Abstract
Objective(s):
Olanzapine, an atypical antipsychotic, causes weight gain and metabolic disorders in humans. Safranal, one of the active components of Crocus sativus (saffron), has been shown to have anti-obesity, lipid and blood pressure lowering and anti-diabetes effects. In this investigation, the effect of safranal on metabolic disorders induced by olanzapine was studied.
Materials and Methods:
Fourty-two female Wistar rats were divided into 7 groups of 6 animals. The two groups were selected as controls, which received olanzapine and safranal solvents, respectively. The third group treated by olanzapine 5 mg/kg. Groups 4, 5 and 6 treated by olanzapine 5 mg/kg plus safranal (2.5, 5 and 10 mg/kg) and the last group received safranal 10 mg/kg. The injections were performed intraperitoneally for 14 days and on the 15th day the rats were killed and their serum were collected to measure metabolic factors including glucose, insulin, triglyceride, total cholesterol and HDL cholesterol. Leptin level in plasma was also measured. Mean systolic blood pressure was measured using tail cuff method at the end of study. The rats were weighed every other day and amount of food consumed was measured daily.
Results:
Olanzapine significantly elevated body weight, food intake, fasting blood glucose, TG, leptin, and mean systolic blood pressure (MSBP). It also significantly decreased HDL cholesterol blood level. Safranal significantly improved all these complications at three doses.
Conclusion:
Based on the results of this study, safranal is thought to be used as an effective combination in controlling metabolic complications caused by olanzapine.
Key Words: Crocus sativus, Metabolic syndrome, Obesity, Olanzapine, Safranal, Saffron
Introduction
Today, second generation antipsychotics (sGA) are the first line treatment for schizophrenia, because of the less extrapyramidal side effects, and more effectiveness on negative symptoms of schizophrenia. One of the drugs which is used widely in treatment of schizophrenia, bipolar disorder and some other psychotic disorders, is olanzapine. It has metabolic adverse effects such as weight gain, increase in adipose tissue mass, blood leptin, insulin levels, insulin resistance, type 2 diabetes, high blood pressure and disruption of fat profile (1, 2). Investigations have shown that olanzapine has the most risk of weight gain between other SGAs (3, 4). The incidence of obesity, which is considered as an important health problem has been increasing worldwide (5). The role of olanzapine and clozapine in the initiation or intensification of type 2 diabetes more than other antipsychotics has been established (6-8), and the risk of type 2 diabetes with olanzapine increases with elevating doses (9). To reduce the adverse effects of olanzapine, especially metabolic complications and weight gain, many studies have been carried out, and the effects of herbal drugs (10, 11) and chemical drugs (12-15) were investigated.
Because of safety, efficacy, cultural acceptability and lesser side effects of herbal medicine, recently, they are considered as a good candidate for the therapy of various diseases such as metabolic syndrome (16). These herbs and their active constituents include Vitis vinifera (17, 18), Nigella sativa (19), Allium sativum (20), Rosmarinus officinalis (21), Crocus sativus (16), Persea americana (22), Cinnamomum verum (23), Camellia sinensis (11), thymoquinone (19) and rutin (24).
Crocus sativus L., which is known as saffron, is a perennial herb of the Iridaceae family (25, 26). Due to its powerful smell and natural yellow color, saffron is a remarkable medicinal herb and spice (26-28). Safranal, with systematic name: 2,6,6-trimethylcyclohexa-3,1-din-1-carboxaldehyde, is a volatile carboxaldehyde compound (29), it is easily obtained by deglycosilation of picocrocin , one of the main organoleptic constituents of saffron (25). Safranal exhibited useful effects on the central nervous system (30) and respiratory system (31, 32). This compound is a potent antioxidant that has many useful effects due to scavenging of free radicals. These effects include regulation of the antioxidant properties of myocardium, modulation of the level of nitrotyrosin and cardioprotective markers (LDH and CK-MB) (33), and it can act as a potent anti-apoptotic chemical agent (32). This compound could reduce blood pressure with a decrease in heart rate and vascular relaxation (34). Safranal also has beneficial metabolic effects, these effects include decrease of fasting blood glucose (FBS), and HbA1c serum level, increase sensitivity to insulin (35) and it also improves the altered serum lipid profile in diabetic patients (36-38).
According to this fact that many schizophrenic patients receiving olanzapine are at increased risk of weight gain and other adverse effects, and safranal is also known to be effective in modulating and regulating these metabolic disorders, this study evaluated the effects of safranal on metabolic disorders induced by olanzapine in female rats.
Materials and Methods
Animals
To evaluate the effects of safranal on metabolic complications induced by olanzapine, 42 female Wistar rats weighing 170 to 200 g from the Buali Institute and the Animal Department of the Mashhad Faculty of Pharmacy were obtained. The age of the animals was 6 to 8 weeks. Animals were exposed to a temperature of 21+2 °C and in 12 hr period of darkness and brightness. Two rats were kept in a box during the study. All animals experiment were conducted according to the rules of the ethics committee of Mashhad University of Medical Sciences (project code: 941401).
Study design
To evaluate the effects of safranal on metabolic complications induced by olanzapine, 42 female Wistar rats were divided into 7 groups of 6. Group 1: rats receiving olanzapine solvent (injectable normal saline with a small amount of glacial acetic acid); group 2: rats receiving safranal solvent (injectable normal saline with a small amount of DMSO and a drop of tween 80) once daily for 14 days; group 3: rats receiving 5 mg/kg of olanzapine once daily for 14 days via intraperitoneal injection (39); group 4: rats receiving 5 mg/kg olanzapine once daily for 14 days via intraperitoneal injection with safranal (2.5 mg/kg/day) for 14 days (32); group 5: rats receiving olanzapine 5 mg/kg once daily for 14 days via intraperitoneal injection with safranal (5 mg/kg/day) for 14 days (34); group 6: rats receiving olanzapine 5 mg/kg once daily for 14 days via intraperitoneal injection with safranal (10 mg/kg/day) for 14 days (34); group 7: rats receiving 10 mg/kg of safranal once daily for 14 days via intraperitoneal injection.
Plasma metabolic factors
On the 15th day of study, the rats were killed under fasting condition and their serum was isolated using a 20 min centrifuge at 4500 rpm, sent to the laboratory for measurement of metabolic factors including glucose, insulin, triglyceride, total cholesterol and HDL cholesterol. Plasma leptin concentration was analyzed using the Abcam-Cambridge (uk) ELISA kit.
Food intake and body weight
Food intake was calculated daily by measuring the difference in the amount of food placed in the hopper and remaining amount at the end of the 24 hrs. Body weight was measured every other day during the study.
Systolic blood pressure (SBP)
At the end of study, SBP was measured by non-invasive tail cuff method (31). The blood pressure was measured from tail vein using NIBP controller. SBP was measured 5 times and the mean SBP was reported.
Statistical analysis
One-way and two-way ANOVA and Post test Tukey-Kramer tests were utilized to compare the different groups. P<0.05 was considered statistically significant. Results were expressed as mean±SEM.
Results
We had two solvent control groups, but because the results of these two groups were not statistically significant, just one of them was used.
Weight changes
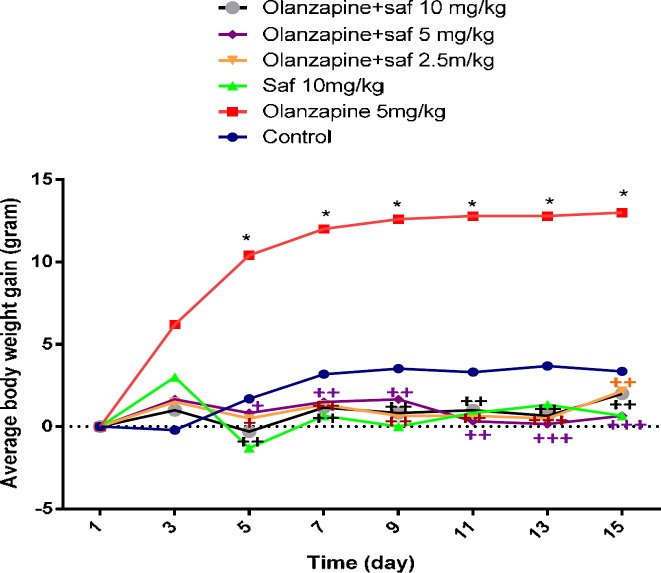
The mean weight changes in the olanzapine group, 5 mg/kg alone (grope 3), were significantly higher than the control group from the fifth day until the last day of the study (P<0.05). Safranal was able to significantly decrease the weight gain induced by olanzapine in all three doses of 2.5, 5 and 10 mg/kg.The mean weight in the group received olanzapine alone increased by 6.48% at the end of the 14-day administration compared to control group, this weight gain in the groups receiving 2.5, 5 and 10 mg/kg of safranal plus olanzapine was 4.04%, 3.76% and 3.51%, respectively compared to olanzapine group (Figure 1).
Figure 1.
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on daily weight changes in female rats during 14 days of intraperitoneal administration. For statistical comparison, two-way ANOVA and Tukey-Kramer Post hoc test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.05(+,*), and P<0.01(++), and P<0.001 (+++) (saf: safranal, olan: olanzapine). N=6
Food intake
Average food intake (g) in the olanzapine group, was significantly higher than the control group (P<0.001). The amount of food intake in safranal group alone was not significantly different from that of the control group. In this study, safranal was able to significantly preventing of increase the food intake induced by olanzapine) 5 mg /kg( in all three doses of 2.5, 5 and 10 mg/kg (P<0.001). Mean food intake in safranal groups at 2.5, 5 and 10 mg/kg plus olanzapine 5 mg/kg compared to the olanzapine group was decreased 28.55%, 29.65% and 30.40% respectively (Figure 2).
Figure 2.
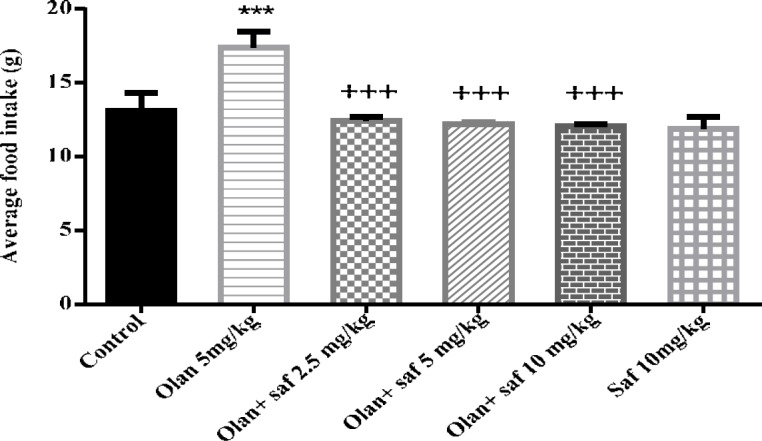
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on food intake in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.001 (+++ , ***) (saf: safranal, olan: olanzapine)
Mean systolic blood pressure (MSBP)
MSBP in the olanzapine group (5 mg/kg), was significantly higher than the control group (P<0.001). MSBP in safranal group alone was not significantly different from that of the control group (group 1). Also, in all three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg), there was a significant decrease in MSBP compared to the olanzapine group.
The results showed that MSBP in group receiving 5 mg/kg olanzapine increased 24.71% compared to the control group; and the amount of reduction in MSBP in group receiving olanzapine plus safranal in doses of 2.5, 5 and 10 mg/kg compared to the group receiving olanzapine alone were 15.96%, 17.77%, and 23.32 mg, respectively (Figure 3).
Figure 3.
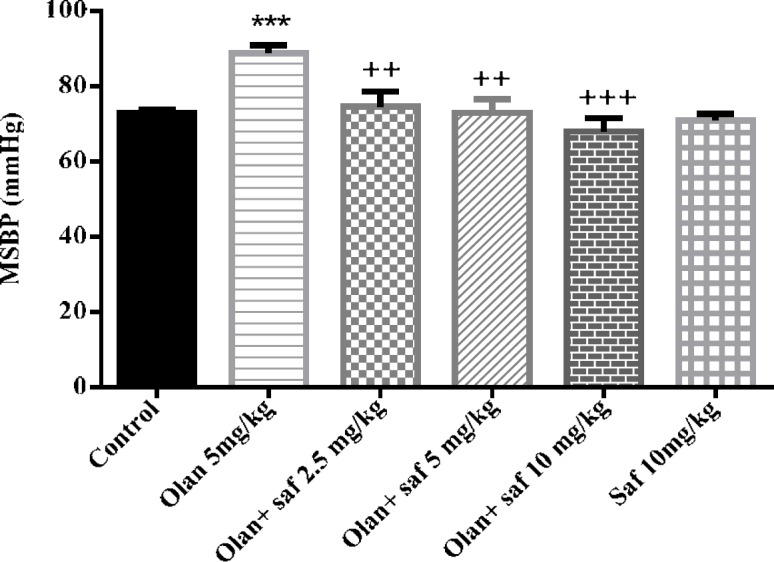
Investigating the effect of olanzapine 5 mg / kg with safranal and alone on mean systolic blood pressure (MSBP) changes in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.01(++) and P<0.001 (+++ , ***) (saf: safranal, olan: olanzapine)
Fasting blood glucose (FBS)
Fasting blood glucose level in group receiving olanzapine showed a significant increase compared to the control group (P<0.001). FBS did not show any significant difference in safranal group alone compared to control group. All three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg) showed a significant decrease in FBS levels compared to the group receiving olanzapine alone (P<0.001). The results of this study showed a decrease of 37.41%, 28.57% and 23.79% in FBS levels in groups received olanzapine plus safranal (2.5, 5 and 10 mg/kg), respectively, compared to the group receiving olanzapine (Figure 4).
Figure 4.
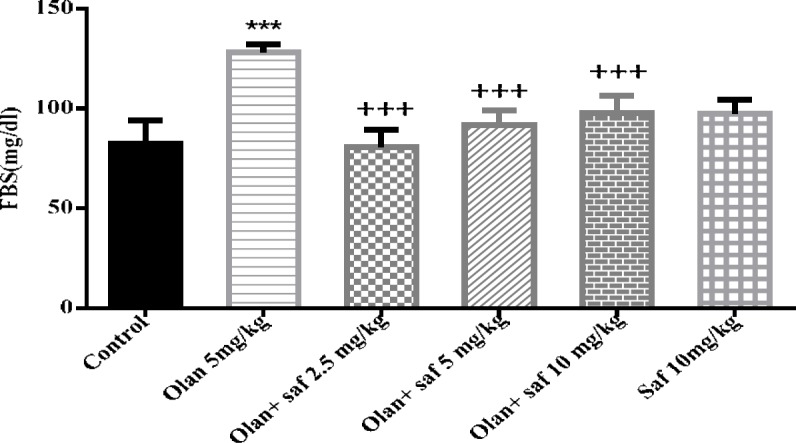
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on fasting blood sugar (FBS) in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.001 (+++ , ***) (saf: safranal, olan: olanzapine)
Insulin serum level
Insulin serum level in group receiving olanzapine showed 1.91% decrease compared to the control group, but it was not significant. In insulin serum level any significant difference in safranal group alone compared to the control group was not observed. The level of insulin was increased significantly in all three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg) P< 0.001, P<0.001 and P<0.01; respectively) (Figure 5).
Figure 5.
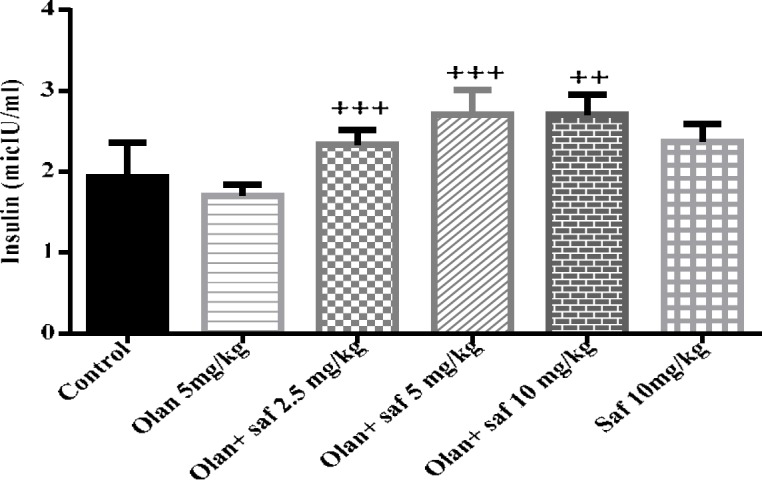
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on insulin serum level in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.001 (+++ , ***) (saf: safranal, olan: olanzapine)
Leptin serum level
Plasma leptin level was significantly increased in olanzapine group compared to the control group (P<0.001). In all three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg), there was a significant decrease in leptin level compared to the olanzapine group (P<0.01, P<0.01 and P<0.001, respectively) (Figure 6).
Figure 6.
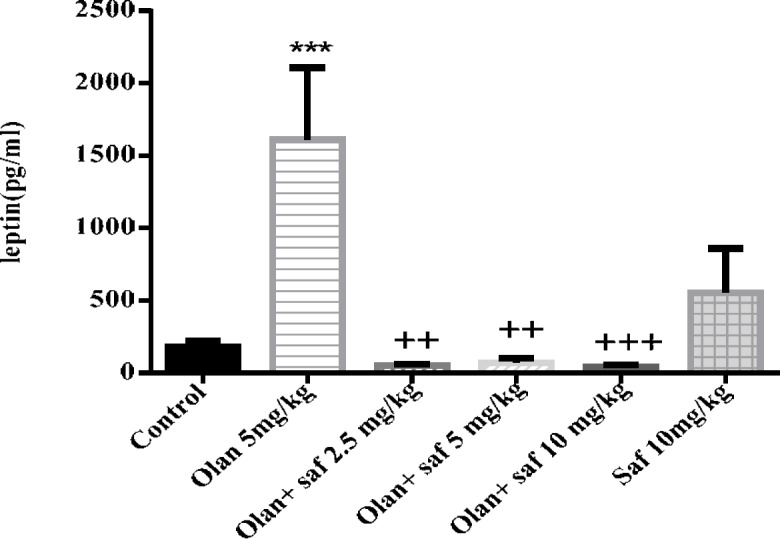
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on leptin serum level in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.01(++) and P <0.001 (+++, ***) (saf: safranal, olan: olanzapine)
Triglyceride serum level
The level of triglyceride in the olanzapine group, was significantly higher than the control group (P<0.001). The level of triglyceride in the safranal group alone did not show any significant difference compared to the control group. Also, in all three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg), there was a significant decrease in triglyceride level compared to olanzapine (P<01, P<0.001 and P<0.001).Increasing triglyceride level in the olanzapine group compared to the control group was 77.51%. Reduced triglyceride level in the olanzapine group plus safranal (2.5, 5 and 10 mg/kg) compared to the olanzapine group was 38.8%, 48.33% and 39.83%, respectively (Figure 7).
Figure 7.

Investigating the effect of olanzapine 5 mg/kg with safranal and alone on triglyceride serum level in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.001 (+++ , ***) (saf: safranal, olan: olanzapine)
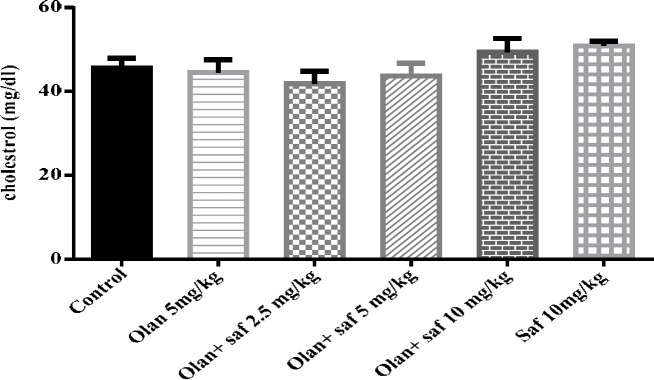
Figure 9.
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on cholesterol in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+ Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used
HDL-C serum level
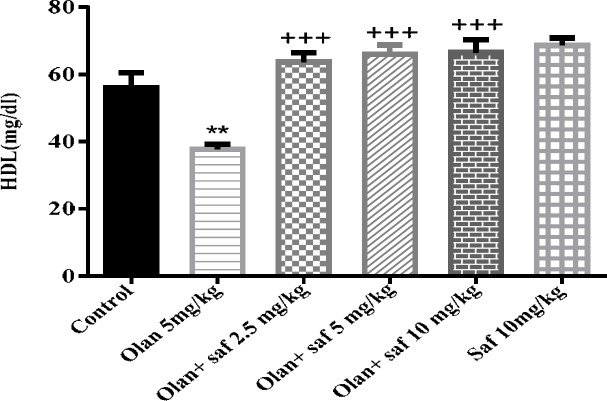
HDL-C level in the olanzapine group was significantly lower than the control group (P<0.01). HDL-C did not show a significant difference in safranal group alone compared to the control group. All three groups receiving olanzapine plus safranal (2.5, 5 and 10 mg/kg) showed a significant increase in HDL-C in comparison with the olanzapine group (P<0.001).The results of this study showed an increase of 68.88%, 75% and 76.37% in HDL-C in groups received olanzapine plus safranal (2.5, 5 and 10 mg/kg), respectively, compared to the group receiving olanzapine (Figure 8).
Figure 8.
Investigating the effect of olanzapine 5 mg/kg with safranal and alone on HDL level in female rats during 14 days of intraperitoneal administration. Values are displayed as SEM+Mean. For statistical comparison, one-way ANOVA and Tukey-Kramer test were used. Comparison with control (*) and comparison with olanzapine (+); P<0.01(**) and P<0.001 (+++) (saf: safranal, olan: olanzapine)
Total cholesterol serum level
Serum levels of cholesterol in all tested groups were within a range and no significant difference was observed.
Discussion
Safranal was able to prevent the increase of food intake, FBG, triglyceride, serum levels of leptin and MSBP and also reduced HDL-C induced by olanzapine in all three doses (2.5, 5 and 10 mg/kg). Safranal also significantly increased insulin serum levels in all three doses compared to those receiving olanzapine. The level of cholesterol was not affected by olanzapine.
In the present study, like many other animal studies (40-42), administration of olanzapine increased food intake and weight gain significantly in female rats (32.80% and 6.48% respectively). Olanzapine also increases the weight and appetite in human studies (3, 4, 43-45). Increased hunger with a decrease in satiety has been reported consistently with the administration of olanzapine (45). Despite the introduction of several mechanisms, the precise mechanisms of olanzapine induced weight gain have not yet been determined, but in addition to increased appetite (40, 46, 47); insulin resistance (48), and increased serum leptin, are proposed (49-51).
Leptin is one of the cytokine-like molecules synthesized from adipose tissue, which is actively transmitted to the hypothalamus, where its activity restricts food intake (52). It also directly affects the secretion of insulin (53). Therefore, leptin has been investigated for its association with changes in body weight and glucose metabolism during treatment with various anti-psychotic drugs (54). In previous studies, increase in the level of leptin has been reported in patients receiving olanzapine (49, 51, 55). In previous studies on saffron, authors determined that this plant reduces appetite (56, 57), decreases insulin resistance (58, 59), and reduces serum leptin levels (60). The results of measurement of food intake, serum level of insulin and leptin in this study also confirmed this. In previous studies, the role of saffron extract was mostly investigated. In this study, the effects of pure safranal as a major part of saffron extract were studied, which showed that safranal prevented olanzapine induced weight gain, which could be due to reduce appetite, decrease serum leptin levels, and increase insulin sensitivity.
Another metabolic complication caused by olanzapine is the initiation or intensification of type 2 diabetes (7, 8). Diabetes can be caused by impaired glucose tolerance and decreased insulin secretion from beta cells of the pancreas, due to the antagonistic effects of olanzapine. Studies on dog have shown that beta cell regression for insulin resistance may be reduced or even eliminated in the presence of olanzapine (48). This lack of increased insulin secretion can facilitate hyperglycemia (6). Saffron is used in diabetes because it has potent antioxidant effects (59, 61). The antioxidant effects reduce the levels of active oxygen species leading to the increase in insulin secretion from pancreatic beta cells, hypoglycemic effects, and increased tissue hypersensitivity to insulin (59). Safranal reduces inflammation of the pancreas and plasma and also decreases the oxidative stress of type 2 diabetes in plasma and pancreas by reducing TNF-α and IL-β levels (62).
The common clinical side effects of olanzapine are its effects on fat profile. In clinical and basic studies, the harmful effects of this drug on the fat profile have been proven (3, 63). One study found that olanzapine increased triglycerides and LDL, and the authors believed that triglyceride increases may be due to a reduction in the metabolic cleansing of triglycerides, or equivalent to an increase in liver lipogenesis (64). Saffron consumption can improve fat profile using antioxidant mechanisms (37). Saffron also increases the amount of fatty stools (65). It also selectively inhibits the activity of the pancreatic lipase as a competitive inhibitor (36). Safranal could have beneficial effects on altered lipid profile with olanzapine in rat and adjusted serum triglyceride and HDL levels. This result was obtained in previous studies by aqueous extract of saffron (36, 37) and we proved in this study that safranal could also have this effect.
Previous studies confirmed that saffron consumption reduced the high blood pressure in humans and rodents (66, 67). The antioxidant, diuretic and vasodilator effects are the three main mechanisms of anti-hypertensive effect of saffron (34). Recently, it has been shown that administering safranal for 5 weeks at 1, 2, 4 mg/kg/day, dose dependently decreases the systolic blood pressure in hypertensive rats receiving desoxycorticosterone acetate (DOCA) salts, but it does not change the blood pressure in rats with normal blood pressure (68). Similar to the tested rats in the present study, the mean systolic blood pressure increased in most patients receiving olanzapine (69).
Conclusion
According to the results of this study, safranal was effective to alleviate different metabolic disorders induced by olanzapine such as obesity, hyperglycemia, hypertriglyceridemia and hypertension. Therefore, it can be used as an effective combination in controlling the metabolic effects of olanzapine as a preventive treatment with olanzapine.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for financial support. The results described in this paper are part of a Pharm D thesis.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Newcomer JW. Metabolic risk during antipsychotic treatment. Clin Ther. 2004;26:1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Lozano P, Delgado D, Gomez D, Rubio M, Iborra JL. A non-destructive method to determine the safranal content of saffron (Crocus sativus L) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. J Biochem Biophys Methods. 2000;43:367–378. doi: 10.1016/s0165-022x(00)00090-7. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Iglesias R, Crespo-Facorro B, Amado JA, Garcia-Unzueta MT, Ramirez-Bonilla ML, Gonzalez-Blanch C, et al. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry. 2007;68:1733–1740. doi: 10.4088/jcp.v68n1113. [DOI] [PubMed] [Google Scholar]
- 4.Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Ejtahed HS, Tabatabaei-Malazy O, Soroush AR, Hasani-Ranjbar S, Siadat SD, Raes J, et al. Worldwide trends in scientific publications on association of gut microbiota with obesity. Iran J Basic Med Sci. 2019;22:65–71. doi: 10.22038/ijbms.2018.30203.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girault EM, Alkemade A, Foppen E, Ackermans MT, Fliers E, Kalsbeek A. Acute peripheral but not central administration of olanzapine induces hyperglycemia associated with hepatic and extra-hepatic insulin resistance. PLoS One. 2012;7:e43244. doi: 10.1371/journal.pone.0043244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianfrancesco FD, Grogg AL, Mahmoud RA, Wang RH, Nasrallah HA. Differential effects of risperidone, olanzapine, clozapine, and conventional antipsychotics on type 2 diabetes: findings from a large health plan database. J Clin Psychiatry. 2002;63:920–930. doi: 10.4088/jcp.v63n1010. [DOI] [PubMed] [Google Scholar]
- 8.Gianfrancesco F, White R, Wang RH, Nasrallah HA. Antipsychotic-induced type 2 diabetes: evidence from a large health plan database. J Clin Psychopharmacol. 2003;23:328–335. doi: 10.1097/01.jcp.0000085404.08426.3a. [DOI] [PubMed] [Google Scholar]
- 9.Lambert BL, Chou CH, Chang KY, Tafesse E, Carson W. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf. 2005;14:417–425. doi: 10.1002/pds.1092. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9:e93310. doi: 10.1371/journal.pone.0093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavi B, Lookian F, Hosseinzadeh H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed Pharmacother. 2017;92:726–731. doi: 10.1016/j.biopha.2017.05.113. [DOI] [PubMed] [Google Scholar]
- 12.Lian J, Huang XF, Pai N, Deng C. Preventing olanzapine-induced weight gain using betahistine: a study in a rat model with chronic olanzapine treatment. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40:1385–1403. doi: 10.1093/schbul/sbu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belanoff JK, Blasey CM, Clark RD, Rao RL. Selective glucocorticoid receptor (type II) antagonists prevent weight gain caused by olanzapine in rats. Eur J Pharmacol. 2011;25:117–120. doi: 10.1016/j.ejphar.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Wallingford N, Sinnayah P, Bymaster F, Gadde K, Krishnan R, McKinney A, et al. Zonisamide prevents olanzapine-associated hyperphagia, weight gain, and elevated blood glucose in rats. Neuropsychopharmacology. 2008;33:2922–2933. doi: 10.1038/npp.2008.9. [DOI] [PubMed] [Google Scholar]
- 16.Razavi BM, Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97:1679–1685. doi: 10.1002/jsfa.8134. [DOI] [PubMed] [Google Scholar]
- 17.Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a Potential Candidate for the Therapy of the Metabolic Syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 18.Rameshrad M, Razavi BM, Imenshahidi M, Hosseinzadeh H. Vitis vinifera (grape) seed extract and resveratrol alleviate bisphenol-A-induced metabolic syndrome: Biochemical and molecular evidences. Phytother Res. 2019;33:832–844. doi: 10.1002/ptr.6276. [DOI] [PubMed] [Google Scholar]
- 19.Razavi B, Hosseinzadeh H. A review of the effects of Nigella sativa L and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 21.Hassani F, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 22.Tabeshpour J, Razavi B, Hosseinzadeh H. Effects of avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytother Res. 2017;31:819–837. doi: 10.1002/ptr.5805. [DOI] [PubMed] [Google Scholar]
- 23.Mollazadeh H, Hosseinzadeh H. Cinnamon effects on metabolic syndrome: a review based on its mechanisms. Iran J Basic Med Sci. 2016;19:1258–1270. doi: 10.22038/ijbms.2016.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava R, Ahmed H, Dixit RK, Dharamveer , Saraf SA. Crocus sativus : A comprehensive review. Pharmacogn Rev. 2010;4:200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gohari A, Saeidnia S, Mahmoodabadi M. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev. 2013;7:61–66. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseini A, Razavi BM, Hosseinzadeh H. Saffron (Crocus sativus) petal as a new pharmacological target: a review. Iran J Basic Med Sci. 2018;21:1091–1099. doi: 10.22038/IJBMS.2018.31243.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaee-Khorasany A, Razavi BM, Taghiabadi E, Tabatabaei Yazdi A, Hosseinzadeh H. Effect of saffron (stigma of Crocus sativus L) aqueous extract on ethanol toxicity in rats: A biochemical, histopathological and molecular study. J Ethnopharmacol . 12:286–299. doi: 10.1016/j.jep.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic. 2004:650. [Google Scholar]
- 30.Hosseinzadeh H, Sadeghnia H. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 31.Rezaee R, Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16:12–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Zeinali M, Zirak MR, Rezaee SA, Karimi G, Hosseinzadeh H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran J Basic Med Sci. 2019;22:334–344. doi: 10.22038/ijbms.2019.34365.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharti S, Golechha M, Kumari S, Siddiqui K, Arya D. Akt/GSK-3beta/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur J Nutr. 2012;51:719–727. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- 34.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of safranal on systolic blood pressure in rats. Iran J Pharm Res. 2015;14:585–590. [PMC free article] [PubMed] [Google Scholar]
- 35.Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants. 2011;3:82–89. [Google Scholar]
- 36.Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed Res Int. 2014;2014:920857. doi: 10.1155/2014/920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, Heydari S, et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in vitro and in vivo. Sci Rep. 2016;6:25139. doi: 10.1038/srep25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollazadeh H, Mahdian D, Hosseinzadeh H. Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. Phytomedicine. 2018;53:43–52. doi: 10.1016/j.phymed.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Jassim G, Skrede S, Vazquez MJ, Wergedal H, Vik-Mo AO, Lunder N, et al. Acute effects of orexigenic antipsychotic drugs on lipid and carbohydrate metabolism in rat. Psychopharmacology (Berl) 2012;219:783–794. doi: 10.1007/s00213-011-2397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity (Silver Spring) 2006;14:36–51. doi: 10.1038/oby.2006.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 42.Arjona AA, Zhang SX, Adamson B, Wurtman RJ. An animal model of antipsychotic-induced weight gain. Behav Brain Res. 2004;152:121–127. doi: 10.1016/j.bbr.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 43.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 44.Fountaine RJ, Taylor AE, Mancuso JP, Greenway FL, Byerley LO, Smith SR, et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity (Silver Spring) 2010;18:1646–1651. doi: 10.1038/oby.2010.6. [DOI] [PubMed] [Google Scholar]
- 45.Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27:662–666. doi: 10.1097/jcp.0b013e31815a8872. [DOI] [PubMed] [Google Scholar]
- 46.Patil BM, Kulkarni NM, Unger BS. Elevation of systolic blood pressure in an animal model of olanzapine induced weight gain. Eur J Pharmacol. 2006;551:112–115. doi: 10.1016/j.ejphar.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Tagami K, Kashiwase Y, Yokoyama A, Nishimura H, Miyano K, Suzuki M, et al. The atypical antipsychotic, olanzapine, potentiates ghrelin-induced receptor signaling: An in vitro study with cells expressing cloned human growth hormone secretagogue receptor. Neuropeptides. 2016;58:93–101. doi: 10.1016/j.npep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Bergman RN, Ader M. Atypical antipsychotics and glucose homeostasis. J Clin Psychiatry. 2005;66:504–514. doi: 10.4088/jcp.v66n0414. [DOI] [PubMed] [Google Scholar]
- 49.Kim BJ, Sohn JW, Park CS, Hahn GH, Koo J, Noh YD, et al. Body weight and plasma levels of ghrelin and leptin during treatment with olanzapine. J Korean Med Sci. 2008;23:685–690. doi: 10.3346/jkms.2008.23.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murashita M, Kusumi I, Inoue T, Takahashi Y, Hosoda H, Kangawa K, et al. Olanzapine increases plasma ghrelin level in patients with schizophrenia. Psychoneuroendocrinology. 2005;30:106–110. doi: 10.1016/j.psyneuen.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Kraus T, Haack M, Schuld A, Hinze-Selch D, Kuhn M, Uhr M, et al. Body weight and leptin plasma levels during treatment with antipsychotic drugs. Am J Psychiatry. 1999;156:312–314. doi: 10.1176/ajp.156.2.312. [DOI] [PubMed] [Google Scholar]
- 52.Prolo P, Wong M, Licinio J. Leptin. Int J Biochem Cell Biol. 1998;30:1285–1290. doi: 10.1016/s1357-2725(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 53.Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50:443–459. [PubMed] [Google Scholar]
- 54.Monteleone P, Fabrazzo M, Tortorella A, La Pia S, Maj M. Pronounced early increase in circulating leptin predicts a lower weight gain during clozapine treatment. J Clin Psychopharmacol. 2002;22:424–426. doi: 10.1097/00004714-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Atmaca M, Tezcan E, Ustundag B. Plasma nitric oxide and leptin values in patients with olanzapine-induced weight gain. J Psychiatric Res. 2007;41:74–79. doi: 10.1016/j.jpsychires.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. 2010;30:305–313. doi: 10.1016/j.nutres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Razi M. Al-Hawi fi’l-tibb (Comprehensive book of medicine) Hyderabad: Osmania Oriental Publications Bureau. 1968;20:548–553. [Google Scholar]
- 58.Maeda A, Kai K, Ishii M, Ishii T, Akagawa M. Safranal, a novel protein tyrosine phosphatase 1B inhibitor, activates insulin signaling in C2C12 myotubes and improves glucose tolerance in diabetic KK-Ay mice. Mol Nutr Food Res. 2014;58:1177–1189. doi: 10.1002/mnfr.201300675. [DOI] [PubMed] [Google Scholar]
- 59.Konstantopoulos P, Doulamis IP, Tzani A, Korou ML, Agapitos E, Vlachos IS, et al. Metabolic effects of Crocus sativus and protective action against non-alcoholic fatty liver disease in diabetic rats. Biomed Rep. 2017;6:513–518. doi: 10.3892/br.2017.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mashmoul M, Azlan A, Mohtarrudin N, Nisak B, Yusof M, Khaza’ai H. Saffron extract and crocin reduced biomarkers associated with obesity in rats fed a high-fat diet. Mal J Nutr. 20017;23:117–127. [Google Scholar]
- 61.Hosseinzadeh H, Mehri S, Abolhassani MM, Ramezani M, Sahebkar A, Abnous K. Affinity-based target deconvolution of safranal. Daru. 2013;21 doi: 10.1186/2008-2231-21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hazman O, Ovali S. Investigation of the anti-inflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Inflammation. 2015;38:1012–1019. doi: 10.1007/s10753-014-0065-1. [DOI] [PubMed] [Google Scholar]
- 63.Graham K, Perkins D, Edwards L, Barrier R, Lieberman J, Harp J. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. Am J Psychiatry. 2005;162:118–123. doi: 10.1176/appi.ajp.162.1.118. [DOI] [PubMed] [Google Scholar]
- 64.Teff K, Kim S. Atypical antipsychotics and the neural regulation of food intake and peripheral metabolism. Physiol Behav. 2011;104:590–598. doi: 10.1016/j.physbeh.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Javadi B, Sahebkar A, Emami S. A survey on saffron in major islamic traditional medicine books. Iran J Basic Med Sci. 2013;16:1–11. [PMC free article] [PubMed] [Google Scholar]
- 66.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 68.Boskabady MH, Shafei MN, Shakiba A, Sefidi HS. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother Res. 2008;22:330–334. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 69.Fabrazzo M, Monteleone P, Prisco V, Perris F, Catapano F, Tortorella A, et al. Olanzapine is faster than haloperidol in inducing metabolic abnormalities in schizophrenic and bipolar patients. Neuropsychobiology. 2015;72:29–36. doi: 10.1159/000437430. [DOI] [PubMed] [Google Scholar]