/j.meatsci.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Zapelena M.J., Astiasarán I., Bello J. Dry fermented sausages made with a protease from Aspergillus oryzae and/or a starter culture. Meat Sci. 1999;52:403–409. doi: 10.1016/s0309-1740(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hu P., Lou L., Zhan J., Fan M., Li D. Antioxidant activities of lactic acid bacteria for quality improvement of fermented sausage. J. Food Sci. 2017;82:2960–2967. doi: 10.1111/1750-3841.13975. [DOI] [PubMed] [Google Scholar]