Abstract
Trauma remains one of the leading causes of death in the United States in patients younger than 45 years. Blunt trauma is most commonly a result of high-speed motor vehicular collisions or high-level fall. The liver and spleen are the most commonly injured organs, with the liver being the most commonly injured organ in adults and the spleen being the most affected in pediatric blunt trauma. Liver injuries incur a high level of morbidity and mortality mostly secondary to hemorrhage. Over the past 20 years, angiographic intervention has become a mainstay of treatment of hepatic trauma. As there is an increasing need for the interventional radiologists to embolize active hemorrhage in the setting of blunt and penetrating hepatic trauma, this article aims to review the current level of evidence and contemporary management of hepatic trauma from the perspective of interventional radiologists. Embolization techniques and associated outcome and complications are also reviewed.
Keywords: liver trauma, injury, hepatic, embolization, interventional radiology
The liver is the most commonly injured organ in adult blunt abdominal injury. 1 The spleen is the most commonly injured organ in children. According to the most recent National Trauma Data Bank (NTDB) 2 data, abdominal injuries made up 11% of the 100,996 adult admissions for trauma in 2016. Motor vehicle accidents and falls accounted for the majority of blunt trauma mechanisms in both adults and children. 2
In the pediatric trauma population, 10 to 15% of all patients with trauma have an associated abdominal injury. 3 More than 90% of abdominal injuries in pediatrics are caused by blunt trauma. Of those patients with abdominal injuries, splenic trauma is the most common, followed by hepatic injury. 4 In a cohort of pediatric patients from the NTDB, approximately 30% of pediatric patients with abdominal trauma had an isolated injury to the liver, whereas 50% had isolated injuries of the spleen. 4
Currently, the options for the management of hepatic trauma in adults and children are open surgery, angioembolization, and nonoperative management (NOM). Traditionally, NOM included careful monitoring of the hemodynamically stable patient and radiological interventions such as embolization. 5 Over the past 20 years, NOM has become the standard of care in the hemodynamically stable patient. 6 Although an invasive intervention, many studies have historically grouped angioembolization under the umbrella of NOM. As the implementation of angioembolization has evolved over this time period, this article is defining NOM as admission with serial abdominal examinations and serial hemoglobin measurements without embolization. 7 In a 2011 study, 94% of NOM patients were successfully managed without open surgery or embolization. 7 With the advent of hybrid operating rooms, a new category of management (angioembolization with surgery) has emerged. This combined approach has been demonstrated to reduce mortality in severely injured patients. 8 9
Although the landscape in the management of hepatic trauma continues to evolve, this article aims to review the most up-to-date available evidence on the management of adult and pediatric patients, discuss the efficacy of different treatments (surgery, embolization, NOMt), and outline the essentials of what interventional radiologists (IRs) need to know in this arena.
Grades of Liver Injury
Understanding the numerous classification schemes in liver injury is of paramount importance in understanding the treatment options for these patients. There are four major classification systems which stratify various traumatic injuries by severity: the Abbreviated Injury Score (AIS), 10 the American Association for Surgery of Trauma (AAST) grading system, the Injury Severity Score, 11 12 and World Society of Emergency Surgery (WSES) grading system. 9 The AIS is an anatomically based global severity system that classifies injury severity by body region. 10 Initially designed for classifying injuries due to motor vehicle and airplane crashes, it has been widely adopted into a coding system to classify injuries taking into account survival data. 13 The AIS is internationally adopted into medical coding and these data have been collected as part of the NTDB. Although the AIS and Injury Severity Score are used in research data collection, the AAST grading system and now the WSES are mainstays of classification in the current clinical settings. The AAST is a radiographic scoring system which categorizes injury from grade I to VI based on hematoma, laceration, and vascular compromise 14 ( Fig. 1 ). The higher the grade, the greater the CT evidence of hepatic compromise ( Table 1 ). While widely adopted, the AAST fails to take into account the clinical presentation of the patient. The 2016 WSES guidelines aim to rectify this by stratifying patients based on AAST score and hemodynamic stability. Patients are mild, moderate, or severe based on AAST grade. Any patient will be categorized as severe (WSES grade IV) if hemodynamically unstable. 15
Fig. 1.
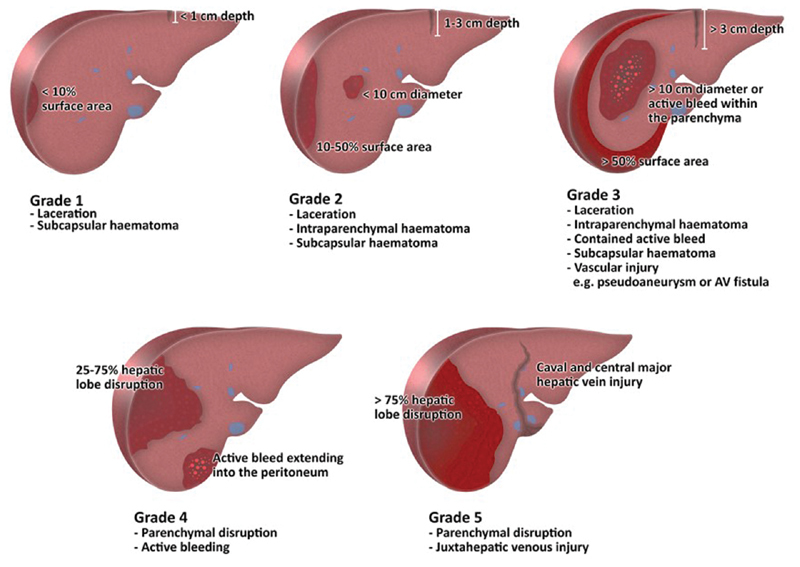
Summary of American Association for the Surgery of Trauma (AAST) Grading of Hepatic Trauma.
Table 1. American Association for the Surgery of Trauma (AAST) grading system for hepatic injury.
| Injury type | Injury description | WSES grade 15 | |
|---|---|---|---|
| I a | Hematoma | Subcapsular <10% of surface | Grade I Minor if hemodynamically stable—includes blunt and penetrating lesions |
| Laceration | Capsular tear <1 cm depth | ||
| II a | Hematoma | Subcapsular 10–50% of surface area, intraparenchymal hematoma, <10cm in diameter | |
| Laceration | 1–3 cm parenchymal depth, <10 cm in length | ||
| III | Hematoma | Subcapsular > 50% of the surface area or expanding, ruptured subcapsular or parenchymal hematoma, intraparenchymal hematoma >10 cm | Grade II If hemodynamically stable in blunt or penetrating lesion |
| Laceration | >3 cm parenchymal depth | ||
| IV | Laceration | Parenchymal disruption 25–75% of hepatic lobe | Severe Grade III—hemodynamically stable Grade IV a —AAST grades I–VI if hemodynamically unstable |
| Vascular | Hepatic injury near major hepatic vein or near the hepatic IVC | ||
| VI | Vascular | Hepatic avulsion |
Abbreviations: IVC, inferior vena cava; WSES, World Society of Emergency Surgery.
Advance grade I for multiple injuries up to grade III.
Overview of Management of Hepatic Trauma in Adults
Current recommendations for managing adult hepatic trauma are supported by the World Society of Emergency Surgery (WSES) 2016 classification of liver injury. 15 Most recent guidelines stratify liver injury according to the WSES classification scheme, which utilizes the AAST grading and takes hemodynamic stability into account. For the hemodynamically stable patient with AAST grade I–IV, NOM is the most evidence-based treatment. 16 Multiple institutional studies and case reviews have established NOM as a safe and highly effective management option. Most studies show a success rate of 80 to 100% for NOM of the adult patient with blunt hepatic trauma. NOM is achievable in an estimated 80% of all hepatic trauma cases. 11 To qualify for NOM, patients must be hemodynamically stable, have an adequate response to initial resuscitation attempts, and have no peritoneal signs. These patients should be imaged with multiphase CT immediately and treated according to imaging findings.
NOM of stable patients suffering from penetrating trauma from gunshot wounds is being carefully explored in select patients. 17 In this very select group, NOM is independently associated with improved mortality and fewer complications. 17
Failure of NOM following blunt traumatic injury is infrequent, with failures reported secondary to hemorrhage, peritonitis, or abdominal compartment syndrome. 7 Hemorrhage is the most common cause of failure of NOM and can be managed with open surgery alone or combined open surgery and embolization or embolization alone.
According to WSES' most recent guideline published in 2016, patients with hepatic trauma who are hemodynamically stable with no signs of free air; localized bowel thickening; evisceration; and impalement on contrast-enhanced CT of the chest, abdomen, and pelvis without evidence of active arterial extravasation/blush are appropriate candidate for NOM. Those who demonstrate active arterial extravasation/blush are recommended to undergo angioembolization. If embolization is effective and the patient remains hemodynamically stable, no further invasive intervention is required. If not, open surgery with or without intraoperative angioembolization is recommended.
In patients who are hemodynamically unstable to tolerate NOM, open surgery alone or in combination with embolization is recommended. Intraoperative embolization is associated with fewer units of blood transfused, fewer complications, and decreased mortality. 9
Overview of Management of Hepatic Trauma in Pediatrics
Management of pediatric hepatic trauma, like splenic trauma, has shifted over the last few decades from primarily surgical to successful NOM in greater than 90% of cases. 11 NOM is more successful in pediatric patients than in adults, likely due to differences in physiology and ability to compensate for blood loss as well as differences in mechanism of injury. 4 To date, there is no consensus on the role of angioembolization in pediatric trauma patients with hepatic injury. Indications and frequency of use vary greatly by center. Case series have demonstrated the safety and efficacy of embolization in patients with evidence of ongoing hepatic bleeding and hemodynamic instability. 10
As in adult patients, initial management of pediatric trauma patients should focus on injuries associated with the airway, breathing, and circulation according to the Advanced Trauma Life Support guidelines. Following stabilization, management is based on history and physical exam as well as on imaging evaluation of intra-abdominal injuries. Initial efforts to standardize management of pediatric patients with blunt solid organ injury by the American Pediatric Society Association (APSA) focused on radiologic grading. 18 Length of stay, intensive care unit admission, and other management were determined by grade of hepatic or splenic injury on contrast-enhanced CT scan. 3 Subsequently, a consortium of level I pediatric trauma centers known as the Arizona-Texas-Oklahoma-Memphis-Arkansas Consortium (ATOMAC) released another set of guidelines, de-emphasizing focus on radiologic grade and advocating for hemodynamic status as the primary criteria for management. 5 Per this guideline, surgical intervention should be considered based on failure of fluid resuscitation and recurrent hypotension. 5 To date, none of these guidelines establishes well-defined indications for angiography and embolization. Many advise that the use of angiography and embolization should be based on available hospital resources and expertise as well as clinical judgement. 5 6
Mortality and Morbidity
Mortality secondary to liver trauma is variable by severity and overall it has decreased from over 50% in 1970 to 10–20% in recent years. 19 Some centers are seeing mortality rates between 2 and 8%. 20 This is largely due to advances in catheter-based techniques and selective nonoperative NOM. 20 The leading cause of mortality in patients with the most severe injuries is hemorrhage. 21 Prior to the routine use of arterial embolization, hemorrhage accounted for 54% of all hepatic mortality after trauma. 21 The increasing use of embolization has been paralleled by decreasing mortality. 20 As embolization has been demonstrated as a safe route of managing patients, there is an ever-increasing role for interventional radiologists to facilitate optimal patient care via angiography. Furthermore, a 2015 study evaluating more than 6,000 high-grade liver injuries in the United States showed hepatic angiointervention as an independent determinant of survival from severe blunt hepatic injury. 22
Unlike adults, mortality in pediatric patients with abdominal trauma is relatively low, and the most frequent cause of death is head injury. 23 In pediatric patients with blunt hepatic injury, splenic injuries are often part of the injury complex. 10 These patients are preferentially managed nonoperatively. A prospective study demonstrated a 3% failure rate due to hepatic or splenic bleeding. However, the mortality for pediatric patients who failed NOM secondary to bleeding was 24%. 10
Current Standard of Care in Stratifying Hepatic Injuries in Adults
Although AAST grading is useful in imaging, there is no precise correlation between survival and AAST grade. 15 This is largely driven by the fact that AAST grading does not take hemodynamic stability into account. At present, the WSES recommends using AAST grading in conjunction with hemodynamic status in evaluating the optimal treatment. 24 Using this diagnostic paradigm, we will evaluate the treatment algorithm in patients based on hemodynamic stability.
A. Hemodynamically Stable Adult Patient
The mainstay of management of the hemodynamically stable adult patient is NOM. 25 In the early 2000s, this was seen as a paradigm shift from exploratory laparotomy, which had previously been the standard of care for blunt hepatic trauma. 25 Furthermore, multiple prospective studies have demonstrated that NOM is highly effective with failure rates of less than 10%. 1 As a result of aforementioned success rates, the standard of care for blunt hepatic trauma is NOM. 18 NOM includes close monitoring, supportive therapy, and angiographic interventions. The most recent international surgical guidelines recommend NOM for hepatic injuries irrespective of grade, mandating a significant role for the interventional radiologist to manage these patients. 10 Of note, there has been no reported correlation between AAST grade and failure of NOM. 19 Due to the success in NOM, only 13% of liver trauma patients are currently managed surgically. 26
B. Hemodynamically Unstable Adult Patient
Regardless of AAST grade, guidelines still recommend surgical management immediately. 24 There are decreased mortality rates by treating with angiography in addition to open laparotomy. 27 Most previously studied cases have used physically separate angiography suites from operating rooms, and patients are triaged to either the operating room or the angiography suite. In one study, 63% of unstable patients underwent hepatic embolization as a first intervention. 28 A more recently and evolving technique involves using c-arm angiography in the operating room for the management of unstable patients. In these instances, femoral access is established in the operating room and angiography is attained intraoperatively. 29 Several studies find the combined approach superior to either surgery alone or surgery with delayed angioembolization, with decreased total time and decreased mortality rate. 30
Standard of Care in Managing Pediatric Patient
Pediatric management for blunt trauma is now based on ATOMAC criteria. 31 NOM in the treatment of hemodynamically stable pediatric patients has been well established in blunt trauma. 18 In these patients, there is a significant role for CT angiography to evaluate for active bleeding prior to angiography. Notably, there is a higher success rate of NOM in children as compared with adults. 18 Pediatric patients can be successfully managed nonoperatively in approximately 95% of cases, whereas adult patients can be managed with NOM in 80% of cases. The driving factors for this discrepancy are not well established. As for adults, surgery is the recommended treatment in hemodynamically unstable patients.
Effective Management of Patients with Embolization
To maximize the likelihood of a successful hepatic artery embolization in the setting of trauma, the following clinical and technical aspects should be addressed:
A. Preoperative Evaluation
Emphasis on patient hemodynamic stability: Hemodynamic stability should be the first determinant of next steps. Even in patients with immediate plans for open surgery, trauma CT can be helpful in operative planning. The rapid acquisition of images in trauma CT has allowed for a minimal delay in definitive management. 32 Although historically angiography and embolization were reserved for patients who were hemodynamically stable, as explained earlier, there is increasing role for intraoperative angioembolization in unstable patients.
Role of single-phase versus multiphase abdominal and pelvic CT: While single-phase CT is more than 90% specific at confirming a parenchymal injury, it can detect only approximately 30% of cases of active hemorrhage. 33 To increase the sensitivity of detection of active hemorrhage, one method is using a split bolus technique where two or three boluses of contrast are given sequentially with a time delay or saline bolus in between followed by a single-pass CT acquisition. The resultant images are a combination of the arterial phase and the portal venous phase. 32 This technique is noninferior to conventional multiphase techniques in diagnosing traumatic injuries in the spleen but variable results in liver trauma. 34 Hence, for patients who are hemodynamically stable, multiphase CT has emerged as a preferred imaging to asses liver trauma and hemorrhage. The major drawback to multiphase imaging is the radiation exposure and time to acquire the images. Although potentially devastating consequences of inaction in arterial bleeding, multiphase CT is preferred in cases of suspected hemorrhage.
Antibiosis: Even in the absence of trauma, liver procedures incur an especially high risk of infection. Skin flora and enteric flora create a clean-contaminated surgical environment. With a competent sphincter of Oddi, the most recent Society of Interventional Radiology (SIR) guidelines recommend a choice of 1.5 to 3 g ampicillin/sulbactam IV, 1 g cefazolin and 500 mg metronidazole IV, or 1 g ceftriaxone IV perioperatively for hepatic arterial embolization. 20 35 If the patient is allergic to penicillin, consider vancomycin plus an aminoglycoside. 35
B. Procedure Nuances
Arterial access: Increasingly, centers are adopting a radial approach for arterial work in general. However, femoral access is the mainstay of treatment in the emergency setting. Utility of the radial versus femoral approach in the setting of trauma has yet to be evaluated in the literature. In acute hepatic trauma, arterial access is obtained by the common femoral artery. Once access is established, upsize the sheath to the same size or 1 Fr beyond the expected base catheter size which is usually 5 Fr. 36 Using a 5-Fr catheter, the superior mesenteric artery should be selected. 9 Digital subtraction angiography (DSA) should exclude an accessory or replaced right hepatic artery and exclude active arterial extravasation or pseudoaneurysm (PSA). The celiac trunk should then be selected. DSA should be performed to look for the same abnormalities described earlier. Using a 2.4- to 2.9-Fr microcatheter coaxially, access to proper hepatic artery is established, and DSA is again performed to evaluate for extravasation or PSA.
Variant anatomy: Recognizing variation in hepatic arterial anatomy is paramount to success in angioembolization. Because variant hepatic arterial anatomy is common, familiarity of the variations can reduce unintended nontargeted embolization. Only approximately 60% of patients have standard hepatic arterial anatomy with the celiac axis giving off the left gastric artery, splenic artery, and common hepatic artery ( Fig. 2 ). After the origin of the gastroduodenal artery, the common hepatic artery becomes the proper hepatic artery, which bifurcates into the right hepatic artery and the left hepatic artery. 37 Approximately 4 to 10% of patients have a replaced left hepatic artery, 10% of patients have a replaced right hepatic artery, 10% have an accessory left hepatic artery from the left gastric artery, and 2 to 7% have an accessory right hepatic artery from the superior mesenteric artery. 37 38 Less than 1% of patients have replaced left and right hepatic arteries. 37
Fig. 2.
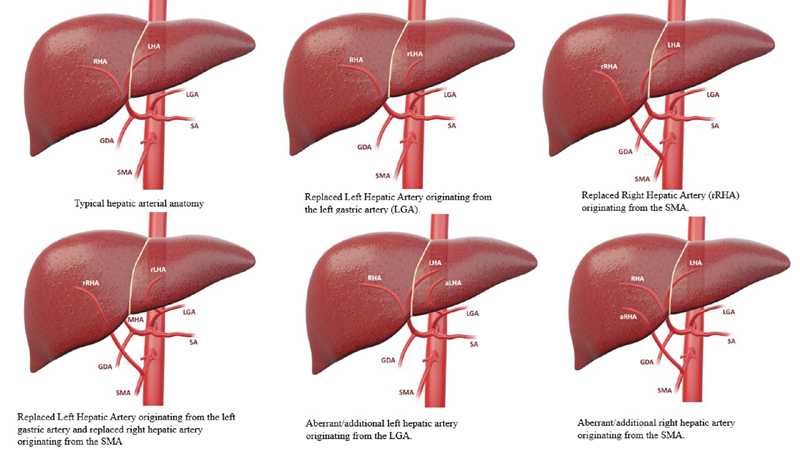
Summary of variant hepatic anatomy.
C. Potential Angiographic Findings
No active extravasation: In the case of a known traumatic injury, with CT evidence of perihepatic hemorrhage such as a subcapsular hemorrhage, with or without hemodynamic instability, empiric Gelfoam embolization of the branch supplying the area of bleeding should be considered.
Pseudoaneurysm: Traumatic psuedoaneurysm s occur in up to 15% of patients with liver trauma. 39 Delayed rupture of a pseudoaneurysm can have devastating consequences. Pseudoaneurysms can develop in delayed fashion. Therefore, postinjury contrast-enhanced CT should be performed 5 to 10 days after the initial scan. If a pseudoaneurysm is present, all patients should be treated with embolization immediately, due to the risk of rupture. Coil embolization is preferred to Gelfoam given the permanent nature of coils as compared with Gelfoam ( Fig. 3 ).
Arteriovenous or arterioportal shunting: Arteriovenous shunting occurs in a rare subset of the traumatic liver injury population. Arteriovenous and arterioportal shunting can be assessed on multiphase CT; however, definitive diagnosis is most accurate on angiography 39 ( Fig. 4 ). These patients are managed with coil embolization of the feeding artery. Failure to treat this abnormality may lead to the development of significant portal hypertension.
Late or venous bleeding: Treatment is based on hemodynamic stability. If stable, consider Gelfoam slurry to slow down the arterial pressure head. If unstable, surgical treatment is warranted. Trauma patients are hypothermic, hypotensive/hypovolemic, and coagulopathic. Once patients are warmed and volume resuscitated, bleeding may increase.
Fig. 3.
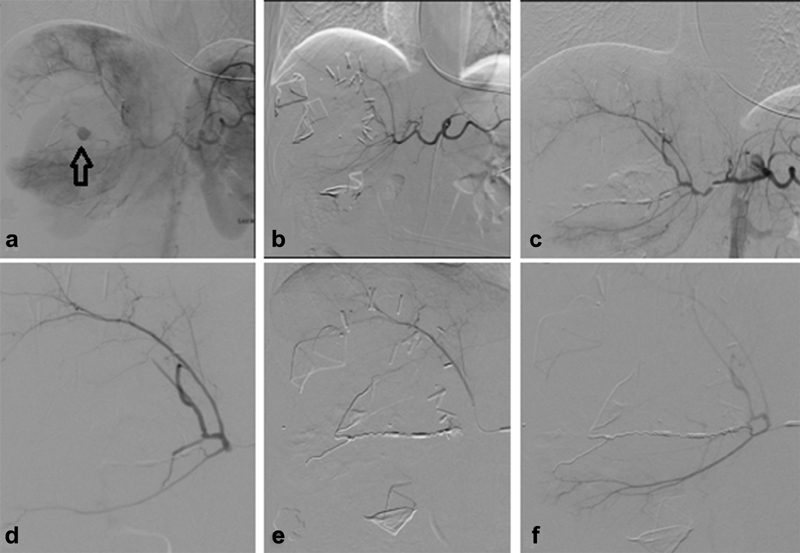
Open surgery followed by embolization. A 30-year-old patient status post gunshot wound taken directly to operating room for hemodynamic instability. Angiography was done intra-operatively after packing was performed due to continued bleeding and patient instability. Blush of contrast demonstrated a pseudoaneurysm (arrow) in area of hepatic laceration ( a ). Post angiography Clips ( b ). Coil embolization of the middle hepatic artery Pseudoaneurysm ( c–e ). Post embolization angiography demonstrated patency of right hepatic artery branches without opacification of the pseudoaneurysm ( f ).
Fig. 4.
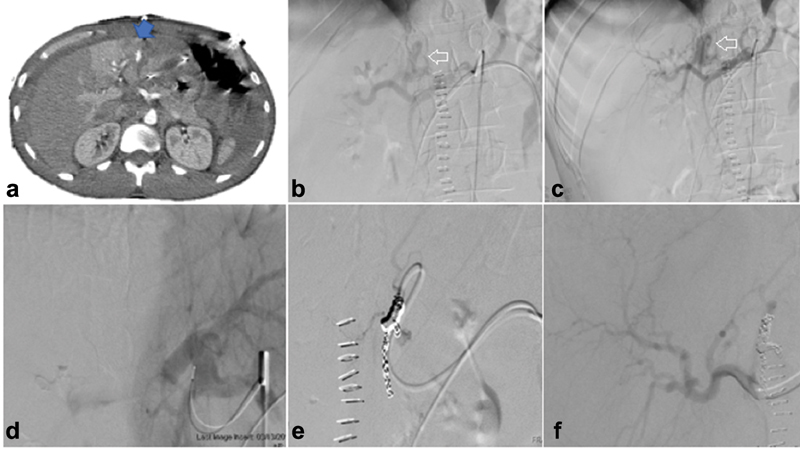
Arterioportal fistula. A 28-year-old patient status post stab wound presenting with acute drop in hemoglobin on postoperative day 2 for second exploratory laparotomy for washout. CTA shows a blush of contrast on arterial phase ( arrow ) ( a ). Diagnostic angiography of the celiac, proper hepatic, and left hepatic arteries ( b and c ) shows large left hepatic artery–left portal venous fistula ( arrows —left portal vein filling during arterial phase). Successful embolization of left hepatic artery using coil embolization with nonopacification of portal venous system on postembolization angiography from the base catheter in the celiac axis ( d–f ).
D. Embolic Choice
The choice of embolic agent should be guided by the presence of active arterial extravasation seen on CT or angiography.
Coil embolization: Coil embolization is best for targeted therapy. In a patient with clear extravasation, coils may be used for downstream targeted embolization of the selected vessel. Coil embolization results in permanent vessel occlusion. To reduce risk of coil displacement, coils should be 20% larger than the target vessel. Undersized coils can result in nontarget embolization. The use of detachable versus pushable coils depends on operators' experience and level of comfort with different types of coils available in their respective institutions.
Glue embolization: N-butyl-2-cyanoacrylate (NBCA) “glue” is fluoroscopically visible when mixed with ethiodized oil or tantalum powder, and used as a flow-directed embolic therapy. 40 This substance polymerizes rapidly when contacting blood or any ionic substance and forms a cast of the vessel. 41 In the instance of a known target, glue embolization can be employed with near-permanent hemostasis independent of coagulation status. Given the flow-dependent and permanent nature, this is not recommended in cases in which no arterial abnormality is identified. Furthermore, due to the rapid casting, the catheter must be withdrawn quickly to prevent adherence to the vessel wall. 41
Ethylene vinyl alcohol copolymer (ONYX): This liquid embolic is similar to the NBCA glue and it produces a permanent embolic effect. Unlike NBCA, ONYX is intrinsically radiopaque. 42 In published literature, onyx has been used in the embolization of vascular malformations and pseudoaneurysms. Onyx is quite expensive and rarely warranted in the treatment of hepatic trauma.
Gelfoam: Gelfoam is a gelatin sponge, which initiates the clotting cascade and provides scaffolding for clot formation. The Gelfoam is in theory temporary, with vessel occlusion lasting 3 to 6 weeks. 41 A Gelfoam slurry may be formed by mixing Gelfoam strips soaked in contrast and saline. A slurry is preferred for nontargeted embolization in smaller vessels, but can be used in targeted embolization of the smaller vessels. In larger vessels, dry pledgets are tightly rolled and loaded into a catheter and subsequently injected into the targeted vessel. Gelfoam embolization is preferred in cases of hemodynamic instability due to the ability to deliver rapid hemostasis. 36 In such cases, the temporary nature of Gelfoam is advantageous in nontargeted embolization. Additionally, Gelfoam may be used in combination with coils in cases where an injury is visualized but the catheter cannot be positioned beyond a PSA or arterioportal fistula. Gelfoam is used for distal occlusion to prevent reperfusion/collateral filling and the coil is placed proximal to the lesion.
Proposed Evidence-Based Management Algorithm for Treatment of Patients with Acute Liver Trauma
Fig. 5 depicts the proposed treatment algorithm for managing adult patients with hepatic trauma based on WSES recommendations and our institutional experience. All patients should be assessed for hemodynamic stability. Hemodynamically stable patients can be assessed with multiphase CT. If there is CT evidence of active extravasation or traumatic pseudoaneurysm, the patient should be further managed with angioembolization. If there is no evidence of active hemorrhage, patient should be managed with NOM. If the patient is hemodynamically unstable and not fluid/blood product responsive, they should be taken to the operating room (preferentially with c-arm capability) immediately. If the patient is fluid responsive/stabilizes with minimal intervention, a split bolus CT should be considered if clinically appropriate. This assessment enables the interventionalist and the surgeon to have a clearer idea of the extent of the trauma and presence of active arterial bleeding. Subsequently, this patient should be taken directly to the operating room with or without angiography.
Fig. 5.
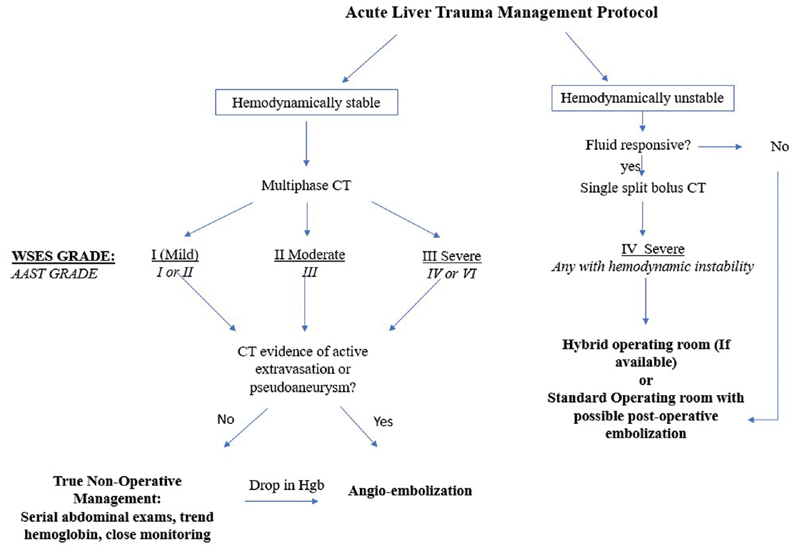
An evidence-based proposed protocol for management of patients with acute liver injury based on WSES guidelines and institutional experience.
Postprocedural Considerations
Role of Imaging Follow-up after embolization
There is no need for aggressive imaging follow-up if the patient is not symptomatic. However, if new symptoms arise, one of the following complications may be the cause. Thus, a multiphase CT is indicated for postprocedural evaluation in the setting of new symptoms.
Expected outcomes
In most cases, angiographic embolization is definitive for establishing hemodynamic control. Although angiographic interventions incur fewer risks than surgical management, there are several postprocedural complications IRs must be aware. According to one study, the most common complications from large-segment angiography and embolization is bile leak and hepatic necrosis as separate complications. 43 Additional complications include sepsis, hepatic abscess, sterile biloma, and gall bladder infarction. 44
Bile leak: This is among the most common complications in hepatic arterial intervention. 43 With embolization, there can be focal necrosis of the bile ducts, which can leak as a result. Leak can cause a biloma. Furthermore, posttraumatic cholecystectomy, when there is a concurrent cystic duct injury, incurs a high risk of bile leak. Current guidelines on the management of bile leak recommend treating any biloma if present with percutaneous drainage and subsequent selective coil embolization of the cystic duct. 45
Intrahepatic/Contained biloma: When a bile leak is contained within the liver parenchyma, it can form a biloma ( Fig. 6 ). This fluid can provide a nidus for infection with potentially devastating consequences. It is therefore recommended to percutaneously drain the intrahepatic biloma and control the source of the biliary collection.
Hepatic necrosis: An estimated 15% of angioembolization cases result in some degree of hepatic necrosis. 44 Furthermore, patients with liver failure at baseline are at an increased risk. Although the liver derives the majority of its blood supply from the portal circulation, the combination of trauma and embolization confers an increased risk of hepatic necrosis. Major hepatic necrosis involving a segment usually requires surgical debridement. 46
Hepatic abscess: Abscess can be a sequela of trauma alone, intervention, or a tertiary complication of hepatic necrosis. Risk factors for abscess development include poor portal flow, elevated transaminases, and a high-grade injury. 47 The etiology is almost universally contamination from the bile ducts or the vasculature. 48 Management requires a multidisciplinary approach between interventional radiology and trauma surgery to ensure appropriate antibiosis and adequate source control. CT-guided percutaneous drainage is the treatment of choice.
Fig. 6.
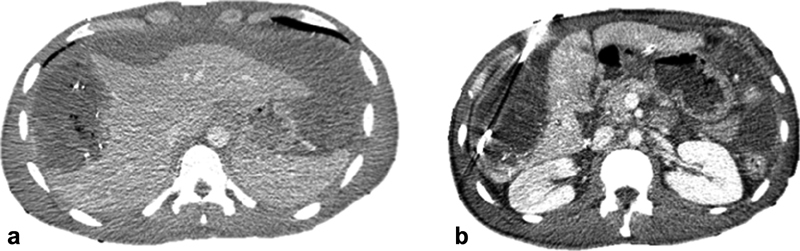
Contained/intrahepatic biloma in patient with grade V hepatic laceration treated with surgical packing followed by Gelfoam embolization due to uncontrolled bleeding ( a ). The biloma was treated with percutaneous drainage ( b ) and resolved after 6 months not shown here.
Conclusions
As interventional radiology catheter-based techniques have improved, the role of embolization in managing patients with hepatic trauma has increased. Given the improvement in mortality, embolization will be a mainstay in the treatment of hepatic injury for the foreseeable future.
Footnotes
Conflict of Interest None declared.
References
- 1.Boese C K, Hackl M, Müller L P, Ruchholtz S, Frink M, Lechler P. Nonoperative management of blunt hepatic trauma: a systematic review. J Trauma Acute Care Surg. 2015;79(04):654–660. doi: 10.1097/TA.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 2.Chang M Cet al. National Trauma Data Bank 2016 Pediatric Annual ReportAmerican College of Surgeons2016. Available at:https://www.facs.org/~/media/files/quality%20programs/trauma/ntdb/ntdb%20pediatric%20annual%20report%202016.ashx. Accessed January 30, 2020 [Google Scholar]
- 3.Vo N J, Althoen M, Hippe D S, Prabhu S J, Valji K, Padia S A. Pediatric abdominal and pelvic trauma: safety and efficacy of arterial embolization. J Vasc Interv Radiol. 2014;25(02):215–220. doi: 10.1016/j.jvir.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Gates R L, Price M, Cameron D B et al. Non-operative management of solid organ injuries in children: an American Pediatric Surgical Association Outcomes and Evidence Based Practice Committee systematic review. J Pediatr Surg. 2019;54(08):1519–1526. doi: 10.1016/j.jpedsurg.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Fodor M, Primavesi F, Morell-Hofert D et al. Non-operative management of blunt hepatic and splenic injury: a time-trend and outcome analysis over a period of 17 years. World J Emerg Surg. 2019;14:29. doi: 10.1186/s13017-019-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hommes M, Navsaria P H, Schipper I B, Krige J E, Kahn D, Nicol A J. Management of blunt liver trauma in 134 severely injured patients. Injury. 2015;46(05):837–842. doi: 10.1016/j.injury.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Parks N A, Davis J W, Forman D, Lemaster D. Observation for nonoperative management of blunt liver injuries: how long is long enough? J Trauma. 2011;70(03):626–629. doi: 10.1097/TA.0b013e31820d1c69. [DOI] [PubMed] [Google Scholar]
- 8.Letoublon C, Morra I, Chen Y, Monnin V, Voirin D, Arvieux C.Hepatic arterial embolization in the management of blunt hepatic trauma: indications and complications J Trauma 201170051032–1036., discussion 1036–1037 [DOI] [PubMed] [Google Scholar]
- 9.Carver D, Kirkpatrick A W, D'Amours S, Hameed S M, Beveridge J, Ball C G.A prospective evaluation of the utility of a hybrid operating suite for severely injured patients: overstated or underutilized?Ann Surg2018. Doi: 10.1097/SLA.0000000000003175. [Epub ahead of print] [DOI] [PubMed]
- 10.Linnaus M E, Langlais C S, Garcia N M et al. Failure of nonoperative management of pediatric blunt liver and spleen injuries: a prospective Arizona-Texas-Oklahoma-Memphis-Arkansas Consortium study. J Trauma Acute Care Surg. 2017;82(04):672–679. doi: 10.1097/TA.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 11.Letoublon C, Amariutei A, Taton Net al. Management of blunt hepatic trauma J Visc Surg 2016153(4, Suppl):33–43. [DOI] [PubMed] [Google Scholar]
- 12.Cook A, Weddle J, Baker Set al. A comparison of the injury severity score and the trauma mortality prediction model J Trauma Acute Care Surg 2014760147–52., discussion 52–53 [DOI] [PubMed] [Google Scholar]
- 13.Loftis K L, Price J, Gillich P J. Evolution of the abbreviated injury scale: 1990–2015. Traffic Inj Prev. 2018;19:S109–S113. doi: 10.1080/15389588.2018.1512747. [DOI] [PubMed] [Google Scholar]
- 14.Moore E E, Cogbill T H, Jurkovich G J, Shackford S R, Malangoni M A, Champion H R. Organ injury scaling: spleen and liver (1994 revision) J Trauma. 1995;38(03):323–324. doi: 10.1097/00005373-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Coccolini F, Catena F, Moore E E et al. WSES classification and guidelines for liver trauma. World J Emerg Surg. 2016;11(01):50. doi: 10.1186/s13017-016-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodor M, Primavesi F, Morell-Hofert D et al. Non-operative management of blunt hepatic and splenic injuries-practical aspects and value of radiological scoring systems. Eur Surg. 2018;50(06):285–298. doi: 10.1007/s10353-018-0545-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schellenberg M, Benjamin E, Piccinini A, Inaba K, Demetriades D. Gunshot wounds to the liver: no longer a mandatory operation. J Trauma Acute Care Surg. 2019;87(02):350–355. doi: 10.1097/TA.0000000000002356. [DOI] [PubMed] [Google Scholar]
- 18.Inchingolo R, Ljutikov A, Deganello A, Kane P, Karani J. Outcomes and indications for intervention in non-operative management of paediatric liver trauma: a 5 year retrospective study. Clin Radiol. 2014;69(02):157–162. doi: 10.1016/j.crad.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Prichayudh S, Sirinawin C, Sriussadaporn S et al. Management of liver injuries: predictors for the need of operation and damage control surgery. Injury. 2014;45(09):1373–1377. doi: 10.1016/j.injury.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.David Richardson J, Franklin G A, Lukan J K et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000;232(03):324–330. doi: 10.1097/00000658-200009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asensio J A, Demetriades D, Chahwan S et al. Approach to the management of complex hepatic injuries. J Trauma. 2000;48(01):66–69. doi: 10.1097/00005373-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Sivrikoz E, Teixeira P G, Resnick S, Inaba K, Talving P, Demetriades D. Angiointervention: an independent predictor of survival in high-grade blunt liver injuries. Am J Surg. 2015;209(04):742–746. doi: 10.1016/j.amjsurg.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Kiankhooy A, Sartorelli K H, Vane D W, Bhave A D. Angiographic embolization is safe and effective therapy for blunt abdominal solid organ injury in children. J Trauma. 2010;68(03):526–531. doi: 10.1097/TA.0b013e3181d3e5b7. [DOI] [PubMed] [Google Scholar]
- 24.Coccolini F, Montori G, Catena F et al. Liver trauma: WSES position paper. World J Emerg Surg. 2015;10(01):39. doi: 10.1186/s13017-015-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra A K, Fabian T C, Croce M A et al. Blunt hepatic injury: a paradigm shift from operative to nonoperative management in the 1990s. Ann Surg. 2000;231(06):804–813. doi: 10.1097/00000658-200006000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinkoff G, Esposito T J, Reed J et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008;207(05):646–655. doi: 10.1016/j.jamcollsurg.2008.06.342. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Cantrell E, Jung K, Smith A, Coimbra R. Influence of postoperative hepatic angiography on mortality after laparotomy in Grade IV/V hepatic injuries. J Trauma Acute Care Surg. 2018;85(02):290–297. doi: 10.1097/TA.0000000000001906. [DOI] [PubMed] [Google Scholar]
- 28.Samuels J M, Urban S, Peltz Eet al. A modern, multicenter evaluation of hepatic angioembolization - Complications and readmissions persistAm J Surg2019. Doi: 10.1016/j.amjsurg.2019.06.021. [Epub ahead of print] [DOI] [PubMed]
- 29.Martin J G, Shah J, Robinson C, Dariushnia S. Evaluation and management of blunt solid organ trauma. Tech Vasc Interv Radiol. 2017;20(04):230–236. doi: 10.1053/j.tvir.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka Y, Minehara H, Kashimi F et al. Hybrid treatment combining emergency surgery and intraoperative interventional radiology for severe trauma. Injury. 2016;47(01):59–63. doi: 10.1016/j.injury.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Kohler J E, Chokshi N K. Management of abdominal solid organ injury after blunt trauma. Pediatr Ann. 2016;45(07):e241–e246. doi: 10.3928/00904481-20160518-01. [DOI] [PubMed] [Google Scholar]
- 32.Jeavons C, Hacking C, Beenen L F, Gunn M L. A review of split-bolus single-pass CT in the assessment of trauma patients. Emerg Radiol. 2018;25(04):367–374. doi: 10.1007/s10140-018-1591-1. [DOI] [PubMed] [Google Scholar]
- 33.Iacobellis F SM, Brillantino A, Scuderi M G et al. The additional value of the arterial phase in the CT assessment of liver vascular injuries after high-energy blunt trauma. Emerg Radiol. 2019;26(06):647–654. doi: 10.1007/s10140-019-01714-y. [DOI] [PubMed] [Google Scholar]
- 34.Marovic P, Beech P A, Koukounaras J, Kavnoudias H, Goh G S. Accuracy of dual bolus single acquisition computed tomography in the diagnosis and grading of adult traumatic splenic parenchymal and vascular injury. J Med Imaging Radiat Oncol. 2017;61(06):725–731. doi: 10.1111/1754-9485.12619. [DOI] [PubMed] [Google Scholar]
- 35.Monzer A, Chehab A S, Tulin-Silver S et al. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol. 2011;22(02):263. [Google Scholar]
- 36.Stainken B. Wolters Kluwer Health; 2016. Trauma management. [Google Scholar]
- 37.Covey A M, Brody L A, Maluccio M A, Getrajdman G I, Brown K T. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224(02):542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 38.Winter T C, III, Nghiem H V, Freeny P C, Hommeyer S C, Mack L A. Hepatic arterial anatomy: demonstration of normal supply and vascular variants with three-dimensional CT angiography. Radiographics. 1995;15(04):771–780. doi: 10.1148/radiographics.15.4.7569128. [DOI] [PubMed] [Google Scholar]
- 39.Durkin N, Deganello A, Sellars M E, Sidhu P S, Davenport M, Makin E. Post-traumatic liver and splenic pseudoaneurysms in children: diagnosis, management and follow-up screening using contrast-enhanced ultrasound (CEUS) J Pediatr Surg. 2016;51(02):289–292. doi: 10.1016/j.jpedsurg.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 40.Darcy M. Wolters Kluwer Health; 2019. Acute Gastrointestinal hemorrhage; p. 232. [Google Scholar]
- 41.Medsinge A, Zajko A, Orons P, Amesur N, Santos E. A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol. 2014;203(04):699–708. doi: 10.2214/AJR.14.12480. [DOI] [PubMed] [Google Scholar]
- 42.Vanninen R L, Manninen I. Onyx, a new liquid embolic material for peripheral interventions: preliminary experience in aneurysm, pseudoaneurysm, and pulmonary arteriovenous malformation embolization. Cardiovasc Intervent Radiol. 2007;30(02):196–200. doi: 10.1007/s00270-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 43.Mohr A M, Lavery R F, Barone Aet al. Angiographic embolization for liver injuries: low mortality, high morbidity J Trauma 200355061077–1081., discussion 1081–1082 [DOI] [PubMed] [Google Scholar]
- 44.reen C S, Bulger E M, Kwan S W. Outcomes and complications of angioembolization for hepatic trauma: a systematic review of the literature. J Trauma Acute Care Surg. 2016;80(03):529–537. doi: 10.1097/TA.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nezami N, Jarmakani H, Arici M et al. Selective trans-catheter coil embolization of cystic duct stump in post-cholecystectomy bile leak. Dig Dis Sci. 2019;64(11):3314–3320. doi: 10.1007/s10620-019-05677-5. [DOI] [PubMed] [Google Scholar]
- 46.Sacks D, Ong A, Fernandez F. Percutaneous debridement of posttraumatic infected major hepatic necrosis. J Vasc Interv Radiol. 2014;25(08):1273–1277. doi: 10.1016/j.jvir.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Hsu C P, Wang S Y, Hsu Y P et al. Risk factors for liver abscess formation in patients with blunt hepatic injury after non-operative management. Eur J Trauma Emerg Surg. 2014;40(05):547–552. doi: 10.1007/s00068-013-0346-7. [DOI] [PubMed] [Google Scholar]
- 48.Lardière-Deguelte S, Ragot E, Amroun K et al. Hepatic abscess: diagnosis and management. J Visc Surg. 2015;152(04):231–243. doi: 10.1016/j.jviscsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]


