Abstract
The success of Staphylococcus aureus as a pathogen is due to its capability of fine‐tuning its cellular physiology to meet the challenges presented by diverse environments, which allows it to colonize multiple niches within a single vertebrate host. Elucidating the roles of energy‐yielding metabolic pathways could uncover attractive therapeutic strategies and targets. In this work, we seek to determine the effects of disabling NADH‐dependent aerobic respiration on the physiology of S. aureus. Differing from many pathogens, S. aureus has two type‐2 respiratory NADH dehydrogenases (NDH‐2s) but lacks the respiratory ion‐pumping NDHs. Here, we show that the NDH‐2s, individually or together, are not essential either for respiration or growth. Nevertheless, their absence eliminates biofilm formation, production of α‐toxin, and reduces the ability to colonize specific organs in a mouse model of systemic infection. Moreover, we demonstrate that the reason behind these phenotypes is the alteration of the fatty acid metabolism. Importantly, the SaeRS two‐component system, which responds to fatty acids regulation, is responsible for the link between NADH‐dependent respiration and virulence in S. aureus.
Keywords: NADH dehydrogenase, NADH/NAD+, respiratory chain, Staphylococcus aureus, two‐component system
Subject Categories: Metabolism; Microbiology, Virology & Host Pathogen Interaction
NADH‐dependent respiration regulates fatty acid metabolism in Staphylococcus aureus. Changes in the concentration of free fatty acids are sensed by the SaeRS two‐component system, which controls virulence.

Introduction
Methicillin‐resistant Staphylococcus aureus (MRSA) is one of the principal multiple drug‐resistant bacterial pathogens causing serious life‐threatening infections 1. Staphylococcus aureus innocuously colonizes the anterior nares of approximately 30% of the human population 2. However, when protective barriers are compromised, S. aureus can cause a variety of distinct infections, including cellulitis, endocarditis, osteomyelitis, bacteremia, and septic shock 3, 4. The success of S. aureus as a pathogen is largely due to the evolution of multidrug resistance and the ability of the bacterium to adapt its metabolism and bioenergetics to infect nearly every site of the human body 5, 6, 7, 8, 9. Infections by S. aureus are notoriously difficult to prevent and treat, as this bacterium has many ways to evade the host's immune response 10, 11. This includes the formation of biofilms on native and implanted surfaces, which not only hinder the immune response but also render S. aureus more resistant to antibiotic treatment 12. Staphylococcus aureus biofilm formation plays an important role in many diseases such as native valve endocarditis 13, osteomyelitis 14, chronic wound infections 15, and chronic lung infections in cystic fibrosis patients 16. Bacteria within biofilms have a wide variety of metabolic states, further emphasizing the important contributions of metabolic adaptability to staphylococcal pathogenesis 17. However, despite the importance of metabolism and metabolic adaptability to infection, our understanding of it is still limited.
The survival of S. aureus, indeed of all pathogens and in any environment, requires the generation of both ATP and a proton motive force (PMF) across the cytoplasmic membrane. Staphylococcus aureus can use aerobic respiration, anaerobic/nitrate respiration fermentation, or a combination of these three metabolic strategies to harvest energy from the environment. During aerobic respiration, membrane‐bound enzymes plus menaquinone 18 make up a branched electron transport chain, with two oxygen reductases (Qox and Cyd) that can use multiple electron donors (such as NADH, l‐lactate, succinate, pyruvate, and malate) 5, 19, 20, 21, 22. Both terminal oxygen reductases drive charges/protons across the membrane to generate a transmembrane voltage (ΔΨ), positive outside. In hypoxic environments when nitrate is present, the terminal oxygen reductases are replaced by nitrate reductase (Nar) 23, 24, 25. Differing from many pathogens, S. aureus does not contain Complex I (the energy‐coupled NADH:quinone oxidoreductase) or Nqr (sodium pumping NADH:quinone oxidoreductase) 26. The only respiratory NADH dehydrogenases present in this organism are the two type‐2 NADH dehydrogenases (NDH‐2s) NdhF and NdhC 20. NDH‐2s are monotopic membrane proteins and neither pump protons across the membrane nor contribute directly to the generation of the PMF. They do, however, reduce menaquinone to menaquinol, which is oxidized by either of the two terminal oxygen reductases or Nar 23, 24, 25. Thus while NDH‐2s do not directly translocate protons, they still can contribute to the PMF 22. Distinct from the l‐lactate (Lqo), succinate (SDH), pyruvate (CidC), and malate (Mqo) menaquinone oxidoreductases, NDH‐2s enable the cell to regenerate NAD+ from NADH 26, 27, 28, 29. The physiological need to maintain redox balance by oxidation of NADH can take precedence over the need to conserve energy, as demonstrated in Mycobacterium tuberculosis 30.
There are critical gaps in our knowledge of how metabolic pathways influence S. aureus virulence inside the host and the production of virulence factors in vitro. Over the years, researchers have studied the involvement of diverse metabolic pathways in S. aureus virulence 31, 32; however, the involvement of its branched and flexible respiratory chain has not been extensively investigated 5, 19, 20, 33, 34. Most studies have been centered on how the complete deficiency of aerobic respiration (small colony variant, SCV) affects virulence and biofilm formation 6, 35, 36. It is also important to address the more nuanced question of how the different components of the highly flexible S. aureus respiratory chain help the pathogen invade and colonize the different organs inside the host.
Previously, we purified and characterized both NDH‐2s, NdhC and NdhF, from S. aureus and demonstrated that they are able to catalyze NADH oxidation with the concomitant reduction of long‐chain quinones within the lipid bilayer 20. There are significant differences between the two NDH‐2s in k cat and K m for NADH 20, suggesting that they may have unique physiological roles. Given the importance of respiration to staphylococcal pathogenesis and the potential for non‐redundant function, we investigated the contribution of NdhF and NdhC to infection. Here, our studies revealed that aerobically in rich media with non‐glycolytic carbon sources while both NDH‐2s are expressed, NdhC contributes significantly to growth but NdhF is largely dispensable. Importantly, deleting both ndh genes together, though reducing growth, does not result in the small colony variant (SCV) phenotype. Further investigation revealed that NdhC is vital for the production of α‐toxin and that both NdhC and NdhF, individually, are critical for biofilm formation and systemic infection in mice. These phenotypes were dependent on the accumulation of fatty acids and regulated by the SaeRS two‐component system. Altogether, these results highlight for the first time the important contribution of NADH‐dependent respiration to S. aureus biofilm formation and virulence.
Results
Each of the Staphylococcus aureus NDH‐2s is important for virulence in mice
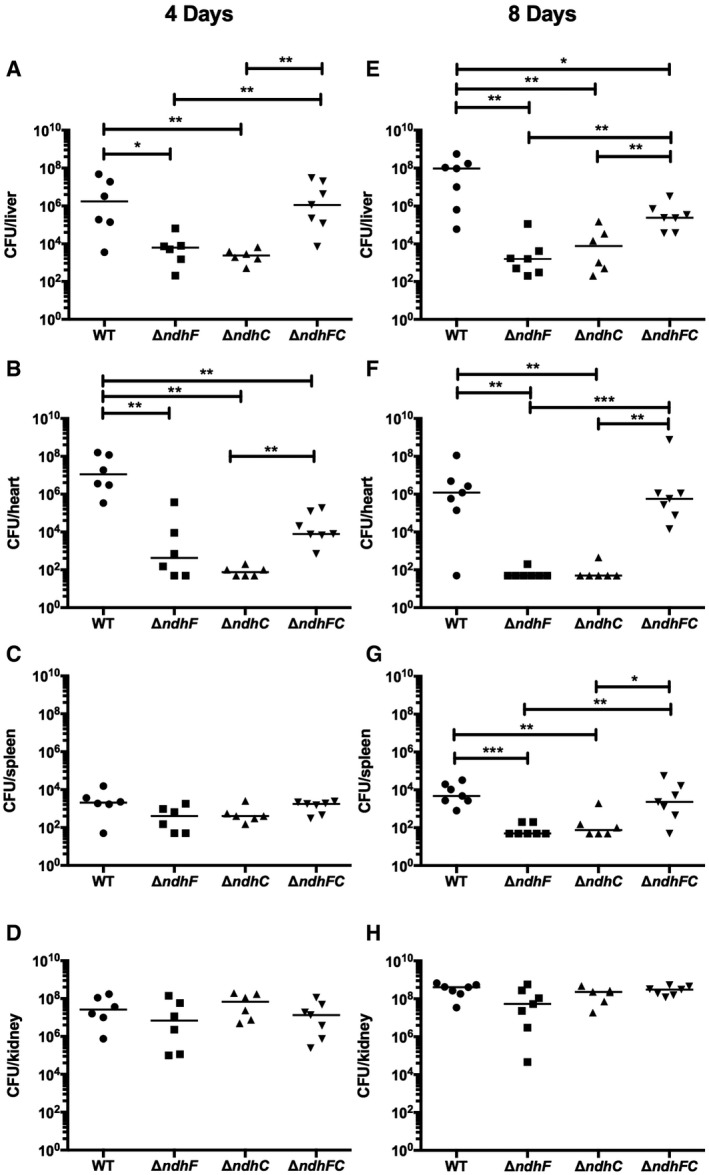
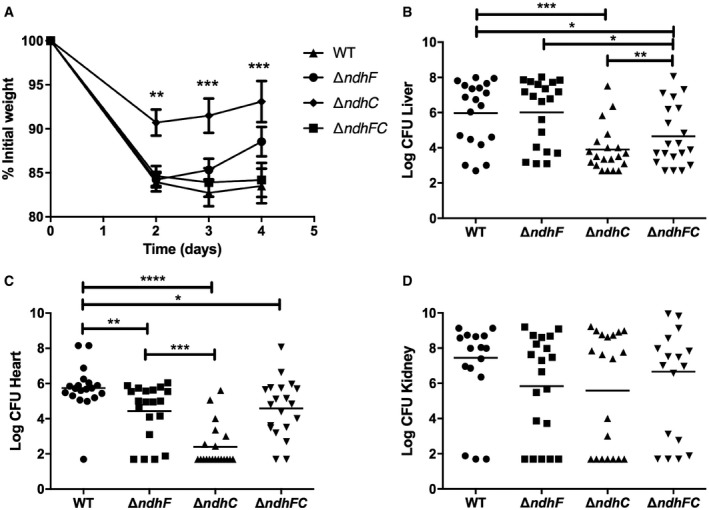
Given the demonstrated importance of aerobic and anaerobic/nitrate respiration to S. aureus pathogenesis 5, 6, 24, 33, 35, 36 and the lack of information on the respiratory enzymes in this pathogen that regenerate NAD+, we investigated the contribution of NdhC and NdhF to the development of invasive disease. We have compared the virulence of the ΔndhC, ΔndhF, and ΔndhC/ΔndhF strains using a murine model of systemic infection, quantifying the ability to colonize different organs. At 4 and 8 days postinfection, bacterial burdens within each organ were determined by counting CFU obtained from serial dilutions of the organ homogenate. Independent determinations using different strains of mice (CD‐1 or C57BL/6) and protocols (tail vein or retro‐orbital infection) were performed, and data are shown in Figs 1 and EV1. Bacterial burdens are decreased in the heart by approximately five logs following challenge with single ndh knockouts (Fig 1B and F), whereas the bacterial burden in the liver is decreased by approximately two logs at 4 days postinfection and four logs at 8 days postinfection when either ndhC or ndhF is inactivated (Fig 1A and E). Moreover, bacterial burdens in spleen, with both single ndh knockouts, are decreased by approximately two logs just at 8 days postinfection (Fig 1C and G). However, no significant changes in the bacterial burdens were observed with any knockout in kidney (Figs 1D and H, and EV1D). Overall, both NDH‐2s contribute to virulence, although tissue‐specific differences were observed between the two mouse models of infection tested (Figs 1 and EV1). A more pronounced weight loss was found in mice infected with the ΔndhF or WT strains compared to mice infected with the ΔndhC strain (Fig EV1A), consistent with the bacterial burdens in liver and heart in this set of experiments (Fig EV1B and C). This indicates that NdhC may play a more important role in establishing systemic disease.
Figure 1. Effects of ndhC and ndhF deletions on Staphylococcus aureus virulence.
-
A–HSix‐ to eight‐week‐old female outbred CD‐1 mice were infected via tail vein injection with 5 × 107 CFU of WT S. aureus Newman or isogenic mutant strains grown in TSB medium to an OD600 of 0.5. Organs were harvested after (A–D) 4 days or (E–H) 8 days postinfection, and bacterial burdens in the (A and E) liver, (B and F) heart, (C and G) spleen, and (D and H) kidney were determined. Data represent mean ± SEM (n = 7 biological replicates). *P < 0.05, **P < 0.005, and ***P < 0.0007, using the Mann–Whitney test.
Figure EV1. NdhC and NdhF are both required for full Staphylococcus aureus virulence.
-
A–DNine‐week‐old female C57BL/6 mice were infected retro‐orbitally with approximately 1 × 107 CFU of WT S. aureus Newman or isogenic mutant strains grown in TSB medium to an OD600 of 0.4. (A) Mean weight loss and (B–D) bacterial burdens in the (B) liver, (C) heart, and (D) kidneys were assessed after 4 days of infection. Data represent mean ± SEM (n = 20 biological replicates). *P < 0.05, **P < 0.005, ***P < 0.0009, and ****P < 0.0001, using the Mann–Whitney test.
Remarkably, deleting both genes (ΔndhC/ΔndhF) partially restores the ability to colonize heart, liver, and spleen, compared to the single deletion strains, (ΔndhC and ΔndhF) (Figs 1A–C and E–G, and EV1B and C). The lack of both NdhC and NdhF seems to trigger a metabolic switch that is not triggered by the lack of either of these enzymes under the conditions of growth in the host. Thus, the two NDH‐2s in S. aureus contribute significantly to overall virulence in a non‐additive manner. These findings also suggest that targeting individual NDH‐2s might be a viable antimicrobial strategy.
NDH‐2s are critical for maintaining proper redox balance, respiration status, and growth in Staphylococcus aureus
To gain better insight into the function of NDH‐2s, we initially evaluated the growth of strains lacking NdhC (ΔndhC) and NdhF (ΔndhF) and assessed their gene expression levels using transcriptional reporter fusions (Fig 2). Loss of NdhC, but not NdhF, significantly impaired staphylococcal growth when compared to wild‐type bacteria in Mueller Hinton Broth cation‐adjusted (MHB‐ca) medium. Interestingly, the ΔndhC/ΔndhF double mutant resembles ΔndhC (Fig 2A), suggesting that NdhF even in the absence of NdhC does not significantly contribute to growth in this medium. Importantly, these differences in growth are not due to cell lysis or cell death since CFU/ml is similar for all the strains. WT and ndh knockouts grown for 24 h in MHB‐ca medium have ~ 5 × 107 CFU/ml with an OD600 of 0.5. As S. aureus lacks amylolytic activity 37, in MHB‐ca, the bacterium is functionally forced to rely on amino acids as the sole carbon source. To determine the extent to which available carbon source influences the importance of NDH‐2s, glucose was added to the growth media. This addition largely, but not completely, reverses the growth defects of ΔndhC and ΔndhC/ΔndhF when compared to MHB‐ca lacking glucose (Fig 2A). This is consistent with the prior observation that an ndhC mutant is unable to grow aerobically in chemically defined medium with amino acids as the sole carbon source, but can grow when glucose is added 38. Analysis of ndhC and ndhF expression indicates that both genes are expressed in MHB‐ca and MHB‐ca supplemented with glucose but at a higher level when cells are grown in MHB‐ca compared to MHB‐ca supplemented with glucose, which generally suppresses respiration in favor of glycolysis. Moreover, loss of ndhC leads to increased expression of ndhF at 24 h, but the reverse was not observed (Fig 2B). Cumulatively, these observations suggest that the growth defects of the strain lacking NdhC are not due to the absence NdhF. In total, these results suggest that NADH‐dependent respiration is particularly important for S. aureus to catabolize amino acids and that NdhC is the primary dehydrogenase used under these conditions. The fact that deleting ndhC is much more consequential to growth than deleting ndhF, which catalyzes the same biochemical reaction, indicates that, under the growth conditions used, NdhC is more effective than NdhF at generating NAD+, possibly due to the differences in the expression of the ndhC and ndhF (Fig 2B) and the lower specific activity of NdhF 20.
Figure 2. Bacterial growth and ndhC and ndhF expression in Newman WT strain and isogenic ndh mutants.
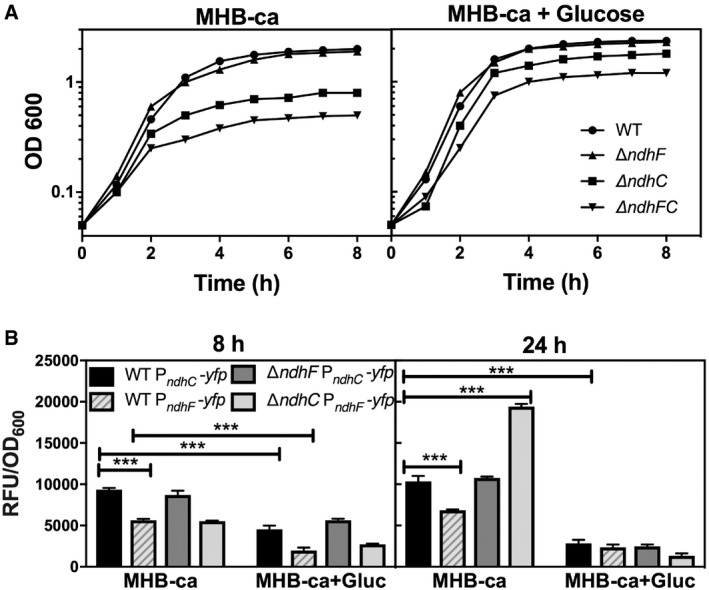
- Bacterial growth was measured by optical density at 600 nm. Data are representative of five independent experiments.
- PndhC and PndhF YFP reporter constructs were used for following gene expression, and data were normalized by growth. Data are expressed as average ± SEM of three independent experiments. ***P < 0.001 compared to ndhC expression in WT strain grown in MHB‐ca medium via two‐way ANOVA with Tukey's posttest.
The growth defects of the ΔndhC and ΔndhC/ΔndhF mutants (Fig 2A) could be readily explained whether the strains have a reduced respiratory activity and, therefore, reduced ability to generate ATP. Notably, these mutations do not result in SCV phenotype, which is the etiological agent of persistent infections 6, 39, 40. Therefore, the rate of oxygen utilization was measured. Unexpectedly, the rates of oxygen consumption in late stationary phase (24 h) of strains lacking NdhC are significantly greater than wild type (Fig 3A), indicating that other respiratory dehydrogenases must be functioning under these conditions, pointing out the shift in metabolic pathways. Loss of NdhF in these assays had no effect on oxygen consumption in either exponential phase or stationary phase bacteria. Notably, the complemented ΔndhC strain restores the low rate of oxygen consumption after 24 h of growth as observed with the WT strain (Fig EV2A). Respiration rates were also measured after the addition of different electron donors (succinate, glucose, pyruvate, and dl‐lactate) (Fig EV3). The addition of succinate, glucose, and pyruvate shows similar data to that reported with glycerol (Figs EV3 and 3A). However, dl‐lactate induces about twice the respiratory activity in all the strains and maintains high respiration rates at 24 h (Fig EV3D). Oxygen consumption assays with isolated membranes of WT, ndhF, and ndhC knockout strains were performed as previously described 20. The ndhF knockout strain has 75% of the WT NADH respiratory activity and the ndhC knockout only 20%.
Figure 3. Effect of NDH‐2s on respiration status and redox balance in Staphylococcus aureus .
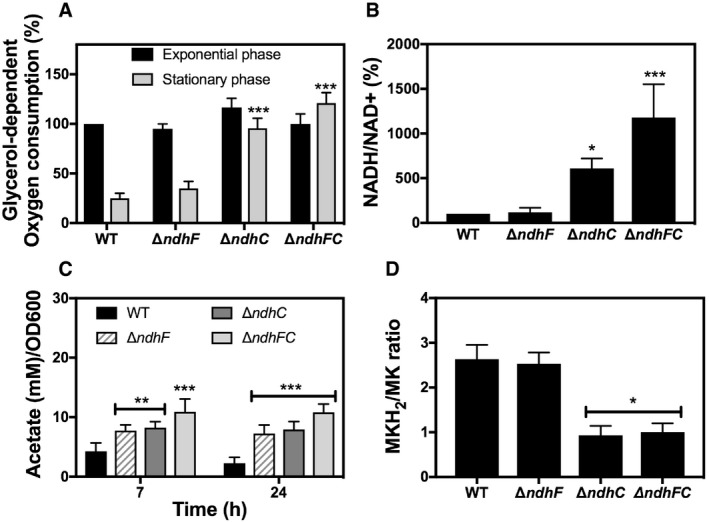
- Oxygen consumption of WT and isogenic mutant strains was measured with Clark oxygen electrode. Cells were grown in MHB‐ca medium until exponential phase (OD600 = 0.6) or until late stationary phase (24 h). Cells were washed with PBS and respiration initiated by the addition of 20 mM glycerol. Data are expressed as average ± SD of four independent experiments. ***P < 0.001 compared to WT via two‐way ANOVA with Tukey's posttest.
- NADH/NAD+ ratios were measured in cells grown aerobically in MHB‐ca medium for 20 h. 100% corresponds to a NADH/NAD+ ratio of 0.003. Data are expressed as average ± SD of four independent experiments. *P < 0.05 and ***P < 0.001 compared to WT via one‐way ANOVA with Dunnett's posttest.
- Acetate concentration was monitored in the supernatants of WT and isogenic ndh strains at 7 and 24 h of growth in MHB‐ca medium. Data are expressed as average ± SD of three independent experiments. **P < 0.005 and ***P < 0.001 compared to WT via two‐way ANOVA with Tukey's posttest.
- MKH2/MK ratios were measured in cells grown aerobically for 20 h in MHB‐ca medium. Data are expressed as average ± SD of three independent experiments. *P < 0.05 compared to WT via one‐way ANOVA with Dunnett's posttest.
Figure EV2. The ΔndhC and ΔndhF phenotypes are complemented by plasmid‐encoded copies of ndhC and ndhF, respectively.
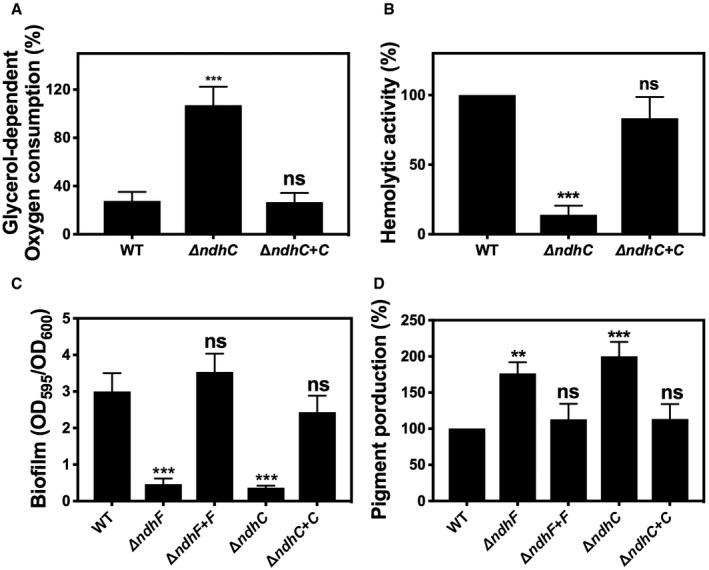
- Comparison of whole cell respiration. Data are expressed as average ± SD of three independent experiments. ***P < 0.001 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
- Hemolytic activity was measured by incubation of rabbit blood cells with serially diluted supernatants from 20‐h cultures grown in MHB‐ca medium. Data are expressed as average ± SD of three independent experiments. ***P < 0.001 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
- Comparison of biofilm formation. Data are expressed as average ± SD of three independent experiments performed by octuplicates. ***P < 0.001 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
- Comparison of pigment production. Data are expressed as average ± SD of three independent experiments. ***P < 0.001, **P < 0.005, and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
Figure EV3. Effect of different carbon sources on WT and ndh mutant strains respiration activity.
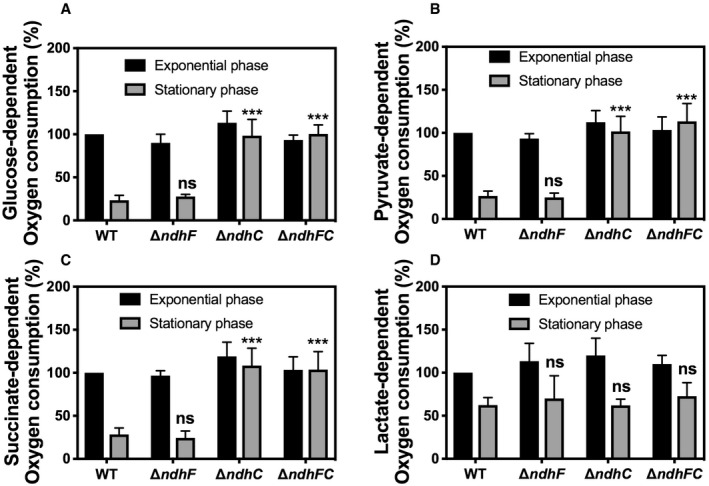
-
A–DOxygen consumption of WT and isogenic mutant strains was measured with Clark oxygen electrode. Cells were grown in MHB‐ca medium until exponential phase (OD600 = 0.6) or until late stationary phase (24 h). Cells were washed with PBS and respiration initiated by the addition of (A) 20 mM glucose, (B) 40 mM sodium pyruvate, (C) 30 mM sodium succinate, or (D) 50 mM dl‐lactate. Data are expressed as average ± SD of three independent experiments. ***P < 0.001 and ns denotes no significant compared to WT via two‐way ANOVA with Tukey's posttest.
The importance of NDH‐2s in S. aureus becomes evident by the comparison of the NADH/NAD+ ratios (Fig 3B). The absence of NdhC in cells grown aerobically in MHB‐ca medium substantially increases the amount of NADH in stationary phase, showing that this enzyme is primarily responsible for recycling NADH under these conditions. This is not observed with the ΔndhF strain, consistent with lack of growth defect caused by ΔndhF (Fig 2A). The high respiration rate in the ΔndhC strains also implies that growth is not impeded by a shortfall in the PMF but that the critical feature behind the alteration in metabolism is the increase in the NADH/NAD+ ratio. The low level of NAD+ in the ΔndhC and ΔndhC/ΔndhF strains should inhibit the TCA cycle, which in S. aureus is indicated by accumulation of acetate since this organism lacks a glyoxylate cycle and requires the TCA cycle for acetate catabolism. Figure 3C shows that this is the case, as previously shown for an sdh‐knockout strain 21. Surprisingly, acetate accumulation is also observed with the ΔndhF strain, showing that this mutation is not entirely benign. The data clearly show that the NDH‐2s are the major routes for NAD+ regeneration in S. aureus under aerobic conditions in MHB‐ca medium. Our results from in vitro cultures as well as prior experiments 20 suggest that the ΔndhC, ΔndhF, and ΔndhC/ΔndhF genotypes could result in an altered steady‐state concentration of reduced menaquinone (MK) in the membrane. Therefore, MKH2/MK ratios were determined for cells grown aerobically. Figure 3D shows that ndhC mutant strains have a lower MKH2/MK ratio compared to wild type, which correlates with the high respiratory activity of these mutants (Fig 3A). It is possible that the inability to maintain the supply of NAD+ due to ΔndhC results in converting pyruvate to l‐lactate, generating NAD+, and increasing respiration using Lqo 33 with l‐lactate as the substrate. In total, our data show that these two redox ratios, NADH/NAD+ and MKH2/MK, can vary independently under certain circumstances.
Inactivation of NdhC and NdhF alters the activity of factors that contribute to staphylococcal virulence
Pigment (staphyloxanthin) production by the ndh knockouts was studied since dramatic effects on pigment production have been observed upon disrupting metabolic pathways such as the TCA cycle and oxidative phosphorylation (Δqox) 41. Moreover, mutant strains of S. aureus lacking staphyloxanthin are less virulent, less able to survive in whole blood, and more oxidant‐sensitive than pigmented strains 42, 43, 44. Contrary to our expectations, single and double ndh knockouts produced increased levels of staphyloxanthin compared to WT (Fig 4A) and, consistent with this, they were more resistant to peroxide stress (Fig 4B), suggesting that the defects in virulence observed with the ndh knockouts (Figs 1 and EV1) are not due to increased susceptibility to reactive oxygen species during infection. Complementation of the missing gene (ndhC or ndhF) restores the WT pigment production (Fig EV2D).
Figure 4. Pigment production and peroxide sensitivity of the ndh mutants.
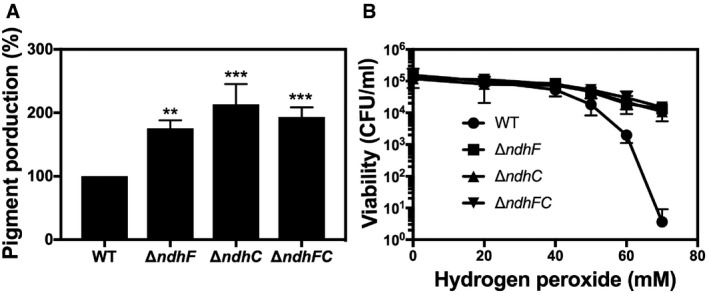
- The carotenoid pigment of different Staphylococcus aureus strains was extracted by methanol and quantified by the optical density at 465 nm, and the values were normalized to WT Newman strain, which was set at 100. Data are expressed as the average ± SD of five independent experiments. **P < 0.005 and ***P < 0.001 compared to WT via one‐way ANOVA with Dunnett's posttest.
- Hydrogen peroxide sensitivity was measured by incubating WT and isogenic strains, previously grown for 20 h in MHB‐ca, with different concentrations of hydrogen peroxide. Serial dilutions were plated in TSB medium for measuring CFU. Data are expressed as the average ± SD of three independent experiments.
An increased concentration of menaquinol (MKH2) in the membrane has previously been suggested to be a signal leading to biofilm formation 35, 36. Additionally, ndhC and sdh are upregulated in S. aureus biofilms 45. Further supporting this idea is the observation that both ndhC and ndhF are expressed under conditions that stimulate biofilm formation (Fig 5A). Therefore, we initially assessed whether biofilm formation was affected by loss of NdhC and NdhF individually or in combination. In contrast to wild type, which formed robust biofilms after 20 h, neither of the single deletion strains nor the double ΔndhC/ΔndhF strain forms any biofilm in a 96‐well plate assay (Fig 5B). Complementation of the missing gene (ndhC or ndhF) restores the WT phenotype and biofilm formation (Fig EV2C). These biofilm data performed with Newman strain were reproduced when the mutations were moved to either LAC (Fig EV4A) or UAMS‐1 strains. Cumulatively, these results reveal that the staphylococcal NDH‐2s individually contribute to biofilm development in a critical manner. Moreover, our data show that the absence of either or both NDH‐2s results in a decrease of the menaquinol pool in the membrane (Fig 5C) by lowering the rate of reduction of menaquinone due probably to the inability to reoxidize NADH, as demonstrated by the increased NADH/NAD+ ratios in the ndh mutant strains (Fig 5D). The same phenotype could result from an increase in activity of the respiratory oxygen reductases (e.g., Cyd) as anticipated from the high respiratory activity of the ΔndhC strains (Fig 3A).
Figure 5. ndhC and ndhF expression, biofilm formation, and redox balance of ndh mutants under biofilm growth conditions.
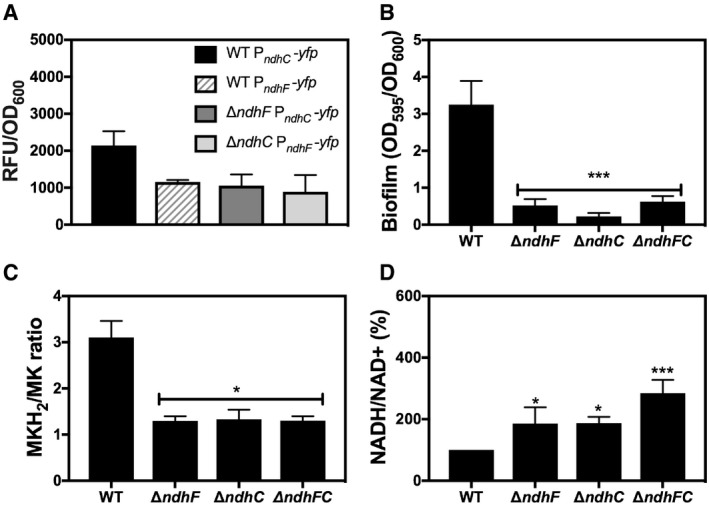
- PndhC and PndhF YFP reporter constructs were used to follow gene expression under the biofilm growth conditions. Data collected after 24 h of static incubation were normalized by optical density at 600 nm. Data are expressed as average ± SEM of three independent experiments. No significant differences were found when ndhC expression in WT strain was compared via one‐way ANOVA with Dunnett's posttest.
- Biofilm formation of Newman WT and ndh‐deficient mutants after static incubation at 37°C for 20 h. The histogram shows the amount of biofilm biomass attached to the microtiter plate when cells were grown in MHB‐ca supplemented with 10% human plasma, 0.5% glucose, and 2% NaCl. Data are expressed as average ± SD of four independent experiments performed by octuplicates. ***P < 0.001 compared to WT via one‐way ANOVA with Dunnett's posttest.
- MKH2/MK ratios were measured in cells grown under the same conditions as for biofilm formation. Data are expressed as average ± SD of three independent experiments. *P < 0.05 compared to WT via one‐way ANOVA with Dunnett's posttest.
- NADH/NAD+ ratios were measured in cells grown under the same conditions as for biofilm formation. 100% corresponds to a NADH/NAD+ ratio of 0.09. Data are expressed as average ± SD of four independent experiments performed by duplicates. *P < 0.05 and ***P < 0.001 compared to WT via one‐way ANOVA with Dunnett's posttest.
Figure EV4. Biofilm formation and hemolytic activity are dependent on the presence of NDH‐2s in LAC strain.
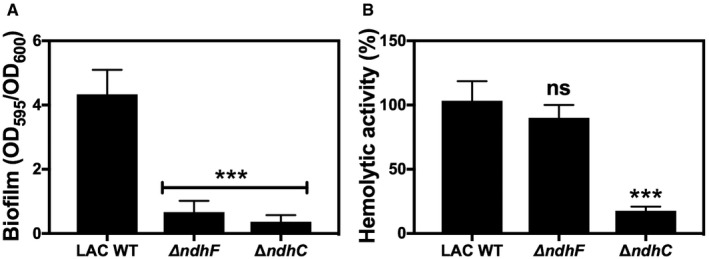
- Biofilm formation of LAC WT and ndh‐deficient mutants after static incubation at 37°C for 20 h. The histogram shows the amount of biofilm biomass attached to the microtiter plate when cells were grown in MHB‐ca supplemented with 10% human plasma, 0.5% glucose, and 2% NaCl. Data are expressed as average ± SD of four independent experiments performed by octuplicates. ***P < 0.001 compared to WT via one‐way ANOVA with Dunnett's posttest.
- Hemolytic activity of the LAC WT and isogenic mutant strains was measured by incubation of rabbit blood cells with serially diluted supernatants from 20‐h cultures grown in MHB‐ca medium. LAC WT has about 50% less hemolytic activity than Newman WT strain. Data are expressed as average ± SD of four independent experiments. ***P < 0.001 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
Based on the in vivo information gained with the ndh knockouts, we decided to expand our investigations and evaluate the involvement of NDH‐2s in the production of the best‐characterized and most potent membrane‐damaging toxin of S. aureus (α‐toxin). Mutants lacking α‐toxin are less virulent in a number of organs and tissues 46, 47. Assessment of in vitro α‐toxin production was done by quantitative analysis of lysis of rabbit red blood cells, which are very susceptible to hemolysis by α‐toxin 48. The ΔndhC strain exhibits drastically reduced hemolytic activity, whereas ΔndhF is similar to the WT (Fig 6). Meanwhile, the double mutant ΔndhC/ΔndhF strain shows intermediate hemolytic activity. As expected from previous studies 49, glucose represses the production of α‐toxin. In contrast, the addition of pyruvate or acetate stimulated hemolytic activity in the ndhC mutants (Fig 6), consistent with recent observations about the role of pyruvate in regulating virulence 50. Similar results to the ones shown in Fig 6 were also observed with LAC strain (Fig EV4B). Complementation of the missing ndhC gene restores the WT phenotype and hemolytic activity (Fig EV2B).
Figure 6. Hemolytic activity is dependent on the presence of NDH‐2 and carbon source.
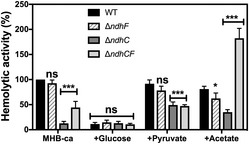
Hemolytic activity of the Newman WT and isogenic mutant strains was measured by incubation of rabbit blood cells with serially diluted supernatants from 20‐h cultures grown in MHB‐ca medium without and with 25 mM glucose, 40 mM sodium pyruvate, or 60 mM sodium acetate. Lysis was monitored by the release of hemoglobin. Data are expressed as average ± SD of five independent experiments. *P < 0.05, ***P < 0.001, and ns denotes no significant compared to WT grown in MHB‐ca and WT grown in MHB‐ca + glucose via two‐way ANOVA with Tukey's posttest.
In total, these observations suggest that loss of staphylococcal NADH dehydrogenase activity not only impairs/modifies metabolism but also alters the activity of important staphylococcal virulence determinants.
The SaeRS two‐component system links respiratory NADH oxidation and virulence in Staphylococcus aureus
We hypothesized that the lack of NDH‐2s triggers metabolic changes that must alter the activity of global regulators, changing the metabolic flux patterns and altering the expression of virulence factors and, hence, S. aureus pathogenesis.
The SaeRS and SrrAB two‐component systems (TCS) have long been recognized as a major regulator of metabolism, biofilm formation, and virulence factors like α‐toxin in S. aureus 35, 36, 51, 52, 53, 54, 55. As with most TCS regulators, the signal to which SaeRS and SrrAB respond is not known, but for both has been speculated to be the redox state of the menaquinol pool in the membrane 35, 36. This speculation is partially based on genetic studies 35, 36 and for SrrB in particular by its analogy to the Escherichia coli ArcAB TCS, which regulates transcription of a similar set of genes and also responds to impaired aerobic respiration 56, 57. Since our data show a decrease of the MKH2/MK ratio (Figs 3D and 5C) in the ndh mutants, single and double knockouts in the TCS and ndh were constructed. Figure 7A and B shows that under our assay conditions, SrrAB is not required for neither α‐toxin activity nor biofilm production. Double ΔsrrAB/ΔndhC strains reproduce the same phenotypes as the single ΔndhC strain demonstrating that SrrAB is not involved in the regulation of virulence by NDH‐2s. On the other hand, the double ΔsaeRS/ΔndhC strain has impaired hemolytic activity and the capacity to form biofilm as the single ΔsaeRS and ΔndhC strains, which does not eliminate the possibility of SaeRS as being responsible for NDH‐2s’ regulation of virulence (Fig 7A and B).
Figure 7. Global regulators connecting virulence and fatty acid homeostasis in the ndh mutants.
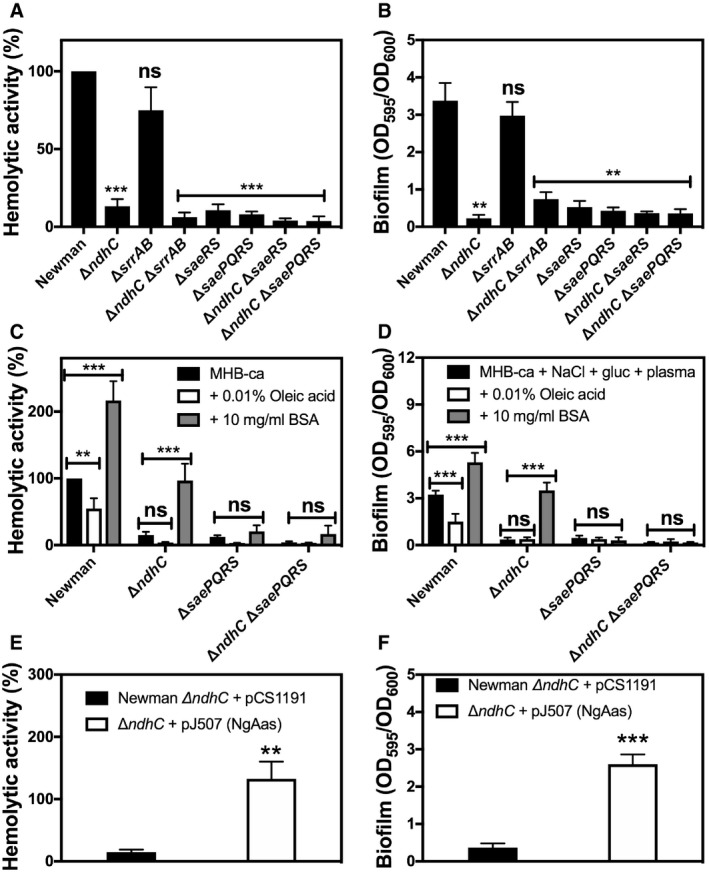
- Hemolytic activity of the Newman WT and isogenic mutant strains was measured by incubation of rabbit blood cells with serially diluted supernatants from 20‐h cultures grown in MHB‐ca medium. Lysis was monitored by the release of hemoglobin. Data are expressed as average ± SD of four independent experiments. ***P < 0.001 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
- Biofilm formation of Newman WT and isogenic mutant strains after static incubation at 37°C for 20 h. The histogram shows the amount of biofilm biomass attached to the microtiter plate when cells were grown in MHB‐ca supplemented with 10% human plasma, 0.5% glucose and 2% NaCl. Data are expressed as average ± SD of four independent experiments performed by octuplicates. **P < 0.005 and ns denotes no significant compared to WT via one‐way ANOVA with Dunnett's posttest.
- Hemolytic activity of the Newman WT and isogenic mutant strains grown aerobically for 20 h in MHB‐ca medium, in the absence or presence of 10 mg/ml FA‐deficient BSA and 0.01% oleic acid. Data are expressed as average ± SD of five independent experiments. **P < 0.005 and ***P < 0.001 compared to each strain without any BSA or oleic acid addition via two‐way ANOVA with Tukey's posttest.
- Biofilm formation of Newman WT and isogenic mutant strains after static incubation for 20 h in MHB‐ca supplemented with 10% human plasma, 0.5% glucose, and 2% NaCl, in the absence or presence of 10 mg/ml FA‐deficient BSA and 0.01% oleic acid. Data are expressed as average ± SD of four independent experiments performed by octuplicates. ***P < 0.001 compared to each strain without any BSA or oleic acid addition via two‐way ANOVA with Tukey's posttest.
- Hemolytic activity of Newman ΔndhC strain overexpressing the Neisseria gonorrhoeae acyl‐ACP synthetase and grown aerobically for 20 h in MHB‐ca medium. Data are expressed as average ± SD of four independent experiments. **P < 0.005 compared to ΔndhC strain with the empty plasmid via Student's t‐test.
- Biofilm formation of Newman ΔndhC strain overexpressing the N. gonorrhoeae acyl‐ACP synthetase and grown under biofilm producing conditions. Data are expressed as average ± SD of three independent experiments performed by octuplicates. ***P < 0.001 compared to ΔndhC strain with the empty plasmid via Student's t‐test.
It was previously demonstrated that saeRS gene expression responds to fatty acids and connects lipid metabolism homeostasis and regulation of α‐toxin production and transcription 58, 59. Therefore, the possibility that in the absence of NdhC fatty acid (FA) metabolism is altered and responsible for the inactivation of the SaeRS signaling was evaluated. Initially, bacterial cells were grown in the absence and presence of exogenous FA (oleic acid) or FA‐free bovine serum albumin (BSA), and both hemolytic activity and biofilm formation were evaluated in the WT, ndhC/saeRS and ndhC/saePQRS knockouts (Fig 7C and D). SaeP and SaeQ are auxiliary proteins that operate in conjunction with the SaeRS TCS 53 and are encoded in the saePQRS operon 55. FA‐deficient BSA has been previously demonstrated to extract a significant portion of the intracellular FA into the medium 58. The data here presented show that the addition of BSA to the medium increases hemolytic activity and biofilm formation in WT and ΔndhC strains but not in the double ΔndhC/ΔsaePQRS strain, which demonstrates that SaeRS is necessary for inducing α‐toxin activity and biofilm formation in response to FA accumulation. Oleic acid, on the other hand, did not improve hemolysis or biofilm formation in any of the knockout strains (Fig 7C and D). Furthermore, the overexpression of Neisseria gonorrhoeae acyl‐ACP synthetase (NgAas), which activates cellular FA for incorporation into phospholipids, established that FA accumulation in the Δndh strains is the reason for an altered SaeRS regulation of virulence. Figure 7E and F shows that cells containing the NgAas restore the hemolytic activity and biofilm formation lost in the ΔndhC strain. Additionally, the overexpression of NgAas also restores the cell density reached in stationary phase by the ΔndhC strain grown aerobically and the pigmentation to wild‐type levels (Figs 2A and 4A).
One other background strain (LAC) was tested and yielded similar results to those shown in Fig 7A–D. Since most of our assays were done in the Newman strain background, it is important to mention that although this strain contains a mutation in saeS that makes it highly active 60, the production of α‐toxin should not be altered. This is due to the fact that SaeR‐phosphorylation has high affinity for the hla gene encoding for α‐toxin 55 and hence basal levels of SaeR‐P are sufficient for its transcription. This would explain why different strain backgrounds (e.g., Newman or LAC) still show the same phenotype when NdhC is absent. Altogether, our results point at NADH‐dependent respiration as an essential regulatory pathway for FA homeostasis, which in turn regulates S. aureus virulence through the SaeRS TCS.
Discussion
The two respiratory NADH dehydrogenases in S. aureus are positioned to play important roles in energy production as well as redox balance and the regeneration of NAD+, an oxidant critical to numerous biochemical reactions and for the function of the TCA cycle. Therefore, the ndh knockouts provide opportunities to determine the molecular mechanism of how S. aureus alters its metabolism in response to imposed constraints. Notably, the loss of NdhC and NdhF does not result in the same phenotypes, further emphasizing that despite apparent redundancy in their biochemical activity the staphylococcal NDH‐2s each uniquely contribute to virulence. Ultimately, the different phenotypes observed upon loss of NdhC and/or NdhF must be attributed to the net effects on the steady‐state concentrations of the redox agents NADH, NAD+, MK, and MKH2. The consequences of changing the concentrations (or ratios) of these redox metabolites are presumed to be mediated by global regulatory systems.
Changes in the concentrations of the redox metabolites are integrated with an array of internal and environmental signals to remold the metabolic pathways utilized by S. aureus. The magnitude of the changes in the concentrations of the redox metabolites by inactivation of either or both NdhF and NdhC depends on how much of each enzyme would normally be present in the WT strain as well as the activity of other enzymes or enzyme networks that contribute to changing the redox state of the NADH/NAD+ and MKH2/MK pools. Hence, growth conditions are critical determinants of the consequences of the ndhF and ndhC knockouts. Broadly speaking, the results presented can be summed up as follows.
In vivo growth (organ colonization) and virulence
The results presented add to the growing body of literature demonstrating that central metabolism plays a key role during host–pathogen interactions and to the link between S. aureus metabolic activity with virulence, survival, and persistence 5, 32, 61, 62. The importance of different metabolic strategies used by S. aureus in different organs of the infected host depends on the available carbon and nitrogen sources as well as the concentrations of respiratory substrates, oxygen, and nitrate. The dramatic and selective effects of the deletion of either ndhC or ndhF on colonization of the heart, liver, and spleen reflect the distinct environmental niches presented in each organ. The changes in the redox status by deleting one of the ndh genes are not compensated by having the second gene being present. Most remarkable is that single ΔndhC and ΔndhF mutants have dramatically diminished virulence but the double ΔndhF/ΔndhC mutant is as virulent as the WT strain in a murine model of systemic infection. In the double ΔndhF/ΔndhC mutant, the increased accumulation of NADH is more extreme than for either single knockout and must trigger metabolic changes under the growth conditions encountered in the heart and liver, whose consequence is to restore virulence to the level of the WT strain. One possibility is that the impaired generation of NAD+ due to the ndh mutations suppresses function of the TCA cycle and increases the expression of either l‐lactate dehydrogenase (Lqo) and/or pyruvate oxidase (CidC). NADH oxidation could result from the sequential reaction of the NAD+‐dependent lactate dehydrogenase (Ldh1/Ldh2), converting pyruvate to l‐lactate, followed by Lqo that regenerates pyruvate and feeds electrons to the respiratory chain by reducing menaquinone 63. Pyruvate oxidase converts pyruvate directly to acetate plus CO2 and directly reduces menaquinone 34. The activity of Lqo and CidC would explain the counterintuitive increased respiration (oxygen utilization) in the ΔndhF/ΔndhC strain compared to WT (Fig 3A), and the increased acetate excreted into the supernatant (Fig 3C). Supporting this assumption, it has been shown that the presence of Lqo helps S. aureus overcome NO stress in myocarditis in mice. Lqo should be present at high levels in the heart 33.
In vitro formation of biofilm
In contrast to the differences in planktonic growth observed with the ΔndhF and ΔndhC knockouts, NhdF and NdhC are each critical for biofilm formation. Eliminating either of these enzymes changes the status of the redox mediators to prevent reaching the threshold required to switch from planktonic growth to form biofilm. It has been suggested that nitrate respiration is important for biofilm formation in S. epidermidis 64 and it has been shown in S. aureus that the nitrate reductase genes are upregulated under the growth conditions that induce biofilm formation 65. It is important to note that rich media, like the one employed in the work presented here (MHB‐ca), contain nitrate 66. Of physiological importance, nitrate is present in different infection sites, such as cystic fibrosis sputum 67, 68, 69 and nasal fluid 70. Moreover, it has been recently shown that nitrate respiration is essential for S. aureus nasal colonization. If NADH is the main electron donor for nitrate respiration, this could explain why the S. aureus ndhC and ndhF genes are required for biofilm formation. If correct, the data presented could inform the development of new treatment strategies for biofilm‐related infections.
In vitro growth and metabolism
Growth in MHB‐ca in the absence of glucose is drastically reduced by deletion of ndhC but not ndhF. This reflects the greater abundance and higher specific activity of NdhC under these growth conditions. The importance of NdhC for production of α‐toxin under these growth conditions is explained similarly. Growth impairment observed with the ΔndhC strain when grown in MHB‐ca or in chemically defined medium with amino acids as sole carbon source 38 may result from the restricted capacity of cells to use the TCA cycle, due to an accumulation of NADH. This could also explain the enhanced pigmentation observed in the ndh deletion mutants. It was previously postulated that inactivation of the TCA cycle may direct acetyl‐CoA flux to the mevalonate pathway, which could result in increased pigment biosynthesis 41 and synthesis of fatty acids 71. Hence, our data indicate that NAD+ generation by the NDH‐2s under aerobic growth conditions is essential for the TCA cycle to function and for acetate and lipid catabolism. This suggests that NADH‐dependent respiration and TCA cycle function must be essential for bacterial survival in abscesses where amino acid catabolism is required 38.
Molecular mechanism
The work presented here strongly suggests that the NDH‐2‐deficient strains accumulate excessive free FA in stationary phase which, in a SaePQRS‐dependent mechanism, results in a decrease in α‐toxin production and biofilm formation (Fig 8). The direct biochemical consequence of the deficiency of NADH‐dependent respiration is the perturbation of both the NADH/NAD+ ratio and the redox status of the menaquinone pool in the membrane. The cellular response almost certainly is driven by one or both of these measures of the redox poise of the cell. The redox status of the menaquinone pool has been suggested as regulating the function of the SaePQRS TCS 35, and this regulator has been demonstrated to be essential for the cellular response to the ndh knockouts. Interestingly, the SaePQRS TCS has also been suggested to be negatively regulated by free FAs 58, 59. A critical role of the accumulation of free FA in the current work is indicated by the fact that the decreased hemolysis and inhibition of biofilm formation that are observed in the NDH‐2‐deficient strains are reversed either upon the addition of BSA to the medium or by the expression of the N. gonorrhoeae acyl‐ACP synthetase (NgAas). Each of these treatments results in lowering the pool of free FA in the cells 58.
Figure 8. Proposed link between NADH‐dependent respiration and virulence in Staphylococcus aureus .
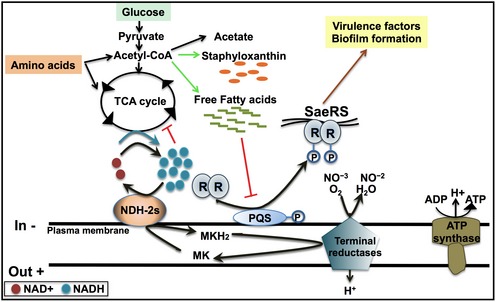
NADH‐dependent respiration (either aerobic or anaerobic/nitrate respiration) allows cells to keep and active TCA cycle and proper acetate catabolism by oxidizing NADH. Free fatty acids accumulate under NADH‐deficient respiration and repress the expression of SaeRSPQ, which regulates the production of virulence factors and biofilm formation.
The mechanism of how the ndh knockout strains result in excess accumulation of free FAs in stationary phase may be due to an inactivation of the TCA cycle by an inefficient reoxidation of NADH. However, the accumulation of free FA could also be due to a decreased rate of incorporation of fatty acids into membrane phospholipids or an increased rate of degradation of membrane phospholipids. Staphylococcus aureus differs from many bacteria in that free FAs are not recycled through the β‐oxidation 72 and that FAs can be incorporated extracellularly membrane phospholipids by pathway utilizing a fatty acid kinase 73.
The current work suggests that the redox imbalance resulting from the perturbation of the respiratory chain in S. aureus is read as a stressor and, in a manner to be determined, results in the accumulation of free FA in the cells. The perturbation of free FA homeostasis drives further consequences, as demonstrated here, through the SaePQRS TCS. The responses of pathogen metabolism to mutations (as in the current work) or to changes in environmental factors (carbon sources, oxygen, etc.) are critical determinants of virulence. Specifically, NADH‐dependent respiration is clearly of importance in S. aureus virulence and is likely to be of importance in other pathogens. The significance of fatty acid homeostasis to virulence requires further clarification in both S. aureus and more generally. Understanding the metabolic adaptation strategies of S. aureus to environmental and bioenergetics challenges can provide clues to therapeutic strategies to interfere with the ability of the pathogen to successfully adapt when it invades different niches within its host.
Materials and Methods
Ethics statement
All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Illinois Urbana‐Champaign (IACUC license number 15059) and of the University of Wisconsin‐Madison (protocol #M02501), and performed according to NIH guidelines, the Animal Welfare Act, and US Federal law.
Bacterial strains and growth conditions
The strains used in this study are described in Table 1. All strains are derivatives of the human clinical isolate, S. aureus Newman, the human clinical isolate USA300 LAC JE2 adapted for laboratory use or the osteomyelitis clinical isolated UAMS‐1 74. Bacteria were routinely grown in TSB and MHB‐ca at 37°C, 200 rpm. All strains were diluted to an OD600 = 0.05 from overnight cultures into fresh medium‐containing 96‐well flat bottom plates, and growth was measured by following optical density at 600 nm. 20 mM glucose was supplemented as carbon source when indicated. Escherichia coli was routinely cultivated in Luria Broth (LB) and on LB agar plates and also grown at 37°C. As needed for plasmid maintenance, 20 μg/ml of chloramphenicol was included in the media used. All strains were stored at −80°C in media containing 25% glycerol.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| NE1801 | USA300 LAC JE2 ndhF::erm | NARSA |
| NE1884 | USA300 LAC JE2 ndhC::erm | NARSA |
| Newman | Staphylococcus aureus wild type | 81 |
| ndhF | ΔndhF | This study |
| ndhF | ΔndhF::erm | This study |
| ndhC | ΔndhC::erm | This study |
| ndhF ndhC | ΔndhF ndhC::erm | This study |
| CYL1171 | ΔsaeRS | 82 |
| CYL12367 | ΔsaePQRS::Km | 55 |
| ndhC saeRS | ΔndhC::erm ΔsaeRS | This study |
| ndhC saePQRS | ΔndhC::erm ΔsaePQRS::Km | This study |
| ndhC srrAB | ΔndhC::erm ΔsrrAB::tet | This study |
| ndhC pCS119 | ΔndhC::erm carrying pCS119 | This study |
| ndhC pJ507 | ΔndhC::erm carrying pJ507 | This study |
| ndhF + ndhF | ΔndhF::erm carrying pOS1‐Plgt ndhF | This study |
| ndhC + ndhC | ΔndhC::erm carrying pOS1‐Plgt ndhC | This study |
| Newman pEmpty | Carrying pEmpty | This study |
| Newman PndhF | Carrying p‐PndhF | This study |
| Newman PndhC | Carrying p‐PndhC | This study |
| ndhF PndhC | ΔndhF::erm carrying p‐PndhC | This study |
| ndhC PndhF | ΔndhC::erm carrying p‐PndhF | This study |
| LAC | Staphylococcus aureus wild type | 83 |
| ndhF | ΔndhF::erm | This study |
| ndhC | ΔndhC::erm | This study |
| JMB 2047 | ΔsrrAB::tet | 36 |
| JMB 2057 | ΔndhF::tet | 36 |
| ndhC srrAB | ΔndhC::erm ΔsrrAB::tet | This study |
| saePQRS | ΔsaePQRS::Km | This study |
| ndhC saePQRS | ΔndhC::erm ΔsaePQRS::Km | This study |
| UAMS‐1 | Osteomyelitis clinical isolate | 74 |
| ndhF | ΔndhF::erm | This study |
| ndhC | ΔndhC::erm | This study |
| pOS1 Plgt | Complementation plasmid containing lgt promoter | 77 |
| pOS1‐Plgt ndhF | ndhF cloned into pOS1‐Plgt | This study |
| pOS1‐Plgt ndhC | ndhC cloned into pOS1‐Plgt | This study |
| pEmpty | pAH5 without a promoter driving YFP expression | 84 |
| p‐PndhF | pEmpty with the ndhF promoter driving YFP expression | This study |
| p‐PndhC | pEmpty with the ndhC promoter driving YFP expression | This study |
| pCS1191 | pCM28SarAP1promoter | 58 |
| pJ507 | pCM28NgAas | 58 |
Construction of staphylococcal mutants and plasmids
The Newman ΔndhF isogenic non‐polar in‐frame deletion mutant was constructed using allelic replacement, as described previously 75. The 5′ and 3′ flanking regions (~ 1 kb up‐ and downstream) of ndhF were amplified using the indicated primers (Table 2). 5′ and 3′ fragments were cloned into the pIMAY knockout vector via site‐specific recombination. The ndhF and ndhC transposon mutants were obtained from the Nebraska transposon library (NE1801 and NE1884, respectively). PCR confirmed that the transposon insertion mapped to the respective gene. Backcross strains of the transposon‐disrupted ndhC, ndhF, and srrAB alleles into WT S. aureus Newman, LAC, or UAMS‐1 were produced via phage Φ85‐mediated transduction 76. Double ndhC/ndhF knockout was constructed by in‐frame deletion of ndhF on top of the single ndhC knockout produce via phage transduction (Table 1). For complementation studies, the ndhC and ndhF genes were PCR‐amplified and cloned into the pOS1‐Plgt plasmid 77 using the primers in Table 2 and the restriction enzymes XhoI and BamHI. BamHI and XhoI were also used to clone the ndhF and ndhC genes into pOS1‐Plgt. Antibiotic selection of erythromycin cassette‐containing resistant recipient cells was achieved with 10 μg/ml erythromycin. Antibiotic selection of strains containing the pOS1‐Plgt plasmid was achieved by using 10 μg/ml of chloramphenicol. To generate the YFP reporter plasmids, the promoters for ndhC and ndhF were amplified with the primers listed in Table 2 and then cloned using standard techniques into pAH5 78. Promoters were cloned upstream yellow fluorescent protein gene in pAH5 using PstI and KpnI cut sites.
Table 2.
PCR primers used in this study
| Name | Sequence |
|---|---|
| ndhF K/O 3′ Fwd | GTATCCGCTATCACGATCATTTAAAATAC |
| ndhF K/O 3′ Rev | CCGCCTAACAAAACTAAGTTTTTCAT |
| ndhF K/O 5′ Fwd | GCACTGTAACAGGACGACTCG |
| ndhF K/O 5′ Rev | TGATCAAACATCGTTGTATCTTCAGTAGG |
| PndhF Fwd | AACTGCAGTACTTCACCTTCTTATTTCATTTGTT |
| PndhF Rev | GGGGTACCAATGAAGTACCCCTTTTATATGTTAATA |
| PndhC Fwd | AACTGCAGATAAAATAACTTTTTAATGGTTAACCCAATT |
| PndhC Rev | GGGGTACCTTAATTTCACCTAAGCTTTCATATTTTTTTA |
| ndhF Comp Fwd | CCGCTCGAGAAAAACTTAGTTTTGTTAGGCGG |
| ndhF Comp Rev | CGGGATCCTTAACCATTATGATATTTATATAACCAAAGTACG |
| ndhC Comp Fwd | CCGCTCGAGGCTCAAGATCGTAAAAAAGTACT |
| ndhC Comp Rev | CGGGATCCCTAGAATTTACCTTTTTTGAATGCTAAAC |
Oxygen consumption
Oxygen concentration was monitored using a dual‐channel respirometer system (model 782) from Strathkelvin Instruments, equipped with a temperature‐controlled 0.5‐ml electrode chamber at 37°C. Mid‐exponential (OD600 = 0.6) and stationary phase cells (24 h) were washed three times and resuspended in phosphate buffered saline (PBS) buffer to an OD600 = 0.3. The concentration of oxygen in the air‐saturated PBS buffer at this temperature was assumed to be 237.5 μM, and the reaction was initiated by injecting 20 mM glycerol.
Microtiter plate analysis of biofilm formation
Traditional static assay for biofilm formation 79 was performed with some modification. Overnight S. aureus cultures grown in MHB‐ca were diluted to an OD600 = 0.05 in new medium with 20 mM glucose, 10% human plasma (Sigma), and 2% NaCl. Control wells contained only the described media. Plates were incubated for 20 h at 37°C without shaking. Afterward, OD600 was measured for each well. Bacterial cultures were then removed by pipetting and washed three times with sterile PBS. The wells were then fixed by incubating the plate at 60°C for 1 h. The biofilm was then stained with 1% crystal violet for 10 min and later removed by washing three times with PBS. The crystal violet was eluted with 30% acetic acid for 10 min, the eluted stain was gently transferred to a new microtiter plate, and the absorbance was measured at 595 nm using a plate reader.
Hemolytic activity
Quantification of blood hemolysis was performed as described before 80 with some modifications. Staphylococcus aureus cells were grown in MHB‐ca for 20 h at 37°C, 200 rpm. Cells were harvested and separated by centrifuging at 8,000 × g for 10 min. The supernatant was collected, filter sterilized using a 0.2‐μm syringe filter, and serial dilutions were used to inoculate rabbit blood previously washed with PBS buffer. MHB‐ca medium was used as a negative control, and 1% sodium dodecyl sulfate was used as a positive control. Solutions were incubated at 37°C without shaking for 15 min. The unlysed blood cells were precipitated by centrifugation for 3 min at 14,000 × g, and the absorbance at 405 nm was measured. WT hemolysis was calculated as 100%.
Pigmentation assay
Cultures of S. aureus cells grown in MHB‐ca for 20 h were harvested and separated by centrifugation at 8,000 × g for 10 min. Pigment quantification was adapted from Lan et al 41. Briefly, cells were washed with 1 ml of PBS buffer and concentrated to an OD600 of 10, and the final pellet was then resuspended in 200 μl of methanol. The solution was heated at 55°C for 3 min and centrifuged at 8,000 × g for 10 min to remove cell debris. The supernatant was collected, and methanol washing was repeated. Finally, the final volume was adjusted to 300 μl with methanol and the absorbance at 405 nm was measured. WT pigment was calculated as 100%.
Exposure of bacteria to hydrogen peroxide and viability assays
Oxidative stress resistance of the S. aureus WT, ndhF, ndhC, and ndhF/ndhC double mutant was assessed by exposing cells to increasing concentrations of hydrogen peroxide (0–70 mM). Cells were grown in MHB‐ca for 20 h at 37°C, 200 rpm and diluted in the same medium to an OD600 of 0.005 (~ 106 CFU). Samples were taken prior to oxidative stress exposure (time zero) and after 30 min. Incubation time was stopped by the addition of 5,000 U/ml of catalase, and then, serial dilutions in MHB‐ca were performed. Dilutions were stamped onto TSB agar (TSA) plates, and after overnight incubation at 37°C CFUs were counted.
Animal infections
Six‐ to eight‐week‐old female outbred CD‐1 mice were infected via tail vein injection with 5 × 107 CFU of WT S. aureus Newman or isogenic mutant strains grown in TSB medium to an OD600 of 0.5. Organs (spleen, heart, liver, and kidney) were harvested at 4 and 8 days postinfection, and bacterial burdens were determined by plating serial dilutions and later counting CFU. Otherwise, 9‐week‐old female C57BL/6 mice were infected retro‐orbitally with approximately 1 × 107 CFU of WT S. aureus Newman or isogenic mutant strains grown in TSB medium to an OD600 of 0.4. Following injection, the infection was allowed to proceed for 96 h before the mice were euthanized with CO2. Livers, hearts, and kidneys were removed, the organs were homogenized, and bacterial burden was determined by plating serial dilutions in TSA and later counting CFU.
Measurement of acetate concentration
Bacterial cells were grown in MHB‐ca medium and cell cultures were collected (1‐ml aliquots), OD600 recorded, and then cell cultures were centrifuged at 14,000 × g for 5 min. The supernatants were removed and stored at −20°C until use. Acetic acid concentrations were determined according to the instructions of the manufacturer using kit purchased from R‐Biopharm.
Determination of the NADH/NAD+ ratio
Cells grown under aerobic or biofilm conditions were collected at 20 h of growth by centrifugation at 14,000 × g for 10 min at 4°C. Pellets were washed twice with PBS and resuspended in 400 μl of extraction buffer at an OD600 of ~ 30. Samples were homogenized twice in a FastPrep‐24 Beadbeater at 6 m/s for 45‐s cycles with 2 min of incubation on ice in between. Homogenized samples were centrifuged at 4°C in a microcentrifuge at 14,000 × g for 10 min. The supernatants were filtered through 10 kDa spin columns, and intracellular levels of NADH and NAD+ were measured using the NAD/NADH quantitation kit (MAK037; Sigma‐Aldrich, St. Louis, MO) according to the manufacturer's instructions. NADH and NAD levels were normalized to milligrams of protein.
Determination of the menaquinol/menaquinone ratio
Cells grown under aerobic or under biofilm conditions were collected at 20 h of growth by centrifugation at 14,000 × g for 10 min at 4°C. Pellets were resuspended with 1:2 isopropanol:n‐hexane and vortex for 2 min. One‐third of water was added to the suspension and vortex for another minute. The mixture was then centrifuged at 7,000 × g for 2 min. Upper phase was evaporated with nitrogen gas, and the residue was dissolved immediately with ethanol. Sodium acetate buffer pH 5.4 was added to the menaquinone ethanol solution to a final concentration of 0.5 mM to avoid rapid oxidation of the reduced menaquinone in solution. Reduced menaquinone was determined by recording the absorption difference at 247–262 nm and total menaquinone by the addition of KBH4 powder. Oxidized menaquinone was calculated by subtracting the reduced menaquinone from the total amounts of menaquinone.
Statistical analyses
Statistical tests were performed using GraphPad Prism version 7.0 software. All statistical tests are listed in the figure legends, and significance was determined if P < 0.05.
Author contributions
LAS‐B and RBG conceived and designed the experiments. LAS‐B performed the experiments and analyzed the data. PKPS, JNR, and GYC performed the animal assays. PKPS and AML performed the gene expression assays. LAS‐B, RBG, J‐DS, and TEK‐F analyzed the data. LAS‐B and RBG supervised the project. LAS‐B and RBG wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank members of the Gennis laboratory for their help and useful discussions. We thank Dr. C. Rock for sharing his valuable insights and helpful discussions, and providing the plasmids pCS1191 and pJ507. We also thank Drs. J. Boyd for kindly providing the strains JMB2047 and JMB2057, C. Lee the strains CYL1171 and CYL1171, and A. Richardson the wild‐type strains LAC and UAMS‐1. This work was supported by operating grants for the National Institutes of Health to RBG (R01 GM095600 and R01 HL16101), TEKF (K22 AI104805 and R01 AI118880), and JDS (R01 CA188034), a Basil O'Connor Starter Scholar Award from March of Dimes to TEKF. Strains NE1801 and NE1884 were obtained through Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources, NIAID, NIH: Nebraska Transposon Mutant Library (NTML) Screening Array.
EMBO Reports (2020) 21: e45832
References
- 1. Fischbach MA, Walsh CT (2009) Antibiotics for emerging pathogens. Science 325: 1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuehnert MJ, Kruszon‐Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK et al (2006) Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis 193: 172–179 [DOI] [PubMed] [Google Scholar]
- 3. Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB (2005) Methicillin‐resistant‐Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis 11: 868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM et al (2007) Invasive methicillin‐resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771 [DOI] [PubMed] [Google Scholar]
- 5. Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, Indriati Hood M, Skaar EP (2013) Two heme‐dependent terminal oxidases power Staphylococcus aureus organ‐specific colonization of the vertebrate host. MBio 4: e00241‐13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Proctor RA, Kriegeskorte A, Kahl BC, Becker K, Loffler B, Peters G (2014) Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections. Front Cell Infect Microbiol 4: 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD, Westbrock‐Wadman S, Hufnagle WO, Folger KR, Bayer AS, Stover CK (1998) Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol 30: 393–404 [DOI] [PubMed] [Google Scholar]
- 8. Carreau A, El Hafny‐Rahbi B, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15: 1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Movahed MR, Hashemzadeh M, Jamal MM (2007) Increased prevalence of infectious endocarditis in patients with type II diabetes mellitus. J Diabetes Complications 21: 403–406 [DOI] [PubMed] [Google Scholar]
- 10. Richardson AR, Dunman PM, Fang FC (2006) The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 61: 927–939 [DOI] [PubMed] [Google Scholar]
- 11. Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR et al (2009) The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus . J Infect Dis 199: 1698–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon AS, Park GC, Ryu SY, Lim DH, Lim DY, Choi CH, Park Y, Lim Y (2008) Higher biofilm formation in multidrug‐resistant clinical isolates of Staphylococcus aureus . Int J Antimicrob Agents 32: 68–72 [DOI] [PubMed] [Google Scholar]
- 13. Chambers HF, Korzeniowski OM, Sande MA (1983) Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 62: 170–177 [PubMed] [Google Scholar]
- 14. Priest DH, Peacock JE Jr (2005) Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J 98: 854–862 [DOI] [PubMed] [Google Scholar]
- 15. Siddiqui AR, Bernstein JM (2010) Chronic wound infection: facts and controversies. Clin Dermatol 28: 519–526 [DOI] [PubMed] [Google Scholar]
- 16. Rajan S, Saiman L (2002) Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect 17: 47–56 [DOI] [PubMed] [Google Scholar]
- 17. Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6: 199–210 [DOI] [PubMed] [Google Scholar]
- 18. Wakeman CA, Hammer ND, Stauff DL, Attia AS, Anzaldi LL, Dikalov SI, Calcutt MW, Skaar EP (2012) Menaquinone biosynthesis potentiates haem toxicity in Staphylococcus aureus . Mol Microbiol 86: 1376–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammer ND, Schurig‐Briccio LA, Gerdes SY, Gennis RB, Skaar EP (2016) CtaM is required for menaquinol oxidase aa3 function in Staphylococcus aureus . MBio 7: e00823‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schurig‐Briccio LA, Yano T, Rubin H, Gennis RB (2014) Characterization of the type 2 NADH:menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines. Biochem Biophys Acta 1837: 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaupp R, Schlag S, Liebeke M, Lalk M, Gotz F (2010) Advantage of upregulation of succinate dehydrogenase in Staphylococcus aureus biofilms. J Bacteriol 192: 2385–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayer S, Steffen W, Steuber J, Gotz F (2015) The Staphylococcus aureus NuoL‐like protein MpsA contributes to the generation of membrane potential. J Bacteriol 197: 794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuchs S, Pane‐Farre J, Kohler C, Hecker M, Engelmann S (2007) Anaerobic gene expression in Staphylococcus aureus . J Bacteriol 189: 4275–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinkel TL, Ramos‐Montanez S, Pando JM, Tadeo DV, Strom EN, Libby SJ, Fang FC (2016) An essential role for bacterial nitric oxide synthase in Staphylococcus aureus electron transfer and colonization. Nat Microbiol 2: 16224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke KA, Lascelles J (1975) Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol 123: 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kerscher S, Drose S, Zickermann V, Brandt U (2008) The three families of respiratory NADH dehydrogenases. Results Probl Cell Differ 45: 185–222 [DOI] [PubMed] [Google Scholar]
- 27. Rao SP, Alonso S, Rand L, Dick T, Pethe K (2008) The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis . Proc Natl Acad Sci USA 105: 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML (2005) Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci USA 102: 15629–15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lencina AM, Franza T, Sullivan MJ, Ulett GC, Ipe DS, Gaudu P, Gennis RB, Schurig‐Briccio LA (2018) Type 2 NADH dehydrogenase is the only point of entry for electrons into the Streptococcus agalactiae respiratory chain and is a potential drug target. MBio 9: e01034‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vilcheze C, Weinrick B, Leung LW, Jacobs WR Jr (2018) Plasticity of Mycobacterium tuberculosis NADH dehydrogenases and their role in virulence. Proc Natl Acad Sci USA 115: 1599–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richardson AR, Somerville GA, Sonenshein AL (2015) Regulating the intersection of metabolism and pathogenesis in gram‐positive bacteria. Microbiol Spectr 3: 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci . Microbiol Mol Biol Rev 73: 233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spahich NA, Vitko NP, Thurlow LR, Temple B, Richardson AR (2016) Staphylococcus aureus lactate‐ and malate‐quinone oxidoreductases contribute to nitric oxide resistance and virulence. Mol Microbiol 100: 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang X, Bayles KW, Luca S (2017) Staphylococcus aureus CidC is a pyruvate: menaquinone oxidoreductase. Biochemistry 56: 4819–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mashruwala AA, Gries CM, Scherr TD, Kielian T, Boyd JM (2017) SaeRS is responsive to cellular respiratory status and regulates fermentative biofilm formation in Staphylococcus aureus . Infect Immun 85: e00157‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mashruwala AA, Guchte AV, Boyd JM (2017) Impaired respiration elicits SrrAB‐dependent programmed cell lysis and biofilm formation in Staphylococcus aureus . Elife 6: e23845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E (2015) An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device‐related infections. J Infect Dis 212: 1883–1893 [DOI] [PubMed] [Google Scholar]
- 38. Halsey CR, Lei S, Wax JK, Lehman MK, Nuxoll AS, Steinke L, Sadykov M, Powers R, Fey PD (2017) Amino acid catabolism in Staphylococcus aureus and the function of carbon catabolite repression. MBio 8: e01434‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baumert N, von Eiff C, Schaaff F, Peters G, Proctor RA, Sahl HG (2002) Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb Drug Resist 8: 253–260 [DOI] [PubMed] [Google Scholar]
- 40. Kriegeskorte A, Konig S, Sander G, Pirkl A, Mahabir E, Proctor RA, von Eiff C, Peters G, Becker K (2011) Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 11: 2476–2490 [DOI] [PubMed] [Google Scholar]
- 41. Lan L, Cheng A, Dunman PM, Missiakas D, He C (2010) Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus . J Bacteriol 192: 3068–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F (2006) Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Gotz F (2005) Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus . J Biol Chem 280: 32493–32498 [DOI] [PubMed] [Google Scholar]
- 44. Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Resch A, Rosenstein R, Nerz C, Gotz F (2005) Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microbiol 71: 2663–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berube BJ, Bubeck Wardenburg J (2013) Staphylococcus aureus alpha‐toxin: nearly a century of intrigue. Toxins (Basel) 5: 1140–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen TS, Hilliard JJ, Jones‐Nelson O, Keller AE, O'Day T, Tkaczyk C, DiGiandomenico A, Hamilton M, Pelletier M, Wang Q et al (2016) Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med 8: 329ra331 [DOI] [PubMed] [Google Scholar]
- 48. Glenny AT, Stevens MF (1935) Staphylococcus toxins and antitoxins. J Pathol Bacteriol 40: 201–210 [Google Scholar]
- 49. Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger‐Bachi B, Bischoff M (2006) Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob Agents Chemother 50: 1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harper L, Balasubramanian D, Ohneck EA, Sause WE, Chapman J, Mejia‐Sosa B, Lhakhang T, Heguy A, Tsirigos A, Ueberheide B et al (2018) Staphylococcus aureus responds to the central metabolite pyruvate to regulate virulence. MBio 9: e02272‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM (2004) Characterization of virulence factor regulation by SrrAB, a two‐component system in Staphylococcus aureus . J Bacteriol 186: 2430–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ulrich M, Bastian M, Cramton SE, Ziegler K, Pragman AA, Bragonzi A, Memmi G, Wolz C, Schlievert PM, Cheung A et al (2007) The staphylococcal respiratory response regulator SrrAB induces ICA gene transcription and polysaccharide intercellular adhesin expression, protecting Staphylococcus aureus from neutrophil killing under anaerobic growth conditions. Mol Microbiol 65: 1276–1287 [DOI] [PubMed] [Google Scholar]
- 53. Liu Q, Yeo WS, Bae T (2016) The SaeRS two‐component system of Staphylococcus aureus . Genes 7: 81 [Google Scholar]
- 54. Pragman AA, Ji Y, Schlievert PM (2007) Repression of Staphylococcus aureus SrrAB using inducible antisense srrA alters growth and virulence factor transcript levels. Biochemistry 46: 314–321 [DOI] [PubMed] [Google Scholar]
- 55. Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C (2010) Differential target gene activation by the Staphylococcus aureus two‐component system saeRS. J Bacteriol 192: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Georgellis D, Kwon O, Lin EC (2001) Quinones as the redox signal for the arc two‐component system of bacteria. Science 292: 2314–2316 [DOI] [PubMed] [Google Scholar]
- 57. Malpica R, Franco B, Rodriguez C, Kwon O, Georgellis D (2004) Identification of a quinone‐sensitive redox switch in the ArcB sensor kinase. Proc Natl Acad Sci USA 101: 13318–13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ericson ME, Subramanian C, Frank MW, Rock CO (2017) Role of fatty acid kinase in cellular lipid homeostasis and SaeRS‐dependent virulence factor expression in Staphylococcus aureus .MBio 8: e00988‐17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krute CN, Rice KC, Bose JL (2017) VfrB is a key activator of the Staphylococcus aureus SaeRS two‐component system. J Bacteriol 199: e00828‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schafer D, Lam TT, Geiger T, Mainiero M, Engelmann S, Hussain M, Bosserhoff A, Frosch M, Bischoff M, Wolz C et al (2009) A point mutation in the sensor histidine kinase SaeS of Staphylococcus aureus strain Newman alters the response to biocide exposure. J Bacteriol 191: 7306–7314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chatterjee I, Schmitt S, Batzilla CF, Engelmann S, Keller A, Ring MW, Kautenburger R, Ziebuhr W, Hecker M, Preissner KT et al (2009) Staphylococcus aureus ClpC ATPase is a late growth phase effector of metabolism and persistence. Proteomics 9: 1152–1176 [DOI] [PubMed] [Google Scholar]
- 62. Zhu Y, Xiong YQ, Sadykov MR, Fey PD, Lei MG, Lee CY, Bayer AS, Somerville GA (2009) Tricarboxylic acid cycle‐dependent attenuation of Staphylococcus aureus in vivo virulence by selective inhibition of amino acid transport. Infect Immun 77: 4256–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fuller JR, Vitko NP, Perkowski EF, Scott E, Khatri D, Spontak JS, Thurlow LR, Richardson AR (2011) Identification of a lactate‐quinone oxidoreductase in Staphylococcus aureus that is essential for virulence. Front Cell Infect Microbiol 1: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Uribe‐Alvarez C, Chiquete‐Felix N, Contreras‐Zentella M, Guerrero‐Castillo S, Pena A, Uribe‐Carvajal S (2016) Staphylococcus epidermidis: metabolic adaptation and biofilm formation in response to different oxygen concentrations. Pathog Dis 74: ftv111 [DOI] [PubMed] [Google Scholar]
- 65. Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS (2004) Global gene expression in Staphylococcus aureus biofilms. J Bacteriol 186: 4665–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu J, Xu X, Verstraete W (2000) Adaptation of E. coli cell method for micro‐scale nitrate measurement with the Griess reaction in culture media. J Microbiol Methods 41: 23–33 [DOI] [PubMed] [Google Scholar]
- 67. Grasemann H, Ioannidis I, Tomkiewicz RP, de Groot H, Rubin BK, Ratjen F (1998) Nitric oxide metabolites in cystic fibrosis lung disease. Arch Dis Child 78: 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palmer KL, Brown SA, Whiteley M (2007) Membrane‐bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol 189: 4449–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kolpen M, Kragh KN, Bjarnsholt T, Line L, Hansen CR, Dalboge CS, Hansen N, Kuhl M, Hoiby N, Jensen PO (2015) Denitrification by cystic fibrosis pathogens – Stenotrophomonas maltophilia is dormant in sputum. Int J Med Microbiol 305: 1–10 [DOI] [PubMed] [Google Scholar]
- 70. Sato M, Fukuyama N, Sakai M, Nakazawa H (1998) Increased nitric oxide in nasal lavage fluid and nitrotyrosine formation in nasal mucosa–indices for severe perennial nasal allergy. Clin Exp Allergy 28: 597–605 [DOI] [PubMed] [Google Scholar]
- 71. Parsons JB, Rock CO (2013) Bacterial lipids: metabolism and membrane homeostasis. Prog Lipid Res 52: 249–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parsons JB, Frank MW, Subramanian C, Saenkham P, Rock CO (2011) Metabolic basis for the differential susceptibility of Gram‐positive pathogens to fatty acid synthesis inhibitors. Proc Natl Acad Sci USA 108: 15378–15383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parsons JB, Frank MW, Jackson P, Subramanian C, Rock CO (2014) Incorporation of extracellular fatty acids by a fatty acid kinase‐dependent pathway in Staphylococcus aureus . Mol Microbiol 92: 234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS (1995) Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63: 3373–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sadykov MR, Mattes TA, Luong TT, Zhu Y, Day SR, Sifri CD, Lee CY, Somerville GA (2010) Tricarboxylic acid cycle‐dependent synthesis of Staphylococcus aureus type 5 and 8 capsular polysaccharides. J Bacteriol 192: 1459–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Novick RP (1991) Genetic systems in staphylococci. Methods Enzymol 204: 587–636 [DOI] [PubMed] [Google Scholar]
- 77. Bubeck Wardenburg J, Williams WA, Missiakas D (2006) Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci USA 103: 13831–13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR (2009) Fluorescent reporters for Staphylococcus aureus . J Microbiol Methods 77: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp 47: e2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blevins JS, Beenken KE, Elasri MO, Hurlburt BK, Smeltzer MS (2002) Strain‐dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus . Infect Immun 70: 470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Duthie ES, Lorenz LL (1952) Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6: 95–107 [DOI] [PubMed] [Google Scholar]
- 82. Luong TT, Sau K, Roux C, Sau S, Dunman PM, Lee CY (2011) Staphylococcus aureus ClpC divergently regulates capsule via sae and codY in strain newman but activates capsule via codY in strain UAMS‐1 and in strain Newman with repaired saeS. J Bacteriol 193: 686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vitko NP, Grosser MR, Khatri D, Lance TR, Richardson AR (2016) Expanded glucose import capability affords Staphylococcus aureus optimized glycolytic flux during infection. MBio 7: e00296‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garcia YM, Barwinska‐Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl‐Fie TE (2017) A superoxide dismutase capable of functioning with iron or manganese promotes the resistance of Staphylococcus aureus to calprotectin and nutritional immunity. PLoS Pathog 13: e1006125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


