Summary
There is a growing appreciation that the regulation of the melatonergic pathways, both pineal and systemic, may be an important aspect in how viruses drive the cellular changes that underpin their control of cellular function. We review the melatonergic pathway role in viral infections, emphasizing influenza and covid‐19 infections. Viral, or preexistent, suppression of pineal melatonin disinhibits neutrophil attraction, thereby contributing to an initial “cytokine storm”, as well as the regulation of other immune cells. Melatonin induces the circadian gene, Bmal1, which disinhibits the pyruvate dehydrogenase complex (PDC), countering viral inhibition of Bmal1/PDC. PDC drives mitochondrial conversion of pyruvate to acetyl‐coenzyme A (acetyl‐CoA), thereby increasing the tricarboxylic acid cycle, oxidative phosphorylation, and ATP production. Pineal melatonin suppression attenuates this, preventing the circadian “resetting” of mitochondrial metabolism. This is especially relevant in immune cells, where shifting metabolism from glycolytic to oxidative phosphorylation, switches cells from reactive to quiescent phenotypes. Acetyl‐CoA is a necessary cosubstrate for arylalkylamine N‐acetyltransferase, providing an acetyl group to serotonin, and thereby initiating the melatonergic pathway. Consequently, pineal melatonin regulates mitochondrial melatonin and immune cell phenotype. Virus‐ and cytokine‐storm‐driven control of the pineal and mitochondrial melatonergic pathway therefore regulates immune responses. Virus‐and cytokine storm‐driven changes also increase gut permeability and dysbiosis, thereby suppressing levels of the short‐chain fatty acid, butyrate, and increasing circulating lipopolysaccharide (LPS). The alterations in butyrate and LPS can promote viral replication and host symptom severity via impacts on the melatonergic pathway. Focussing on immune regulators has treatment implications for covid‐19 and other viral infections.
Keywords: aryl hydrocarbon receptor, covid‐19, immune, influenza, melatonin, metabolism, mitochondria, sirtuin, treatment, viral infection
1. INTRODUCTION
Viruses, including SARS, and influenza, can change quickly, thereby negating the efficacy of developed vaccines and targetted antiviral drugs. 1 Consequently, it is important to utilize any substance that can more widely limit viral effects. Often, as in the case of covid‐19, there is little likelihood of developing a vaccine or antiviral within a year of the emergence of such a devastating infection. Utilizing or developing more generic antivirals is a significant challenge, given that viruses can vary considerably as to the their effects, including on the immune system. For example, a general antiviral requirement for influenza and covid‐19 would be for a treatment that limits the initial innate immune‐associated “cytokine storm” where evident, while also acting to increase the efficacy of the adaptive immune response. 2 However, for the Ebola virus, such initial dampening of the innate immune response is not so clinically relevant. Allowing for such variability in virus effects, melatonin has emerged as a top candidate for protection against an array of different viruses, including the coronavirus.3, 4, 5 This would seem to arise from the close evolution of melatonin within the emerging mitochondria in the first single‐cellular organsims, allowing melatonin to be intimately associated with mitochondrial function, and thereby with immune system regulation. 6 The interactions of our “bacterial‐driven” coexistence with viruses have provided a “War of the Worlds” from which plant and animal evolution have emerged, and in which evolution has acted to tolerate and limit rather than annihilate. Melatonin is an important aspect of our interactions with viruses.
As data pertaining to the pathophysiology of covid‐19 have still to emerge, the current article focusses primarily on the role of melatonergic pathway in the management of influenza viruses, as well as highlighting its potential utility in supporting people at high risk of fatality from influenza viruses and covid‐19. At the time of writing, data from China and Italy indicate that the elderly are at far higher risk of covid‐19 linked mortality, primarily as a consequence of ageing‐associated conditions, such as cardiovascular, pulmonary, and diabetic disorders as well as in immune‐compromised conditions. Melatonin has clinical utility in all of these health conditions, suggesting its utility in buffering against covid‐19 interactions with these medical conditions. However, the interactions of the melatonergic pathway with viruses may be more complex than ameliorative effects on ageing‐associated preexisting medical conditions. First, the melatonergic pathway is reviewed and how this pathway may interact with the effects of the influenza, covid‐19, and other viruses. This has treatment implications as well as providing indications as to important future research on how an understanding of human physiological systems can provide treatment targets that regulate the impact of viral infections, including influenza, and covid‐19 driven pathophysiology.
2. MELATONERGIC PATHWAY
Melatonin is classically associated with its night‐time release from the pineal gland, where its effects on circadian entrainment have long been recognized. Melatonin modulates cellular function via a number of processes, including the activation of an intracellular signalling pathways and transcription factors, which act to dampen inflammatory activity. Melatonin is also a powerful antioxidant and antiinflammatory that mediates many of its effects via the optimization of mitochondrial function. Emerging data indicate that the melatonergic pathway is evident in all cells, not only those of the pineal gland, and that it may be predominantly present within mitochondria. Figure 1 shows the enzymes and factors forming the melatonergic pathway, and how different processes can modulate this pathway.
FIGURE 1.
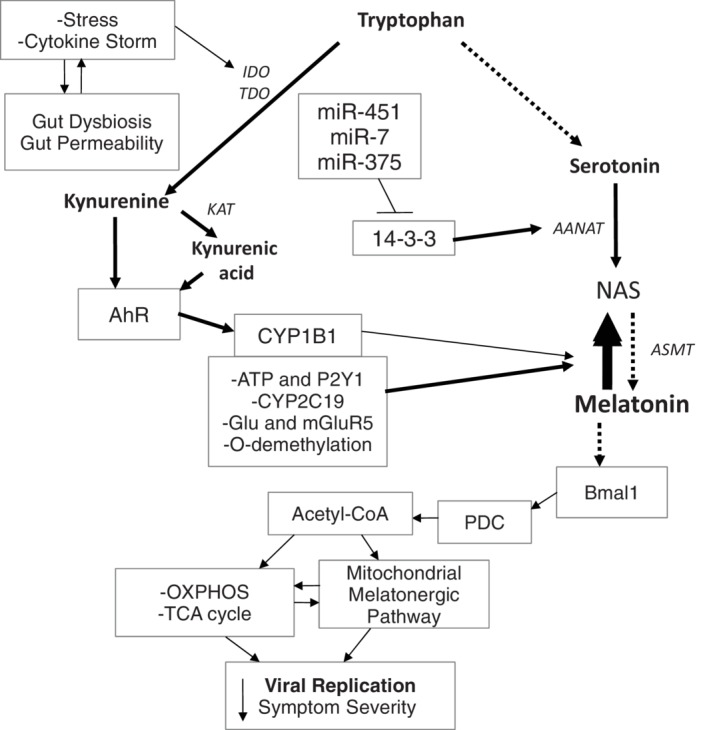
The cytokine storm and stress can increase gut dysbiosis/permeability, further contributing to cytokine induced IDO and TDO, leading to kynurenine and kynurenic acid, which activate the AhR to increase CYP1B1 and regulate the NAS/Melatonin ratio. Other factors, including CYP2C19, mGluR5, P2Y1, and O‐demethylation can also regulate the NAS/melatonin ratio. The miRNAs, miR‐7, miR‐375, and miR‐451 are increased following many viral infections, thereby suppressing 14‐3‐3 and the stabilization of AANAT, leading to melatonergic pathway inhibition. The suppression of melatonin, including from an increase in the NAS/melatonin ratio, attenuates melatonin's induction of Bmal1 and therefore the circadian regulation of mitochondria. Bmal1 induces PDC, leading to an increase in OXPHOS, the TCA cycle and the acetyl‐CoA that is a necessary co‐substrate for AANAT and melatonergic pathway activation. The decrease in pineal and mitochondrial melatonin contributes to an increase in the replication and severity of many viral infections. The arrows indicate “stimulation”, with a crossed‐line indicating “inhibitory”
Melatonin is derived from tryptophan, via its conversion to serotonin. Arylalkylamine N‐acetyltransferase (AANAT) converts serotonin to N‐acetylserotonin (NAS), which is then converted to melatonin by acetylserotonin methyltransferase (ASMT). However, variations in the NAS/melatonin ratio occur and have been largely ignored in previous research, with melatonin synthesis having been seen as the ultimate aim of the melatonergic pathway. A number of factors may act to regulate the NAS/melatonin ratio by a variety of mechanisms in different cell types, including O‐demethylation/cytochrome P450 (CYP)2C19, CYP1B1, ATP, and the purinergic receptor (P2Y1), as well as intercellular glutamate and metabotropic glutamate receptor (mGluR5) activation.7, 8, 9, 10, 11, 12, 13 Variations in the NAS/melatonin ratio exist may be of significant clinical importance in a range of medical conditions, including glioblastoma 14 and multiple sclerosis. 15 Although NAS and melatonin can have similar antioxidant and antiinflammatory effects, the NAS/melatonin ratio may be of importance given that NAS activates the brain derived neurotrophic factor (BDNF) receptor, TrkB, as well as inducing BDNF. Two products of the kynurenine pathway, kynurenic acid, and kynurenine, can activate the aryl hydrocarbon receptor (AhR), which then induces CYP1B1. This allows proinflammatory cytokines, cortisol and oxidative stress, which increase indoleamine 2,3‐dioxygenase (IDO) and tryptophan 2,3‐dioxygenase (TDO), to increase AhR ligands, as well as other kynurenine pathway products. Consequently, proinflammatory conditions as well as heightened levels of psychological stress and oxidative stress not only decrease tryptophan availability for the serotonergic and melatonergic pathways, but may also modulate the NAS/melatonin ratio, including via AhR activation (Figure 1). Such data would indicate that the NAS/melatonin ratio may be an important sensor of wider body systems, with consequences for intracellular and extracellular function.
2.1. Interactions of pineal and mitochondrial melatonin
The circadian entrainment induced by pineal melatonin is strongly driven by melatonin's upregulation of the circadian gene, Bmal1, which seems to mediate many of melatonin's effects in mitochondria. Bmal1 inhibits pyruvate dehydrogenase kinase (PDK), leading to the disinhibition of the pyruvate dehydrogenase complex (PDC). PDC drives the conversion of pyruvate to acetyl‐coenzyme A (acetyl‐CoA) in mitochondria, thereby increasing the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS) and ATP production. Acetyl‐CoA is also a necessary co‐substrate for AANAT, and therefore allows pineal melatonin via the Bmal1‐PDK‐PDC path to increase the activation of the mitochondrial melatonergic pathway. For this to happen, AANAT needs to be stabilized by 14‐3‐3 protein, which is present within the mitochondrial matrix.16, 17 It has been proposed that AANAT may also be stabilized by the mitochondrial ionic channel (LETM1), which has a 14‐3‐3‐like motif in its C‐terminal within the mitochondrial matrix. 18
The pineal melatonin/mitochondrial melatonin path may be most important in immune cells, although relevant in all cells subject to circadian regulation by pineal melatonin. The daytime activation of immune cells is associated with glycolytic metabolism, which pineal melatonin resets to OXPHOS, thereby inducing more quiescent immune cell phenotyes. It is by such processes that immune cells are “dampened” at night. However, in the presence of indicants of required immune‐inflammatory activity at night, proinflammatory cytokines can “switch off” pineal melatonin production, thereby forming another branch of the immune‐pineal axis. 19 As such, the circadian rhythm, as driven by pineal melatonin, is a powerful immune regulator, with effects that can include the upregulation of the mitochondrial melatonergic pathway. A decrease in pineal melatonin, as often occurs in the elderly and in conditions associated with high susceptibility to severe viral infection, can therefore have a significant impact on the mitochondrial metabolism and phenotype of immune cells, as well as of other cell types, including CNS glial cells. The suppression of pineal melatonin is proposed to increase the susceptibility to an array of medical conditions, including cancers,16, 17 via the loss of this night‐time shift from glycolytic metabolism to OXPHOS. Such interactions of pineal melatonin with mitochondrial metabolism and the melatonergic pathway provide important points of impact by viral infections.
2.2. Viral pathophysiology
Viruses can have quite distinct interactions with the host over the course of an infection, making generalizations across different viruses impossible. However, some factors have been found to regulate the effects of many different viruses. One such factor is the AhR.
2.3. Aryl hydrocarbon receptor
The AhR has a number of endogenous and exogenous ligands, including 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin (TCDD), commonly found in cigarette smoke; polychlorinated biphenyl congener 126 (PCB126), a common pollutant; the novel pharmaceutical, 2‐(1′H‐indole‐3′‐carbonyl)‐thiazole‐4‐carboxylic acid methyl ester (ITE); and the endogenous AhR ligand, 6‐formylindolo[3,2‐b]carbazole (FICZ), which is a tryptophan degradation product. All of these ligands can modulate immune responses to viral infection.20, 21 However, it is the activation of the AhR by kynurenine and kynurenic acid that more readily links the AhR to immune inflammatory activity, as shown in Figure 1. AhR activation by different ligands can result in some differential effects, complicating the understanding of AhR activation. The AhR is also differentially expressed over the circadian rhythm, thereby linking the AhR to variations in the immune response over the circadian rhythm. 22 As noted, AhR activation induces an increase in mitochondrial CYP1B1, thereby contributing to an elevated NAS/melatonin ratio. Given proinflammatory cytokine induction of AhR ligands, AhR activation is therefore an aspect of the “cytokine storm” that can follow viral infection, thereby contributing to suppressed serotonin and melatonin availability, whilst also having consequences for the regulation of the mitochondrial melatonergic pathway, including the NAS/melatonin ratio.
A number of studies show the AhR modulates the antiviral immune response, including the prototypical coronavirus, murine hepatitis virus. 23 These authors show that the AhR is an important mediator of how this coronavirus modulates the host's immune response, leading to the expression of several effector genes, including TCDD‐inducible poly(ADP‐ribose) polymerase (TiPARP), which is required for maximal coronavirus replication. This study also showed that the AhR modulates macrophage and dendritic cell responses, including the levels of interleukin (IL)‐1β, IL‐10, and tumor necrosis factor (TNF)‐α. 23 Other data show the different AhR ligands differentially modulate immunoglobulin (Ig)G and CD8+ T‐cell responses to the influenza virus, with the loss of CYP1 attenuating AhR‐driven effects on virus‐driven immune responses. 20 Whether such AhR‐driven effects are regulated by variations in the mitochondrial melatonergic pathway and NAS/melatonin ratio requires investigation, including as to the relevance of these AhR‐associated pathways in covid‐19 infection. Overall, the AhR may be an important link between the initial “cytokine storm” and alterations in mitochondrial and immune cell function, including via alterations in the melatonergic pathway.
2.4. Circadian rhythm
The influence of the circadian rhythm is also seen following influenza vaccination where the timing of vaccination and of sample collection modulates the B‐cell response measures, especially in the elderly. 24 The host circadian rhythm is a significant regulator of the response to viral infection. Preclinical data show that irrespective of the viral burden, survival following influenza viral infection is determined by the circadian influence on the patterning of the immune response. 25 The circadian gene, Bmal1, is a significant inhibitor of herpes simplex virus (HSV)‐1 and influenza viruses, highlighting the role of circadian factors in the regulation of viral infections. 26 As the loss of the circadian rhythm can increase viral replication, viruses can act to sabotage circadian regulation, exemplified by HSV‐1 suppression of Bmal1. 26 This overlaps to data in the lung, where pulmonary airway epithelial cell Bmal1 regulates the response to the influenza virus, 27 with the influenza virus also decreasing Bmal1, thereby contributing to the circadian disruption induced by this virus. 28 Such data highlight the circadian nature of host physiology, including in many virus‐relevant pathways, upon which viruses act to promote their survival and proliferation.
2.5. Circadian rhythm and mitochondrial metabolism
As noted, the effects of pineal melatonin include Bmal1 induction, which is a major driver of pineal melatonin's influence on circadian rhythms, with Bmal1 acting to regulate mitochondrial metabolism. The effects of melatonin‐induced Bmal1 include the suppression of PDK and the disinhibition of PDC, thereby increasing the conversion of pyruvate to acetyl‐CoA for the TCA cycle, OXPHOS and ATP production. Concurrently, acetyl‐CoA acts to upregulate the mitochondrial melatonergic pathway, with consequences that include the stimulation of sirtuin‐3 and superoxide dismutase 2 (SOD2). The circadian impacts on mitochondrial metabolism have been most thoroughly investigated in immune cells. However, this would seem relevant to the regulation of metabolism in all cells. As such, the effects of viral infection on the circadian rhythm are intimately linked to alterations in mitochondrial metabolism and the nature of the immune response. Within this framework, the viral suppression of pineal melatonin will be intimately associated with concurrent alterations in the mitochondrial melatonergic pathway, and thereby with key aspects of mitochondrial metabolism. Consequently, some viruses act to inhibit both pineal and mitochondrial melatonin production.
The importance of such processes is given some support by data in humans with a severe influenza infection and associated poor survival rates. Severe influenza and covid‐19 infections seem driven by an initial “cytokine storm”, with the increase in proinflammatory cytokines acting to suppress pineal melatonin production. 29 This is the essence of the immune‐pineal axis, 19 whereby proinflammatory cytokines act to signal the need for an ongoing immune response and thereby prevent its suppression by pineal melatonin. The major driver of the multiorgan failure that is evident in severe influenza infection is the dysregulation of mitochondrial metabolism, 30 and the lost influence of decreased pineal melatonin on such metabolic dysregulation. An elevation in cellular trypsin, as a hemagglutinin processing protease for viral multiplication, is often an aspect of this, leading to the “influenza virus‐cytokine‐trypsin” cycle, which is coupled to a decrease in mitochondrial ATP. 30
Work on the “influenza virus‐cytokine‐trypsin” cycle has led to new proposed treatments, which target the ATP crisis and multiorgan failure that occur during the late phase of infection. This contrasts to the utilization of antiviral treatments with neuraminidase inhibitors, which are targeted to processes that are evident in the initial phase of influenza infection. Treatment options proposed include the restoration of mitochondrial OXPHOS, thereby countering infection‐induced PDK4 activation and therefore PDC inhibition. Clearly, the reestablishment of the effector of the circadian rhythm, by melatonin treatment, would more naturally target an upregulation of Bmal1 and mitochondrial OXPHOS. As to how viruses that suppress Bmal1, interact with exogenous melatonin will be important to determine, including as to the most effective melatonin dose, if applicable.
As IL‐1β seems an important proinflammatory cytokine in the upregulation of the “influenza virus‐cytokine‐trypsin” cycle, this would indicate a role for the induction of the NOD‐, LRR‐, and pyrin domain‐containing protein (NLRP)3 inflammasome. 31 Most viruses increase their proliferation and survival via an increase in the NLRP3 inflammasome, 32 with the regulation of the NLRP3 inflammasome being taken over by viruses. 33 As melatonin is a significant inhibitor of the NLRP3 inflammasome, 34 the suppression of melatonin production, both pineal and mitochondrial, by viruses is likely to be an important aspect of how viruses disengage the NLRP3 inflammasome from physiological processes. It should also be noted that IL‐18 is induced along with IL‐1β from NLRP3 inflammasome activation, with IL‐18 being relatively under‐investigated in viral research, as in many other medical conditions. 35 IL‐18 and IL‐1β pathway interactions may also be relevant to the interactions of preexisting bacterial infection with new covid‐19 or influenza infection in the regulation of disease severity and patient survival. 36 Overall, the viral inhibition of melatonin may contribute to increased NLRP3 inflammasome, and thereby to viral proliferation and survival.
2.6. Sirtuins
Sirtuins are an important aspect of mitochondrial metabolism, both directly via mitochondria‐located sirtuins (sirtuin‐3, −4, −5) and indirectly via cytosolic sirtuin‐1 induction of peroxisome proliferator‐activated receptor gamma (PPAR‐γ)/PPAR‐γ coactivator1α (PGC‐1 α), which is a major regulatory path of mitochondrial metabolism. The sirtuins are also an aspect of the circadian rhythm. 37 Alterations in the levels and effects of cytosolic, nuclear, and mitochondrial sirtuins are also evident in a number of viral infections, including within dendritic cells, which have an important role in regulating the patterned immune response. 38 Generally, the sirtuins seem to be evolutionary conserved antiviral agents. 39 Like the circadian rhythm, viruses may act to disengage sirtuins from their regular physiological processes. The evolutionary conserved relationship of melatonin‐induced sirtuins is important to the maintenance of mitochondrial function, 40 highlighting another important aspect of normal mitochondrial function that viruses can disengage via the suppression of pineal and mitochondrial melatonin.
2.7. microRNAs
microRNAs (miRNA) are important mediators of cellular co‐ordination, with each miRNA regulating up to 100 genes. Consequently, alterations in the regulation of miRNAs are another aspect of how viruses modulate immune and wider cell function. A number of miRNAs act to suppress YWHAZ/14‐3‐3ζ, including miR‐7, miR‐375, and miR‐451, thereby suppressing the 14‐3‐3ζ stabilization of AANAT and leading to a general inhibition of the melatonergic pathway. Increased miR‐451 is one of the mechanisms whereby the influenza virus acts to regulate dendritic cells, and therefore patterned immune activity. 41 The influenza virus also increases miR‐7, 42 whilst miR‐375 can have contrasting effects of viral survival and replication. 43 Although miRNAs can influence multiple genes, the impact of quite distinct viruses on these mitochondrial melatonergic pathway regulating miRNAs would indicate an important role for this pathway in to how viruses can determine cellular function.
2.8. Gut microbiome
There is a growing interest in the role of the gut microbiome across a host of diverse medical conditions, including neurodegenerative and psychiatric conditions, 44 but also immune‐mediated conditions such as multiple sclerosis and arthritis.15, 45 In line with this, recent data show that many viral infections, including influenza, drive changes in the gut and lung microbiomes, 46 with viral‐mediated changes in the gut including gut dysbiosis and increased gut permeability. These authors also show how such changes in the gut microbiome/permeability can contribute to secondary bacterial pneumonia. 46 The high levels of circulating proinflammatory cytokines that are evident in many viral infections, especially during the “cytokine storm” increase gut permeability both directly and via mucosal mast cells. Mucosal mast cell TNF‐α, as increased by stress‐induced corticotropin releasing hormone (CRH), 47 also mediates psychological stress‐induced gut dysbiosis/permeability. These are two mechanisms whereby infection induced increases in gut dysbiosis/permeability can occur, with consequences for mitochondria and immune cell function, as well as for the severity of viral infections.
There are a number of ways that the gut microbiome/permeability can be relevant to viral infection pathophysiology, including from a reduction in the gut microbiome‐derived short‐chain fatty acid, butyrate, to an increase in the levels of circulating lipopolysaccharide (LPS) arising from an increase in gut permeability. 48 As the alterations in butyrate and LPS can impact mitochondrial function and immune system responses, including from changes in the regulation of immune cell mitochondria, such changes in gut processes provide another focus for viral interactions with wider body systems.
Gut dysbiosis and increased gut permeability are intimately linked. Butyrate acts to maintain the gut barrier, at least in part via an increase in the melatonergic pathway in intestinal epithelial cells. 49 Butyrate is also an immune‐suppressant via its capacity to disinhibit PDC and thereby increase mitochondrial OXPHOS, TCA cycle, and acetyl‐CoA for the melatonergic pathway. 17 It seems not unlikey that butyrate therefore acts on the mitochondrial melatonergic pathway to increase melatonin production, as suggested by its induction of the melatonergic pathway in intestinal epithelial cells. 49 Dysregulating the gut microbiome and decreasing gut‐derived butyrate may therefore be another mechanism whereby viruses can act to modulate mitochondrial and immune cell function. Butyrate is also a histone deacetylase (HDAC) inhibitor, thereby allowing it have epigenetic regulatory effects that are relevant to viral infections. A number of HDACs act to regulate viral infections, with the inhibition of HDAC1 leading to a decrease in influenza‐driven pneumonia infections. 50 The role of gut microbiome‐derived butyrate, and the nutriceutical sodium butyrate, in the regulation of viral infections clearly requires further investigation.
The increase in gut permeability driven by proinflammatory cytokines allows for an elevation in the levels of circulating LPS from the early phases of some viral infections. By activating toll‐like receptor (TLR)2/4, circulating LPS can modulate the immune system, as well as many other cells. As most viruses first make contact with their host's mucosal surfaces, which are typically already colonized with bacterial microbials, bacteria‐virus interactions are a long‐established integral aspect of viral infections. 51 TLR4 activation by LPS potentiates the lethality of influenza virus infection in preclinical models by increasing proinflammatory cytokine production and the glycolytic metabolic reprogramming of dendritic cells, which the authors propose to be partly mediated by the induction of the TLR4 ligand, high‐mobility group box (HMGB)1. 52 This is supported by other preclinical data. 53
Under conditions of increased gut permeability, intestinal epithelial cells increase HMGB1 release in exosomes, 54 suggesting that both circulating LPS and exosomal HMGB1 may contribute to viral lethality via increased gut permeability. The roles of LPS and HMGB1 require further investigation as to their influence on the course of different viral infections, 55 as clearly the levels of circulating LPS are far lower than in most preclinical experimental investigations. It also requires investigation as to whether a preexisting increase in gut permeability, as in many preestablished medical conditions or following psychological stress, interact with the pathophysiological changes induced by different viral infections. This has treatment implications, including the use of sodium butyrate, which maintains the gut barrier and attenuates the stress‐induced increase in proinflammatory cytokines from mucosal mast cells that underpin psychological stress‐induced gut permeability. 56
2.9. Autonomic nervous system and alpha 7 nicotinic receptor
The increase in proinflammatory cytokines and the “cytokine storm” that can contribute to viral infection severity and fatality are positively regulated by the activation of the sympathetic nervous system, as shown with influenza virus infection. 57 These authors also showed sympathetic nervous system activation to increase pulmonary pneumonia in a preclinical model, with the inhibition of the sympathetic nervous system increasing survival. 57 Some of the effects of the sympathetic nervous system may be mediated by an increase release of not only catecholamines, but also neuropeptide Y (NPY), the release of which from monocytes also contributes to influenza‐associated fatality. 58
In contrast, the activation of the parasympathetic nervous system following the vagal nerve release of acetylcholine, affords protection against some viral infections via the activation of the alpha 7 acetylcholine nicotinic receptor (α7nAChR), 59 including decreasing macrophage inflammatory responses. 60 It should be noted that the α7nAChR is intimately associated with melatonin, being positively regulated by pineal melatonin over the circadian rhythm, 61 whilst the impact of melatonin in preventing gut permeability under challenge is mediated by its induction of vagal nerve ACh release and subsequent α7nAChR activation. 62 As such, the viral suppression of melatonin will be associated with a decrease in its induction of the α7nAChR and vagal ACh, thereby decreasing the viral‐ and immune‐suppressive activity of vagal activated α7nAChRs. It should also be noted that the α7nAChR is expressed on the mitochondria outer membrane, suggesting more direct impacts on mitochondrial function, including in immune cells and in the regulation of mitochondria Ca2+ influx.63, 64
Overall, shifts in the balance of the sympathetic/parasympathetic nervous systems may be another aspect of viral infections. This is another route whereby the viral regulation of the melatonergic pathway can act on wider systemic regulatory processes in a manner to increase viral severity and decrease host survival.
2.10. Preexisting medical conditions
It is also noteworthy that increased gut dysbiosis/permeability is frequently associated with the preexisting health conditions that increase the risk of fatality from viral infections. Preexisting health conditions that increase fatality risk from influenza and covid‐19 include cardiovascular and lung disorders, diabetes, and cancer. 65 Many of the specific medical conditions that are included within these groups of disorders are linked to an elevation in gut dysbiosis/permeability,66, 67 mitochondrial dysfunction, 68 and circadian dysregulation,69, 70 with melatonin showing utility in their management.71, 72 Ageing, the main risk factor for death from influenza and covid‐19, is highly associated with these pathophysiological changes,73, 74, 75, 76 with melatonin, partly via sirtuin induction as well as circadian and mitochondria regulation, attenuating ageing‐associated processes. 77 Overall, such data would suggest that the pathophysiological changes associated with many viruses overlap with the biological underpinnings of the high‐risk conditions associated with increased risk of virus‐associated fatality.
2.11. Future research
Does melatonin have utility in decreasing symptoms and fatality rates of influenza and covid‐19 viruses? If so, what are the relevant doses? Extrapolating from preclinical data would suggest that doses as high as 500 mg may be necessary to dampen the initial cytokine storm. Such doses are well tolerated in humans, but clearly require investigation in virus‐infected patients.
Does melatonin attenuate the downregulation of Bmal1 by the influenza, and possibly covid‐19, viruses?
Does pineal‐derived melatonin and exogenous melatonin increase the mitochondrial melatonergic pathway in immune cells and other cell types, including during the course of viral infection?
Preclinical data shows that maternal exposure to an AhR ligand can drive alterations in the immune response to viral infection in the offspring. 78 It requires investigation as to whether such transgenerational effects on the viral immune response involve alterations in the mitochondrial melatonergic pathway.
Is the regulation of the NAS/melatonin ratio by the AhR/CYP1B1, CYP2C19, mGluR5, P2Y1 receptor or O‐demethylation relevant to the pathophysiology of different viruses?
Does covid‐19 decrease the Bmal1‐PDC‐acetyl‐CoA‐TCA cycle‐OXPHOS path with consequences that include suppression of the mitochondrial melatonergic pathway and associated changes in sirtuins and mitochondrial antioxdant enzymes?
How important is the induction of gut dysbiosis/permeability to the consequences of the “cytokine storm” at the early stages of severe viral infections?
IL‐18 was formally known as “IFNγ‐inducing factor”, with IFNγ having a significant role in the regulation of immune responses to viral infection. 79 Does NLRP3 inflammasome induction of IL‐18 have a distinct role from NLRP3‐induced IL‐1β in virus‐driven pathophysiology, via their differential regulation of IFNγ?
What is the relevance of elevations in circulating LPS arising from increased gut permeability to proliferation and severity of viral infections, including influenza and covid‐19?
To what extent do variations in the melatonergic pathway and gut dysbiosis/permeability explain the large variance in influenza and covid‐19 symptom severity across the general population?
How relevant are variations in the autonomic nervous system and α7nAChR activation to symptom severity in influenza and covid‐19 viral infections?
2.12. Treatment implications
Given the common viral suppression of melatonin, coupled to melatonin's positive modulation of processes inhibited by most viruses, it is not unreasonable to propose melatonin to have utility in limiting the symptomatology and fatality associated with viral infection, including influenza and covid‐19. Melatonin may also have prophylactic utility, especially in people with preexistent medical conditions associated with suppressed pineal melatonin synthesis.
Given the role of gut microbiome‐derived butyrate and its nutriceutical equivalent, sodium butyrate, in the regulation of mitochondrial function, the melatonergic pathway and HDAC inhibition, does butyrate have a role in pathophysiology and treatment of viral infections?
Are there any additive or synergistic interactions of melatonin and butyrate treatments in the management of viral infections, including in decreasing fatalities from influenza and covid‐19 infections?
3. CONCLUSIONS
Overall, commonly affected processes in viral infections would suggest that the lack of an available vaccine and antiviral, and their impotence in the face of new viral mutations, should not necessarily lead to a management response that is solely dependent upon social isolation in order to decrease human mortality. A number of factors can act to inhibit the key cellular changes upon which viruses act, with melatonin being one such factor. The utility of melatonin is paradoxically under‐appreciated, if not suppressed, by its ready availability, low cost, and very high safety profile. At the time of writing, there has been a significant impact of covid‐19 on global economies. It would seem inappropriate to wait for the pharmaceutical companies to provide a targeted antiviral and vaccine in over a year's time, at which point covid‐19 may well have mutated. As highlighted throughout, viral impacts on human physiology allows for the use of nutriceuticals and pharmaceuticals that may lower infection severity by targeting physiological processes. Melatonin is one such substance. Melatonin is actively inhibited by most viruses, indicating its importance in the regulation of viral infections. A further benefit of melatonin in the management of influenza and covid‐19 arises from its utility in management of the array of preexisting medical conditions associated with viral‐linked fatality. The potential benefits of sodium butyrate in the management of influenza and covid‐19 infections include its immune suppressive and mitochondrial optimization effects as well as its induction of the melatonergic pathway and ability to decrease the gut permeability that is associated with high risk preexisting medical conditions. Overall, there are biomedical, financial, and moral reasons for developing and testing interventions that may immediately limit the impact of viral infections.
ACKNOWLEGEMENTS
Not applicable.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Abbreviations
- AANAT
aralkylamine N‐acetyltransferase
- AhR
aryl hydrocarbon receptor
- ASMT
acetylserotonin methytransferase
- BDNF
brain derived neurotrophic factor
- CRH
corticotrophin releasing hormone
- CYP
cytochrome P450
- Glu
glutamate
- HDAC
histone deacetylase
- HMGB
high‐mobility group box
- HSV
herpes simplex virus
- IDO
indoleamine 2,3‐dioxygenase
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- LPS
lipopolysaccharide
- MCT
monocarboxylate transporters
- mGlur
metabotropic glutamate receptor
- nAChR
nicotinic acetylcholine receptor
- NAS
N‐acetylserotonin
- NLRP
Nod‐like receptor family pyrin domain‐containing
- OXPHOS
oxidative phosphorylation
- P2Y1
purinergic receptor 2Y1
- PDC
pyruvate dehydrogenase complex
- PDK
pyruvate dehydrogenase kinase
- PGC
peroxisome proliferator‐activated receptor‐gamma coactivator
- TCA
tricarboxylic acid
- TCDD
2,3,7,8‐tetrachlorodibenzo‐p‐dioxin
- TDO
tryptophan 2,3‐dioxygenase
- Th
T‐helper
- TLR
toll‐like receptor
- TNF
tumor necrosis factor
Anderson G, Reiter RJ. Melatonin: Roles in influenza, Covid‐19, and other viral infections. Rev Med Virol. 2020;30:e2109 10.1002/rmv.2109
REFERENCES
- 1. Petrova VN, Russell CA. The evolution of seasonal influenza viruses. Nat Rev Microbiol. 2018;16(1):60 10.1038/nrmicro.2017.146. [DOI] [PubMed] [Google Scholar]
- 2. Tan DX, Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation. Melatonin Res. 2020;3(1):120‐43. [Google Scholar]
- 3. Zhou Y, Hou Y, Shen J et al Network‐based repurposing for human coronavirus. MedRxiv 2020.02.03.2020263 (2020). doi: 10.1101/2020.02.03.2020263 [DOI]
- 4. Reiter RJ, Ma Q, Sharma R. Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 2020;3(1):43‐57. 10.32794/mr11250047. [DOI] [Google Scholar]
- 5. Anderson G, Maes M, Markus RP, Rodriguez M. Ebola virus: melatonin as a readily available treatment option. J Med Virol. 2015;87(4):537‐543. 10.1002/jmv.24130. [DOI] [PubMed] [Google Scholar]
- 6. Tan DX, Reiter RJ. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019;2:44‐66. [Google Scholar]
- 7. Anderson G. Integrating pathophysiology in migraine: Role of the gut microbiome and melatonin. Curr Pharm Des. 2019;25(33):3550‐3562. 10.2174/1381612825666190920114611. [DOI] [PubMed] [Google Scholar]
- 8. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33(4):489‐494. 10.1124/dmd.104.002410). [DOI] [PubMed] [Google Scholar]
- 9. Ferreira ZS, Markus RP. Characterisation of P2Y(1)‐like receptor in cultured rat pineal glands. Eur J Pharmacol. 2001;415(2–3):151‐156. 10.1016/s0014-2999(01)00823-8. [DOI] [PubMed] [Google Scholar]
- 10. Mortani Barbosa EJ, Ferreira ZS, Markus RP. Purinergic and noradrenergic cotransmission in the rat pineal gland. Eur J Pharmacol. 2000;401(1):59‐62. 10.1016/s0014-2999(00)00416-7. [DOI] [PubMed] [Google Scholar]
- 11. Souza‐Teodoro LH, Dargenio‐Garcia L, Petrilli‐Lapa CL, et al. Adenosine triphosphate inhibits melatonin synthesis in the rat pineal gland. J Pineal Res. 2016;60(2):242‐249. 10.1111/jpi.12309. [DOI] [PubMed] [Google Scholar]
- 12. Villela D, Atherino VF, Lima Lde S, et al. Modulation of pineal melatonin synthesis by glutamate involves paracrine interactions between pinealocytes and astrocytes through NF‐κB activation. Biomed Res Int. 2013;2013:618432 10.1155/2013/618432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishio S, Yamada H, Craft CM, Moriyama Y. Hydroxyindole‐O‐methyltransferase is another target for L‐glutamate‐evoked inhibition of melatonin synthesis in rat pinealocytes. Brain Res. 1999;850(1–2):73‐78. [DOI] [PubMed] [Google Scholar]
- 14. Anderson G, Reiter RJ. Glioblastoma: Role of mitochondria N‐acetylserotonin/melatonin ratio in mediating effects of miR‐451 and aryl hydrocarbon receptor and in coordinating wider biochemical changes. Int J Tryptophan Res. 2019;12:1178646919855942 10.1177/1178646919855942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson G, Rodriguez M, Reiter RJ. Multiple sclerosis: Melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut‐microbiome‐derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int J Mol Sci. 2019;20(21):E5500 10.3390/ijms20215500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiter RJ, Sharma R, Ma Q, Rosales‐Corral SA, Acuna‐Castroviejo D, Escames G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome aerobic glycolysis, limit tumor growth and reverse insensitivity to chemotherapy. Melatonin Res. 2019;2:105‐119. [Google Scholar]
- 17. Anderson G. Daytime orexin and night‐time melatonin regulation of mitochondria melatonin: roles in circadian oscillations systemically and centrally in breast cancer symptomatology. Melatonin Res. 2019;2(4):1‐8. 10.32794/mr11250037. [DOI] [Google Scholar]
- 18. Bjørklund G, Dadar M, Anderson G, Chirumbolo S, Maes M. Preventive measures to slow down the progression of Parkinson's disease. Pharm Res. In press. [DOI] [PubMed] [Google Scholar]
- 19. Markus RP, Fernandes PA, Kinker GS, da Silveira C‐MS, Marçola M. Immune‐pineal axis—acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br J Pharmacol. 2018;175(16):3239‐3250. 10.1111/bph.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boule LA, Burke CG, Jin GB, Lawrence BP. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep. 2018;8(1):1826 10.1038/s41598-018-20197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franchini AM, Myers JR, Jin GB, Shepherd DM, Lawrence BP. Genome‐wide transcriptional analysis reveals novel AhR targets that regulate dendritic cell function during influenza A virus infection. Immunohorizons. 2019;3(6):219‐235. 10.4049/immunohorizons.1900004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson G, Maes M. Interactions of tryptophan and its catabolites with melatonin and the alpha 7 nicotinic receptor in central nervous system and psychiatric disorders: Role of the aryl hydrocarbon receptor and direct mitochondria regulation. Int J Tryptophan Res. 2017;10:1178646917691738 10.1177/1178646917691738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grunewald ME, Shaban MG, Mackin SR, Fehr AR, Perlman S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3‐dioxygenase‐independent manner, contributing to cytokine modulation and proviral TCDD‐inducible‐PARP expression. J Virol. 2020;94(3):e01743‐19 10.1128/JVI.01743-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurupati RK, Kossenkoff A, Kannan S, et al. The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine. 2017;35(30):3700‐3708. 10.1016/j.vaccine.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 25. Sengupta S, Tang SY, Devine JC, et al. Circadian control of lung inflammation in influenza infection. Nat Commun. 2019;10(1):4107 10.1038/s41467-019-11400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edgar RS, Stangherlin A, Nagy AD, et al. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A. 2016;113(36):10085‐10090. 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Z, Hunter L, Wu G, et al. Genome‐wide effect of pulmonary airway epithelial cell‐specific Bmal1 deletion. FASEB J. 2019;33(5):6226‐6238. 10.1096/fj.201801682R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundar IK, Ahmad T, Yao H, et al. Influenza A virus‐dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci Rep. 2015;4:9927 10.1038/srep09927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pontes GN, Cardoso EC, Carneiro‐Sampaio MM, Markus RP. Pineal melatonin and the innate immune response: the TNF‐alpha increase after cesarean section suppresses nocturnal melatonin production. J Pineal Res. 2007;43(4):365‐371. [DOI] [PubMed] [Google Scholar]
- 30. Kido H, Indalao IL, Kim H, Kimoto T, Sakai S, Takahashi E. Energy metabolic disorder is a major risk factor in severe influenza virus infection: Proposals for new therapeutic options based on animal model experiments. Respir Investig. 2016;54(5):312‐319. 10.1016/j.resinv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 31. Indalao IL, Sawabuchi T, Takahashi E, Kido H. IL‐1β is a key cytokine that induces trypsin upregulation in the influenza virus‐cytokine‐trypsin cycle. Arch Virol. 2017;162(1):201‐211. 10.1007/s00705-016-3093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang W, Li G, Wu D, et al. Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin‐1β secretion. Nat Commun. 2018;9(1):106 10.1038/s41467-017-02645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao C, Zhao W. NLRP3 inflammasome‐A key player in antiviral responses. Front Immunol. 2020;11:211 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu HM, Zhao CC, Xie QM, Xu J, Fei GH. TLR2‐melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front Immunol. 2020;11:172 10.3389/fimmu.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson G, Ojala J. Alzheimer's and seizures: interleukin‐18, indoleamine 2,3‐dioxygenase and quinolinic Acid. Int J Tryptophan Res. 2010;3:169‐173. 10.4137/IJTR.S4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL‐1β/IL‐6/CRP and IL‐18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12(12):e1005973 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foteinou PT, Venkataraman A, Francey LJ, Anafi RC, Hogenesch JB, Doyle FJ 3rd. Computational and experimental insights into the circadian effects of SIRT1. Proc Natl Acad Sci U S A. 2018;115(45):11643‐11648. 10.1073/pnas.1803410115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elesela S, Morris SB, Narayanan S, Kumar S, Lombard DB, Lukacs NW. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog. 2020;16(2):e1008319 10.1371/journal.ppat.1008319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koyuncu E, Budayeva HG, Miteva YV, et al. Sirtuins are evolutionarily conserved viral restriction factors. mBio. 2014;5(6):e02249‐14 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reiter RJ, Tan DX, Rosales‐Corral S, Galano A, Jou MJ, Acuna‐Castroviejo D. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J Mol Sci. 2018;19(8):E2439 10.3390/ijms19082439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenberger CM, Podyminogin RL, Navarro G, et al. miR‐451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189(12):5965‐5975. 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buggele WA, Johnson KE, Horvath CM. Influenza A virus infection of human respiratory cells induces primary microRNA expression. J Biol Chem. 2012;287(37):31027‐31040. 10.1074/jbc.M112.387670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang X, Jia Y, Wang X, et al. MiR‐375 has contrasting effects on newcastle disease virus growth depending on the target gene. Int J Biol Sci. 2019;15(1):44‐57. 10.7150/ijbs.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson G, Maes M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr Top Med Chem. 2020;20 10.2174/1568026620666200131094445. [DOI] [PubMed] [Google Scholar]
- 45. Picchianti‐Diamanti A, Rosado MM, D'Amelio R. Infectious agents and inflammation: the role of microbiota in autoimmune arthritis. Front Microbiol. 2018;8:2696 10.3389/fmicb.2017.02696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory viral infection‐induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin‐releasing hormone increase intestinal permeability in humans by a mast cell‐dependent mechanism. Gut. 2014;63(8):1293‐1299. 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 48. Anderson G. Gut dysbiosis dysregulates central and systemic homeostasis via decreased melatonin and suboptimal mitochondria functioning: pathoetiological and pathophysiological implications. Melatonin Res. 2019;2(2):70‐85. 10.32794/mr11250022. [DOI] [Google Scholar]
- 49. Jin CJ, Engstler AJ, Sellmann C, et al. Sodium butyrate protects mice from the development of the early signs of non‐alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br J Nutr. 2016;23:1‐12. [DOI] [PubMed] [Google Scholar]
- 50. Yagi K, Ishii M, Namkoong H, et al. Histone deacetylase inhibition protects mice against lethal postinfluenza pneumococcal infection. Crit Care Med. 2016;44(10):e980‐e987. 10.1097/CCM.0000000000001821. [DOI] [PubMed] [Google Scholar]
- 51. Shi Z, Gewirtz AT. Together forever: bacterial‐viral interactions in infection and immunity. Viruses. 2018;10(3):E122 10.3390/v10030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perrin‐Cocon L, Aublin‐Gex A, Sestito SE, et al. TLR4 antagonist FP7 inhibits LPS‐induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci Rep. 2017;7:40791 10.1038/srep40791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koch RM, Diavatopoulos DA, Ferwerda G, Pickkers P, de Jonge MI, Kox M. The endotoxin‐induced pulmonary inflammatory response is enhanced during the acute phase of influenza infection. Intensive Care Med Exp. 2018;6(1):15 10.1186/s40635-018-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Y, Sun H, Bai Y, Zhi F. Gut dysbiosis‐derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem Biophys Res Commun. 2019;509(3):767‐772. 10.1016/j.bbrc.2018.12.180. [DOI] [PubMed] [Google Scholar]
- 55. Bandoro C, Runstadler JA. Bacterial lipopolysaccharide destabilizes influenza viruses. mSphere. 2017;2(5):e00267‐17 10.1128/mSphere.00267-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang CC, Wu H, Lin FH, et al. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018;24(1):40‐46. 10.1177/1753425917741970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grebe KM, Takeda K, Hickman HD, et al. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol. 2010;184(2):540‐544. 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fujiwara S, Hoshizaki M, Ichida Y, et al. Pulmonary phagocyte‐derived NPY controls the pathology of severe influenza virus infection. Nat Microbiol. 2019;4(2):258‐268. 10.1038/s41564-018-0289-1. [DOI] [PubMed] [Google Scholar]
- 59. Li‐Sha G, Jing‐Lin Z, Li L, Guang‐Yi C, Xiao‐Wei L, Yue‐Chun L. Nicotine inhibits the production of proinflammatory cytokines of mice infected with coxsackievirus B3. Life Sci. 2016;148:9‐16. 10.1016/j.lfs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 60. Cui WY, Zhao S, Polanowska‐Grabowska R, et al. Identification and characterization of poly(I:C)‐induced molecular responses attenuated by nicotine in mouse macrophages. Mol Pharmacol. 2013;83(1):61‐72. 10.1124/mol.112.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Markus RP, Silva CL, Franco DG, Barbosa EM Jr, Ferreira ZS. Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol Ther. 2010;126(3):251‐262. 10.1016/j.pharmthera.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 62. Sommansson A, Nylander O, Sjöblom M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor‐dependent pathway in rats in vivo. J Pineal Res. 2013;54(3):282‐291. 10.1111/jpi.12013. [DOI] [PubMed] [Google Scholar]
- 63. Anderson G. Linking the biological underpinnings of depression: role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt C):255‐266. 10.1016/j.pnpbp.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 64. Gergalova G, Lykhmus O, Kalashnyk O, et al. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS One. 2012;7(2):e31361 10.1371/journal.pone.0031361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tekin S, Keske S, Alan S, et al. Predictors of fatality in influenza A virus subtype infections among inpatients in the 2015‐2016 season. Int J Infect Dis. 2019;81:6‐9. 10.1016/j.ijid.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 66. Noval Rivas M, Wakita D, Franklin MK, et al. Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity. 2019;51(3):508‐521.e6. 10.1016/j.immuni.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bindels LB, Neyrinck AM, Loumaye A, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018. Apr 6;9(26):18224‐18238. 10.18632/oncotarget.24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Geto Z, Molla MD, Challa F, Belay Y, Getahun T. Mitochondrial dynamic dysfunction as a main triggering factor for inflammation associated chronic non‐communicable diseases. J Inflamm Res. 2020;13:97‐107. 10.2147/JIR.S232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chellappa SL, Vujovic N, Williams JS, Scheer FAJL. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019;30(10):767‐779. 10.1016/j.tem.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A. Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer. Ann Am Thorac Soc. 2016;13(7):1144‐1154. 10.1513/AnnalsATS.201602-125FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anderson G, Mazzoccoli G. Left ventricular hypertrophy: roles of mitochondria CYP1B1 and melatonergic pathways in co‐ordinating wider pathophysiology. Int J Mol Sci. 2019;20(16):E4068 10.3390/ijms20164068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hosseinzadeh A, Javad‐Moosavi SA, Reiter RJ, Hemati K, Ghaznavi H, Mehrzadi S. Idiopathic pulmonary fibrosis (IPF) signaling pathways and protective roles of melatonin. Life Sci. May 2018;201:17‐29. 10.1016/j.lfs.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 73. Mok JX, Ooi JH, Ng KY, Koh RY, Chye SM. A new prospective on the role of melatonin in diabetes and its complications. Horm Mol Biol Clin Investig. 2019;40(1). 10.1515/hmbci-2019-0036. [DOI] [PubMed] [Google Scholar]
- 74. Liu A, Lv H, Wang H, Yang H, Li Y, Qian J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J Gerontol A Biol Sci Med Sci. 2020;glz263 10.1093/gerona/glz263. [DOI] [PubMed] [Google Scholar]
- 75. Zole E, Ranka R. Mitochondria, its DNA and telomeres in ageing and human population. Biogerontology. 2018;19(3–4):189‐208. 10.1007/s10522-018-9748-6. [DOI] [PubMed] [Google Scholar]
- 76. Froy O. Circadian rhythms, nutrition and implications for longevity in urban environments. Proc Nutr Soc. 2018;77(3):216‐222. 10.1017/S0029665117003962. [DOI] [PubMed] [Google Scholar]
- 77. Reiter RJ, Tan DX, Rosales‐Corral S, Galano A, Zhou XJ, Xu B. Mitochondria: central organelles for melatonin's antioxidant and anti‐aging actions. Molecules. 2018;23(2):E509 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Post CM, Boule LA, Burke CG, O'Dell CT, Winans B, Lawrence BP. The ancestral environment shapes antiviral CD8+ T cell responses across generations. iScience. 2019;20:168‐183. 10.1016/j.isci.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee AJ, Ashkar AA. The dual nature of type I and type II interferons. Front Immunol. 2018;9:2061 10.3389/fimmu.2018.02061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


