Abstract
Background
Oral tranexamic acid (TXA), if effective in reducing blood loss after delivery for women experiencing primary PPH, could be administered where parenteral administration is not feasible. This trial assessed the efficacy, safety, and acceptability of oral TXA when used as an adjunct to sublingual misoprostol to treat postpartum hemorrhage (PPH) following vaginal delivery.
Methods
From October 2016 to January 2018, women presenting at four hospitals in Senegal and Vietnam for vaginal delivery were screened for enrollment in the trial. Women diagnosed with postpartum hemorrhage (defined as blood loss ≥700 ml) were randomized to receive either oral TXA (1950 mg) or placebo in addition to 800 mcg sublingual misoprostol. Postpartum blood loss was measured using a calibrated drape. Blood loss for all PPH cases was recorded for 2 h after administration of the drugs. The primary outcome measure was the proportion of women with bleeding controlled with the trial regimen without recourse to further treatment. Secondary outcomes including the rate of severe PPH, mean/median blood loss, use of additional uterotonics and/or interventions side effects, and acceptability were also recorded.
Results
Of the 258 women who received treatment for PPH, 128 received placebo and misoprostol and 130 received TXA and misoprostol. The proportion of women who had active bleeding controlled with trial drugs alone and no additional interventions was similar in both groups: 77(60.2%) placebo; 74 (56.9%) TXA, p = 0.59). Use of other interventions to control bleeding, including uterotonics, did not differ significantly between groups. Median blood loss at PPH diagnosis was 700 ml in both groups. Uterine atony alone or in addition to another cause contributed to over 90% of PPH cases reported (92.2% placebo vs. 91.5% TXA), other causes included perineal and cervical lacerations and retained placenta. Reports of side effects and acceptability were similar in the two groups.
Conclusion
Adjunct use of oral TXA with misoprostol to treat PPH resulted in similar clinical and acceptability outcomes when compared to treatment with misoprostol alone.
Trial registration
This trial was registered with ClinicalTrials.gov, number NCT02805426. Registered on 3 September 2016.
Keywords: Postpartum hemorrhage (PPH), Tranexamic acid, Misoprostol
Plain English summary
Excessive bleeding after childbirth – postpartum hemorrhage (PPH) – is a complication that can occur without warning and can quickly lead to death. Timely treatment strategies are urgently needed wherever women deliver. Tranexamic acid (TXA) is a blood clot stabilizer used routinely for reduction of blood loss in surgery and trauma. It has shown promise in reducing the risk of death from bleeding after childbirth when given by intravenous (IV) administration within 3 h of delivery, and is recommended in clinical guidelines (WHO 2017). The route and time-dependent administration make TXA out of reach for most women who experience primary PPH in settings where IV administration is not feasible and transfer within 3 h is unlikely. TXA is widely available in tablet form at low cost and is stable at room temperature, creating a potential opportunity for its use as part of a PPH management package in lower level health facilities and home births. This trial explored the potential benefit of oral TXA when used as an adjunct to sublingual misoprostol to treat PPH following vaginal delivery. Two hundred and fifty-eight women diagnosed with PPH were randomly assigned to receive sublingual misoprostol and either oral TXA or placebo. Providers measured blood loss for 2 h after administration of the medicines and recorded suspected cause of PPH, blood loss, additional interventions, and side effects. The findings suggest that the addition of oral TXA did not confer any substantial advantage in treating primary PPH when compared to treatment with misoprostol alone.
Introduction
Obstetric hemorrhage contributes to approximately 25% of maternal deaths worldwide [1]. Despite systematic use of prophylaxis, PPH still occurs in 3–10% [2, 3], of deliveries.
Tranexamic acid, a synthetic derivative of the amino acid lysine, is an anti-fibrinolytic agent that acts by blocking the lysine binding sites on plasminogen [4]. TXA significantly reduces postoperative blood loss and the need for transfusions following surger y[5–8]..A multi-site trial demonstrated that IV TXA (1 g) administered immediately after the onset of postpartum bleeding and within 3 h of birth reduces death among women with PPH [9]. In fact, WHO recommends that IV TXA should be administered to all diagnosed cases of PPH, regardless of cause [10].. While these findings confirm that IV TXA is effective in in helping manage PPH, questions remain as to whether a tablet formulation administered orally is also an effective treatment option. Currently, no information is available about its effectiveness in oral formulation for PPH treatment. Oral TXA has been explored for PPH prophylaxis and may be effective when combined with misoprostol when administered postpartum [11]. If oral TXA were helpful in reducing blood loss after delivery for women experiencing PPH, it could be administered by mid and low level providers at lower level health facilities or home births, where parenteral administration is not feasible and transfer to higher level of care may be delayed. TXA could then serve as a complement to misoprostol, an E1 prostaglandin which is effective in controlling PPH [2, 3] cause by atony. The two drug regimen of oral TXA and misoprostol might improve the efficacy of treatment of PPH in low resource settings, where a large proportion of deaths from PPH occur [1]. This trial aimed to assess the proportion of women with bleeding controlled when oral tranexamic acid is used in conjunction with misoprostol for treatment of PPH.
Methods
This individually, randomized, double-blind, placebo-controlled trial enrolled participants from October 25, 2016 to January 19, 2018 in four secondary and tertiary level hospitals in Senegal (2) and Vietnam (2). During the course of the trial, due to slower than expected enrollment, one site in Senegal was replaced with a new site in Vietnam in June 1, 2017. To be eligible, women had to deliver vaginally and give written informed consent prior to delivery. Women were excluded if there was a history of thrombosis or a clear contraindication for tranexamic acid such as a known allergy. Per standard of care, women received (either 5 or 10 IU) oxytocin prophylactically. After delivery, blood loss was measured using a plastic drape with a calibrated funnel (MEDIPRO©, Vietnam) that was placed under the woman’s buttocks immediately after delivery of the baby for a minimum of 30 min or until active bleeding ceased. PPH was diagnosed if blood loss reached 700 ml on the drape. Providers could also diagnose PPH based on clinical signs and symptoms (such as heart rate or blood pressure). Women diagnosed with PPH were randomized to receive the next sequential trial drug packet, which consisted of 1950 mg oral tranexamic acid (3 × 650 mg, AMRING Pharmaceuticals) or placebo and 800 mcg (four × 200 mcg) misoprostol (GyMiso®, HRA Pharma, France) administered sublingually. Women were asked to swallow the oral TXA or oral placebo tablets immediately after diagnosis with water before placing the four misoprostol tablets under the tongue.
The oral dose of tranexamic acid selected for this study was based on available pharmacokinetic data in the literature including a previous study reporting on the safety of administering 2000 mg dose [12]. While much higher doses have been shown to be safe and are recommended for heavy bleeding during menses (1300 mg × 3 daily), the selected dose for this study was also based on practical considerations, taking into account the number of pills that would be needed based on the tablet dose available in Vietnam, where study drugs were procured. .
The randomization scheme was computer-generated in blocks of ten and maintained by Gynuity Health Projects. The allocation ratio between placebo and TXA was equal overall but randomly varied within blocks. Trial staff including providers and women were masked to treatment assignment. The trial was unblinded after all trial data were collected. Periodic monitoring ensured that each hospital followed the numerical sequence of the boxes and that masking was successful.
For all PPH cases, blood loss was recorded at five intervals: at treatment, 20 min-, 40 min-, 1 h-, and 2 h- after treatment. If the woman was stable, providers were asked to wait 20 min after administering trial treatment before considering additional interventions for the PPH, although the administration of any additional intervention at any time point was documented. Additional interventions and the cause of PPH (as determined by the provider) were documented. At the time of discharge from the hospital, participants were asked about side effects, acceptability and satisfaction with the trial treatment.
The primary outcome was the proportion of women for whom bleeding was controlled with just the trial regimen (placebo or 1950 mg TXA, followed by 800 mcg misoprostol) without recourse to additional treatment. Controlled bleeding was subject to provider assessment. We hypothesized that bleeding would stop among 89% of women in the placebo group (misoprostol alone), as demonstrated in previous trials on the efficacy of misoprostol to treat PPH [2]. We estimated that an additional 8% of women who received the TXA in addition to the misoprostol would experience cessation of bleeding with no other intervention. Based on these assumptions (89% vs 97%), a sample of 250 PPH cases (125 per group) was required for a one-sided test with 80% power, alpha = 0.05.
Data were collected and recorded by staff trained in trial procedures and reviewed by coordinators at each hospital. Data were entered in SPSS 15 software (IBM, Chicago, IL, USA). All data were entered and analyzed using SPSS 19 software (IBM, Chicago, IL, USA).
The trial was planned and analyzed as intent to treat (ITT). Univariate analysis reported on demographic variables. Bivariate analyses, stratified by trial arm (ITT) and by actual receipt of and compliance with the intervention tested the primary and secondary outcomes.
The protocol was approved by National Council on Health Research, National Ethical Committee, Ministry of Health and Prevention, Senegal and in Vietnam; Ethics Committee in Biomedical Research of Hung Vuong Hospital and Ethics Committee in Biomedical Research of the National Hospital and is reported in accordance with the revised CONSORT statement [13]. An independent Data Safety Monitoring Board (DSMB) reviewed the dataset for safety concerns when two-thirds of the PPH cases had been enrolled.
Results
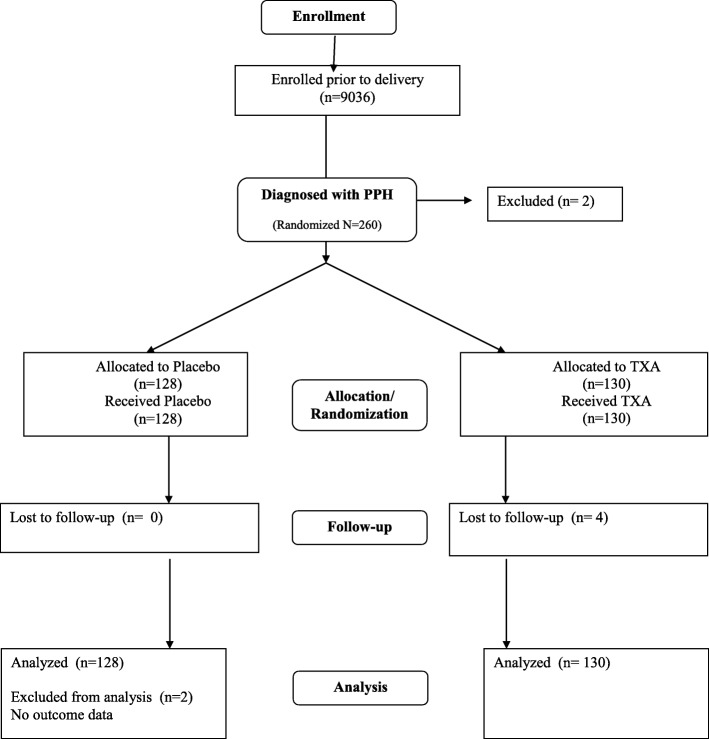
Of the 9036 participants, 260 (3%) were diagnosed with PPH. A total of 258 women were randomized and included in the analysis: 128 in the placebo arm and 130 in the TXA group. Two PPH cases were eligible but not randomized: in one case the woman was unconscious and could not take oral medication, and the second woman experienced secondary PPH 9 days after the delivery. All 258 received the 800mcg sublingual misoprostol as part of the study intervention. (Fig. 1). There were no differences in baseline or delivery characteristics between the two groups (Table 1). Among women who received placebo, active bleeding was controlled without recourse to additional interventions in 77 (60.2%) of cases compared to 74 (56.9%) in the TXA group (p = 0.59) (Table 2) RR = 0.936, 95% CI 0.734–1.194) Of the women who received interventions in addition to the trial regimen, approximately one-third received additional uterotonics (placebo: 39 (30.5%); TXA treatment: 45 (34.6%) p = 0.51) (Table 2). Receipt of additional IV TXA (placebo: 16 (12.5%); TXA: 17 (13.1%)) was similar across groups. In most instances the additional interventions were administered within 20 min after administration of trial regimen (placebo: 39 (78%); TXA: 43 (76.8%)) (data not shown). Disaggregated results show no significant differences in outcomes associated with cause of PPH or uterotonics received prior to third stage of labor (data not shown).
Fig. 1.
Consort flow chart
Table 1.
Baseline and delivery characteristics among vaginal births (PPH cases only)
| Miso + Placebo N = 128 |
Miso + TXA N = 130 |
|
|---|---|---|
| Age Median (range) | 28 (19–48) | 28 (17–40) |
| Parity | 0.61 | 0.85 |
| Woman experience previous PPH | 3 (2.3) | 7 (5.4) |
| Singleton birth | 125 (97.7) | 125 (96.2) |
| Neonatal deaths | 1 (0.8) | 0 (0.0) |
| Episiotomy | 117 (91.4) | 113 (86.9) |
| Uterotonic to induce labor | 11 (8.6) | 13 (10.0) |
| Uterotonic to augment labor | 39 (30.5) | 39 (30.0) |
| Oxytocin prophylaxis | 128 (100) | 130 (100) |
| IM | 123 (96.1) | 118 (90.8) |
| Manual removal of placentaa | 5 (3.9) | 11 (8.5) |
There were no statistically significant differences between the two groups for any of these variables
a did not involve transfer to theater. No other complications were noted for these women
Table 2.
Primary Outcome, treatment outcomes and interventions
| Placebo N = 128 |
TXA N = 130 |
|
|---|---|---|
| Bleeding controlled with treatment only –(no additional intervention)a | 77 (60.2) | 74 (56.9) |
| Bleeding controlled with treatment only –(no additional serious intervention)b | 102 (79.7) | 108 (83.1) |
| Additional interventions | ||
| Oxytocin | 37 (28.9) | 39 (30.0) |
| IV | 25 (19.5) | 25 (19.2) |
| IM | 12 (9.4) | 14 (10.8) |
| Ergometrine | 34 (26.6) | 34 (26.2) |
| Syntocinon | 1 (0.8) | 1 (0.8) |
| Carbetocin IV | 10 (7.8) | 14 (10.8) |
| Misoprostol | 0 (0.) | 1 (0.8) |
| TXA IV | 16 (12.5) | 17 (13.1) |
| Uterine evacuation (MVA) | 0 (0.0) | 1 (0.8) |
| Bimanual compression | 8 (6.3) | 8 (6.2) |
| Suturing | 111 (86.7) | 108 (83.1) |
| Uterine packing | 15 (11.7) | 10 (7.7) |
| Blood transfusion | 13 (10.2) | 12 (9.2) |
| Uterine artery ligature | 1 (0.8) | 0 (0) |
| Hysterectomy | 1 (0.8) | 0 (0) |
| Tissue repair | 2 (1.6) | 1 (0.8) |
| Plasma expanders | 4 (3.1) | 3 (2.3) |
There were no statistically significant differences between the two groups for any of these variables
aadditional interventions include: uterotonics, TXA, bimanual compression, uterine evacuation, uterine packing, blood transfusion, uterine artery ligation, hysterectomy, tissue repair, plasma expanders. Suturing and administration of IV fluids were excluded
b additional serious interventions calculated as all interventions excluding uterotonics, TXA, bimanual compression, suturing and fluids (serious interventions only)
Median blood loss at diagnosis for women who received study treatment was 700 ml (range 500-1500 ml) (Table 3). Only 6 women (2.3%) were diagnosed with severe PPH (blood loss ≥1000 ml).
Table 3.
PPH diagnosis and blood loss
| Miso + Placebo N = 128 |
Miso + TXA N = 130 |
|
|---|---|---|
| Median Blood loss at PPH diagnosis | 700 (500–1200) | 700 (500–1500) |
| Reason for PPH | ||
| Uterine atony (among causes) | 118 (92.2) | 119 (91.5) |
| Uterine atony alone | 91 (71.1) | 89 (69.5) |
| Uterine atony + other causea | 27 (21.3) | 30 (23.4) |
| Non atonic cause | 9 (7.1) | 9 (7.0) |
| Median blood loss at treatment | 700 (500–2000) | 700 (500–1500) |
| Median blood loss at 20 min post treatment | 750 (500–2200) | 750 (550–1600) |
| Median blood loss at 40 min post treatment | 800 (500–2300) | 800 (550–2000) |
| Median blood loss at 1 h post treatment | 800 (500–2300) | 800 (550–2000) |
| Median blood loss at 2 h post treatment | 800 (500–2300) | 800 (550–2000) |
| Time to bleeding controlled post treatment | ||
| Mean | 33 min (0-2 h) | 33 min (0-2 h) |
| Median | 20 min | 20 min |
| Mean time to bleeding controlled post | 23 min (N = 74) | 28 min (N = 74) |
| treatment- TXT DRUG ONLY | Median: 20 min | Median: 20 min |
| Mean time to bleeding controlled post | 48 min (N = 52) | 41 (N = 54) |
| treatment- ADDITIONAL INTERVENTIONS | Median: 30 min | Median:30 min |
There were no statistically significant differences between the two groups for any of these variables
aOther causes included cervical or perineal lacerations and retained placenta
Uterotonics had been used to induce labor in approximately 9% of the cases and augment in 30% of the cases. Over 90% of women had episiotomy, and all women were given oxytocin prophylaxis during the third stage of labor. Characteristics of the enrolled PPH cases were comparable in the two study groups. Uterine atony either alone or in addition to other causes cited contributed to the majority of PPH cases (placebo: 118 (92.2%); TXA: 119 (91.5%)). Approximately 70% of women experienced uterine atony as the sole cause of PPH (placebo: 91 (71.7%); TXA: 89 (69.5%)) while 21.3% (n = 27) and 23.4% (n = 30) for placebo and TXA respectively, had PPH due to uterine atony in addition to perineal lacerations, cervical lacerations or retained placenta. 7.1% (n = 9) and 7.0% (n = 9) in the placebo and TXA groups respectively, had a reported PPH due to non-atonic causes (Table 1). Median time to PPH treatment after birth was 10 min (same in each arm) with only 6.3% (placebo) and 7.7% (TXA) administered treatment more than 1 h after childbirth (data not shown).
The side effect profile was similar in the two trial groups. Shivering was reported as the most frequent side effect experienced [placebo: 81 (63.8%); TXA: 82 (63.6%)] followed by fever [placebo: 40 (31.3%); TXA: 33 (25.4%)] (Table 4). In both groups, women reported that treatment was acceptable (Table 4). One participant in the placebo arm who experienced atonic PPH underwent a hysterectomy 4 days post-delivery. In this case, oxytocin was used to induce and augment the delivery. Approximately an hour after PPH diagnoses, the placenta was removed manually and the uterus packed. The woman received IM oxytocin, ergometrine, IV TXA and 2 units of blood. Shortly after receipt of these interventions a uterine artery ligation and B-lynch were performed. After her 2-day hospital stay, the woman was transferred to a cardiac unit because of cardiac concerns where the hysterectomy was performed 2 days later after diagnosis of uterine necrosis. She was discharged in stable condition. There were no deaths reported among trial participants.
Table 4.
Side effects
|
Miso + Placebo N = 128 |
Miso + TXA N = 130 |
|
| Side Effects experienced: | ||
| Shivering | 81 (63.8) | 82 (63.6) |
| Fever | 40 (31.3) | 33 (25.4) |
| Nausea | 13 (10.2) | 9 (7.0) |
| Vomiting | 7 (5.5) | 6 (4.7) |
| Diarrhea | 1 (0.8) | 1 (0.8) |
| Fainting | 1 (0.8) | 2 (1.6) |
| Acceptability (reported by women) | N = 119 | N = 121 |
| Very acceptable or acceptable | 107 (89.9) | 113 (93.3) |
| Neutral | 7 (5.7) | 4 (3.3) |
| Very unacceptable or unacceptable | 5 (4.2) | 4 (3.3) |
There were no statistically significant differences between the two groups for any of these variables
Discussion
This trial was designed to explore whether the addition of oral TXA to misoprostol treatment of PPH would produce improved outcomes. The rate of additional interventions employed to curb bleeding and mean blood loss post-treatment was not significantly different among women who received oral TXA in addition to misoprostol compared to women who did not receive TXA. These findings suggest that the addition of oral TXA did not confer any substantial advantage in treating PPH. It may be possible that TXA offers limited additional clinical benefit related to serious outcomes.
Previous trials have documented that 9 out of 10 women experience cessation of bleeding within 20 min after administration of misoprostol without recourse to additional interventions [3]. Although median time to bleeding cessation for both groups was also 20 min in our study, almost 80% of women who received additional interventions received them less than 20 min after study treatment, possibly limiting the observed effect of trial drugs alone. Additional analysis, excluding women who received treatment prior to 20 min, continues to show no significant differences between treatment groups [placebo 78/89 (87.6%); TXA 74/87 (85.1%)].
Whereas IV TXA is recommended for use in the treatment of PPH, oral TXA may not confer the same effect as IV administration, which allows for much greater bioavailability [11]. Therefore, it is possible that the misoprostol treatment (in addition to other interventions), may have effectively controlled blood loss before oral TXA could take effect. Moreover, studies have highlighted the importance of early use to treat PPH [14].Although in the the large multisite WOMAN trial, IV TXA was shown to reduce death due to blood loss [9], there was no benefit if TXA was administered more than 3 h postpartum. Given the later onset of action, oral TXA may be most effective when administered earlier in delivery.
The oral TXA dose administered in this study was based on the established safety a 2000 mg dose [12]. Given current recommendations for higher doses (used for management of heavy menstrual bleeding) the lower study dose used may have constituted a limitation in examining the true potential of oral TXA. Nevertheless, a study testing 1000 mg oral TXA at the end of the first stage of labor followed by misoprostol showed that this regimen was associated with lower blood loss when compared to prophylactic IV oxytocin [11].
Another limitation of our study is that the rates of intervention were very different from our assumptions for sample size calculation (above). We estimated that bleeding would be controlled with no additional intervention in 89% of cases in the placebo group, when in fact this was the case for only 60%. We aimed to conduct an exploratory study of a pill-based regimen in a location where other treatments were readily available in case they were needed. The unintended consequence of the high use of additional interventions at these sites may have made it difficult to assess the true effect of the study medicines. As in the WOMAN trial, where the authors argue that interventions carried out prior to TXA treatment may have diluted its effect, it is possible that outcomes might be different with a larger sample and in other delivery settings with more limited options for PPH management.
Consistent with the literature [15], atony contributed to over 90% of the PPH cases and was the primary cause of PPH for approximately 70% of women in this cohort. These findings reconfirm the importance of ensuring good access to high quality uterotonics for management of hemorrhage. The management practices documented in this study, however, also reveal the potential for over-use of PPH medicines that are becoming increasingly available. For example, 80.4% (n = 86) of the women who received additional interventions received additional uterotonics, and 76.7% (n = 66) of these women received 2 or more uterotonic drugs for PPH treatment. This tally does not include the uterotonics also given for induction/augmentation and prophylaxis or the misoprostol treatment all women received. Administration of multiple doses and potential overuse of available medicines deserve greater attention and evaluation in relation to women’s quality of care, clinical outcomes (additional treatments with uterotonics did not appear to have an overall effect on blood loss), and resources and costs to health systems.
Conclusion
While trial findings suggest that oral TXA may not have a significant effect in addressing PPH, options that treat non-atonic causes deserve consideration. International guidelines, clearly recommend that IV TXA be administered to women diagnosed with PPH and should be made available as part of a standard treatment package. It nevertheless remains imperative to explore simple options to manage all causes of PPH and to manage PPH related morbidity, especially when access to IV therapy or surgical interventions is limited.
Acknowledgements
Not applicable.
Abbreviations
- PPH
Postpartum hemorrhage
- TXA
Tranexamic acid
Authors’ contributions
AD analyzed and interpreted the data. AD and DA contributed to writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Merck for Mothers and the Bill and Melinda Gates Foundation. Merck for Mothers reviewed final study protocol prior to implementation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The protocol was approved by National Council on Health Research, National Ethical Committee, Ministry of Health and Prevention, Senegal and in Vietnam; Ethics Committee in Biomedical Research of Hung Vuong Hospital and Ethics Committee in Biomedical Research of the National Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayisha Diop, Email: adiop@mail.com.
Dina Abbas, Email: dabbas@gmail.com.
Nguyen thi Nhu Ngoc, Email: nhungoccrcrh@gmail.com.
Roxanne Martin, Email: rmartin@gynuity.org.
Ange Razafi, Email: razafy.dia@gmail.com.
Hoang Thi Diem Tuyet, Email: tuyethoang05@yahoo.com.vn.
Beverly Winikoff, Email: bwinikoff@gynuity.org.
References
- 1.Department of Reproductive Health and Research. World Health Organization . WHO recommendations for the prevention and treatment of postpartum haemorrhage. WHO Library Cataloguing-in-Publication Data NLM classification. 2012. [PubMed] [Google Scholar]
- 2.Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:217–223. doi: 10.1016/S0140-6736(09)61923-1. [DOI] [PubMed] [Google Scholar]
- 3.Winikoff B, Dabash R, Durocher J, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labour: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375:210–216. doi: 10.1016/S0140-6736(09)61924-3. [DOI] [PubMed] [Google Scholar]
- 4.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;6:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet. 2013;287:463–468. doi: 10.1007/s00404-012-2593-y. [DOI] [PubMed] [Google Scholar]
- 6.Henry DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(3):CD001886. 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed]
- 7.Gai M, Wu L, Su Q, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. Eur J Obstet Gynaecol. 2004;112:154–157. doi: 10.1016/S0301-2115(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 8.Shakur H, Beaumont D, Pavord S, Gayet-Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964. doi: 10.1002/14651858.CD012964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shakur Haleema, Roberts Ian, Fawole Bukola, Chaudhri Rizwana, El-Sheikh Mohamed, Akintan Adesina, Qureshi Zahida, Kidanto Hussein, Vwalika Bellington, Abdulkadir Abdulfetah, Etuk Saturday, Noor Shehla, Asonganyi Etienne, Alfirevic Zarko, Beaumont Danielle, Ronsmans Carine, Arulkumaran Sabaratnam, Grant Adrian, Afsana Kaosar, Gülmezoglu Metin, Hunt Beverley, Olayemi Oladapo, Roberts Ian, Chalmers Iain, Lumbiganon Pisake, Piaggio Gilda, Brady Tony, Shakur Haleema, Roberts Ian, Alfirevic Zarko, Elbourne Diana, Gülmezoglu Metin, Ronsmans Carine, Balogun Eni, Pepple Tracey, Prowse Danielle, Quashi Nigel, Barneston Lin, Barrow Collette, Beaumont Danielle, Cook Lisa, Frimley Lauren, Gilbert Daniel, Gilliam Catherine, Jackson Rob, Kawahara Taemi, Miah Hakim, Kostrov Sergey, Ramos Maria, Roberts Ian, Shakur Haleema, Edwards Phil, Godec Tom, Huque Sumaya, Fawole Bukola, Okunade Olujide, Adetayo Olusade, Chaudhri Rizwana, Kayani Aasia, Javaid Kiran, Fawole Bukola, Chaudhri Rizwana, Biryabarema Chrstine, Qureshi Zahida, Tchounzou Robert, El-Sheikh Mohamed, Kidanto Hussein, Regmi Mohan, Vwalika Bellington, Dallaku Kastriot, Sahani Mateus, Akhter Sayeba, Abdulkadir Abdulfetah, Meda Nicolas, Dah Anthony Kwame, Akintan Adesina, Odekunle Olufemi, Monehin Oluwabusola, Ojo Austin, Akinbinu Grace, Offiah Ifeoma, Etuk Saturday, Akpan Ubong, Udofia Uduak, Okon Useneno, Omoronyia Ezukwa, James Okpe, Olayemi Oladapo, Bello Nike, Adeyemi Blessed, Aimakhu Chris, Akinsanya Olufemi, Adeleye Bamidele, Adeyemi Oluwaseun, Oluwatosin Kayode, Aboyeji Abiodun, Adeniran Abiodun, Adewale Adebayo, Olaomo Noah, Omo-Aghoja Lawrence, Okpako Emmanuel, Oyeye Lucky, Alu Francis, Ogudu John, Ladan Ezekiel, Habib Ibrahim, Okusanya Babasola, Onafowokan Olatunde, Isah David, Aye Abalaka, Okogbo Felix, Aigere Egbaname, Ogbiti Mark, Onile Temitope, Salau Olaide, Amode Yinka, Shoretire Kamil, Owodunni Adebola, Ologunde Kehinde, Ayinde Akintunde, Alao Moses, Awonuga Olalekan, Awolaja Babatunde, Adegbola Omololu, Habeebu-Adeyemi Fatimah, Okunowo Adeyemi, Idris Hadiza, Okike Ola, Madueke Nneka, Mutihir Josiah, Joseph Nankat, Adebudo Babatunde, Fasanu Adeniyi, Akintunde Olugbenga, Abidoye Olufemi, Opreh Owigho, Udonwa Sophia, Dibia Gladys, Bazuaye Simeon, Ifemeje Arafat, Umoiyoho Aniefiok, Inyang-Etoh Emmanuel, Yusuf Sununu, Olayinka Kayode, Adeyemi Babalola, Ajenifuja Olusegun, Ibrahim Umar, Adamu Yusuf Baffah, Akinola Oluwarotimi, Adekola-Oni Grace, Kua Paul, Iheagwam Roseline, Idrisa Audu, Geidam Ado, Jogo Andrea, Agulebe Joseph, Ikechebelu Joseph, Udegbunam Onyebuchi, Awoleke Jacob, Adelekan Oluseyi, Sulayman Hajaratu, Ameh Nkeiruka, Onaolapo Nurudeen, Adelodun Affiss, Golit William, Audu Dachollom, Adeniji Adetunji, Oyelade Folasade, Dattijo Lamaran, Henry Palmer, Adeyemi Babalola, Loto Olabisi, Umeora Odidika, Onwe Abraham, Nzeribe Emily, Okorochukwu Bartthy, Adeniyi Augustine, Gbejegbe Emmanuel, Ikpen Akpojaro, Nwosu Ikemefuna, Sambo Abdulrasaq, Ladipo Olubunmi, Abubakar Sola, Okike Ola Nene, Nduka Enyinnaya Chikwendu, Ezenkwele Eziamaka Pauline, Onwusulu Daniel, Irinyenikan Theresa Azonima, Singh Swati, Bariweni Amaitari, Galadanci Hadiza, Achara Peter, Osayande Osagie, Gana Mohammed, Chaudhri Rizwana, Jabeen Kiran, Mobeen Ayesha, Mufti Sadaf, Zafar Maliha, Noor Shehla, Ahmad Basharat, Munawar Maimoona, Gul Jeharat, Usman Naseema, Shaheen Fehmida, Tariq Mariam, Sadiq Nadia, Batool Rabia, Ali Habiba Sharaf, Jaffer Manahil, Baloch Asma, Mukhtiar Noonari, Ashraf Tasneem, Asmat Raheela, Khudaidad Salma, Taj Ghazala, Qazi Roshan, Dars Saira, Sardar Faryal, Ashfaq Sanobar, Majeed Saeeda, Jabeen Sadaqat, Karim Rukhsana, Burki Farzana, Bukhari Syeda Rabia, Gul Fouzia, Jabeen Musarrat, Sherin Akhtar, Ain Qurratul, Rao Shahid, Shaheen Uzma, Manzoor Samina, Masood Shabween, Rizvi Shabana, Ali Anita, Sajid Abida, Iftikhar Aisha, Batool Shazia, Dar Lubna, Sohail Shahenzad, Rasul Shazia, Humayun Shamsa, Sultana Rashida, Manzoor Sofia, Mazhar Syeda, Batool Afshan, Nazir Asia, Tasnim Nasira, Masood Hajira, Khero Razia, Surhio Neelam, Aleem Samana, Israr Naila, Javed Saba, Bashir Lubna, Iqbal Samina, Aleem Faiza, Sohail Rubina, Iqbal Saima, Dojki Samina, Bano Alia, Saba Naseem, Hafeez Maimoona, Akram Nishat, Israr Naila, Shaheen Riffat, Hashmi Haleema, Arshad Sharmeen, Hussain Rubina, Khan Sadia, Shaheen Nighat, Khalil Safia, Sachdev Pushpa, Arain Gulfareen, Zarreen Amtullah, Saeed Sara, Hanif Shamayela, Tariq Nabia, Jamil Mahwish, Chaudhry Shama, Rajani Hina, Wasim Tayyiba, Aslam Summera, Mustafa Nilofar, Quddusi Huma, Karim Sajila, Sultana Shazia, Harim Misbah, Chohan Mohd, Salman Nabila, Waqar Fareesa, Sadia Shamsunnisa, Kahloon Lubna, Manzoor Shehla, Amin Samar, Akram Umbreen, Ikram Ambreen, Kausar Samina, Batool Tahira, Naila Brigadier, Kyani Tahir, Biryabarema Christine, Bulime Ruth, Akello Regina, Lwasa Bernadette Nakawooya, Ayikoru Joselyn, Namulwasira Christine, Komagum Patrick, Rebecca Isabirye, Annet Nayiga, Nuulu Nakirigya, Nionzima Elizabeth, Bwotya Rose, Nankya Margret, Babirye Sarah, Ngonzi Joseph, Sanchez Cesar, Innocent Nkonwa, Anitah Kusasira, Jackson Ayiko, Ndagire Elizabeth, Nanyongo Christine, Drametu Dominic, Meregurwa Grace, Banya Francis, Atim Rita, Byaruhanga Emmanuel, Felix Lema, Iman Hussein, Oyiengo Vincent, Waigi Peninah, Wangui Rose, Nassir Faiza, Soita Musimbi, Msengeti Rophina, Zubier Zeinab, Mabeya Hillary, Wanjala Antony, Mwangi Henry, Liyayi Brian, Muthoka Evelyn, Osoti Alfred, Otara Amos, Ongwae Veronicah, Qureshi Zahida, Wanjohi Victor, Musila Bonface, Wekesa Kubasu, Bosire Alex Nyakundi, Asonganyi Etienne, Ntem Alice, Njoache Angeline, Ashu Alice, Simo André, Tchounzou Robert, Keka Dorothy, Bruno Kenfack, Ndouoya Amadou, Saadio Martin, Tchana Mesack, Gwan Odel, Assomo Pauline, Mutsu Venantius, Eric Nji, Foumane Pascal, Nsem Philemon, Fouedjio Jeanne, Fouelifack Ymele, Tebeu Pierre Marie, Nko'ayissi Georges, Mbong Eta Ngole, Nabag Wisal, Desougi Riham, Mustafa Hadia, Eltaib Huida, Umbeli Taha, Elfadl Khalid, Ibrahim Murwan, Mohammed Abdalla, Ali Awadia, Abdelrahiem Somia, Musa Mohammed, Awadalla Khidir, Ahmed Samirra, Bushra Mahdi, Babiker Omer, Abdullahi Hala, Ahmed Mohamed, Safa Elhassan, Almardi Huida, Rayis Duria, Abdelgabar Saeed Abdelrahman, Alfirevic Zarko, Houghton Gillian, Sharpe Andrew, Thornton Jim, Grace Nick, Smith Carys, Hinshaw Kim, Edmundson Dawn, Ayuk Paul, Bates Alison, Bugg George, Wilkins Joanne, Tower Clare, Allibone Alysha, Oteng-Ntim Eugene, Kidanto Hussein, Kazumari Ahmad, Danford Anna, Ngarina Matilda, Abeid Muzdalifat, Mayumba Khadija, Zacharia Magreth, Mtove George, Madame Leonard, Massinde Anthony, Mwambe Berno, Onesmo Rwakyendela, Ganyaka Sebastian Kitengile, Regmi Mohan, Gupta Shyam, Bhatt Rabindra, Agrawal Ajay, Pradhan Pramila, Dhakal Nikita, Yadav Punita, Karki Gyanendra, Shrestha Bhola Ram, Vwalika Bellington, Lubeya Mwansa, Mumba Jane, Silwimba Willies, Hansingo Isaiah, Bopili Noojiri, Makukula Ziche, Kawimbe Alexander, Lubeya Mwansa Ketty, Mtambo Willard, Ng'ambi Mathew, Dallaku Kastriot, Cenameri Saimir, Tasha Ilir, Kruja Aferdita, Brahimaj Besnik, Tola Armida, Kaza Leon, Sahani Mateus, Tshombe Desire, Buligho Elizabeth, Paluku-Hamuli Roger, Kacha Charles, Faida Kato, Musau Badibanga, Kalyana Herman, Simisi Phanny, Mulyumba Serge, Jason Nzanzu Kikuhe, Lubamba Jean Robert, Missumba Willis, Islam Ferdousi, Begum Nazneen, Akhter Sayeba, Chowdhury Ferdousi, Begum Rokeya, Basher Farjana, Nargis Nazlima, Kholdun Abu, Jesmin Shahela, Paul Shrodha, Segni Hailemariam, Ayana Getachew, Haleke William, Abdulkadir Abdulfetah, Hussien Hassen, Geremew Fikre, Bambara Moussa, Somé Adolphe, Ly Amadou, Pabakba Roamba, Fletcher Horace, Samuels Leslie, Opare-Addo Henry, Larsen-Reindorf Roderick, Nyarko-Jectey Kwadwo, Mola Glen, Wai Malts, El Rahman Magdy, Basta Wafaa, Khamis Hussein, Escobar Maria Fernanda, Vallecilla Liliana, Faye Gabriel Essetchi. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. The Lancet. 2017;389(10084):2105–2116. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) Updated WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage. Geneva: WHO; 2017. [PubMed] [Google Scholar]
- 11.Shady Nahla W., Sallam Hany F., Elsayed Ahmed H., Abdelkader Abdelrahman M., Ali Shymaa S., Alanwar Ahmed, Abbas Ahmed M. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2017;32(11):1806–1812. doi: 10.1080/14767058.2017.1418316. [DOI] [PubMed] [Google Scholar]
- 12.Pibrant, et al. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20(1):65–72. doi: 10.1007/BF00554669. [DOI] [PubMed] [Google Scholar]
- 13.Murphy JF. Consort 2010 statement on randomised controlled trials. Ir Med J. 2010;103(5):132. [PubMed] [Google Scholar]
- 14.Gayet-Ageron Angèle, Prieto-Merino David, Ker Katharine, Shakur Haleema, Ageron François-Xavier, Roberts Ian, Kayani Aasia, Geer Amber, Ndungu Bernard, Fawole Bukola, Gilliam Catherine, Adetayo Cecelia, Barrow Collette, Beaumont Danielle, Prowse Danielle, I'Anson David, Balogun Eni, Miah Hakim, Shakur Haleema, Roberts Ian, Brooks Imogen, Onandia Julio, Ker Katharine, Javaid Kiran, Suncuan Laura, Frimley Lauren, Reid Mia, Arribas Monica, Benyahia Myriam, Okunade Olujide, Edwards Phil, Chaudhri Rizwana, Kostrov Sergey, Kansagra Sneha, Pepple Tracey. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. The Lancet. 2018;391(10116):125–132. doi: 10.1016/S0140-6736(17)32455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman BT, Berman MF, Riley LE, Leffert LR. The Epidemiology of Postpartum Hemorrhage in a Large, Nationwide Sample of Deliveries. Anesth Analg. 2010;110:1368–1373. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.