Abstract
The ongoing pandemic coronavirus disease 19 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a matter of global concern. Environmental factors such as air pollution and smoking and comorbid conditions (hypertension, diabetes mellitus and underlying cardio-respiratory illness) likely increase the severity of COVID-19. Rheumatic manifestations such as arthralgias and arthritis may be prevalent in about a seventh of individuals. COVID-19 can result in acute interstitial pneumonia, myocarditis, leucopenia (with lymphopenia) and thrombocytopenia, also seen in rheumatic diseases like lupus and Sjogren’s syndrome. Severe disease in a subset of patients may be driven by cytokine storm, possibly due to secondary hemophagocytic lymphohistiocytosis (HLH), akin to that in systemic onset juvenile idiopathic arthritis or adult-onset Still’s disease. In the absence of high-quality evidence in this emerging disease, understanding of pathogenesis may help postulate potential therapies. Angiotensin converting enzyme 2 (ACE2) appears important for viral entry into pneumocytes; dysbalance in ACE2 as caused by ACE inhibitors or ibuprofen may predispose to severe disease. Preliminary evidence suggests potential benefit with chloroquine or hydroxychloroquine. Antiviral drugs like lopinavir/ritonavir, favipiravir and remdesivir are also being explored. Cytokine storm and secondary HLH might require heightened immunosuppressive regimens. Current international society recommendations suggest that patients with rheumatic diseases on immunosuppressive therapy should not stop glucocorticoids during COVID-19 infection, although minimum possible doses may be used. Disease-modifying drugs should be continued; cessation may be considered during infection episodes as per standard practices. Development of a vaccine may be the only effective long-term protection against this disease.
|
Key Points • Patients with coronavirus disease 19 (COVID-19) may have features mimicking rheumatic diseases, such as arthralgias, acute interstitial pneumonia, myocarditis, leucopenia, lymphopenia, thrombocytopenia and cytokine storm with features akin to secondary hemophagocytic lymphohistiocytosis. • Although preliminary results may be encouraging, high-quality clinical trials are needed to better understand the role of drugs commonly used in rheumatology like hydroxychloroquine and tocilizumab in COVID-19. • Until further evidence emerges, it may be cautiously recommended to continue glucocorticoids and other disease-modifying antirheumatic drugs (DMARDs) in patients receiving these therapies, with discontinuation of DMARDs during infections as per standard practice. |
Keywords: COVID-19, Epidemiology, Hydroxychloroquine, Hypothesis, Immunity, Pathogenesis, Vaccines, Vitamin D
Introduction
The pandemic resulting from coronavirus disease 19 (COVID-19) caused by the ribonucleic acid (RNA) virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged a few months ago in Wuhan, China, and has claimed more than 30,000 victims worldwide [1–3]. After an initial explosive outbreak of pneumonia of unknown etiology in China, the disease spread first to neighboring Asian countries and then worldwide. The trajectory of COVID-19 spread may remind us of the ancient Silk Route disease, now called Behcet’s syndrome, which affected certain ethnicities, transformed into multisystem neutrophilic disorder and migrated from Asia to Europe millennia ago. The United States of America, Italy, Spain, China, Germany and Iran are now the leading countries with most confirmed cases and related deaths [4]. The detection of SARS-CoV-2 infection relies on a polymerase chain reaction (PCR) analysis of samples from throat and nasal swabs [2]. Reporting of the results of the PCR tests to the World Health Organization (WHO) has resulted in mapping the disease transmission and distinguishing continental Europe and North America as the current hotbed of this pandemic [4]. The dynamics of this pandemic raises concerns over the safety of the global human population and necessitates responsible actions of the whole society. Patients affected with rheumatic diseases represent a particularly vulnerable group, considering that they might be on immunosuppressive therapy. In this article, we provide the perspectives of a rheumatologist on COVID-19, including rheumatic manifestations associated with this disease, and special considerations for this group of patients. We also discuss potential therapeutic targets for management of COVID-19, some of which are commonly used in rheumatology practice.
Risk factors for severe COVID-19
Crude fatality rates vary globally from 5.6 to 15.2% [5], with a greater risk of dying for elderly individuals and those with comorbid conditions such as hypertension and diabetes mellitus [6]. A pooled analysis of 46,248 cases revealed that hypertension (17%, 95% confidence interval [CI] 14–22%), diabetes mellitus (8%, 95% CI 6–11%), cardiovascular disease (5%, 95% CI 4–7%) and respiratory morbidity (2%, 95% CI 1–3%) are the most prevalent comorbidities [7]. Smoking appears to increase the risk of adverse outcomes in COVID-19 [8]. It may increase the expression of angiotensin-converting enzyme 2 (ACE2) in Asian current smokers and make them prone to COVID-19 [9]. Air pollution may also increase the risk of severe COVID-19. High mortality due to COVID-19 in some countries and air pollution are possibly associated [10]. Rheumatologists should be aware of these risk factors for severe COVID-19 as these are also likely to be operational in their patients with rheumatic diseases.
Manifestations of COVID-19 which may mimic rheumatic diseases
Clinical presentation of COVID-19 ranges from asymptomatic to severe pneumonia. Based on the WHO analysis of 55,924 confirmed cases, fever (87.9%), dry cough (67.7%) and fatigue (38.1%) are common symptoms of the infection [11]. Myalgia or arthralgia (14.8%) are also among typical symptoms [11]. Acute bilateral interstitial pneumonia is the major cause of morbidity and mortality in COVID-19. Early diagnosis of pneumonia is preferably based on high-resolution computed tomography since chest radiography can miss pulmonary infiltrates at early stages of the disease [12]. The dynamics of platelet count and its low level may predict severe course and lethal outcome in COVID-19 pneumonia. A recent systematic review identified greater odds of severe COVID-19 in those patients with thrombocytopenia (odds ratio 5.1, 95% CI 1.8–14.6) [13]. Also, severe course is typically associated with low lymphocyte, high leucocyte counts and elevated neutrophil-lymphocyte ratio [14]. Acute interstitial pneumonia, lymphopenia and thrombocytopenia may also be seen with disease activity in systemic lupus erythematosus and Sjogren’s syndrome. Hence, the development of such features in patients with these underlying diseases might result in clinical confusion as to whether this is infective or autoimmune in origin.
Uncontrolled release of inflammatory cytokines such as interleukin (IL)-1ß, IL-6, monocyte chemoattractant protein 1, associated with increased serum ferritin levels and decreased natural killer cell function may result in cytokine storm syndromes [14, 15]. In conjunction with profound immunosuppression, this may suggest secondary hemophagocytic lymphohistiocytosis (HLH), an entity also associated with rheumatic diseases such as systemic lupus erythematosus and systemic onset juvenile idiopathic arthritis. Emerging evidence suggests that a subset of patients (up to one-fourth of patients in some instances) with severe COVID-19 can have myocardial dysfunction or myocarditis [16, 17]. Anecdotal reports suggest benefit with intravenous immunoglobulin with resolution of myocarditis and recovery of cardiogenic shock by 3 weeks [18]. Similarly, patients with COVID-19 have been found to have elevated D-dimer levels and may be at greater risk of venous thromboembolism, although this needs to be prospectively studied and validated in the future [16, 17]. Table 1 summarizes the manifestations associated with COVID-19 which may mimic rheumatic syndromes.
Table 1.
Manifestations associated with coronavirus disease 19 (COVID-19) mimicking rheumatic syndromes
| 1. Arthralgias and Myalgias | |
| 2. Cytopenias: leucopenia (predominantly lymphopenia); thrombocytopenia | |
| 3. Acute interstitial pneumonia-like presentation | |
| 4. Myocarditis | |
| 5. Secondary hemophagocytic lymphohistiocytosis and cytokine storm | |
| 6. Possible greater risk of venous thromboembolism |
Potential drug targets for COVID-19
Various drugs are being investigated for their potential utility in COVID-19. Improved understanding of molecular mechanisms regulating infection with this virus and the subsequent immune responses can pave the way for empirical approaches and trials at this stage.
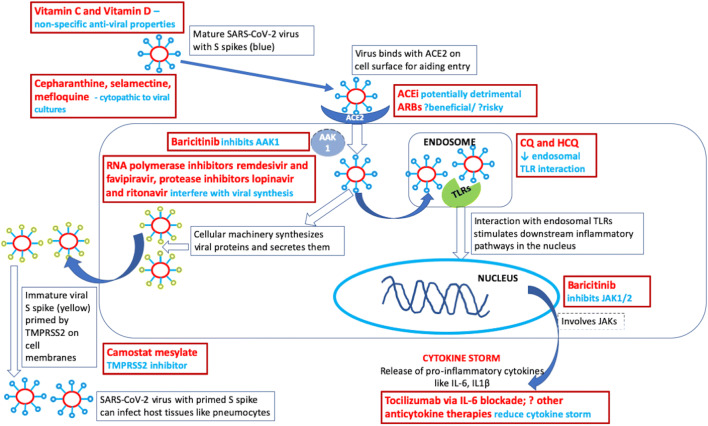
Molecular evidence suggests that SARS-CoV-2 utilizes ACE2 receptor for cellular entry (Fig. 1). ACE2 is expressed on numerous cells, including the epithelial cells of oral mucosa and surfactant-producing type 2 pneumocytes [19, 20]. Type 2 pneumocytes are targets in severe COVID-19 [19]. Presumably, ACE2 is more expressed in Asians than in Whites or African Americans [19]. Likewise, patients with hypertension and those treated with ACE inhibitors and angiotensin receptor blockers may have overly expressed ACE2, inviting the virus into the lower airways and resulting in severe COVID-19. In this regard, soluble recombinant human ACE2 protein may be a promising therapeutic agent in subjects contracting the virus [19]. Also, angiotensin II receptor antagonist losartan is postulated to exert a protective action [19]. At this stage, it must be emphasized that current evidence does not favour either addition or discontinuation of ACE inhibitors or angiotensin receptor antagonists in patients with COVID-19, as recommended presently by cardiology societies [16, 17]. From the rheumatologist’s perspective, there exists a school of thought that the commonly used non-steroidal anti-inflammatory drug ibuprofen is believed to increase the expression of ACE2 [6] and result in severe course and complications in COVID-19 [21, 22]. Such a belief is based on anecdotal reports, pointing to paracetamol as a safer option for those in need of non-steroidal anti-inflammatory therapy. While this is an evolving area of knowledge and contradictory opinions are expressed in the published literature [21, 22], the authors opine that until clearer evidence emerges, it may be prudent to rely on specific circumstances and, if possible, avoid using ibuprofen during this pandemic.
Fig. 1.
Potential therapeutic targets for SARS-CoV-2 and COVID-19. AAK1–AP2-associated protein kinase 1; ACE–angiotensin-converting enzyme; ARB–angiotensin receptor blocker; COVID-19–coronavirus disease 19; CQ–chloroquine; HCQ–hydroxychloroquine; IL–interleukin; JAK–Janus kinase; SARS-CoV-2–severe acute respiratory syndrome coronavirus 2; TLR–toll-like receptor; TMPRSS2–serine protease enzyme
Once the SARS-COV-2 enters the cell, it utilizes the cellular machinery to generate viral proteins and RNA, synthesize and then secrete a mature virion. The maturation of the S spike on the virion (conferring its infectivity) relies on the cellular serine protease enzyme TMPRSS2. Hence, inhibitors of this enzyme such as camostat mesylate may be useful [23]. Inhibitors of RNA polymerase such as remdesivir [24] and favipiravir [25] and protease inhibitors lopinavir and ritonavir [26] affect intracellular virion assembly, and there remains a rationale to further evaluate these drugs in COVID-19 [27]. A recent open-label, randomized, controlled trial evaluated the role of lopinavir and ritonavir in 199 COVID-19 patients with hypoxemia (99 treated with lopinavir/ritonavir and 100 with standard of care). Those treated with lopinavir/rotinavir did not demonstrate any significant benefit in hazard ratio for earlier clinical improvement (1.24 [95%CI 0.9–1.72]) or reduction in mortality at 28 days (− 5.8% [95%CI − 17.3% to 5.7%]). Secondary outcomes revealed that those patients treated with lopinavir/ritonavir demonstrated clinical improvement 1 day earlier than the control group in a modified intention-to-treat analysis, as well as were discharged from the ICU a median of 5 days earlier [28]. While the trial failed to meet its primary objective, it possibly included patients with severe disease alone, and future studies may consider evaluating the role of lopinavir/ritonavir earlier in the course of COVID-19 [29]. Also, the potential for demonstrating a beneficial effect with a larger sample size cannot be ruled out based on the findings of this study. This drug may yet merit further evaluation in clinical trials of COVID-19. Another recent small open-label clinical trial compared favipiravir (35 patients) with lopinavir/ritonavir (45 patients) on a background of interferon alpha therapy in patients from China with milder COVID-19 without hypoxemia or respiratory distress and demonstrated earlier viral clearance in those on favipiravir. Chest radiology showed better improvement in favipiravir-treated patients than those treated with lopinavir/ritonavir at day 14, but not at earlier time points [30].
The mature virion enters the endosome and interacts with toll-like receptors (TLR) to stimulate downstream inflammatory pathways; chloroquine and hydroxychloroquine use interferes with endosomal acidification and inhibits such TLR activation. These drugs also interfere with downstream inflammatory pathways and resultant cytokine production, as well as dampen the inflammatory response via upregulation of regulatory anti-inflammatory molecules [31–33]. A systematic review of available expert opinion pieces and 23 ongoing trials in China supported the notion that chloroquine limits the replication of SARS-CoV-2 [34]. Preliminary reports suggest that chloroquine and hydroxychloroquine are probably equally beneficial in COVID-19 and that a loading dose of the drug should be followed by a maintenance dose, prescribed for a long period [35]. A recent clinical trial evaluated hydroxychloroquine (with or without azathioprine) versus placebo in severe COVID-19 and demonstrated potential benefit for reducing viral replication. The trial reported outcomes for 20 (out of 26 patients) who received active treatment, compared to 16 controls who refused such active treatment. The former group demonstrated negative PCR in 70% at day 6 of treatment (versus 12.5% in the control group). However, this trial had a limited sample size of 36 patients, allocation to active treatment or control was not randomized, only a small proportion of patients had severe disease (lower respiratory tract infection) and clinical outcomes were not reported for all patients. These considerations `potentially undermine the quality of the study [36]. Also, concerns exist regarding the potential for cardiac arrhythmias with chloroquine (and its analogs) as well as with azithromycin and protease inhibitors; hence, due care and careful monitoring for cardiac arrhythmias are essential while using these drugs alone or in combination [37]. Nevertheless, the rationale driving the potential benefits with hydroxychloroquine merits its exploration as a therapeutic agent in COVID-19. Another suggestion has been to provide prophylaxis with hydroxychloroquine (400 mg twice daily for a day, followed by 400 mg once a week for 7 weeks) for healthcare workers dealing with COVID-19 as well as close contacts of these patients, as recommended empirically recently by a national society [38]. There exists a need to generate evidence regarding the role of such prophylactic therapy in COVID-19. The exact clinical scenarios where chloroquine or hydroxychloroquine may be useful in COVID-19 remain to be ascertained, unlike their use in rheumatic diseases where their indications are widely understood [39].
Janus kinases (JAK) 1 and 2 are involved in inflammation, and the enzyme AP-2-associated protein kinase 1 (AAK1) plays a role in viral cellular entry. Based on information generated by bioinformatics analysis, baricitinib may help reduce SARS-CoV-2 infection by inhibiting AAK1, and also possibly dampening the resulting inflammation by JAK1/2 inhibition [40]. The resultant cytokine storm responsible for severe COVID-19 and associated secondary HLH may respond to immunosuppressive agents used for HLH such as tocilizumab (IL-6 blockade) and anakinra (IL-1 blockade) [15]. A recent pre-print retrospectively evaluated the use of a single dose of intravenous tocilizumab 400 mg in 21 patients with COVID-19 from China who either had respiratory distress, hypoxemia or required intensive care support. Nineteen of these 21 patients demonstrated clinical improvement with discharge from hospital by 2 weeks. The findings of this study need to be cautiously interpreted in the context of small numbers, the lack of a control group for comparison and background treatment with antiviral therapies and corticosteroids [41]. Ongoing clinical trials are further evaluating the role of IL-6 blockade with tocilizumab and sarilumab in severe COVID-19; the former drug is also approved in China for this indication [42, 43].
Cepharanthine, selamectine and mefloquine hydrochloride are other drugs that have been demonstrated to be cytopathic to viral cultures of SARS-CoV-2, but their precise mechanisms of action are unclear [44]. Related clinical research studies are warranted. Based on numerous studies of other viruses [45, 46], it is reasonable to hypothesize that vitamins C and D supplementation may boost immunity and help human organism fight COVID-19 and its aggressive effects on all organ systems (Fig. 1). High-dose vitamin D supplementation may be considered for subjects with laboratory-confirmed deficiency, particularly the elderly, obese, those with dark skin and those individuals living at higher latitudes [47]. Based on its protective effects in subjects at risk of chronic diseases, including cancers, cardiovascular disease, respiratory tract infections, diabetes mellitus and hypertension, experts hypothesize that vitamin D supplementation and associated increase of serum 25-hydroxyvitamin D concentration above 50 ng/ml (125 nmol/l) may substantially reduce the incidence and severity of various viral diseases, including COVID-19 [48]. Arguably, there is little evidence base for most of the potential drug therapies in COVID-19 (Table 2). More clinical trials and cohort studies are urgently needed to evaluate the efficacy of preventive and curative agents before evidence-based recommendations can be drafted by professional societies.
Table 2.
Potential drugs for different phases of coronavirus disease 19 (COVID-19), whose role will need to be evaluated in future clinical trials
| Phase of disease | Drugs meriting exploration |
|---|---|
| Early phase/pre-clinical phase | Preventative strategies with chloroquine, hydroxychloroquine, vitamin D, vitamin C. |
| Viremic phase | Antiviral drugs (lopinavir/ritonavir, favipiravir, remdesivir), chloroquine, hydroxychloroquine |
| Cytokine storm phase | Tocilizumab, sarilumab, baricitinib. |
Considerations for patients with rheumatic diseases on immunosuppression
Glucocorticoids have an overall immunosuppressive effect, and these are often used in patients with inflammatory and autoimmune diseases. While glucocorticoids are also often prescribed in sepsis, available evidence does not favor their use to ameliorate the deleterious effect of lung inflammation caused by viral infections [49]. On this basis, the authors opine that it may be cautiously recommended to manage patients on long-term corticosteroid therapy by gradually tapering doses to 5–7.5 mg/daily during this pandemic. It is also advisable to stick to the recommendations of rheumatology societies, warning against abrupt cessation of corticosteroid therapy. There exists little evidence to guide the course of COVID-19 in patients on immunosuppressive therapy. Until more data emerges, it is considered prudent to continue any ongoing immunosuppressive therapy. Such drugs (other than glucocorticoids) are often discontinued during an episode of infection, and it seems reasonable to pursue a similar strategy for the time being even in the case of COVID-19 infection (Table 3) [50–53].
Table 3.
Summary of recommendations from rheumatology societies (EULAR, ACR, BSR, ARA) for patients with rheumatic diseases during coronavirus disease 19 (COVID-19) outbreak [50–53]
| 1. Practising sneeze/cough hygiene, regular hand washing, avoiding touching the face, keeping away from crowded places, social distancing, avoiding busy public transport and cancelling unnecessary travel is recommended. | |
| 2. Use of a mask is recommended for those with suspected and confirmed infection. In such instances, N95 respirators with appropriate fit to the face are advisable. | |
| 3. Abrupt discontinuation of glucocorticoid therapy should be avoided, even during active infection. | |
| 4. If patients are on disease-modifying antirheumatic drugs, including biologics, small molecules, and other immunosuppressive agents, standard practices may be followed to discontinue them should one develop infection. | |
| 5. Routine face-to-face appointments should be delayed until the outbreak settles. Both patients and healthcare personnel should consider substituting face-to-face appointments with video appointments if feasible. | |
| 6. Patients should be updated about appropriate flu and pneumococcal vaccination practices. |
ACR American College of Rheumatology, ARA Australian Rheumatology Association, BSR British Society for Rheumatology, EULAR European League against Rheumatism
Measures at a societal level and preparing for future outbreaks
It is also important to implement restrictive measures at a societal level to mitigate the spread of the virus. Emerging evidence suggests that the virus spreads by droplets and fomites [54]. Therefore, the importance of regular hand washing, sneeze and cough hygiene and the use of personal protective equipment for healthcare workers managing COVID-19 patients are likely to help mitigate the spread of this infection [50–53].
Many afflicted countries are now under lockdown or have severe travel restrictions. This is an effort to break the chain of transmission of the virus. Controlling the infection by imposing severe restrictions on travel and social contacts can be viewed as the main strategy, primarily benefiting the elderly and those with chronic comorbid conditions [55]. Screening for the virus safely and enforcing isolation of confirmed cases can also prevent short- and long-term adverse outcomes of this pandemic [56]. However, an analysis of the transmission of COVID-19 in China in January–February 2020 revealed that 86% of subjects potentially contracted the virus from patients with no or minimal symptoms, pointing to the difficulties of containing the virus spread [57]. Recently, the prolonged excretion of SARS-CoV-2 in feces for a mean period of 4 weeks has been documented, which further present difficulties in attempts to limit the community spread of disease [58]. These recent findings also point to the urgent need to develop and experiment with SARS-CoV-2 vaccines that may prevent further seasonal outbreaks of the infection. Experts also proposed a hypothesis that measles and rubella vaccines may generate cross-resistance against SARS-CoV-2, which is based on the observations that Chinese children vaccinated against measles are less vulnerable to COVID-19 than the elderly; these merit further exploration [59]. Given the dynamics of the influenza outbreaks in the early twentieth century [60], it is possible to predict a temporary remission in the COVID-19 pandemic in the coming summer months. High temperatures and heat waves are expected to minimize virulence of SARS-CoV-2. Other factors such as increased daytime hours and increased ultraviolet exposure may also exert additive beneficial effects [61].
To sum up, rheumatologists should closely monitor current COVID-19 pandemic, raise the global awareness of the virus characteristics and its targets, and explore strategies to minimize the incidence of severe cases. While waiting for evidence-based recommendations, empirical preventive measures should be implemented. Relying on expert opinion and rapidly accumulating evidence is currently the best way to avoid major mistakes. Balanced and well-informed actions by all specialists and the whole society are urgently needed. It is also important to be aware of the ongoing postulates regarding potential immune-mediated injury in severe COVID-19 akin to secondary HLH. In such situations, rheumatologists may be called upon to decide on appropriate immunosuppressive strategies, and such decisions may need to be taken on a case-to-case basis, in the face of limited evidence to guide them.
Authors’ contributions
The conception and design of the study–DPM, AYG; acquisition of data, analysis and interpretation of data–DPM, VA, OZ, AYG.
Drafting the article–DPM, VA; revising it critically for important intellectual content–OZ, AYG.
Final approval of the version to be submitted–DPM, VA, AYG, OZ.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved–DPM, VA, AYG, OZ.
Compliance with ethical standards
Conflict of interest
Durga Prasanna Misra declares that he has no conflict of interest, including no relationship with pharmaceutical companies.
Vikas Agarwal declares that he has no conflict of interest, including no relationship with pharmaceutical companies.
Olena Zimba declares that she has no conflict of interest, including no relationship with pharmaceutical companies.
Armen Yuri Gasparyan declares that he has no conflict of interest, including no relationship with pharmaceutical companies.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclaimer
All views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any institution or association.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 cases worldwide. https://www.worldometers.info/coronavirus/. Accessed on 29 Mar 2020
- 2.Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed]
- 3.Habibzadeh P, Stoneman EK. The novel coronavirus: a Bird's eye view. Int J Occup Environ Med. 2020;11:65–71. doi: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Situation report 68 by the World Health Organization. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200328-sitrep-68-covid-19.pdf?sfvrsn=384bc74c_2.2. Updated on 28 March 2020. Accessed on 29 Mar 2020
- 5.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G (2020) Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 10.1016/s1473-3099(20)30195-x [DOI] [PMC free article] [PubMed]
- 6.Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 10.1016/s2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed]
- 7.Yang J, Zheng Y, Gou X et al (2020) Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 10.1016/j.ijid.2020.03.017
- 8.Liu W, Tao ZW, Lei W et al (2020) Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 10.1097/cm9.0000000000000775 [DOI] [PMC free article] [PubMed]
- 9.Cai H (2020) Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 10.1016/s2213-2600(20)30117-x [DOI] [PMC free article] [PubMed]
- 10.Environmental issues in Iran. https://www.theguardian.com/world/iran-blog/2014/nov/21/iran-environmental-consequences-of-sanctions. Updated 2014. Accessed on 19 Mar 2020
- 11.Report of the WHO-China Joint Mission on Coronavirus Disease 19 (COVID-19). Available at https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19%2D%2D-final-report-1100hr-28feb2020-11mar-update.pdf?sfvrsn=1a13fda0_2&download=true. Updated 28 February 2020. Accessed on 24 Mar 2020
- 12.Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35:e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G, Plebani M, Michael Henry B (2020) Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed]
- 14.Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed]
- 15.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 10.1016/s0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed]
- 16.Clerkin Kevin J, Fried Justin A, Raikhelkar J et al (2020) Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 10.1161/CIRCULATIONAHA.120.046941
- 17.Driggin E, Madhavan MV, Bikdeli B et al (2020) Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 27204. 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed]
- 18.Hu H, Ma F, Wei X, Fang Y (2020) Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed]
- 19.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. doi: 10.1042/cs20200163. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day M (2020) Covid-19: European drugs agency to review safety of ibuprofen. 368:m1168. 10.1136/bmj.m1168 [DOI] [PubMed]
- 22.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. Bmj. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed]
- 24.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 26.Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emanuel EJ, Persad G, Upshur R et al (2020) Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 10.1056/NEJMsb2005114 [DOI] [PubMed]
- 28.Cao B, Wang Y, Wen D et al (2020) A trial of Lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed]
- 29.Baden LR, Rubin EJ (2020) Covid-19 — the search for effective therapy. N Engl J Med. 10.1056/NEJMe2005477 [DOI] [PMC free article] [PubMed]
- 30.Cai Q, Yang M, Liu D et al (2020) Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed]
- 31.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Tian Z, Yang X (2020) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 10.5582/bst.2020.01047 [DOI] [PubMed]
- 33.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 34.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (2020) A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 10.1016/j.jcrc.2020.03.005 [DOI] [PMC free article] [PubMed]
- 35.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 105932. 10.1016/j.ijantimicag.2020.105932 [DOI] [PMC free article] [PubMed]
- 36.Gautret P, Lagier J-C, Parola P et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed]
- 37.Mason JW. Antimicrobials and QT prolongation. J Antimicrob Chemother. 2017;72:1272–1274. doi: 10.1093/jac/dkw591. [DOI] [PubMed] [Google Scholar]
- 38.Indian Council of Medical Research guidelines for use of empirical use of hydroxychloquine prophylaxis in COVID-19. https://icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommendation_22March_final_MM_V2.pdf. Updated 23 March 2020, Accessed on 24 Mar 2020
- 39.Ramos-Casals M, Brito-Zeron P, Bombardieri S, et al. EULAR recommendations for the management of Sjogren's syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79:3–18. doi: 10.1136/annrheumdis-2019-216114. [DOI] [PubMed] [Google Scholar]
- 40.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/s0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Han M, Li T et al (2020) Effective treatment of severe COVID-19 patients with Tocilizumab.[ChinaXiv:202003.00026]. Available at http://www.chinaxiv.org/abs/202003.00026. Updated on 05 March 2020. Accesed on 29 Mar 2020
- 42.Approval for tocilizumab use in severe Covid-19 in China. https://www.pharmaceutical-technology.com/news/roche-actemra-coronavirus-complications/. Updated 05 March 2020. Accessed on 19 Mar 2020
- 43.Trial in interleukin-6 blockade with sarilumab for Covid-19. https://www.clinicaltrialsarena.com/news/sanofi-regeneron-trial-kevzara-covid-19/. Updated 16 March 2020. Accessed on 19 Mar 2020
- 44.Fan HH, Wang LQ, Liu WL et al (2020) Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin Med J. 10.1097/cm9.0000000000000797 [DOI] [PMC free article] [PubMed]
- 45.Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti-Infect Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 46.Goncalves-Mendes N, Talvas J, Duale C, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant WB, Al Anouti F, Moukayed M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur J Clin Nutr. 2020;74:366–376. doi: 10.1038/s41430-020-0564-0. [DOI] [PubMed] [Google Scholar]
- 48.Grant WB, Lahore H, McDonnell SL et al (2020) Vitamin D supplementation could prevent and treat influenza, coronavirus, and pneumonia infections. Preprints 2020030235 10.20944/preprints202003.0235.v1
- 49.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.EULAR guidance for patients during Covid-19 outbreak. https://www.eular.org/eular_guidance_for_patients_covid19_outbreak.cfm. Updated 17 March 2020. Accessed on 19 Mar 2020
- 51.BSR guidance for patients during Covid-19 outbreak. https://www.rheumatology.org.uk/News-Policy/Details/Covid19-Coronavirus-update-members. Updated 25 March 2020. Accessed on 29 Mar 2020
- 52.ACR guidance for patients during Covid-19 outbreak. https://www.rheumatology.org/announcements. Updated 28 March 2020. Accessed on 29 Mar 2020
- 53.Australian Rheumatology Association guidance for patients during Covid-19 outbreak. https://arthritisaustralia.com.au/advice-regarding-coronavirus-covid-19-from-the-australian-rheumatology-association/. Updated 17 March 2020. Accessed on 19 Mar 2020
- 54.van Doremalen N, Bushmaker T, Morris DH et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed]
- 55.Wilder-Smith A, Freedman DO (2020) Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med 27. 10.1093/jtm/taaa020 [DOI] [PMC free article] [PubMed]
- 56.Kwon KT, Ko J-H, Shin H, Sung M, Kim JY (2020) Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci 35:e123. 10.3346/jkms.2020.35.e123 [DOI] [PMC free article] [PubMed]
- 57.Li R, Pei S, Chen B, et al. (2020) Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science eabb3221. 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed]
- 58.Wu Y, Guo C, Tang L et al (2020) Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 10.1016/s2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed]
- 59.Adnan Shereen M, Khan S, Kazmi A, Bashir N, Siddique R (2020) COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed]
- 60.Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–278. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hindawi SI, Hashem AM, Damanhouri GA, el-Kafrawy SA, Tolah AM, Hassan AM, Azhar EI. Inactivation of Middle East respiratory syndrome-coronavirus in human plasma using amotosalen and ultraviolet a light. Transfusion. 2018;58:52–59. doi: 10.1111/trf.14422. [DOI] [PMC free article] [PubMed] [Google Scholar]