Abstract
Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) is a rare and highly aggressive ovarian malignancy. In almost all cases, it is associated with somatic and often germline pathogenic variants in SMARCA4, which encodes for the SMARCA4 protein (BRG1), a subunit of the SWI/SNF chromatin remodeling complex. Approximately 20% of human cancers possess pathogenic variants in at least one SWI/SNF subunit. Because of their role in regulating many important cellular processes including transcriptional control, DNA repair, differentiation, cell division and DNA replication, SWI/SNF complexes with mutant subunits are thought to contribute to cancer initiation and progression.
Fewer than 500 cases of SCCOHT have been reported in the literature and approximately 60% are associated with hypercalcemia. SCCOHT primarily affects females under 40 years of age who usually present with symptoms related to a pelvic mass. SCCOHT is an aggressive cancer, with long term survival rates of 30% in early-stage cases. Although various treatment approaches have been proposed, there is no consensus on surveillance and therapeutic strategy. An international group of multidisciplinary clinicians and researchers recently formed the International SCCOHT Consortium to evaluate current knowledge and propose consensus surveillance and therapeutic recommendations, with the aim of improving outcomes. Here, we present an overview of the genetics of this cancer, provide updates on new treatment targets and propose management guidelines for this challenging cancer.
Introduction
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is a rare and aggressive cancer which mainly occurs in adolescents and young women. It represents less than 0.01% of all ovarian malignancies (1), with fewer than 500 cases reported to date in the medical literature. The clinical and pathologic aspects of this tumor were initially described by Robert E Scully in 1979 (2). In describing these neoplasms, he noted: 1) the characteristic morphological appearance of small hyperchromatic cells with scant cytoplasm and brisk mitotic activity, 2) the occurrence in young females and 3) the presence of hypercalcemia. Although the mechanism underlying the commonly observed serum hypercalcemia is not well established, one study found that in 4 of 7 cases, the tumor cells expressed parathyroid hormone-related protein (PTHrp) (3). It has long been postulated that some cases could be familial (4) and in 2014, multiple groups discovered that SCCOHT is characterized by both germline and somatic deleterious mutations (henceforth termed pathogenic variants, PVs) in SMARCA4 (5-8). Studies have shown that SMARCA4 appears as the only recurrently mutated gene in SCCOHT (6-8). Therefore, PVs in SMARCA4 likely serve as the driver mutation for almost all cases of SCCOHT (8). The discovery of SMARCA4 PVs in >95% of SCCOHTs has been the first step in the development and implementation of potential targeted treatment options (9). With these discoveries in mind we brought together international experts and formed the International SCCOHT Consortium consisting of researchers, clinical scientists and clinicians. We held the first a symposium on SCCOHT in London in July 2018 to lay out the current state of knowledge regarding genetics and consider potential treatment targets. There have been 2-3 monthly follow up conference calls since then to foster collaborative research and to develop a consensus guideline for the diagnosis and management of SCCOHT.
SWI/SNF chromatin remodeling complex
Recent sequencing studies have identified mutations in subunits of the SWI/SNF chromatin remodeling complexes in over 20% of human cancers (10). Multiple configurations of the SWI/SNF complex exist, each consisting of ~15 proteins (11). With several isoforms existing for many of these proteins, theoretically, one hundred or more different combinations may exist (12). While all SWI/SNF complexes contain one of the two mutually exclusive ATPase subunits, SMARCA4 (BRG1) or SMARCA2 (BRM), functional differences appear among them (13). This is likely due to the differential complex compositions and the cell type in which they exist, as some SWI/SNF subunits specifically target certain genomic regions and transcription factors (14). Depending on the cell type and time point in development, these complexes can both repress and activate gene expression (15). Thus, SWI/SNF alterations play important and varied roles in driving tumorigenesis.
Normal SMARCA4 functions
SMARCA4 is involved in a plethora of cellular processes including transcriptional regulation, DNA damage repair, differentiation and mitosis, all of which may contribute to SCCOHT phenotypes. As a part of the diverse SWI/SNF (BAF, PBAF and ncBAF) chromatin remodeling complexes, SMARCA4 utilizes energy from ATP hydrolysis to mobilize nucleosomes and remodel chromatin. This remodeling activity commonly makes DNA accessible for loading of transcriptional regulators or repressors. Thus, SMARCA4 is normally found at promoters and enhancers of actively transcribed genes. Because SCCOHTs do not possess complex genomes, transcriptional and epigenetic deregulation induced by SMARCA4 loss that remain to be uncovered likely play a central role in driving tumorigenic pathways.
Clinical Management
The clinical management of SCCOHT has varied widely, although some clinical guidelines have previously been published (16). Outcome remains poor, with estimated long-term survival reported as 33% in stage I disease, and 10–20% overall. The International SCCOHT Consortium here present consensus guidelines for the diagnosis and management of women and their families affected by this condition, summarized in Table 1. We discuss potential strategies in diagnosis, genetic counselling, surveillance and treatment for SCCOHT as well as many issues arising from the lack of established data on this very rare malignancy. The Consortium unreservedly recommends further research to explore the effectiveness of current recommendations for this rare cancer. We also recommend conducting all work in liaison with specialist support groups, such as the Small Cell Ovarian Cancer Foundation, that provide psychosocial support and advocacy for affected families. (8)
Table 1.
Summary of the ISC guidelines
| Oncologic Management: Newly Diagnosed Disease | Oncologic Management: Recurrent Disease |
|---|---|
|
|
| Genetic Counseling | Familial Surveillance |
|
|
The role of cytoreductive surgery is not well defined for SCCOHT, but may be considered before initiating chemotherapy or at the time of recurrence in cases where residual disease appears completely resectable (such as in referral cases where attempted cytoreduction was never performed).
Contact members of the ISC if patients want to contribute to international research efforts
SCCOHT Pathology and Diagnosis
SCCOHT is currently classified as a miscellaneous neoplasm in the 2014 World Health Organization (WHO) Classification of Tumours of Female Reproductive Organs (17). The discovery of recurrent SMARCA4 mutations has resulted in alternative terminologies such as malignant rhabdoid tumor of the ovary (8) and SMARCA4-deficient ovarian neoplasm being proposed. However, the term SCCOHT will be retained in the upcoming 2020 WHO classification given that this term is well established in the literature. SCCOHT is the prototypical ovarian neoplasm composed predominantly or exclusively of small round cells with scant cytoplasm (so-called “small round blue cell tumor”). Because of the wide range of differential diagnoses of the various neoplasms in this broad group, pathologists commonly struggle with these tumors due to overlapping morphology and immunohistochemistry (IHC) (18). In diagnosing the various tumor types, IHC and molecular studies are of value (19). While typical SCCOHT is part of the differential diagnosis of a small round blue cell tumor, the large cell variant of SCCOHT may be confused with other neoplasms composed of large cells (Figure 1). Older studies investigated the immunophenotype of SCCOHT to try to elucidate the histogenesis but were inconclusive. The neoplastic cells are sometimes focally positive with epithelial membrane antigen (EMA), broad spectrum cytokeratins, calretinin, and CD10 (20), while desmin, S100 and inhibin are consistently negative. Occasional neoplasms are focally positive with neuroendocrine markers. Most cases also exhibit diffuse nuclear positivity with an antibody against the N-terminal of WT1 (20); this may be of some diagnostic use, although many other tumors, including some in the differential diagnosis of SCCOHT, are also positive. While estrogen receptor (ER) and progesterone receptor (PR) have not been widely investigated in SCCOHT, these neoplasms invariably do not stain using antibodies directed against hormone receptors.
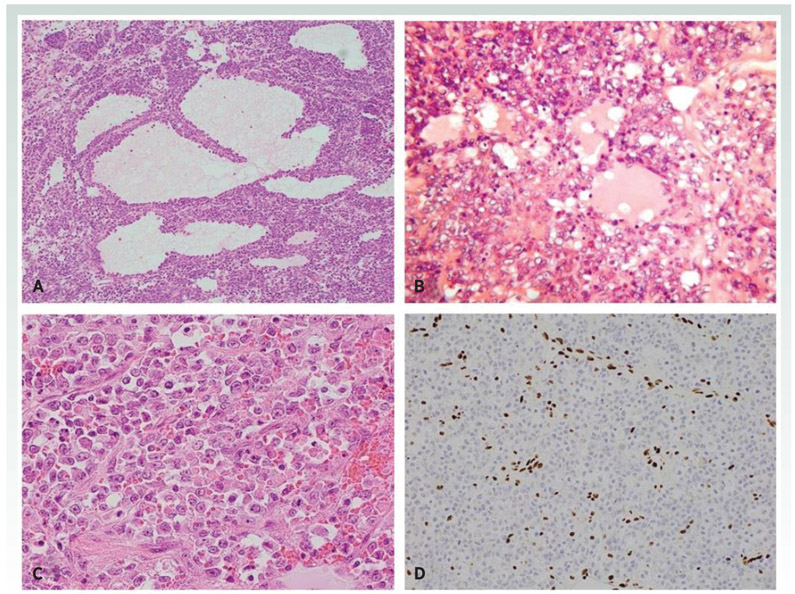
Figure 1.
SCCOHT is composed of predominantly diffuse arrangement of cells with follicle-like structures (A). On higher power, the tumor cells have hyperchromatic nuclei and scant cytoplasm (B). Large cell variant of SCCOHT composed of tumor cells with abundant eosinophilic cytoplasm (C). There is loss of nuclear immunoreactivity with SMARCA4 (BRG1) with a positive internal control in the form of nuclear staining of endothelial cells (D).
The discovery of SMARCA4 mutations in almost all SCCOHT tumors resulted in the development of a SMARCA4 (also known as BRG1) antibody which is highly useful in the diagnosis of this neoplasm and distinction from its many mimics (21). One of the original publications describing germline and somatic SMARCA4 mutations in these neoplasms showed loss of SMARCA4 nuclear immunoreactivity in 51/54 (94%) cases (8). Subsequent studies showed that over 95% of these neoplasms exhibit loss of nuclear immunoreactivity with this marker, making it an important diagnostic tool, although not all cases are negative (22,23). Occasional tumors exhibit loss of SMARCB1 (INI1) or SMARCA2 (BRM) immunohistochemical staining with retention of SMARCA4 (BRG1) (7). Dual loss of SMARCA4 and SMARCA2 (the latter is a subunit of the SWI/SNF complex mutually exclusive with SMARCA4) occurs in many SCCOHT (23); SMARCA2 loss occurs through epigenetic inactivation as mutations or deletions rarely occur in human tumors and have not been demonstrated in SCCOHT (23-27) Potentially consistent with this notion, treatment with epigenetic inhibitors such as DNMTi or HDACi, leads to re-expression of SMARCA2 in cancer cell lines (23,28).
It should be stressed that SMARCA4 loss through mutations, deletions and other mechanisms is observed in several other tumor types; for example, 10-37% of primary non-small cell lung cancers (NSCLC) exhibit loss of immunohistochemical expression of SMARCA4 (29-32). Dual loss of SMARCA4 and SMARC2 is also found in SMARCA4-deficient thoracic sarcomas (33), undifferentiated and dedifferentiated endometrial carcinomas and rare undifferentiated uterine sarcomas (23,34). Although the diagnosis of SCCOHT can usually be made on the basis of morphology and loss of SMARCA4 staining, SMARCA4 tumor sequencing could be considered in problematic cases of suspected SCCOHT with the minimum requirements to cover all exons and splice sites. SMARCA4 sequencing can help to distinguish SCCOHT from other cancers with SMARCA4 loss when combined with histologic features or in the context of few other somatic mutations, which is much more frequent in SCCOHTs compared to most other tumors with SMARCA4 loss.
The age range at diagnosis is quite wide and has ranged from 7 months to 56 years, with an average age of 23.9 years (35) . In one study, 26 of 60 patients (43%) had a germline SMARCA4 PV, including all patients diagnosed before the age of 15 years. Women with germline PVs present at a significantly younger age than those without (p=0.02) (36). We recommend caution in diagnosing SCCOHT in females under ten or over 50 years of age and although one publication reported a SCCOHT in a 71-year-old female, the lack of modern diagnostic markers used (immunostaining or SMARCA4 mutation analysis) makes the validity of this diagnosis unclear (9). Given the importance of establishing a correct diagnosis and the wide differential diagnosis (18), we strongly recommend an expert opinion from a specialist gynecological pathologist and SMARCA4 immunohistochemical staining to establish the diagnosis in an ovarian neoplasm in which SCCOHT is considered in the differential diagnosis and where a firm diagnosis of an alternative neoplasm cannot be established. At present, most pathology laboratories do not perform SMARCA4 immunohistochemistry
Genetic counselling and screening in patients and at-risk family members
Germline and somatic SMARCA4 PVs causing SCCOHT are generally nonsense or frameshift, although in-frame indels and missense mutations have been reported (6-8,33,36). The penetrance of these PVs remains uncertain , and interpretation of risk is complicated by our observation that germline PVs are often paternally inherited, with only two known cases caused by a de novo SMARCA4 PV (37,38). While SMARCA4 PVs occur across the entire gene, with no obvious predilection for certain domains, loss of function variants in some exons may not be pathogenic for SCCOHT due to their lack of expression in transcripts expressed in the ovary (39). Furthermore, we do not know whether individuals with a germline SMARCA4 PV associated with SCCOHT possess an increased risk for development of other types of cancers.
The risk of cancer in females with germline SMARCA4 PVs remains uncertain but may be considerable. Only one publication reports a female with a SMARCA4 germline mutation who remained cancer-free past her sixth decade (36). On the other hand, there is likely to be ascertainment bias in the literature, resulting in overestimation of cancer risk. Prospective studies will be needed to provide more accurate cancer risk estimates. Thus, one of the top priorities of this Consortium effort is the establishment of an international registry of patients with SCCOHT to facilitate follow up of families and provide opportunities for participation in research and clinical trials. Please see https://smallcellovariancancer.com/contact-us/ for more details.
The Consortium recommends referral of all patients with SCCOHT to a clinical genetics service or provider, with an offer for testing for germline SMARCA4 PVs (Table 1). It is important to use a clinical laboratory that offers full gene sequencing, including copy number calling, as PVs are typically scattered throughout the gene, and whole or partial gene deletions have been reported (40). The incidence of germline pathogenic PVs could be high (up to 43%), and the family history is not generally informative, especially if the germline PV was inherited from the proband’s father (36). There are several different approaches to genetic testing for diagnostic confirmation and with the increasing use of matched tumor-normal sequencing, both germline and somatic mutations can be identified. If no SMARCA4 mutation is detected, the diagnosis of SCCOHT should be reconsidered, along with sequencing of other related genes as a SMARCB1 mutation has been reported in one case of SCCOHT (41). When there is a confirmed diagnosis of SCCOHT (appropriate histological findings plus loss of SMARCA4 expression), germline testing is strongly recommended, regardless of somatic testing.
We have recently identified a molecularly-confirmed second primary SCCOHT in the right ovary of a young woman initially diagnosed with a left-sided SCCOHT in 2011 . This suggests that the remaining ovary females with SCCOHT and a germline SMARCA4 PV. Therefore, we recommend discussion of risk-reducing removal of the other ovary if a germline SMARCA4 PV is detected. Such variants are inherited in an autosomal dominant manner. All at-risk relatives of those with SCCOHT due to a germline SMARCA4 PV should receive genetic counselling and be offered predictive testing which should be covered by personal or national health insurance. Males with germline SMARCA4 PVs will not develop SCCOHT, but their daughters will have a 50% chance of inheriting the PV. Confidence in the pathogenicity of a germline variant can be enhanced by either IHC showing loss of SMARCA4 protein expression in the tumor, or by identifying a second PV in the tumor.
Surveillance for at-risk family members
This remains controversial considering the lack of proven efficacy and the potential risks including a false sense of security, risk of false positive screens, and the potential exclusion of effective risk-reducing surgery. Although early detection methods using imaging offer an appealing alternative to risk-reducing bilateral salpingo-oophorectomy (RRBSO), this approach remains unproven and has been ineffective to date for other more common ovarian malignancies (42,43). While RRBSO performed prior to malignant transformation may prove more effective, its benefits remain unproven. Determining the optimal age for RRBSO is extremely challenging considering the early age of disease onset and the uncertain penetrance of germline SMARCA4 PVs (36). RRBSO has been offered, on a highly selective basis, to females with germline SMARCA4 PVs, typically for siblings in an affected family (42,44). Counselling for such a procedure needs sensitivity, including a discussion of surgical-based risks, onset of surgical menopause, estrogen replacement therapy, and reproductive preservation options including oocyte cryopreservation and preimplantation genetic diagnosis (PGD). These considerations are highly relevant to families affected by SCCOHT that also carry germline SMARCA4 PVs. RRBSO should be only be considered with extreme caution where a germline SMARCA4 PVs is identified incidentally during genetic testing for another indication and where there is no personal or family history of SCCOHT as the penetrance for these variants remains uncertain . There are no published studies that suggest any form of surveillance can prevent death from SCCOHT and therefore we do not recommend it. The similarities identified between rhabdoid tumors (RTs) and SCCOHT (45) suggest that infant carriers of SMARCA4 PVs may be at risk for both. However, the risk of SMARCA4-related RTs is likely confined to very young children because of its absence in children older than 46 months (36). We recommend the development of standard guidelines for the care of unaffected individuals with germline SMARCA4 PVs.
In certain cases, testing other cancers by sequencing and/or IHC for SMARCA4 can help to determine pathogenicity. Testing female relatives over the age of 60 years may also help to better estimate penetrance and cancer risk. Classifications of variants (46), particularly variants of uncertain significance (VUS), will require periodic review because they are likely to change over time.
Recommendations for Oncological Management
Given the lack of prospective studies, treatment recommendations are based on small case series and management strategies remain heterogeneous. Despite the absence of data, the general principles of primary cytoreductive surgery for epithelial ovarian cancer apply to patients with SCCOHT with the goal of complete surgical resection leaving no visible disease. A multimodal approach including radical surgery, chemotherapy, radiotherapy after discussion at an in-person or virtual multidisciplinary tumor board is essential (47). Fertility sparing surgery may be considered in comprehensively surgically staged Stage IA patients without germline SMARCA4 PVs if they desire future pregnancies, although radical surgery for all patients remains the norm.
Adjuvant chemotherapy is recommended for all stages, generally cisplatin and etoposide-based combination regimens (e.g. BEP: bleomycin, etoposide, cisplatin; VPCBAE: vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin and etoposide; PAVEP: cisplatin, doxorubicin, etoposide cyclophosphamide). For patients where initial surgery is not feasible (e.g. stage IV disease, unresectable disease, medically unfit patient), administration of chemotherapy and interval cytoreductive surgery may be considered on an individual basis. High dose chemotherapy (HDC) with autologous stem cell transplantation rescue following a complete response to initial chemotherapy, with or without surgery, may also be considered (48). Recently updated survival data suggest that multi-modality therapy including surgery and multi-agent chemotherapy with possible stem cell transplantation and radiotherapy are most effective (36). However, these non-randomized studies may suffer from selection bias. Early stage patients will more likely meet the criteria for HDC, which requires a complete response to initial chemotherapy, with the expectation of improved outcomes compared to patients with more advanced stage disease (16). Although SCCOHT is often chemosensitive initially, a substantial risk for relapse persists and the effectiveness of additional chemotherapy is limited. Reported options for chemotherapy in the recurrent setting include combinations of cyclophosphamide, doxorubicin and vincristine, or carboplatin in combination with paclitaxel and topotecan (47). Subsequent responses are often short-lived, emphasizing the need for more clinical trials.
Identification of SCCOHT therapeutic candidates
To date, a paucity of approved or investigational agents exists for SWI/SNF-mutant tumors. For SCCOHT specifically, there is no agreed standard of care and the usual care management includes surgery, platinum-based chemotherapy plus high-dose chemotherapy with stem cell rescue, and consideration of radiation in select cases (36). Under the current usual care, SCCOHT portends a poor outcome, with ~30% survival, even following early diagnosis. Clinical trials have not yet been performed in SCCOHT patients as a single entity, in large part because of the rarity of the disease, although some genetic basket studies have included SCCOHT patients. This means that most efforts have focused on identifying effective targeted treatment strategies that can translate rapidly into clinical trials. Cancer therapeutic approaches aimed at restoring expression of inactivated tumor suppressor genes, such as TP53, RB1 or BRCA1, have not proven successful. Therefore, targeted drug development for SCCOHT has focused on several approaches, including exploiting known synthetic lethal interactions of SMARCA4 loss and identifying novel targets through unbiased genetic screens. We discuss the most promising results from mainly preclinical studies below (summarized in Figure 2 and Table 2).
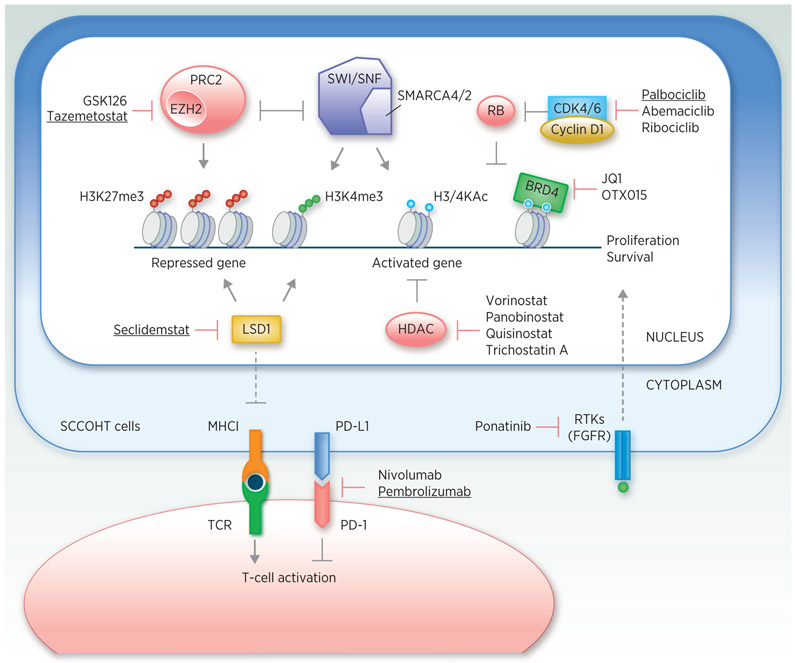
Figure 2.
Graphic summary of SCCOHT therapeutic candidates and their corresponding drugs. Agents that are being tested in clinical trials available to SCCOHT patients are underlined. See Table 2 for more details.
Table 2.
Potential treatments and trials for SCCOHT. EZH2, Enhancer of zeste homolog 2; LSD1, Lysine-specific histone demethylase 1; HDAC, Histone deacetylase: BET, Bromodomain and Extra-Terminal motif; CDK, Cyclin-dependent kinase; PD-1, Programmed cell death protein 1
| Class | Target | Drug | Activities against SCCOHT |
FDA-approved application | Clinical trial available for SCCOHT |
Reference | |
|---|---|---|---|---|---|---|---|
| in vitro | in vivo | ||||||
|
Epigenetic therapeutics |
EZH2 | GSK126 | X | X | (55) | ||
| Tazemetostat | X | X | Phase II NCT02601950 | (53,55) | |||
| LSD1 | Seclidemstat | Phase I, NCT03895684 | (77) | ||||
| HDAC | Vorinostat | X | Cutaneous T-cell lymphoma | (56) | |||
| Panobinostat | X | Multiple myeloma | (56) | ||||
| Quisinostat | X | X | (56) | ||||
| Trichostatin A | X | (23) | |||||
| BET proteins | JQ1 | X | (61) | ||||
| OTX015 | X | X | (61) | ||||
|
Kinase Inhibitors |
Multi-kinases | Ponatinib | X | X | ALL, CML | (63) | |
| CDK4/6 | Palbociclib | X | X | Breast cancer | Phase II, NCT03297606 | (66) | |
| Abemaciclib | X | (66) | |||||
| Ribociclib | X | (66) | |||||
|
Immuno- therapies |
PD-1 | Pembrolizumab | X | Multiple cancers | Phase II, NCT03012620 | (70) | |
| PD-1 | Nivolumab | X | Multiple cancers | (70) | |||
Epigenetic therapeutics
Since tumor suppressor loss is not directly druggable, most investigators have searched for therapeutic vulnerabilities by exploiting the concomitant changes in gene expression and signaling pathways. Cancer cells are often dependent on these alterations induced by tumor suppressor loss, resulting in a targetable synthetic lethality (49,50). For example, suppression of SMARCA2 is synthetic lethal with SMARCA4 loss in non-small cell lung carcinoma (NSCLC) cells (30,51,52) likely driven by paralogous subunit compensation. However, SMARCA2 is currently undruggable and cancer cells with SMARCA4/2-dual loss, such as SCCOHT and most lung adenocarcinomas, are unlikely to respond to SMARCA2 inhibition.
The best-developed therapeutic target comes from studies demonstrating that SWI/SNF complexes oppose the repressor function of PRC1 and PRC2 in regulating gene expression (24,26). SWI/SNF loss leads to elevated PRC2 activity with a concomitant increase in H3K27me3 levels, providing a viable approach for treatment interventions. Indeed, SMARCA4-deficient cancer cells display sensitivity to suppression of EZH2 (53-55), the catalytic subunit of PRC2. In SCCOHT cells, EZH2 inhibitors induce re-expression of SMARCA2 (53,56) and neuronal-like proteomic signatures, as well as potently inhibiting growth of SCCOHT cell line xenografts (55). The only trial (NCT02601950) to include SCCOHT patients by name are investigating the most studied EZH2 inhibitor, tazemetostat (EPZ-6438) and early results from these Phase I/II trials reported on two SCCOHT patients, one with stable disease and one with a partial response after treatment. A tazemetostat trial sponsored by the National Cancer Institute was recently suspended due to observations of secondary lymphomas. Of interest, one report has shown that growth inhibition of SMARCA4-deficient NSCLC appears primarily dependent on a non-catalytic role of EZH2 for stabilizing the PRC2 complex, which current EZH2 inhibitors do not target (54).
Targeting other histone modification complexes has also shown promise for treatment of SCCOHT patients. Histone deacetylase inhibitors (HDACi) have been clinically approved for the treatment of several hematological malignancies but have proved less effective for solid tumors (57). Several studies have shown that histone deacetylase inhibitors in the context of SCCOHT result in re-expression of SMARCA2, which strongly suppresses growth of SCCOHT cells (23,56). One of these reports also showed in vivo sensitivity of SCCOHT cells to the HDACi quisinostat (56). They further demonstrated that quisinostat acts synergistically with EPZ-6438 in vitro and further reduces tumor growth in vivo (56). While HDACi may offer an attractive treatment option for SCCOHT patients, a single case report did not find efficacy with this approach (58). A Phase I trial (NCT03895684) of seclidemstat, a lysine-specific demethylase inhibitor, will open specifically for SWI/SNF-mutant gynecologic cancers, with an emphasis on SCCOHT, ovarian clear cell carcinomas, and endometrial carcinomas that show SMARCA4 or ARID1A mutation or loss. Following dose escalation with the single-agent, combination with pembrolizumab will be examined.
In addition to targeting EZH2 and HDAC, bromodomain and extra-terminal motif containing protein inhibitors (BETi) have been explored in SCCOHT models, based on previous studies showing the dependency of SMARCA4-mutant esophageal cancer models for BET protein BRD4 (59) and the coregulation of an oncogenic network by BRD4 and SMARCA4 in acute leukemia (60). Consistent with these findings, SCCOHT cells were highly sensitive to BETi JQ1 and OTX015, the latter of which showed strong anti-tumor activities in an orthotopic xenograft model of SCCOHT (61). In addition to BRD4, other BET proteins have been linked to SWI/SNF function. Inactivation of another key SWI/SNF subunit SMARCB1, also known as INI1 or SNF5, occurs in synovial sarcomas and rhabdoid tumors (RTs) show as well as in a small fraction of SCCOHTs. Recent evidence suggests that both synovial sarcomas and RTs require a non-canonical SWI/SNF complex (ncBAF, as opposed to BAF and PBAF), carrying BRD9 as an essential subunit, for their survival (10). Supporting this, CRISPR knockout screens uncovered BRD9 as a therapeutic target in these cancers (62). However, pharmacological inhibition of BRD9 did not recapitulate this phenotype, indicating ncBAF function requires protein domains beyond the BRD9 bromodomain. While these studies suggest that BRD9 inhibitors may prove effective for the treatment of SCCOHT patients, Michel et al. showed that BRD9 forms complexes with SMARCA4 but not SMARCB1 (10). Therefore, the effects of BRD9 inhibition on SCCOHT remains untested. Currently, there is no available clinical study to investigate the effect of BETi in SCCOHT patients.
Kinase Inhibitors
Functional genetic screening approaches have proven to be a powerful tool to uncover novel drug targets in cancers. In this context, the kinome is often chosen because pharmacological inhibitors targeting kinases identified from the screens are often available, providing the highest chance of clinical implementation. Using an arrayed kinome-focused siRNA screen, Lang et al. showed sensitivity of SCCOHT cell lines in culture and in xenografts as well as PDX models to the clinically available multi-targeted tyrosine kinase inhibitor ponatinib (63). They also implicated a dependence upon FGFR signaling as the underlying mechanism for this sensitivity (56). These results coincide with similar observations in RTs where re-expression of SMARCB1 resulted in decreased expression of FGFR1 and FGFR2, as well as the relative In vitro and In vivo sensitivity of RT cell lines to RTK inhibitors ponatinib and BGJ-398 (64,65). Ponatinib is FDA-approved for the use in leukemias and warrants further investigation in SCCOHT.
Using a pooled shRNA screening approach also targeting human kinome, Xue et al. found that SCCOHT cells are highly sensitive to CDK4/6 inhibition (66). They showed that SMARCA4 loss causes downregulation of cyclin D1, limiting CDK4/6 kinase activity in SCCOHT cells and leading to In vitro and In vivo susceptibility to CDK4/6 inhibitors. Thus, their findings indicated that CDK4/6 inhibitors, approved for a breast cancer subtype addicted to CDK4/6 activation, could be repurposed to treat SCCOHT. They also observed this synthetic lethal interaction between SMARCA4-loss and CDK4/6 inhibition in SMARCA4-deficient NSCLC despite their differences in tissue of origin and mutation landscape (67). Given that SMARCA4 loss occurs in a variety of other cancer types, this common druggable vulnerability, shared by SCCOHT and NSCLC, may also be effective for targeting other SMARCA4-deficient tumors. Furthermore, patients may also benefit from the anti-tumor immunity triggered by CDK4/6 inhibition as recently shown by others (68,69). Canadian Profiling and Targeted Agent Utilization Trial (CAPTUR) (NCT03297606), a pan-Canadian phase 2 basket trial matching cancer patients with different genetic variants to appropriate targeted treatments, has recently approved a new match to treat SMARCA4-mutant tumors with the CDK4/6 inhibitor palbociclib based on the above findings (66,67). SCCOHT patients will be included in this new trial arm.
Immunotherapies
Although the low mutation-burden of SCCOHT would not predict responsiveness to immune checkpoint blockade (ICB) based on neoantigen burden alone, PD-1 inhibitors including pembrolizumab have shown substantial and durable responses in selected patients with recurrent SCCOHT after prior treatment with cytotoxic chemotherapy and also immediately following radiation treatment (70). In addition, one patient, known to these authors, showed near complete treatment response to CDK4/6 inhibition in combination with ICB (see comment above). Further, preclinical data from SWI/SNF-mutant melanoma (71) and clear cell renal carcinoma (72) models demonstrate a causal connection between loss of SWI/SNF components such as PBRM1 and sensitivity to immune checkpoint blockade. Although these reports focus upon loss of the PBRM1 subunit, Pan et al. showed loss of this subunit in SCCOHT cells (73). Rhabdoid tumours, also with low mutation burden, have recently been shown to have high infiltration of immune cells; this immunogenicity is linked to endogenous retrovirus expression induced by SMARCB1 loss (74). Indeed, several recent reports support the efficacy of these inhibitors in patients with rhabdoid tumors (75,76). Checkpoint blockade responses in SWI/SNF-mutant cancers may be associated with over-expression of immune-stimulatory genes (71,72). Given the similarity between SCCOHT and rhabdoid tumors, similar mechanisms may be in place underlying the response of SCCOHT to PD-1 inhibitor. A French phase II basket trial ‘AcSé program with pembrolizumab (NCT03012620) is currently open for women with relapsed SCCOHT. While further investigation will define the utility of checkpoint inhibitors in SCCOHT, mechanistic understanding remains limited based on the complex nature of modeling immune cell interactions in the available SCCOHT models, where the cell of origin is not yet defined. A phase II including pembrolizumab in combination with initial adjuvant chemotherapy will start in Europe in 2020.
As more clinical trials become available for patients with SCCOHT, we suggest that immunotherapy appears to be the best first choice for treatment when eligibility requirements permit. After initial therapy, as described previously, immunotherapy appears to be the most promising non-standard therapy based on activity in selected patients reported to date and data from other related tumor types. The addition of CDK 4/6 inhibitors and epigenetic therapies also have some favorable reported outcomes to date based on very limited, and mostly unpublished, case reports. We hope that additional viable options will become available as more preclinical and translational research is reported.
Conclusions
In the five years since the discovery of SMARCA4 mutations in SCCOHT, this cancer has been transformed from being a little studied, poorly understood cancer for which there was no rational therapeutic options to tumor which is now the focus of much research. This is leading to the discovery of multiple potential treatments meaning there is now a need to carefully evaluate and prioritize the best candidate drugs for future trials. The rarity of SCCOHT creates challenges in the identification and management of affected patients. The true number of women affected worldwide remains unknown, creating barriers to rapidly and systematically identify eligible individuals for new treatments or clinical trials. We have begun establishing an International Registry of well-characterized patients with, or at risk for, SCCOHT. The collation of high quality clinical and epidemiological data will allow us to better understand this disease and act as a catalyst to improve translational research and the overall outcome for patients with SCCOHT.
Females with germline PVs in SMARCA4 likely have a clinically important risk of SCCOHT up to age ~60 years, particularly in the context of a positive family history. Genetic testing for germline SMARCA4 PVs is recommended for all affected individuals with SCCOHT with cascade testing of at-risk family members upon identification of germline PVs. Because surveillance is unproven, we recommend RRBSO for unaffected adult females with germline PVs in the context of a positive family history. Given the lack of defined guidelines for the management of women with SCCOHT, we recommend aggressive cytoreductive surgery followed by adjuvant combination therapy with a cisplatin and etoposide-based regimen. HDC with stem-cell rescue for individuals who have a complete clinical response may be considered (36,48). Patients with progressive or recurrent disease should enroll in a clinical trial or consider non-standard therapy based on the latest data that may come from case reports and limited source material.
SCCOHT is an excellent example of a cancer where the development of novel therapies targeting the cancer vulnerabilities induced by the driver mutation is possible. The key message for patients and family members it to remain in contact with disease experts who have knowledge of the latest clinical trials, seek consultation from academic referral centers, and request input from members of the International SCCOHT Consortium https://www.smallcellovarian.org/consortium.html. The landscape of treatments for SCCOHT will continually change as we improve patient outcomes and work towards prevention and cure for this rare and aggressive malignancy. International prospective multicenter protocols with collection of data are urgently needed to be considered for both first-line and relapsed disease in this particularly rare disease.
Acknowledgments
We acknowledge support from The Eve Appeal research charity who funded the SCCOHT Symposium held on 5th July 2018 in London, UK, that led to the formation of the International SCCOHT Consortium. This was made possible with the support and generosity of families affected by SCCOHT including Linda, Mike and Mark Butcher and their extended family and friends in memory of Angela; The Louise Hartley Memorial Fund and the Hartley Family in memory of Louise; The family and friends of Emma Houlston and Matt Lees; the family and friends of Ailsa Renshaw. We are grateful for assistance from Inga Plaskoncinska and input from Maren Petersen of the Small Cell Ovarian Cancer Foundation, founded in memory of Stephanie Petersen. M. Tischkowitz was funded by the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement n.310018 and Cancer Research UK (CanGene-CanVar Catalyst Award C61296/A27223). S. Huang is supported by CIHR grants MOP-130540 and PJT-156233. J.M. Trent, B.E. Weissman, J.D. Lang, W.P. Hendricks and D. Huntsman receive support from support from the U.S. NCI grant R01CA195670. A. Oza is supported by Princess Margaret Cancer Foundation and Ontario Institute Cancer Research Ovarian Translational Research Initiative. D.A. Levine is supported by U.S. DOD grant W81XWH-15-1-0429. W.D. Foulkes is funded by the Canadian Institutes of Health Research (CHIR; FDN- 148390). These guidelines have been endorsed by the GENTURIS European Reference Network.
Footnotes
Conflicts of Interest
The authors do not have any conflicts of interest to declare
References
- 1.Young RH, Goodman A, Penson RT, Russell AH, Uppot RN, Tambouret RH. Case records of the Massachusetts General Hospital. Case 8-2010. A 22-year-old woman with hypercalcemia and a pelvic mass. N Engl J Med 2010;362(11):1031–40 doi 10.1056/NEJMcpc1000272. [DOI] [PubMed] [Google Scholar]
- 2.Scully RE. Tumors of the ovary and maldeveloped gonads In: Hartmann WH CWR, editor. Atlas of Tumor Pathology Washington, DC: Armed Forces Institute of Pathology; 1979. [Google Scholar]
- 3.Matias-Guiu X, Prat J, Young RH, Capen CC, Rosol TJ, Delellis RA, et al. Human parathyroid hormone-related protein in ovarian small cell carcinoma. An immunohistochemical study. Cancer 1994;73(7):1878–81 doi . [DOI] [PubMed] [Google Scholar]
- 4.Longy M, Toulouse C, Mage P, Chauvergne J, Trojani M. Familial cluster of ovarian small cell carcinoma: a new mendelian entity? J Med Genet 1996;33(4):333–5 doi 10.1136/jmg.33.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupryjanczyk J, Dansonka-Mieszkowska A, Moes-Sosnowska J, Plisiecka-Halasa J, Szafron L, Podgorska A, et al. Ovarian small cell carcinoma of hypercalcemic type - evidence of germline origin and SMARCA4 gene inactivation. a pilot study. Pol J Pathol 2013;64(4):238–46. [DOI] [PubMed] [Google Scholar]
- 6.Jelinic P, Mueller JJ, Olvera N, Dao F, Scott SN, Shah R, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet 2014;46(5):424–6 doi 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos P, Karnezis AN, Craig DW, Sekulic A, Russell ML, Hendricks WP, et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet 2014;46(5):427–9 doi 10.1038/ng.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet 2014. doi 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Shi H. An In-Depth Look at Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT): Clinical Implications from Recent Molecular Findings. J Cancer 2019;10(1):223–37 doi 10.7150/jca.26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel BC, D'Avino AR, Cassel SH, Mashtalir N, McKenzie ZM, McBride MJ, et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat Cell Biol 2018;20(12):1410–20 doi 10.1038/s41556-018-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride MJ, Kadoch C. Disruption of mammalian SWI/SNF and polycomb complexes in human sarcomas: mechanisms and therapeutic opportunities. J Pathol 2018;244(5):638–49 doi 10.1002/path.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann AF, Vakoc CR. A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet 2014;30(8):356–63 doi 10.1016/j.tig.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashtalir N, D'Avino AR, Michel BC, Luo J, Pan J, Otto JE, et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018;175(5):1272–88 e20 doi 10.1016/j.cell.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan R, Tuoc TC. Roles of chromatin remodeling BAF complex in neural differentiation and reprogramming. Cell Tissue Res 2014;356(3):575–84 doi 10.1007/s00441-013-1791-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhan X, Shi X, Zhang Z, Chen Y, Wu JI. Dual role of Brg chromatin remodeling factor in Sonic hedgehog signaling during neural development. Proc Natl Acad Sci U S A 2011;108(31):12758–63 doi 10.1073/pnas.1018510108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed NS, Pautier P, Avall-Lundqvist E, Choi CH, du Bois A, Friedlander M, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian small cell cancers. Int J Gynecol Cancer 2014;24(9 Suppl 3):S30–4 doi 10.1097/IGC.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 17.Kurman RJIAfRoCH CS; Carcangiu ML WHO Classification of Tumours of Female Reproductive Organs (IARC WHO Classification of Tumours) International Agency for Research on Cancer; 2014. [Google Scholar]
- 18.McCluggage WG. Ovarian neoplasms composed of small round cells: a review. Adv Anat Pathol 2004;11(6):288–96. [DOI] [PubMed] [Google Scholar]
- 19.Witkowski L, Goudie C, Foulkes WD, McCluggage WG. Small-Cell Carcinoma of the Ovary of Hypercalcemic Type (Malignant Rhabdoid Tumor of the Ovary): A Review with Recent Developments on Pathogenesis. Surg Pathol Clin 2016;9(2):215–26 doi 10.1016/j.path.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 20.McCluggage WG, Oliva E, Connolly LE, McBride HA, Young RH. An immunohistochemical analysis of ovarian small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol 2004;23(4):330–6. [DOI] [PubMed] [Google Scholar]
- 21.Karanian-Philippe M, Velasco V, Longy M, Floquet A, Arnould L, Coindre JM, et al. SMARCA4 (BRG1) loss of expression is a useful marker for the diagnosis of ovarian small cell carcinoma of the hypercalcemic type (ovarian rhabdoid tumor): a comprehensive analysis of 116 rare gynecologic tumors, 9 soft tissue tumors, and 9 melanomas. Am J Surg Pathol 2015;39(9):1197–205 doi 10.1097/PAS.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 22.Clarke BA, Witkowski L, Ton Nu TN, Shaw PA, Gilks CB, Huntsman D, et al. Loss of SMARCA4 (BRG1) protein expression as determined by immunohistochemistry in small-cell carcinoma of the ovary, hypercalcaemic type distinguishes these tumours from their mimics. Histopathology 2016;69(5):727–38 doi 10.1111/his.12988. [DOI] [PubMed] [Google Scholar]
- 23.Karnezis AN, Wang Y, Ramos P, Hendricks WP, Oliva E, D'Angelo E, et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol 2016;238(3):389–400 doi 10.1002/path.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci Adv 2015;1(5):e1500447 doi 10.1126/sciadv.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 2013;45(6):592–601 doi 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 2011;11(7):481–92 doi 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 27.Jelinic P, Schlappe BA, Conlon N, Tseng J, Olvera N, Dao F, et al. Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Mod Pathol 2016;29(1):60–6 doi 10.1038/modpathol.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaros S, Cirrincione GM, Muchardt C, Kleer CG, Michael CW, Reisman D. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene 2007;26(49):7058–66 doi 10.1038/sj.onc.1210514. [DOI] [PubMed] [Google Scholar]
- 29.Medina PP, Romero OA, Kohno T, Montuenga LM, Pio R, Yokota J, et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Hum Mutat 2008;29(5):617–22 doi 10.1002/humu.20730. [DOI] [PubMed] [Google Scholar]
- 30.Oike T, Ogiwara H, Tominaga Y, Ito K, Ando O, Tsuta K, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res 2013;73(17):5508–18 doi 10.1158/0008-5472.CAN-12-4593. [DOI] [PubMed] [Google Scholar]
- 31.Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res 2003;63(3):560–6. [PubMed] [Google Scholar]
- 32.Rodriguez-Nieto S, Canada A, Pros E, Pinto AI, Torres-Lanzas J, Lopez-Rios F, et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Hum Mutat 2011;32(2):E1999–2017 doi 10.1002/humu.21415. [DOI] [PubMed] [Google Scholar]
- 33.Le Loarer F, Watson S, Pierron G, de Montpreville VT, Ballet S, Firmin N, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47(10):1200–5 doi 10.1038/ng.3399. [DOI] [PubMed] [Google Scholar]
- 34.Kolin DL, Quick CM, Dong F, Fletcher CDM, Stewart CJR, Soma A, et al. SMARCA4-deficient Uterine Sarcoma and Undifferentiated Endometrial Carcinoma are Distinct Clinicopathologic Entities. Am J Surg Pathol 2019. doi 10.1097/PAS.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 35.Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol 1994;18(11):1102–16 doi 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Witkowski L, Goudie C, Ramos P, Boshari T, Brunet JS, Karnezis AN, et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol Oncol 2016;141(3):454–60 doi 10.1016/j.ygyno.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Errichiello E, Mustafa N, Vetro A, Notarangelo LD, de Jonge H, Rinaldi B, et al. SMARCA4 inactivating mutations cause concomitant Coffin-Siris syndrome, microphthalmia and small-cell carcinoma of the ovary hypercalcaemic type. J Pathol 2017;243(1):9–15 doi 10.1002/path.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witkowski L, Lalonde E, Zhang J, Albrecht S, Hamel N, Cavallone L, et al. Familial rhabdoid tumour 'avant la lettre'-from pathology review to exome sequencing and back again. J Pathol 2013;231(1):35–43 doi 10.1002/path.4225. [DOI] [PubMed] [Google Scholar]
- 39.Muppala R, Donenberg T, Huang MS, Schlumbrecht MP. SMARCA4 germline gene mutation in a patient with epithelial ovarian: A case report. Gynecol Oncol Rep 2017;22:45–7 doi 10.1016/j.gore.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goudie C, Witkowski L, Vairy S, McCluggage WG, Foulkes WD. Paediatric ovarian tumours and their associated cancer susceptibility syndromes. J Med Genet 2018;55(1):1–10 doi 10.1136/jmedgenet-2017-104926. [DOI] [PubMed] [Google Scholar]
- 41.Ramos P, Karnezis AN, Hendricks WP, Wang Y, Tembe W, Zismann VL, et al. Loss of the tumor suppressor SMARCA4 in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). Rare Dis 2014;2(1):e967148 doi 10.4161/2167549X.2014.967148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berchuck A, Witkowski L, Hasselblatt M, Foulkes WD. Prophylactic oophorectomy for hereditary small cell carcinoma of the ovary, hypercalcemic type. Gynecol Oncol Rep 2015;12:20–2 doi 10.1016/j.gore.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Administration UFaD. Ovarian Cancer Screening Tests: Safety Communication - FDA Recommends Against Use. 2016. doi https://wayback.archive-it.org/7993/20170111134026/http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm519413.htm.
- 44.Pejovic T, McCluggage WG, Krieg AJ, Xu F, Lee DM, Witkowski L, et al. The dilemma of early preventive oophorectomy in familial small cell carcinoma of the ovary of hypercalcemic type. Gynecol Oncol Rep 2019;28:47–9 doi 10.1016/j.gore.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foulkes WD, Clarke BA, Hasselblatt M, Majewski J, Albrecht S, McCluggage WG. No small surprise - small cell carcinoma of the ovary, hypercalcaemic type, is a malignant rhabdoid tumour. J Pathol 2014;233(3):209–14 doi 10.1002/path.4362. [DOI] [PubMed] [Google Scholar]
- 46.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–24 doi 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Supplement_4):iv1–iv18 doi 10.1093/annonc/mdy001. [DOI] [PubMed] [Google Scholar]
- 48.Pautier P, Ribrag V, Duvillard P, Rey A, Elghissassi I, Sillet-Bach I, et al. Results of a prospective dose-intensive regimen in 27 patients with small cell carcinoma of the ovary of the hypercalcemic type. Ann Oncol 2007;18(12):1985–9 doi 10.1093/annonc/mdm376. [DOI] [PubMed] [Google Scholar]
- 49.Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer 2005;5(9):689–98 doi 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 50.Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med 2015;66:455–70 doi 10.1146/annurev-med-050913-022545. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman GR, Rahal R, Buxton F, Xiang K, McAllister G, Frias E, et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci U S A 2014;111(8):3128–33 doi 10.1073/pnas.1316793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson BG, Helming KC, Wang X, Kim Y, Vazquez F, Jagani Z, et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol Cell Biol 2014;34(6):1136–44 doi 10.1128/MCB.01372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan-Penebre E, Armstrong K, Drew A, Grassian AR, Feldman I, Knutson SK, et al. Selective Killing of SMARCA2- and SMARCA4-deficient Small Cell Carcinoma of the Ovary, Hypercalcemic Type Cells by Inhibition of EZH2: In Vitro and In Vivo Preclinical Models. Mol Cancer Ther 2017;16(5):850–60 doi 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 54.Kim KH, Kim W, Howard TP, Vazquez F, Tsherniak A, Wu JN, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med 2015;21(12):1491–6 doi 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Chen SY, Karnezis AN, Colborne S, Santos ND, Lang JD, et al. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. J Pathol 2017;242(3):371–83 doi 10.1002/path.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Chen SY, Colborne S, Lambert G, Shin CY, Santos ND, et al. Histone Deacetylase Inhibitors Synergize with Catalytic Inhibitors of EZH2 to Exhibit Antitumor Activity in Small Cell Carcinoma of the Ovary, Hypercalcemic Type. Mol Cancer Ther 2018;17(12):2767–79 doi 10.1158/1535-7163.MCT-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah RR. Safety and Tolerability of Histone Deacetylase (HDAC) Inhibitors in Oncology. Drug Saf 2019;42(2):235–45 doi 10.1007/s40264-018-0773-9. [DOI] [PubMed] [Google Scholar]
- 58.Rao V, Bauer F, Vredenburgh JJ. Refractory Small Cell Carcinoma of the Ovary - Hypercalcemic Type (SCCOHT) Treated with Romidepsin and Topotecan: A Case Report and Review of the Literature. Conn Med 2016;80(9):529–32. [PubMed] [Google Scholar]
- 59.Campbell J, Ryan CJ, Brough R, Bajrami I, Pemberton HN, Chong IY, et al. Large-Scale Profiling of Kinase Dependencies in Cancer Cell Lines. Cell Rep 2016;14(10):2490–501 doi 10.1016/j.celrep.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev 2013;27(24):2648–62 doi 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shorstova T, Marques M, Su J, Johnston J, Kleinman CL, Hamel N, et al. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res 2019. doi 10.1158/0008-5472.CAN-18-1545. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Wang S, Troisi EC, Howard TP, Haswell JR, Wolf BK, et al. BRD9 defines a SWI/SNF sub-complex and constitutes a specific vulnerability in malignant rhabdoid tumors. Nat Commun 2019;10(1):1881 doi 10.1038/s41467-019-09891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang JD, Hendricks WPD, Orlando KA, Yin H, Kiefer J, Ramos P, et al. Ponatinib Shows Potent Antitumor Activity in Small Cell Carcinoma of the Ovary Hypercalcemic Type (SCCOHT) through Multikinase Inhibition. Clin Cancer Res 2018;24(8):1932–43 doi 10.1158/1078-0432.CCR-17-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wohrle S, Weiss A, Ito M, Kauffmann A, Murakami M, Jagani Z, et al. Fibroblast growth factor receptors as novel therapeutic targets in SNF5-deleted malignant rhabdoid tumors. PLoS One 2013;8(10):e77652 doi 10.1371/journal.pone.0077652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong JP, Todd JR, Finetti MA, McCarthy F, Broncel M, Vyse S, et al. Dual Targeting of PDGFRalpha and FGFR1 Displays Synergistic Efficacy in Malignant Rhabdoid Tumors. Cell Rep 2016;17(5):1265–75 doi 10.1016/j.celrep.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue Y, Meehan B, Macdonald E, Venneti S, Wang XQD, Witkowski L, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun 2019;10(1):558 doi 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO, Rieker R, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun 2019;10(1):557 doi 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548(7668):471–5 doi 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov 2018;8(2):216–33 doi 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jelinic P, Ricca J, Van Oudenhove E, Olvera N, Merghoub T, Levine DA, et al. Immune-Active Microenvironment in Small Cell Carcinoma of the Ovary, Hypercalcemic Type: Rationale for Immune Checkpoint Blockade. J Natl Cancer Inst 2018;110(7):787–90 doi 10.1093/jnci/djx277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018;359(6377):770–5 doi 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359(6377):801–6 doi 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan J, McKenzie ZM, D'Avino AR, Mashtalir N, Lareau CA, St Pierre R, et al. The ATPase module of mammalian SWI/SNF family complexes mediates subcomplex identity and catalytic activity-independent genomic targeting. Nat Genet 2019;51(4):618–26 doi 10.1038/s41588-019-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leruste A, Tosello J, Ramos RN, Tauziede-Espariat A, Brohard S, Han ZY, et al. Clonally Expanded T Cells Reveal Immunogenicity of Rhabdoid Tumors. Cancer Cell 2019;36(6):597–612 e8 doi 10.1016/j.ccell.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Lowery CD, Dowless M, Renschler M, Blosser W, VanWye AB, Stephens JR, et al. Broad Spectrum Activity of the Checkpoint Kinase 1 Inhibitor Prexasertib as a Single Agent or Chemopotentiator Across a Range of Preclinical Pediatric Tumor Models. Clin Cancer Res 2019;25(7):2278–89 doi 10.1158/1078-0432.CCR-18-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geoerger B, Kang HJ, Yalon-Oren M, Marshall LV, Vezina C, Pappo A, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol 2020;21(1):121–33 doi 10.1016/S1470-2045(19)30671-0. [DOI] [PubMed] [Google Scholar]
- 77.Soldi RW A; Thode T; Lewis R; Kaadige M; Vankayalapati H; Hendricks W; Sharma S Abstract 3869: The reversible LSD1 inhibitor SP-2509 promotes anti-tumor immunity in small cell carcinoma of the ovary-hypercalcemic type (SCCOHT). Cancer Research 2019;79(13 ):3869. [Google Scholar]