Abstract
The influence of dietary vitamin D3 (VD3) levels on growth, bone performance, and duodenal type IIb sodium-dependent phosphate cotransporter (NaPi-IIb) genes in broiler chicken were studied. One-day-old male Ross308 broilers (n = 432) were allocated into 6 treatment groups with each group consisting of 6 cage pens. Each treatment group received diet containing different amounts of VD3 (80, 200, 500, 1,250, 3,125, or 7,813 IU per kg of diet) from a day-old to 31 D of age. Dietary available phosphorus and calcium were kept the same across all treatments in each phase. At 14 D, influence of VD3 on BW gain was found in the birds that received VD3 of 3,125 IU/kg and 200 IU/kg (P < 0.05). Toe ash and tibia ash linearly increased (P < 0.05) at 14 D with increase in dietary VD3. There was no significant influence of dietary VD3 on tibia breaking strength. In both phases, relative expression of duodenal NaPi-IIb linearly increased (P < 0.01) with increase in dietary VD3. At 14 D, highest expression of 3.2 folds was observed in birds treated with VD3 at 7,813 IU/kg of feed. At 31 D, birds that received VD3 levels of 3,125 and 7,813 IU/kg of feed showed 2.9 folds higher in NaPi-IIb expression compared with those fed lowest level of VD3 at 80 IU/kg of feed. When dietary calcium and phosphorus were maintained at the standard requirement, increase in dietary VD3 did not improve growth performance. For optimum growth and bone characteristics, dietary inclusion of VD3 at 500 IU/kg was adequate for both starter and grower broiler diets. Vitamin D3 enhanced the expression of NaPi-IIb at higher doses and thus improving the tibia ash content in high VD3 treatment groups. This study reported for the first time an increased in the expression of duodenal NaPi-IIb in 31-day-old broilers in response to high dietary VD3 levels.
Key words: broiler, vitamin D3, phosphate cotransporter, gene expression, bone characteristics
Introduction
Phosphorus (P) is one of the essential minerals for animal growth and survival. Around 80% of the inorganic P is used for the bone mineralization and the rest in the formation of nucleic acids, nucleotides, phospholipids, and phosphorylated proteins (Veum et al., 2010, Proszkowiec-Weglarz and Angel, 2013). Plasma P levels are mainly regulated by the metabolic pathway that includes parathyroid hormone, Vitamin D3 (VD3), calcitonin, and the associated receptors in the small intestine, bone, and kidneys (Veum, 2010). Vitamin D3 is the active form of Vitamin D, which is one of the fat-soluble secosteroids that is essentially required for optimal absorption of dietary Ca and P (Dusso et al., 2005). In animals, VD3 is either produced in the skin by sunlight exposure or obtained from the dietary supplementation (Holick et al., 1977, Dusso et al., 2005). It is further metabolized into 25-hydroxycholecalciferol (25(OH)D3) and subsequently into 1,25-dihydroxycholecalciferol (1,25(OH)2D3) in the liver and kidneys, respectively (Garcia et al., 2013).
Together with its metabolites, VD3 plays crucial role in poultry by improving weight gain, feed intake, feed efficiency, egg production, egg shell quality, and reproduction (Kahn and Mukhtar, 2013). The chickens hatched from breeders fed with higher VD3 diets and gained higher BW and tibia ash compared with those fed with lower VD3 (Atencio et al., 2005). It was reported that high concentration of dietary VD3 improved BW gain, feed intake, and feed efficiency when broilers were fed with optimal or suboptimal levels of Ca and nonphytate phosphorus (nPP) (Whitehead et al., 2004, Rao et al., 2006, Rama Rao et al., 2009). Dietary VD3 of 1,400 to 2,000 IU/kg for broilers up to 14 D of age and 800 IU/kg after 14 D was recommended (Whitehead et al., 2004). The recommended level of VD3 was up to 10,000 IU/kg for prevention of tibial dyschondroplasia. Rao et al. (2006) recommended 3,600 IU/kg of VD3 for growth performance and bone mineralization while using suboptimal levels of Ca and nPP. Increasing VD3 from 300 IU/kg to 1,200 IU/kg improved BW gain, tibia ash %, and tibia strength in broilers at 17 D and 35 D (Ramo Rao et al., 2019). Nevertheless, the minimum recommended dose of VD3 in broiler diet is 200 ICU/kg or 5 μg/kg (NRC, 1994). In a series of studies that evaluated the efficiency of commercially available VD3 sources, broiler requirements of VD3 ranged from 800 to 1,000 IU/kg of feed depending on the product (Kasim and Edwards, 2000). In commercial practices, VD3 is supplemented between 3,000 and 5,000 IU/kg (Whitehead et al., 2004). For the birds fed with optimum levels of Ca and P, VD3 was required in the range of 1,400 to 2,000 IU/kg up to 14 D for cortical bone quality and up to 10,000 IU/kg of VD3 to eliminate tibial dyschondroplasia (Whitehead et al., 2004). Moreover, VD3 requirements were reported in the range of 1,600 to 3,100 IU/kg to eliminate the rickets (Elliot and Edwards, 1997, Ledwaba and Roberson, 2003).
Sodium-dependent phosphate cotransporters type IIb (NaPi-IIb) are reported to be the responsible for 90% of the Na-dependent intestinal transcellular P transport (Tenenhouse, 2005, Sabbagh et al., 2009). Vitamin D3 and its derivatives are assumed as the major regulators for intestinal NaPi-IIb-related P absorption (Kido et al., 2013). An ex vivo study with chicken intestinal tract revealed that the intestinal mucosal tissues transported P in vitamin D–dependent manner (Peterlik and Wasserman, 1978). Previous studies proved that NaPi-IIb was regulated essentially by dietary P and 1,25(OH)2D3 (Katai et al., 1999, Marks et al., 2010, Chen et al., 2017, Shao et al., 2018). Segawa et al. (2004) reported that 1,25(OH)2D3 regulated the Na-dependent P transport system in the small intestine of mice. For early embryonic development, NaPi-IIb is an essential regulator gene (Ohi et al., 2011). Intestinal NaPi-IIb protein and NaPi-IIb transport activities were lower in NaPi-IIb heterozygous (+/−) mice compared with NaPi-IIb homozygous (+/+) mice (Ohi et al., 2011). Liao et al. (2017) reported the upregulation of duodenal NaPi-IIb in ligated duodenal loops from 22 day-old-broilers fed with 1,25(OH)2D3 diets.
To date, limited reports are available on the effect of dietary VD3 or its metabolites on expression of NaPi-IIb in broilers (Han et al., 2009, Han et al., 2012, Han et al., 2018, Liao et al., 2017, Shao et al., 2018). Data on effect of dietary VD3 on intestinal NaPi-IIb expression in broilers above 21 D of age are lacking. Because broilers are raised above 21 D in commercial practice, it is of great interest to study the intestinal active P transport in growing broilers that would benefit in optimization of P usage in diets. The aim of the current study was to evaluate the influence of VD3 on growth performance, bone characteristics, and intestinal NaPi-IIb expression in both starter (14 D) and growing (31 D) broilers.
Materials and methods
This study was conducted in accordance with the principles and guidelines stipulated in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (NRC, 2010). The study design and procedures involved were approved by the Institutional Animal Care and Use Committee.
Animal and Diets
A total of 432 male Ross308 broilers with average BW of 42 g were collected from Feed Research and Innovation Center hatchery, Charoen Pokphand, Chonburi, at the hatching day. Birds were equally placed into 36 cage pens with 12 birds per pen. Each 6 pens group was randomly assigned to one dietary treatment, totaling 6 treatments. One pen was considered as a replicate. Mash feeds were prepared for starter phase (0–14 D) and grower phase (15–31 D) (Table 1). Birds were raised up to 31 D under standard housing and management conditions with ad libitum of feed and water as previously described (Tay-Zar et al., 2019). Basal feed was formulated to meet minimum nutrient requirements by National Research Council (NRC, 1994) except for VD3. To the basal feed, VD3 was added at 6 different levels (80, 200, 500, 1,250, 3,125, and 7,813 IU/kg of diet), making a total of 6 dietary treatments. Microvit D3 Promix 500 from Adisseo (Adisseo, Antony, France) was used as the source of VD3. Levels of nPP and Ca in all treatments were maintained at 1.00 and 0.45% for starter phase and 0.90 and 0.35% for grower phase, respectively. Prepared diets were sent to the CP Bangna Central Laboratory (Charoen Pokphand Group, Bangkok, Thailand) to analyze the nutrient values.
Table 1.
Diet composition (%) and calculated nutrient values.
| Items | Basal diets1 |
|
|---|---|---|
| 0 to 14 D | 15 to 31 D | |
| Ingredients (%) | ||
| Corn | 57.03 | 65.26 |
| Soybean meal | 34.82 | 27.63 |
| Crude palm oil | 2.62 | 2.02 |
| Mono calcium phosphate | 1.37 | 0.96 |
| Limestone | 1.70 | 1.65 |
| Salt | 0.41 | 0.35 |
| Sodium bicarbonate | 0.10 | 0.10 |
| DL-methionine | 0.33 | 0.33 |
| L-lysine | 0.19 | 0.27 |
| Choline chloride | 0.10 | 0.10 |
| Premixes2 | 0.34 | 0.32 |
| Total | 99.00 | 99.00 |
| Calculated values (%) | ||
| ME (kcal/kg) | 3,018 | 3,067 |
| Crude protein | 21.48 | 18.80 |
| Crude fat | 5.32 | 4.93 |
| Crude fiber | 2.49 | 2.38 |
| Ca | 1.00 | 0.90 |
| Total phosphorus | 0.72 | 0.61 |
| nPP | 0.45 | 0.35 |
| Ca/nPP | 2.22 | 2.57 |
| Sodium | 0.20 | 0.17 |
| Chloride | 0.30 | 0.27 |
| Lysine | 1.33 | 1.20 |
| Methionine | 0.63 | 0.61 |
| Methionine + Cystine | 0.97 | 0.91 |
| Threonine | 0.84 | 0.74 |
| Tryptophan | 0.27 | 0.23 |
Abbreviations: Ca, calcium; nPP, nonphytate phosphorus.
Vitamin D3 was premixed with 1.00% of corn and added to basal diets.
Per kg of diet: Copper, 8 mg; Iodine, 0.35 mg; Iron, 80 mg; Manganese, 60 mg; Selenium, 0.15 mg; Zinc, 40 mg; Vitamin A, 1,500 IU; Vitamin E, 10 IU; Vitamin K 0.50 mg; Vitamin B12, 0.01 mg; Riboflavin 3.6 mg; Choline, 1,000 mg; Thiamin, 1.8 mg; Biotin, 0.15 mg.
Growth Performance
Feed intake was observed weekly. At 14 D and 31 D of age, live BW was checked, and feed conversion ratio (FCR) was calculated for each phase.
Bone Characteristics
At 14 D of age, 4 to 5 birds from each pen were euthanized for bone sample collection. Tibia and middle toes were collected from both sides of the birds. The rest of the birds were kept until 31 D and euthanized for collection of tibia and middle toes. Tibias were deflashed, and patella were separated from tibia bones. Bone samples were stored at 25°C for 24 h followed by ovendrying at 105°C for 24 h. To determine the ash content of the bones, left tibia and toes were subjected to 600°C for 6 h inside the muffle furnace. For bone breaking strength, right tibia bones were tested on Instron Materials tester (Instron 5,965; Instron Corp., Canton, MA) with automated materials test system software, version 8.0 (Shim et al., 2012). Bone breaking strength was determined as described earlier (Tay-Zar et al., 2019). Computerized software (Instron Corp.) was used to calculate the tibia breaking strength from the load-deformation curve.
Tissue Samples
At 14 D and 31 D, one bird from each pen which represented the average BW of the treatment was selected. Duodenum was separated and flashed with sterile cold normal saline. Using clean sterile scalpel blades, duodenal mucosa was scrapped at the middle portion of the duodenum and immediately preserved in cryovials filled with RNAlater (Sigma-Aldrich, St. Louis, MO). Cryovials with samples were incubated overnight at 4°C and subsequently kept at −80°C for RNA extraction.
Real-Time PCR
Total RNAs from samples were extracted with RNeasy mini kit (Qiagen, Germantown, MD) followed by quality check using Nanodrop (ThermoFisher, Waltham, MA) and QIAxcel capillary gel electrophoresis (Qiagen). One micro gram of RNA was used to construct complementary DNA using Omniscript reverse transcription kit (Qiagen). Beta-actin genes were used as endogenous control to calculate relative quantitation. Nucleotide sequences of β-actin and NaPi-IIb primers (Yan et al., 2007) are listed in Table 2. The real-time PCR assay was performed as described previously by Tay-Zar et al., 2019. Briefly, QuantiFast SYBR Green PCR Kit (Qiagen) was used with following cycling conditions: 95°C for 5 min; 95°C for 30 s; 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s; 72°C for 5 min. The assay was performed on Roche Lightcycler96 (Roche, Basel, Switzerland). Relative expression of NaPi-IIb was evaluated by delta CT (2−ΔΔCT) method using β-actin as internal control genes (Pfaffl, 2001).
Table 2.
Polymerase chain reaction primer sequences and product sizes (Yan et al., 2007).
| Name | Sequence (5' - 3′) | Product size (bps) | Accession number | |
|---|---|---|---|---|
| NaPi-IIb | Forward | CTGGATGCACTCCCTAGAGC | 126 | NM_204474.2 |
| Reverse | TTATCTTTGGCACCCTCCTG | |||
| β-actin | Forward | GAGAAATTGTGCGTGACATCA | 152 | NM_205518.1 |
| Reverse | CCTGAACCTCTCATTGCCA | |||
Abbreviations: NaPi-IIb, sodium-dependent phosphate cotransporter type IIb; β-actin, beta actin.
Statistical Analysis
Each cage pen was analyzed as a replicate. Data were analyzed by one-way ANOVA using General Linear Model (GLM) procedure followed by Tukey's Honestly Significant Difference (HSD) test in SAS 9.0 (SAS Inst. Inc., Cary, NC). A value of P < 0.05 was considered statistically significant. Parameters with significant P-value by dietary VD3 levels were further analyzed by orthogonal polynomial contrasts to determine linear and quadratic trends. A value of less than P < 0.05 was considered significant.
Results
Growth Performance
Table 3 summarizes the growth performance of the birds. At 14 D, the effect of dietary VD3 levels on BW gain was mathematically significant (P < 0.05) between the groups that received VD3 200 IU/kg of feed and 3,125 IU/kg of feed. Nevertheless, there was no linear trend in response to VD3. Feed intake, FCR, and mortality were not affected by dietary VD3 levels. Highest weight gain was observed in birds fed with VD3 at 3,125 IU/kg of feed. At 31 D, feed intake was influenced (P < 0.05) by dietary VD3 levels. There was no significant difference on BW gain, feed intake, FCR, and mortality among the groups treated with dietary VD3 80, 500, 1,250, 3,125, and 7,813 IU/kg of diet. Overall (0–31 D), there was no significant improvement for BW gain, FCR, and mortality, when diets were supplied with different VD3 levels. There was no correlation between mortality rates and dietary VD3 levels in both phases.
Table 3.
Growth performance of broilers from 0 to 31 D of age.
| Items | Vitamin D3 (IU/kg) |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 200 | 500 | 1,250 | 3,125 | 7,813 | VD3 | Linear | Quadratic | ||
| At 14 D | ||||||||||
| BW gain (g) | 430a,b | 419b | 443a,b | 445a,b | 457a | 442a,b | 3.51 | 0.039 | 0.215 | 0.007 |
| Feed intake (g) | 482 | 468 | 488 | 496 | 501 | 490 | 3.36 | 0.083 | 0.253 | 0.022 |
| FCR | 1.122 | 1.118 | 1.101 | 1.116 | 1.096 | 1.111 | 0.00 | 0.417 | 0.570 | 0.166 |
| Mortality (%) | 0.00 | 1.67 | 0.00 | 1.39 | 1.67 | 2.78 | 0.51 | 0.600 | 0.336 | 0.720 |
| 15 to 31 D | ||||||||||
| BW gain (g) | 1,160 | 1,128 | 1,176 | 1,184 | 1,216 | 1,181 | 13.28 | 0.346 | 0.321 | 0.065 |
| Feed intake (g) | 1,84a,b | 1,761b | 1,908a,b | 1,900a,b | 1,947a | 1,897a,b | 17.38 | 0.043 | 0.195 | 0.032 |
| FCR | 1.158 | 1.142 | 1.177 | 1.166 | 1.162 | 1.169 | 0.00 | 0.078 | 0.384 | 0.689 |
| Mortality (%) | 0.00 | 0.00 | 0.00 | 0.00 | 1.67 | 0.00 | 0.49 | 0.337 | 0.742 | 0.410 |
| 0 to 31 D | ||||||||||
| BW gain (g) | 1,591 | 1,543 | 1,620 | 1,629 | 1,676 | 1,624 | 10.58 | 0.129 | 0.239 | 0.025 |
| Feed intake (g) | 2,315a,b | 2,224b | 2,395a,b | 2,391a,b | 2,434a | 2,383a,b | 20.99 | 0.026 | 0.176 | 0.028 |
| FCR | 1.455 | 1.442 | 1.479 | 1.468 | 1.453 | 1.468 | 0.01 | 0.128 | 0.538 | 0.916 |
| Mortality (%) | 1.39 | 1.67 | 0.00 | 1.39 | 3.33 | 2.78 | 0.68 | 0.812 | 0.416 | 0.539 |
a-bWithin comparisons, means in a row with no common superscript differs significantly (P < 0.05).
Abbreviation: FCR, feed conversion ratio.
Bone Characteristics
Influence of dietary VD3 levels on tibia and toe ash, and tibia breaking strength are summarized in Table 4. At day 14, dietary VD3 levels affected on toe ash content (P < 0.001) and tibia ash content (P < 0.05). Both tibia and toe ash content did not respond to changes in dietary VD3 levels above 500 IU/kg. For tibia ash, only the group of birds treated with VD3 of 200 IU/kg of feed was significantly affected. Toe ash responded both in linear and quadratic (P < 0.001) trend to dietary VD3 inclusions, whereas tibia ash responded linearly (P < 0.05). At 31 D of age, only tibia ash content was influenced by dietary VD3 levels (P < 0.001). Above 500 IU/kg of dietary VD3, tibia ash did not respond to increase in dietary VD3 contents. Linear trend was observed in tibia ash in response to dietary VD3 levels (P < 0.001).
Table 4.
Toe ash, tibia ash, and tibia breaking strength at 14 D and 31 D of age.
| Items | Vitamin D3 (IU/kg) |
SEM |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 200 | 500 | 1,250 | 3,125 | 7,813 | VD3 | Linear | Quadratic | ||
| At 14 D | ||||||||||
| Toe ash (%) | 10.98b | 10.85b | 12.02a | 12.39a | 12.05a | 12.04a | 0.12 | <0.001 | 0.032 | 0.001 |
| Tibia ash (%) | 39.91a,b | 39.72b | 41.36a,b | 41.89a,b | 42.26a,b | 42.94a | 0.34 | 0.020 | 0.004 | 0.092 |
| Tibia strength (kgf/mm)1 | 8.54 | 8.67 | 8.73 | 8.91 | 9.35 | 9.85 | 0.18 | 0.236 | 0.009 | 0.561 |
| At 31 D | ||||||||||
| Toe ash (%) | 11.94 | 12.21 | 11.94 | 12.00 | 11.97 | 12.01 | 0.07 | 0.890 | 0.92 | 0.767 |
| Tibia ash (%) | 40.86c | 41.41b | 42.99a,b,c | 42.97a,b,c | 43.74a,b | 44.83a | 0.34 | 0.001 | <0.001 | 0.099 |
| Tibia strength (kgf/mm) | 19.32 | 19.73 | 19.92 | 20.18 | 20.69 | 21.26 | 0.27 | 0.321 | 0.021 | 0.441 |
a-cWithin comparisons, means in a row with no common superscript differs significantly (P < 0.05).
Kilogram force per millimeter.
Expression of NaPi-IIb
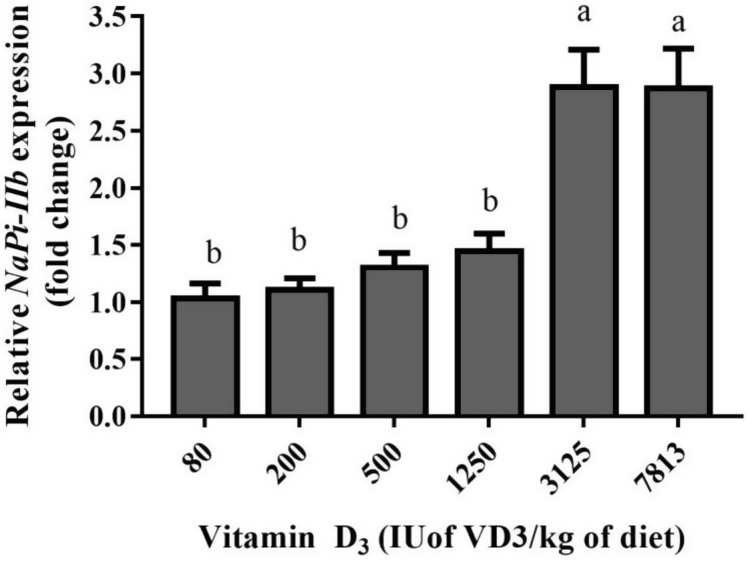
Figures 1 and 2 illustrate the relative expression of NaPi-IIb with reference to β-actin at 14 D and 31 D, respectively. In both phases, the group treated with lowest level of dietary VD3 (80 IU/kg) was assumed as calibrator and compared with other groups within the same period. At 14 D, relative expressions of NaPi-IIb were not significant for the groups treated with VD3 from 80 to 3,125 IU/kg. The highest VD3 group showed 3.2 folds increase in relative NaPi-IIb expression. There was linear response for NaPi-IIb expression (P < 0.001). At 31 D, highest NaPi-IIb expression of 2.8 folds increment was observed in birds fed with VD3 at 3,125 and 7,813 IU/kg. The rest of the groups were not influenced by VD3. There were both linear (P < 0.001) and quadratic (P < 0.05) responses of NaPi-IIb expression to dietary VD3 inclusions.
Figure 1.
Relative quantitation of type IIb sodium-dependent phosphate cotransporter (NaPi-IIb) in broilers at 14 D of age in response to different levels of dietary vitamin D3 (VD3). Mean fold changes in relative expression of NaPi-IIb compared with β-actin were calculated relative to the respective values at the VD3 level of 80 IU/kg of diet (n = 6). Within comparisons, means values of each bar with no common alphabet differs significantly (P < 0.05).
Figure 2.
Relative quantitation of type IIb sodium-dependent phosphate cotransporter (NaPi-IIb) in broilers at 31 days of age in response to different levels of dietary vitamin D3 (VD3). Mean fold changes in relative expression of NaPi-IIb compared with β-actin were calculated relative to the respective values at the VD3 level of 80 IU/kg of diet (n = 6). Within comparisons, means values of each bar with no common alphabet differs significantly (P < 0.01).
Discussion
Owing to the very limited synthesis of endogenous VD3 in commercial broilers, it is a regular practice to add VD3 in diet formulations. Vitamin D3 requirement for broilers is 200 IU/kg of feed as prescribed in NRC (1994). However, some of the studies claimed that the modern-day broilers need higher dose than NRC recommendation (Kasim and Edwards, 2000, Ledwaba and Roberson, 2003, Whitehead et al., 2004). Exposure of birds to UV light could eliminate the requirement of dietary VD3 as birds could synthesize VD3 in the body. In the present study, birds were raised under UV light–free environment. Lowest dietary VD3 inclusion was 80 IU/kg of feed and increased 2.5 times for each treatment up to 7,813 IU/kg of feed. All diets were formulated to contain 1.00% of Ca and 0.45% of nPP for the starter phase and 0.90% of Ca and 0.35% of nPP for grower phase which met or exceeded the requirements for normal physiologic functions of the birds. A recent study on vitamin D requirements for modern broiler breeds suggested that increasing dietary VD3 inclusion from 1,000 to 7,000 IU/kg of feed did not improve the growth performance in 2 modern broiler breeds (Sakkas et al., 2018). Similarly, in this study, there was no significant influence of VD3 on the growth performance when birds were fed with diets containing VD3 at 500 IU/kg and above. There was no improvement in weight gain at 21 D when fed with the supplementation of VD3 in the range of 100 to 3,200 IU/kg of feed, and feed efficiency was dropped in those broilers compared with the birds treated with VD3 in the range of 0 to 50 IU/kg of feed (Leyva-Jimenez et al., 2019). Some studies revealed that the growth performance traits were not very sensitive in response to dietary VD3 levels (Waldroup et al., 1963, Edwards et al., 1994, Angel et al., 2005). Fritts and Waldroup (2003) reported that the broilers treated with different levels of VD3 ranging from 125 to 4,000 IU/kg of feed with adequate Ca and nPP in diets up to 42 D showed no influence of VD3 on feed intake, FCR, and mortality in all treatments. In the same study, weight gain was improved (P < 0.05) at 42 D in birds treated with VD3 at the level of 1,000 IU/kg of feed and above. The broilers (16 D old) treated with different diets including VD3 from 0 to 1,000 IU/kg of feed did not show any significant difference in BW gain and FCR (Kasim and Edwards, 2000).
In the current study, fat was not extracted from the tibias. It has been suggested that bone ash results were reliable and comparable regardless of fat extraction (Yan et al., 2005, Garcia and Dale, 2006). One of the strong influences of VD3 on broilers was seen in bone parameters. Significant impacts of dietary VD3 on growth, tibia ash, and tibia strength were seen in 21 day-old-broilers treated with diets composed of VD3 at 0 to 3,200 IU/kg of feed (Leyva-Jimenez et al., 2019). In the present study, there was no influence of VD3 on tibia strength at both 14 D and 31 D. Toe ash (%) was significantly (P < 0.05) improved with increased VD3 at 14 D. No further improvement of VD3 on toe ash was seen at 31 D. Tibia ash linearly improved with increase in VD3 for both 14-day-old and 31-day-old birds (P < 0.001). Supporting results were found in another study where tibia ash content improved on both 21 D and 42 D with increase in dietary VD3 from 125 to 4,000 IU/kg of feed (Fritts and Waldroup, 2003). When broilers were fed with 0 and 3,500 IU/kg of VD3, there was no significant impact on growth performance; nevertheless, tibia ash was improved in both nPP-deficient groups and control groups (Shao et al., 2018). It was found that one-alpha-hydroxycholecalciferol (1α-OHD3), an analog of VD3 improved growth performance, bone quality, and meat color in 42-day-old broilers feed Ca- and P-deficient diets (Han et al., 2012).
In the present study, duodenal mucosa was collected to determine NaPi-IIb expression. Among the small intestinal segments, the highest expression NaPi-IIb was seen in duodenum (Yan et al., 2007, Han et al., 2012, Han et al., 2018, Shao et al., 2018). The dietary nPP levels influenced on duodenal NaPi-IIb expression in both 14-day-broilers and 31-day-broilers, when VD3 and Ca in diets were at adequate amount (Tay-Zar et al., 2019). Vitamin D3 significantly affected the P absorption in all segments of poultry small intestine, whereas VD3 affected basolateral P transport in duodenum (Wasserman and Taylor, 1973). Effects of VD3 on intestinal P transport were most efficiently observed in duodenum than in jejunum and ileum (Peterlik and Wasserman, 1978). Previous research studies reported the use of different vitamin D metabolites (1α-OHD3, 25(OH)D3, 1,25(OH)2D3 and VD3) as a source of vitamin D and studied their influence on broiler NaPi-IIb expression (Han et al., 2012, Han et al., 2018, Liao et al., 2017 Han et al 2018; Shao et al., 2018). It should be noted that different metabolites of vitamin D influenced differently on animal growth and bone characteristics. In the body, 25(OH)D3 is 20% more efficiently absorbed than VD3, and 1α-OHD3 and 1,25(OH)2D3 do not require renal metabolism (Applegate et al., 2003, Garcia et al., 2013). Influence of VD3 and its metabolites on intestinal NaPi-IIb expression is difficult to predict. In 21-day-old broilers, Shao et al. (2018) revealed the significant impact of VD3 levels (P < 0.001) on intestinal NaPi-IIb expression in ileum. Nevertheless, the highest expression was seen in duodenum among small intestine segments. In the present study, VD3 linearly increased (P < 0.001) the relative expression of intestinal NaPi-IIb in both 14-day-broilers and 31-day-broilers. In rats, 1,25(OH)2D3 upregulated NaPi-IIb expression in suckling age but not in adults suggesting age-dependent response on P metabolism (Xu et al., 2002). Expression of NaPi-IIb was influenced by dietary VD3, and higher expression was observed in the birds fed with nPP diets (Shao et al., 2018). In broiler, highest expression of intestinal NaPi-IIb expression in response to dietary 25(OH)D3 were reported at 21 D of age followed by 35 D and 28 D (Han et al., 2018). Dietary supplementation of 25(OH)D3 in 14-day-old broilers, upregulated duodenal NaPi-IIb expression to 1.86 folds higher (P < 0.05) than those without 25(OH)D3 in diets, while Ca and nPP were kept the same at standard requirement for both diets (Han et al., 2018). This finding supports the current study that VD3 and its metabolites enhanced the expression of intestinal NaPi-IIb expression. However, in the current study, VD3 significantly influenced the intestinal NaPi-IIb expression only at very high doses; 7,813 IU/kg of feed for 14 D and above 1,250 IU/kg of feed for 31 D.
In conclusion, there was no significant influence of VD3 on growth performance of both starter (14 D) and grower (31 D) broilers when supplemented with adequate dietary Ca and nPP. Optimum growth performance was achieved at 500 IU/kg of feed and above for both phases. Tibia ash % linearly increased with increase in dietary VD3 levels; nevertheless, no significant impact was seen above 500 IU/kg of feed. Similarly, VD3 linearly increase the intestinal NaPi-IIb expression in both growth phases.
Acknowledgments
The authors thank the Charoen Pokphand Group for funding this study.
References
- Angel R., Saylor W.W., Dhandu A.S., Powers W., Applegate T.J. Effects of dietary phosphorus, phytase, and 25-hydroxycholecalciferol on performance of broiler chickens grown in floor pens. Poult. Sci. 2005;847:1031–1044. doi: 10.1093/ps/84.7.1031. [DOI] [PubMed] [Google Scholar]
- Applegate T., Angel R., Classen H. Effect of dietary calcium, 25-hydroxycholecalciferol, or bird strain on small intestinal phytase activity in broiler chickens. Poult. Sci. 2003;827:1140–1148. doi: 10.1093/ps/82.7.1140. [DOI] [PubMed] [Google Scholar]
- Atencio A., Edwards H.M., Jr., Pesti G. Effects of vitamin D3 dietary supplementation of broiler breeder hens on the performance and bone abnormalities of the progeny 1. Poult. Sci. 2005;847:1058–1068. doi: 10.1093/ps/84.7.1058. [DOI] [PubMed] [Google Scholar]
- Chen P., Huang Y., Bayir A., Wang C. Characterization of the isoforms of type IIb sodium-dependent phosphate cotransporter (Slc34a2) in yellow catfish, Pelteobagrus fulvidraco, and their vitamin D3-regulated expression under low-phosphate conditions. Fish Physiol. Biochem. 2017;431:229–244. doi: 10.1007/s10695-016-0282-7. [DOI] [PubMed] [Google Scholar]
- Garcia A.R., Dale N.M. Foot ash as a means of Quantifying bone mineralization in chicks. J. Appl. Poult. Res. 2006;151:103–109. [Google Scholar]
- Dusso A.S., Brown A.J., Slatopolsky E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005;2891:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- Edwards H.M., Jr., Elliot M.A., Sooncharernying S., Britton W.M. Quantitative requirement for cholecalciferol in the absence of ultraviolet Light1. Poult. Sci. 1994;732:288–294. doi: 10.3382/ps.0730288. [DOI] [PubMed] [Google Scholar]
- Elliot M., Edwards H., Jr. Effect of 1,25-dihydroxycholecalciferol, cholecalciferol, and fluorescent lights on the development of tibial dyschondroplasia and rickets in broiler chickens. Poult. Sci. 1997;764:570–580. doi: 10.1093/ps/76.4.570. [DOI] [PubMed] [Google Scholar]
- Fritts C.A., Waldroup P.W. Effect of source and level of vitamin D on live performance and bone development in growing Broilers1. J. Appl. Poult. Res. 2003;121:45–52. [Google Scholar]
- Garcia A.F.Q.M., Murakami A.E., Duarte C.R.D.A., Rojas I.C.O., Picoli K.P., Puzotti M.M. Use of vitamin d3 and its metabolites in broiler chicken feed on performance, bone parameters and meat quality. Asian-Australas. J. Anim. Sci. 2013;263:408–415. doi: 10.5713/ajas.2012.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Wang Y., Qu H., Liang F., Zhang J., Shi C., Zhang X., Li L., Xie Q., Wang C. One alpha-hydroxycholecalciferol improves growth performance, tibia quality, and meat color of broilers fed calcium-and phosphorus-deficient diets. Asian-Australas. J. Anim. Sci. 2012;25:267–271. doi: 10.5713/ajas.2011.11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.C., Yang X.D., Zhang T., Li H., Li W.L., Zhang Z.Y., Yao J.H. Effects of 1alpha-hydroxycholecalciferol on growth performance, parameters of tibia and plasma, meat quality, and type IIb sodium phosphate cotransporter gene expression of one- to twenty-one-day-old broilers. Poult. Sci. 2009;882:323–329. doi: 10.3382/ps.2008-00252. [DOI] [PubMed] [Google Scholar]
- Han J.C., Zhang J.L., Zhang N., Yang X., Qu H.X., Guo Y., Shi C.X., Yan Y.F. Age, phosphorus, and 25-hydroxycholecalciferol regulate mRNA expression of vitamin D receptor and sodium-phosphate cotransporter in the small intestine of broiler chickens. Poult. Sci. 2018;974:1199–1208. doi: 10.3382/ps/pex407. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Frommer J.E., McNeill S.C., Richtand N.M., Henley J.W., Potts J.T. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 1977;761:107–114. doi: 10.1016/0006-291x(77)91674-6. [DOI] [PubMed] [Google Scholar]
- Kahn S.H., Mukhtar N. Dynamic role of cholecalciferol in commercial chicken performance. Worlds. Poult. Sci. J. 2013;6903:587–600. [Google Scholar]
- Kasim A.B., Edwards H.M., Jr. Evaluation of cholecalciferol sources using broiler chick bioassays 1. Poult. Sci. 2000;7911:1617–1622. doi: 10.1093/ps/79.11.1617. [DOI] [PubMed] [Google Scholar]
- Katai K., Miyamoto K., Kishida S., Segawa H., Nii T., Tanaka H., Tani Y., Arai H., Tatsumi S., Morita K., Taketani Y., Takeda E. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem. J. 1999;343(Pt 3):705–712. [PMC free article] [PubMed] [Google Scholar]
- Kido S., Kaneko I., Tatsumi S., Segawa H., Miyamoto K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib. Nephrol. 2013;180:86–97. doi: 10.1159/000346786. [DOI] [PubMed] [Google Scholar]
- Ledwaba M., Roberson K. Effectiveness of twenty-five-hydroxycholecalciferol in the prevention of tibial dyschondroplasia in Ross cockerels depends on dietary calcium level. Poult. Sci. 2003;8211:1769–1777. doi: 10.1093/ps/82.11.1769. [DOI] [PubMed] [Google Scholar]
- Leyva-Jimenez H., Khan M., Gardner K., Abdaljaleel R.A., AL-Jumaa Y., Alsadwi A.M., Bailey C.A. Developing a novel oral vitamin D3 intake bioassay to re-evaluate the vitamin D3 requirement for modern broiler chickens. Poult. Sci. 2019;989:3770–3776. doi: 10.3382/ps/pez074. [DOI] [PubMed] [Google Scholar]
- Liao X.D., Suo H.Q., Lu L., Hu Y.X., Zhang L.Y., Luo X.G. Effects of sodium, 1,25-dihydroxyvitamin D3 and parathyroid hormone fragment on inorganic P absorption and Type IIb sodium-phosphate cotransporter expression in ligated duodenal loops of broilers. Poult. Sci. 2017;967:2344–2350. doi: 10.3382/ps/pex033. [DOI] [PubMed] [Google Scholar]
- Marks J., Debnam E.S., Unwin R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Ren. Physiol. 2010;2992:F285–F296. doi: 10.1152/ajprenal.00508.2009. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev.edn. The National Academies Press; 1994. Nutrient Requirements of Poultry. 1994. [Google Scholar]
- National Research Council . National Academies Press; 2010. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Ohi A., Hanabusa E., Ueda O., Segawa H., Horiba N., Kaneko I., Kuwahara S., Mukai T., Sasaki S., Tominaga R., Furutani J., Aranami F., Ohtomo S., Oikawa Y., Kawase Y., Wada N.A., Tachibe T., Kakefuda M., Tateishi H., Matsumoto K., Tatsumi S., Kido S., Fukushima N., Jishage K.-i., Miyamoto K.-i. Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b+/− mice. Am. J. Physiol. Ren. Physiol. 2011;3015:F1105–F1113. doi: 10.1152/ajprenal.00663.2010. [DOI] [PubMed] [Google Scholar]
- Peterlik M., Wasserman R.H. Effect of vitamin D on transepithelial phosphate transport in chick intestine. Am. J. Physiol. Endocrinol. Metab. 1978;2344:E379. doi: 10.1152/ajpendo.1978.234.4.E379. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;299:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility1. J. Appl. Poult. Res. 2013;223:609–627. [Google Scholar]
- Rama Rao S.V., Raju M.V.L.N., Panda A.K., Shyam Sunder G., Sharma R.P. Performance and bone mineralisation in broiler chicks fed on diets with different concentrations of cholecalciferol at a constant ratio of calcium to non-phytate phosphorus. Br. Poult. Sci. 2009;504:528–535. doi: 10.1080/00071660903125826. [DOI] [PubMed] [Google Scholar]
- Rao S.V.R., Raju M.V.L.N., Panda A.K., Sunder G.S., Sharma R.P. Effect of high concentrations of cholecalciferol on growth, bone mineralization, and mineral Retention in broiler chicks fed suboptimal concentrations of calcium and Nonphytate phosphorus. J. Appl. Poult. Res. 2006;154:493–501. [Google Scholar]
- Sabbagh Y., O'Brien S.P., Song W., Boulanger J.H., Stockmann A., Arbeeny C., Schiavi S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009;2011:2348–2358. doi: 10.1681/ASN.2009050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas P., Smith S., Hill T.R., Kyriazakis I. A reassessment of the vitamin D requirements of modern broiler genotypes. Poult. Sci. 2018;981:330–340. doi: 10.3382/ps/pey350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H., Kaneko I., Yamanaka S., Ito M., Kuwahata M., Inoue Y., Kato S., Miyamoto K. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am. J. Physiol. Renal Physiol. 2004;2871:F39–47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- Shao Y., Wen Q., Zhang S., Lu L., Zhang L., Liao X., Luo X. Dietary supplemental vitamin D3 enhances phosphorus absorption and utilisation by regulating gene expression of related phosphate transporters in the small intestine of broilers. Br. J. Nutr. 2018;1211:9–21. doi: 10.1017/S0007114518002763. [DOI] [PubMed] [Google Scholar]
- Shim M.Y., Karnuah A.B., Mitchell A.D., Anthony N.B., Pesti G.M., Aggrey S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012;918:1790–1795. doi: 10.3382/ps.2011-01968. [DOI] [PubMed] [Google Scholar]
- Tay-Zar A.C., Srichana P., Sadiq M.B., Anal A.K. Restriction of dietary non-phytate phosphorus on growth performance and expression of intestinal phosphate cotransporter genes in broilers. Poult. Sci. 2019;9810:4685–4693. doi: 10.3382/ps/pez171. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H.S. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu. Rev. Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- Veum T.L. CAB International; Oxfordshire, UK: 2010. Phosphorus and Calcium Nutrition and Metabolism. Phosphorus and Calcium Utilization and Requirements in Farm Animals. M. S. S. V. Dorinha and E. Kebreab; pp. 94–111. [Google Scholar]
- Waldroup P.W., Ammerman C.B., Harms R.H. The Relationship of phosphorus, calcium, and vitamin D3 in the diet of broiler-type Chicks1. Poult. Sci. 1963;424:982–989. [Google Scholar]
- Wasserman R.H., Taylor A.N. Intestinal absorption of phosphate in the chick: effect of vitamin D3 and other parameters. J. Nutr. 1973;1034:586–599. doi: 10.1093/jn/103.4.586. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., McCormack H.A., McTeir L., Fleming R.H. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus and vitamin A. Br. Poult. Sci. 2004;453:425–436. doi: 10.1080/00071660410001730941. [DOI] [PubMed] [Google Scholar]
- Xu H., Bai L., Collins J.F., Ghishan F.K. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)2 vitamin D3. Am. J. Physiol. Cell Physiol. 2002;2823:C487–C493. doi: 10.1152/ajpcell.00412.2001. [DOI] [PubMed] [Google Scholar]
- Yan F., Angel R., Ashwell C.M. Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult. Sci. 2007;861:67–76. doi: 10.1093/ps/86.1.67. [DOI] [PubMed] [Google Scholar]
- Yan F., Keen C.A., Zhang K.Y., Waldroup P.W. Comparison of methods to evaluate bone Mineralization1. J. Appl. Poult. Res. 2005;143:492–498. [Google Scholar]