Abstract
Background
In the context of global malaria elimination efforts, special attention is being paid to submicroscopic Plasmodium falciparum infections. In pregnant, sub-Saharan African women, such infections are more prevalent than microscopic infections, and are thought to have adverse effects on both mothers’ and newborns’ health. However, no study has studied the dynamics and determinants of these infections throughout pregnancy. Retard de Croissance Intra-uterin et Paludisme (RECIPAL), a preconception cohort study carried out in Benin between 2014 and 2017, represented a unique opportunity to assess this issue.
Methods
We used data from 273 pregnant Beninese women who were followed-up from preconception to delivery. We studied the dynamics of and factors influencing submicroscopic (and microscopic) P. falciparum infections during the 3 trimesters of pregnancy, using an ordinal logistic mixed model.
Results
The incidence rate of submicroscopic P. falciparum infections during pregnancy was 12.7 per 100 person-months (95% confidence interval [CI] 10.8–14.9), compared to 6.7 per 100 person-months (95% CI 5.5–8.1) for microscopic infections. The prevalences were highest in the first trimester for both submicroscopic and microscopic infections. After adjustment for potential confounding factors, we found that those of young age and those with a submicroscopic P. falciparum infection prior to pregnancy were at significantly higher risks of submicroscopic and microscopic infections throughout pregnancy, with a more pronounced effect in the first trimester of pregnancy.
Conclusions
The first trimester of pregnancy is a particularly high-risk period for P. falciparum infection during pregnancy, especially for the youngest women. Malaria prevention tools covering the preconception period and early pregnancy are urgently needed to better protect pregnant women and their newborns.
Keywords: dynamic, submicroscopic P. falciparum infections, pregnancy, preconception cohort, sub-Saharan Africa
In a preconception malaria cohort study, the prevalence of submicroscopic versus microscopic Plasmodium falciparum infections was higher throughout pregnancy, most markedly in the first trimester, and young women with submicroscopic infections before pregnancy had a higher subsequent risk of infection.
(See the Editorial Commentary by Alkan on pages 175–6.)
Approximately 30 million pregnant women are exposed to malaria every year [1, 2] in sub-Saharan Africa. Many studies have highlighted the impact of malaria in pregnancy (MiP), with adverse consequences such as maternal anemia, prematurity, and low birth weight [3–6] associated with a high risk of maternal and infant mortality [7]. To protect women against MiP, the World Health Organization recommends different strategies, such as intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine and insecticide-treated nets, which have led to substantial improvements in birth outcomes [8–10] and reductions of malaria-related mortality and morbidity rates [11]. However, these policies remain suboptimal, in particular because the first trimester of pregnancy currently remains unprotected for different reasons. Firstly, IPTp with sulfadoxine-pyrimethamine is contraindicated in the first trimester due to possible teratogenic effects [12]. Secondly, in sub-Saharan Africa, pregnant women habitually attend their first antenatal consultation only in the second trimester [13, 14]. Therefore, little is known about the actual infection risk during the first trimester, which is likely an under-protected period [15, 16]. This is critical, since malaria in early pregnancy has been shown to be associated with deleterious pregnancy outcomes [17–19].
Moreover, in the last decade, several studies have revealed a high prevalence of carriage of submicroscopic infections, detected by polymerase chain reaction (PCR)-based molecular methods that are more sensitive than the standard malaria detection tools, and shedding new light on the real prevalence of malaria infections [20], especially in pregnant women [21]. In addition, such submicroscopic infections—especially those occurring early in pregnancy [21] are suspected to adversely affect womens’ and newborns’ health [21–23], but are nevertheless usually asymptomatic and, thus, remain untreated during pregnancy.
The Retard de Croissance Intra-uterin et Paludisme (RECIPAL) study, a cohort study of malaria in pregnant women followed-up from preconception to delivery in Benin, was a unique opportunity to assess the dynamics and determinants of submicroscopic (and microscopic) infections throughout pregnancy, particularly in the first trimester.
METHODS
Study Design
RECIPAL is a preconception cohort study that assessed the effects of malaria infection (microscopic and submicroscopic) during pregnancy on the mother and the fetus. The study design has been described elsewhere [15]. Briefly, it was conducted in southern Benin from June 2014 to August 2017. A total of 1214 women of reproductive age (WRA) were recruited (primary cohort). The subsample of women who became pregnant was then followed-up monthly in the study’s health facilities throughout pregnancy, until delivery (secondary cohort). During the study, infections with Plasmodium falciparum were detected by both thick blood smears (TBSs) and PCRs. Only infected women (detected by TBS and rapid diagnostic test [RDT], when applicable) were treated. PCRs were performed later. The project provided all treatments given for any infection occurring during pregnancy [24].
Preconception Follow-up: Primary Cohort
The study was introduced to the local authorities and various approaches were used to recruit WRA, including repeated awareness sessions, participation of the community’s leaders, and door-to-door recruitment. Another study (Clinical development of a VAR2CSA-based Placental Malaria Vaccine [PlacMalVac]), which concerned the development of a Variant surface antigen 2 chondroitin sulfate A-based vaccine against placental malaria, was implemented in the same area and included exclusively primigravidae [25]. The women enrolled in the vaccine-related study were no longer eligible for RECIPAL.
At enrollment, demographics, socioeconomic characteristics, and reproductive histories were collected and all women were screened for malaria. Women were visited at home monthly to record the first day of the last menstrual period and a urinary pregnancy test was performed on all women who did not have a until pregnancy was confirmed with a maximum of 24 months.
Gestational Follow-up: Secondary Cohort
Once the pregnancy was confirmed, the women were followed-up monthly until delivery. Clinical, obstetrical, and anthropometric data, as well as malaria infection screening data, were collected. A gestational age (GA) estimation was based either on the last menstrual period or first ultrasound scan [26]. A TBS and an RDT were performed in the case of a fever or malaria-like symptoms. According to recommendations in Benin (at least 2 doses during pregnancy), IPTp was given from the second trimester.
Laboratory Procedures
P. falciparum was (1) quantified by the Lambaréné technique, with a detection threshold estimated at 5 parasites/µL [24, 27]; and (2) tested by real-time quantitative PCR that targeted the 18S ribosomal ribonucleic acid gene [28, 29]. A negative control with no DNA template was run in all reactions. The RDT used was the Pf + pan rapid test (SD Bioline Ag, IDA Foundation; BioSynex) [15].
Ethics Statement
The Ethics Committee of the Institut des Sciences Biomédicales Appliquées approved this study, as did the Ministry of Health in Benin. Before any enrollment, the study was explained in the local language to the woman, and her freely given consent was obtained.
Statistical Analysis
Our main objective was to study the dynamics and the determinants of submicroscopic (as well as microscopic) P. falciparum infections during pregnancy, with a particular focus on the first trimester.
Our analysis sample was the 273 women followed-up from preconception until delivery, allowing for the definition of their P. falciparum infection status during the 3 trimesters of pregnancy.
Our dependent variable was a time-dependent, ordinal variable with 3 classes (negative, submicroscopic, and microscopic infection status), summarizing the P. falciparum infection status of the woman in each trimester. This variable was built in 2 steps. First, at each visit, the P. falciparum infection was defined as negative if all tests (TBS, PCR, and RDT, when applicable) were negative; as a submicroscopic infection if the TBS (and RDT, when applicable) was negative but the PCR was positive; and as a microscopic infection if the TBS or RDT (when applicable) was positive whatever the PCR was positive or negative.
Second, in each trimester, the P. falciparum infection status was defined as the sum of P. falciparum infections during the visits of the trimester. Thus, for a given trimester, the P. falciparum infection status of a women was either:
Negative (no P. falciparum infection), if the woman was diagnosed as negative at all visits during the trimester;
A submicroscopic P. falciparum infection, if the woman was diagnosed as having a submicroscopic infection during at least 1 visit and was not diagnosed as having a microscopic infection at any visit during the trimester; or
A microscopic P. falciparum infection, if the woman was diagnosed as having a microscopic infection during at least 1 visit during the trimester.
An example is shown (Figure 1) of a hypothetical pregnant women who would have attended 8 visits during the 3 trimesters.
Figure 1.
Construction of the Plasmodium falciparum infection status per trimester variable, in a hypothetical pregnant woman who would have attended 2 visits in the first trimester, 3 visits in the second trimester, and 3 visits in the third trimester. If at the 2 visits in the first trimester the woman was diagnosed as negative, in the second trimester she was diagnosed as negative/submicroscopic/negative, and at least at 1 of the 3 visits in the third trimester she was diagnosed as microscopic, she was therefore classified, respectively, as having a negative infection status, submicroscopic infection status, and microscopic infection status for the first, second and third trimesters. Abbreviations: Micro, microscopic P. falciparum infection at the visit; Neg, no P. falciparum infection (negative) at the visit; Sub, submicroscopic P. falciparum infection at the visit.
As explanatory variables, we considered sociodemographic characteristics, including maternal age (2 classes, according to the median of our sample: 26 years old), marital status, residence area, ethnicity, and education level. We also considered clinical characteristics, including the presence of a P. falciparum infection before pregnancy (ie, at inclusion in the primary cohort; negative, submicroscopic, microscopic); gravidity; number of IPTp doses during pregnancy until the current trimester (time-dependent variable varying according to the trimester); trimesters of pregnancy (≤14 weeks of gestation [wg], 15–27 wg, and ≥28 wg for the first, second, and third trimesters, respectively); P. falciparum infection status from the previous trimester (set to negative for the first trimester); and season at delivery.
Statistical Model
Since our dependent variable was a repeated (at the 3 trimesters) ordinal variable, we performed a classic, ordinal, logistic, mixed model to assess the determinants of P. falciparum infection status’ dynamics according to the trimesters of pregnancy. The hypothesis of the parallel lines was tested and was not violated for any covariate. The variables for which the P values were less than 0.20 in a univariate analysis were introduced in the multivariate model.
A preliminary analysis showed a possible interaction between having a P. falciparum infection before pregnancy and maternal age, which depended on the trimester of pregnancy. For this reason, we introduced in our model a second-order interaction term between P. falciparum infections before pregnancy, maternal age, and trimester.
We performed a step-by-step backward selection to eliminate the nonsignificant, independent variables introduced in the initial multivariate model. The variables for which the P values were less than 0.05 were retained in the final multivariate model.
In an ordinal logistic mixed model, the estimated odds ratios are cumulative, and then do not allow for a comparison of the risk of a submicroscopic (or microscopic) infection status versus a negative status according to the covariates. Therefore, we derived from the model the predicted probabilities (risk) of a P. falciparum infection status according to the submicroscopic infection status before pregnancy and maternal age.
Stata version 13 for Windows (Stata Corp., College Station, TX) was used for all statistical analyses.
RESULTS
During follow-up (Figure 2), 1214 WRA were included. Among them, 411 women (33.8%) became pregnant and 273 (66.4%) delivered with a complete follow-up. We compared the 138 lost to follow-up and the 273 pregnant women included in our analysis according to maternal age, gestational rank, and P. falciparum infection status before pregnancy, and found no significant difference (Supplementary Table 1). Out of the 138 pregnant women lost to follow-up, the main causes were miscarriages (52.17%) and withdrawals of consent (33.33%).
Figure 2.
Flow chart of Retard de Croissance Intra-uterin et Paludisme (RECIPAL) study, June 2014–August 2017, Benin. Abbreviation: WRA, women of reproductive age.
Pregnant Womens’ Characteristics and Malaria Infection During the Follow-up
Table 1 presents the general characteristics of the pregnant women. More than three-quarters of the women were under 30 years of age, and 8.8% of them were primigravidae. The first antenatal consultation occurred, on average, at 7.1 wg and the mean GA at delivery was 39.2 wg. The majority of pregnant women (74.3%) belonged to the Toffin ethnic group. Almost 1 in 4 women (24.9%) were carrying submicroscopic P. falciparum infections before pregnancy. All but 10 women received at least 1 dose of IPTp during the follow-up. Out of the 263 pregnant women who received IPTp, the average GA at the first intake of IPTp was at 23 wg ± 5 wg.
Table 1.
General Characteristics of the Pregnant Women Followed-up Until Delivery in the Retard de Croissance Intra-uterin et Paludisme (RECIPAL) Study, N = 273, Benin, 2014–2017
| Characteristics | Total | Mean ± SD or proportion (95% CI) |
|---|---|---|
| Age, years | 273 | 26.8 ± 4.9 |
| <23 | 55 | 20.1 (15.8–25.4) |
| 23–30 | 165 | 60.4 (54.5–66.1) |
| >30 | 53 | 19.4 (15.1–24.6) |
| Gestational age at the first ANC, wg | 273 | 7.1 ± 2.5 |
| ITN possession | 267 | 97.8 (95.2–99.0) |
| Gravidity | 273 | … |
| Primigravida | 24 | 8.8 (5.9–12.8) |
| Secondigravida | 40 | 14.6 (10.9–19.4) |
| Multigravida | 209 | 76.6 (71.1–81.2) |
| Ethnic group, n | 273 | |
| Toffin | 203 | 74.35 (68.6–77.2) |
| Fon | 21 | 7.7 (5.0–11.5) |
| Aîzo | 39 | 14.28 (12.0–16.5) |
| Others | 10 | 3.7 (1.5–5.8) |
| Education level, n | 273 | |
| Illiterate | 195 | 71.4 (65.7–76.5) |
| Literate | 78 | 28.6 (23.5–34.2) |
| Professional status, n | 273 | |
| Active | 261 | 95.6 (92.4–97.5) |
| Not active | 8 | 2.9 (1.5–5.8) |
| In training | 4 | 1.5 (.5–3.9) |
| Marital status, n | 273 | |
| Cohabitation | 18 | 6.6 (4.2–10.2) |
| Married | 255 | 93.4 (89.7–95.8) |
| P. falciparum infection before pregnancy, n | 273 | |
| Negative | 188 | 68.9 (63.1–74.1) |
| Submicroscopic | 68 | 24.9 (20.1–30.4) |
| Microscopic | 17 | 6.2 (3.9–9.8) |
| Gestational age at delivery, wg | 273 | 39.2 ± 2.2 |
| Number of ANC visits (scheduled and unscheduleded) during pregnancy | 273 | 8.3 ± 1.3 |
| Number of IPTp doses | 273 | |
| 0 | 10 | 3.7 (2.0–6.7) |
| 1 | 54 | 19.8 (15.4–25.0) |
| 2 | 171 | 62.6 (56.7–68.2) |
| 3 | 38 | 13.9 (10.3-18.6) |
Abbreviations: ANC, antenatal consultation; CI, confidence interval; IPTp, intermittent preventive treatment in pregnancy; ITN, insecticide-treated nets; P. falciparum, Plasmodium falciparum; SD, standard deviation; wg, weeks of gestation.
Figure 3 shows the evolution of submicroscopic and microscopic P. falciparum infections from preconception to delivery in the study. At each visit, the proportion of submicroscopic P. falciparum infections was higher than the proportion of microscopic P. falciparum infections. Both submicroscopic and microscopic P. falciparum infections were at their highest level during the first trimester. The overall incidence rates of submicroscopic and microscopic P. falciparum infections during pregnancy were 12.7 (95% confidence interval [CI] 10.8–14.9) and 6.7 (95% CI 5.5–8.1) per 100 persons-months, respectively.
Figure 3.
Dynamics of Plasmodium falciparum infections before and during pregnancy, Retard de Croissance Intra-uterin et Paludisme (RECIPAL) 2014–2017, Benin.
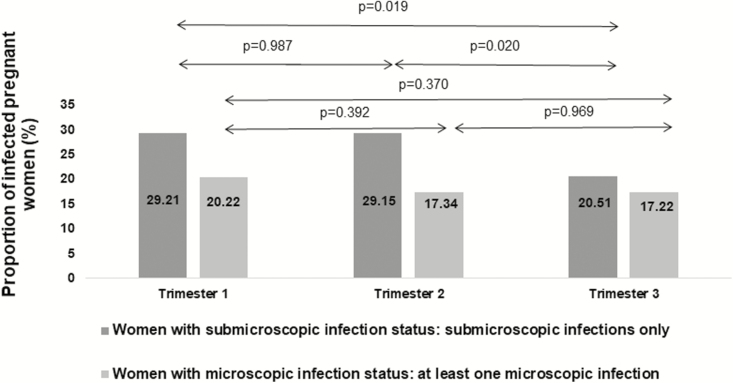
Figure 4 shows the proportions of women with microscopic and submicroscopic infections at each trimester. The proportion with submicroscopic infections was always higher than the proportion with microscopic infections (29.2% vs 20.2% for the first trimester, respectively; 29.1% vs 17.3% for the second trimester, respectively; and 20.5% vs 17.2% for the third trimester, respectively).
Figure 4.
Proportion of women with submicroscopic and microscopic infection status in each trimester of pregnancy, Retard de Croissance Intra-uterin et Paludisme (RECIPAL) 2014–2017, Benin. P values are from Chi-square tests.
Factors Contributing to the Plasmodium falciparum Infection Dynamics
In the final multivariate model, the statistically significant factors contributing to P. falciparum infection status (submicroscopic and microscopic) dynamics were ethnicity (P = .04), cumulative number of IPTp doses (P = .01), and submicroscopic P. falciparum infection status for the previous trimester (P < .001). In addition, the second-order interaction term between maternal age, P. falciparum infection status before pregnancy, and trimester was also significant (P < .001).
The predicted probabilities of having a P. falciparum infection according to maternal age and having a P. falciparum infection before pregnancy were performed (only the results regarding the submicroscopic infection class are shown, in Figures 5 and 6). Figures 5 and 6 show, respectively, the predicted probabilities of having submicroscopic and microscopic infections for each trimester. Younger women with a submicroscopic P. falciparum infection before conception had the highest probability of harboring a submicroscopic or microscopic P. falciparum infection throughout pregnancy. More specifically, this interaction between age and infection status before pregnancy was significant at each trimester. The highest risk was found in young women with a submicroscopic infection before pregnancy at each trimester, and this risk was higher in the first and second trimesters, compared to the third trimester.
Figure 5.
Predicted probabilities from the ordinal logistic mixed model of the occurrences of submicroscopic Plasmodium falciparum infections per trimester, according to maternal age and presence of submicroscopic P. falciparum infection before conception (Retard de Croissance Intra-uterin et Paludisme [RECIPAL] 2014–2017, Benin). P values correspond to the t-test comparisons. *Indicates a t-test comparison between the predicted probabilities of having a submicroscopic P. falciparum infection at the first trimester of pregnancy for the youngest women (P value = .1059). **Indicates a t-test comparison between the predicted probabilities of having a submicroscopic P. falciparum infection at the second trimester of pregnancy for the youngest women (P value = .5212). ***Indicates a t-test comparison between the predicted probabilities of having a submicroscopic P. falciparum infection at the third trimester of pregnancy for the youngest women (P value = .3554).
Figure 6.
Predicted probabilities from the ordinal logistic mixed model of the occurrence of microscopic Plasmodium falciparum infections per trimester, according to maternal age and presence of submicroscopic P. falciparum infection before conception (Retard de Croissance Intra-uterin et Paludisme [RECIPAL] 2014–2017, Benin). P values correspond to the t-test comparisons. *Indicates a t-test comparison between the predicted probabilities of having a microscopic P. falciparum infection at the first trimester of pregnancy for the youngest women (P value < .0001). **Indicates a t-test comparison between the predicted probabilities of having a microscopic P. falciparum infection at the second trimester of pregnancy for the youngest women (P value = .0810). ***Indicates a t-test comparison between the predicted probabilities of having a microscopic P. falciparum infection at the third trimester of pregnancy for the youngest women (P value = .5404).
DISCUSSION
To our knowledge, RECIPAL is the first longitudinal study of malaria in sub-Saharan Africa to follow women from preconception right through to delivery. This was an excellent opportunity to study the dynamics of submicroscopic (as well as microscopic) P. falciparum infections and their determinants throughout pregnancy, starting from the first trimester. We observed that the proportions of women with submicroscopic P. falciparum infections were consistently higher than those of women with microscopic P. falciparum infections during pregnancy, with a cumulative incidence rate for submicroscopic infections that was twice that of microscopic infections. This is consistent with several other studies in pregnant women [21, 30–32]. In addition to this confirmation of previous findings, the study revealed that the proportion of infected women was highest in the first trimester.
We also found that being a young age and having a submicroscopic infection prior to pregnancy were associated with increased risks of both submicroscopic and microscopic infections in the different trimesters of pregnancy. The first result is well known. Indeed, possible explanations could be that (1) younger women, in which primi- and secondigravidae are overrepresented [25], have low or no immunity to the relevant parasite antigens, compared to the older group [12, 33–35]; and (2) women exhibited different behavior, with respect to malaria prevention tools during pregnancy (the use of bed nets, for example), in relation to age. To our knowledge, the relationship between having a submicroscopic infection before pregnancy and the risk of infection during pregnancy has never been shown. This extends preliminary results of the RECIPAL study on microscopic infections [36] that showed that women infected before pregnancy were more at risk of having an infection in the first trimester. This finding is important in public health terms, since it suggests that the preconception period should be considered as a vulnerable period, just as much as pregnancy itself. A recent study that was conducted on another subset of primigravidae [25]in the same area showed that infections occurring during the first trimester were predominantly (70%) composed of persistent P. falciparum genotypes that were contracted before pregnancy. This seems to confirm that infections, even at a submicroscopic level (and then untreated, as most probably not accompanied by symptoms), that have occurred before pregnancy may persist until (at least) the early stage of pregnancy [36], and then be a source of infection during the first trimester.
Additionally, a more thorough analysis showed (1) an interaction between those 2 factors (young age and P. falciparum infection status before pregnancy); and (2) a different impact of the interaction itself, depending on the trimester of pregnancy. Overall, the effects of these factors and their interactions were found to be highest in the first trimester, and then to gradually decrease in the second and third trimesters. Specifically, the youngest women with submicroscopic infections before pregnancy remained at significantly higher risks of P. falciparum infection (both submicroscopic and microscopic) during all 3 trimesters, compared to the oldest women with no infection before pregnancy.
Whereas the importance of P. falciparum infections (submicroscopic and microscopic) during pregnancy was already known [15, 21–23, 31], we found that the first trimester was a period of a higher prevalence of P. falciparum infections than the rest of pregnancy. Due to the critical importance of P. falciparum infections on early pregnancy [3, 16–18, 37] aggravated by the suboptimal protection of pregnant women during this period, this result highlights the excess risks faced by pregnant women and, therefore, the necessity of improved protection in this period.
As expected, we found a significant impact of the number of IPTp doses on the risk of having a P. falciparum infection. The proportions of women with microscopic and submicroscopic infections decreased after the first trimester, and the minimum was reached in the third trimester. Nevertheless, we observed a consistently higher proportion of submicroscopic, compared to microscopic, P. falciparum infections, and the proportion of women with a submicroscopic infection remained non-negligible throughout pregnancy. In this study, more than three-quarters of the women received at least 2 doses of IPTp (only 14% had 3 doses), which is higher than the coverage reported in Benin [38] and Africa [11], probably due to the monthly follow-up.
We also found that ethnicity was associated with a woman’s P. falciparum infection status. This could be related to a higher exposure to Anopheles by the Toffin, who are located in the lake area that is more favorable for transmission of the parasite. A woman with a submicroscopic infection at a given trimester (first or second) was also significantly more susceptible to infection (submicroscopic/microscopic infection) in the next trimester. This suggests that infections frequently persist during pregnancy.
Overall, the results highlight the particular role of submicroscopic infections in the dynamics and persistence of P. falciparum infections during pregnancy.
Our study has some limitations. First, the relatively high number of those lost to follow-up during pregnancy (mainly because of miscarriages, migrations, and withdrawal of consent due to some refusals of blood and/or placental sampling [39]).This could lead to a selection bias. However, a comparison of those lost to follow-up and our analysis sample showed no significant differences. Additionally, the proportion of primigravidae was low, compared to the proportions in other African malaria studies (8.79% vs the 15–25% usually mentioned) [21, 30, 31]. This could be explained by the PlacMalVac vaccine-related study, which was conducted simultaneously in the same area and included exclusively primigravidae [25]. This underrepresentation of primigravidae may have led to a lack of power and may explain why the primigravid group did not show significant differences with multigravidae, but is unlikely to have impacted our results to a large degree.
In conclusion, the RECIPAL study allowed us to study the complete dynamics of submicroscopic (as well as microscopic) infections throughout pregnancy, with a particular focus on the first trimester. It also made it possible to evaluate various factors contributing to these dynamics, and their complex interactions. We demonstrated for the first time the existence of a large reservoir of submicroscopic infections in the first trimester, starting from the very beginning of pregnancy. This is of public health importance, since infections in early pregnancy are known to be associated with serious pregnancy outcomes. In addition, our results seem to confirm the link between submicroscopic infections before conception and infections occurring both in early pregnancy and throughout pregnancy. Finally, we found that the youngest women with submicroscopic infections before pregnancy were a particularly high-risk group for infection not only in the first trimester, but also in the rest of pregnancy. Hence, focusing on research concerning preconception prevention strategies is paramount, including the development of a vaccine for nulligravidae [40], to protect women/fetuses against the damaging consequences of MiP.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. T. N., M. C., and G. C. contributed equally to this work. V. B. was the principal investigator. A. Massougbodji, M. C., and V. B. conceived of and designed the study. M. A., E. Y., N. F., D. S., B. V., and V. B. collected the data. C. P. A. H. and G. C. conducted the statistical analyses. C. P. A. H., V. B., M. C., and G. C. wrote the manuscript. N. T. N., A. Mama, D. S., B. V., and N. F. conducted the biology and molecular analyses. All authors read and approved the final manuscript.
Acknowledgments. The authors thank all the pregnant women and their families who participated in the Retard de Croissance Intra-uterin et Paludisme (RECIPAL) study; the health center and field workers; the local authorities of Sô-Ava and Akassato Districts; the RECIPAL team, including researchers, engineers, technicians, and managers; the Sorbonne Université for PhD scholarship of C. P. A. H.; the doctoral network of École des Hautes Études en Santé Publique; and Mr. Adrian J. F. Luty for proofreading the article before submission.
Financial support. This work was supported by the French Agence Nationale de la Recherche (grant number ANR-13-JSV1-0004) and the Fondation Simone Beer, under the auspices of the Fondation de France (grant number 00074147).
Potential conflicts of interest. No conflict declared. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Des vies en danger: le paludisme pendant la grossesse. Available at: https://www.who.int/features/2003/04b/fr/. Accessed 13 March 2019. [Google Scholar]
- 2. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLOS Med 2010; 7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. Am J Trop Med Hyg 2007; 76:849–54. [PubMed] [Google Scholar]
- 4. Schantz-Dunn J, Nour NM. Malaria and pregnancy: a global health perspective. Rev Obstet Gynecol 2009; 2:186–92. [PMC free article] [PubMed] [Google Scholar]
- 5. Ayoola OO, Whatmore A, Balogun WO, Jarrett OO, Cruickshank JK, Clayton PE. Maternal malaria status and metabolic profiles in pregnancy and in cord blood: relationships with birth size in Nigerian infants. Malar J 2012; 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huynh BT, Cottrell G, Cot M, Briand V. Burden of malaria in early pregnancy: a neglected problem? Clin Infect Dis 2015; 60:598–604. [DOI] [PubMed] [Google Scholar]
- 7. Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 2001; 64:36–44. [DOI] [PubMed] [Google Scholar]
- 8. Briand V, Cottrell G, Massougbodji A, Cot M. Intermittent preventive treatment for the prevention of malaria during pregnancy in high transmission areas. Malar J 2007; 6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004; CD000363. doi:10.1002/14651858.CD000363.pub2 [DOI] [PubMed] [Google Scholar]
- 10. Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLOS Med 2007; 4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Points essentiels: Rapport sur le paludisme dans le monde 2017. Available at: http://www.who.int/malaria/media/world-malaria-report-2017/fr/. Accessed 13 March 2019. [Google Scholar]
- 12. Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 13. Ouédraogo S, Koura GK, Accrombessi MM, Bodeau-Livinec F, Massougbodji A, Cot M. Maternal anemia at first antenatal visit: prevalence and risk factors in a malaria-endemic area in Benin. Am J Trop Med Hyg 2012; 87:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mkandawire P, Atari O, Kangmennaang J, Arku G, Luginaah I, Etowa J. Pregnancy intention and gestational age at first antenatal care (ANC) visit in Rwanda. Midwifery 2019; 68:30–8. [DOI] [PubMed] [Google Scholar]
- 15. Accrombessi M, Yovo E, Cottrell G, et al. Cohort profile: effect of malaria in early pregnancy on fetal growth in Benin (RECIPAL preconceptional cohort). BMJ Open 2018; 8:e019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hounkonnou C, Djènontin A, Egbinola S, et al. Impact of the use and efficacy of long lasting insecticidal net on malaria infection during the first trimester of pregnancy - a pre-conceptional cohort study in southern Benin. BMC Public Health 2018; 18:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valea I, Tinto H, Drabo MK, et al. ; Fonds de Solidarité Prioritaire/MIcronutriments et SAnté de la Mère et de l’Enfant Study Group. An analysis of timing and frequency of malaria infection during pregnancy in relation to the risk of low birth weight, anaemia and perinatal mortality in Burkina Faso. Malar J 2012; 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huynh BT, Fievet N, Gbaguidi G, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am J Trop Med Hyg 2011; 85:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmiegelow C, Matondo S, Minja DTR, Resende M, Pehrson C, Nielsen BB, et al. Plasmodium falciparum infection early in pregnancy has profound consequences for fetal growth. J Infect Dis. 2017;216:1601–10. [DOI] [PubMed] [Google Scholar]
- 20. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 2009; 200:1509–17. [DOI] [PubMed] [Google Scholar]
- 21. Cottrell G, Moussiliou A, Luty AJ, et al. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis 2015; 60:1481–8. [DOI] [PubMed] [Google Scholar]
- 22. Adegnika AA, Verweij JJ, Agnandji ST, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg 2006; 75:798–803. [PubMed] [Google Scholar]
- 23. Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol 2005; 43:3630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Accrombessi M, Yovo E, Fievet N, et al. Effects of malaria in the first trimester of pregnancy on poor maternal and birth outcomes in Benin. Clin Infect Dis Off Publ Infect Dis Soc Am 2018. doi:10.1093/cid/ciy1073 [DOI] [PubMed] [Google Scholar]
- 25. Tuikue Ndam N, Tornyigah B, Dossou AY, et al. Persistent Plasmodium falciparum infection in women with an intent to become pregnant as a risk factor for pregnancy-associated malaria. Clin Infect Dis 2018; 67:1890–6. [DOI] [PubMed] [Google Scholar]
- 26. Papageorghiou AT, Sarris I, Ioannou C, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century. Ultrasound methodology used to construct the fetal growth standards in the INTERGROWTH-21st Project. BJOG 2013; 120(Suppl 2):27–32, v. [DOI] [PubMed] [Google Scholar]
- 27. Swysen C, Vekemans J, Bruls M, et al. ; Clinical Trials Partnership Committee. Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J 2011; 10:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tran TM, Aghili A, Li S, et al. A nested real-time PCR assay for the quantification of Plasmodium falciparum DNA extracted from dried blood spots. Malar J 2014; 13:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diallo A, Ndam NT, Moussiliou A, et al. Asymptomatic carriage of plasmodium in urban Dakar: the risk of malaria should not be underestimated. PLOS One 2012; 7:e31100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rantala AM, Taylor SM, Trottman PA, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J 2010; 9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mockenhaupt FP, Rong B, Till H, et al. Submicroscopic Plasmodium falciparum infections in pregnancy in Ghana. Trop Med Int Health 2000; 5:167–73. [DOI] [PubMed] [Google Scholar]
- 32. Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol Res 2008; 102:333–42. [DOI] [PubMed] [Google Scholar]
- 33. Espinoza E, Hidalgo L, Chedraui P. The effect of malarial infection on maternal-fetal outcome in Ecuador. J Matern Fetal Neonatal Med 2005; 18:101–5. [DOI] [PubMed] [Google Scholar]
- 34. Walker-Abbey A, Djokam RR, Eno A, et al. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. Am J Trop Med Hyg 2005; 72:229–35. [PubMed] [Google Scholar]
- 35. Rogerson SJ, van den Broek NR, Chaluluka E, Qongwane C, Mhango CG, Molyneux ME. Malaria and anemia in antenatal women in Blantyre, Malawi: a twelve-month survey. Am J Trop Med Hyg 2000; 62:335–40. [DOI] [PubMed] [Google Scholar]
- 36. Accrombessi M, Fievet N, Yovo E, et al. Prevalence and associated risk factors of malaria in the first trimester of pregnancy: a preconceptional cohort study in Benin. J Infect Dis 2018; 217:1309–17. [DOI] [PubMed] [Google Scholar]
- 37. Schmiegelow C, Msemo OA, Møller SL, et al. Preconceptional factors associated with haemoglobin concentration in early pregnancy: a community-based cohort study in rural Northeastern Tanzania. Trop Med Int Health 2019; 24:596–607. [DOI] [PubMed] [Google Scholar]
- 38. Enquête Démographique et de Santé au Bénin (EDSB) de 2017-2018. Institut National de la Statistique et de l’Analyse Économique (INSAE). Enquête Démographique et de Santé au Bénin (EDSB) de 2017-2018. Available at: https://www.insae-bj.org/images/docs/insae-statistiques/enquetes-recensements/EDS/Enqu%C3%AAte%20D%C3%A9mographique%20et%20de%20Sant%C3%A9%20au%20B%C3%A9nin%20(EDSB)%20de%202017-2018.pdf. Accessed 13 March 2019. [Google Scholar]
- 39. Abudu EK, Inyang-Etoh EC, Eziagu UB. Pregnant women perception of placenta donation for biomedical research- experience at a Nigerian Tertiary Health Care Institution. Savannah J Med Res Pract. 2015;4:8–14. [Google Scholar]
- 40. Gbédandé K, Fievet N, Viwami F, et al. ; Multi-Centre Research Paper. Clinical development of a VAR2CSA-based placental malaria vaccine PAMVAC: quantifying vaccine antigen-specific memory B & T cell activity in Beninese primigravidae. Vaccine 2017; 35:3474–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.